Abstract
Rationale
The α2a-noradrenergic agonist guanfacine decreases stress-induced smoking in female, but not male, human smokers. It is not known whether these effects are due to effects on mood regulation and/or result from nicotinic-cholinergic interactions.
Objectives
To determine whether there are sex-differences in the effect of guanfacine in tests of anxiolytic- and antidepressant-efficacy in mice at baseline and in a hypercholinergic model of depression induced by the acetylcholinesterase inhibitor physostigmine.
Methods
The effects of guanfacine were measured in the light/dark box, tail suspension and the forced swim test in female and male C57BL/6J mice. In parallel, electrophysiological properties were evaluated in prefrontal cortex, a critical brain region involved in stress responses. c-fos immunoreactivity was measured in other brain regions known to regulate mood.
Results
Despite a baseline sex difference in behavior in the forced swim test (female mice were more immobile), guanfacine had similar, dose-dependent, antidepressant-like effects in mice of both sexes (optimal dose: 0.15 mg/kg). An antidepressant-like effect of guanfacine was also observed following pre-treatment with physostigmine. A sex difference in the paired-pulse ratio in PFC (male: 1.4; female, 2.1) was observed at baseline that was normalized by guanfacine. Other brain regions areas involved in cholinergic control of depression-like behaviors, including basolateral amygdala and lateral septum, showed sex-specific changes in c-fos expression.
Conclusions
Guanfacine has a robust antidepressant-like effect and can reverse a depression-like state induced by increased ACh signaling. These data suggest that different brain areas are recruited in female and male mice, despite similar behavioral responses to guanfacine.
Keywords: norepinephrine, sex difference, depression, amygdala, prefrontal cortex, mouse models
INTRODUCTION
Noradrenaline/norepinephine (NE) is one of the major neurotransmitters involved in arousal and stress reactivity, and can mediate behavioral responses to stressful stimuli. It is therefore not surprising that NE abnormalities are implicated in several psychiatric disorders (Glavin 1985). Noradrenergic reuptake inhibitors are a class of antidepressant medications that are effective on their own or when combined with other antidepressant treatments (Dell’Osso et al. 2011; Sepede et al. 2012). Recent studies have also suggested that NE plays a role in addiction and more specifically, in withdrawal-induced anxiety, a critical element underlying relapse (Aston-Jones and Kalivas 2008). Thus, given the fact that withdrawal from drugs of abuse induces stress and increases NE signaling (Gold 2015; Sofuoglu et al. 2014), it was hypothesized that noradrenergic agents such as guanfacine (a selective α2A receptor agonist) could be useful for the treatment of opiate addiction. Due to its action at α2A autoreceptors, it is reasonable to expect that guanfacine could decrease NE tone through negative feedback which could help relieve some of the negative mood symptoms induced by withdrawal (Fox and Sinha 2014; Fox et al. 2014). Guanfacine can also be helpful for smoking cessation (McKee et al. 2015), potentially because stress is a critical trigger for relapse. Neurobiologically, these effects have been attributed to the ability of guanfacine to influence cognitive control of emotion through regulation of activity in the prefrontal cortex (PFC) during stressful situations, including the nicotine withdrawal that occurs following smoking cessation (Hains et al. 2015; McKee et al. 2015; Schulz et al. 2013).
The cholinergic system also contributes to stress reactivity and emotionality, as well as major depressive disorder (Dilsaver 1986; Janowsky et al. 1972). Increased cholinergic tone induced by agents such as the cholinesterase inhibitor physostigmine can increase anxiety and depressive symptoms in individuals with mood disorders, as well as in control subjects (Janowsky and Overstreet 1990b; Risch et al. 1980). More recent imaging studies have shown that patients who were actively depressed had higher brain acetylcholine levels, as evidenced by competition for tracer binding to high affinity nicotinic acetylcholine receptors (Esterlis et al. 2013a; Hannestad et al. 2013; Saricicek et al. 2012). Interestingly, patients with a history of depression who were not actively depressed showed an intermediate phenotype. These studies demonstrate that excessive activation of the cholinergic system can increase stress-related behaviors, and may therefore be important for stress-induced relapse to smoking. It also indicates that cholinergic dysregulation induced by smoking and nicotine withdrawal could alter mood through alteration of nicotinic receptor signaling.
Both NE and acetylcholine (ACh) regulate the amygdala-prefrontal cortex circuit and other critical brain regions involved in behaviors related to mood and anxiety. These systems can interact (Carrier and Bishop 1972; Devore and Linster 2012) and control basic physiological functions (muscle control, sleep, blood pressure regulation etc.) and recent studies have investigated the connection between central ACh and NE, particularly in the context of mood and addiction. For instance, stimulation of nAChRs can control the release of ACh and NE from the habenulo-interpenducular pathway (Beiranvand et al. 2014), which is critical for withdrawal-related mood dysregulation. Likewise, in the hippocampus, a region implicated in ACh-dependent mood dysregulation (Mineur et al. 2013), it was established that cholinergic terminals could be regulated by NE and ACh (Milusheva et al. 1994). Together, these results highlight the tight connection that exists between central ACh and NE and how modulation of either system can contribute to mood regulation.
Recent clinical studies have shown that guanfacine can decrease stress-induced cigarette smoking in women, but not men, although it can lead to smoking cessation in both sexes (McKee et al. 2015; Verplaetse et al. 2015). The neurobiological substrates of these behavioral effects remain unclear, however. It is not known whether the effects of guanfacine on smoking cessation are due to normalization of stress symptoms induced by withdrawal or whether guanfacine can counteract the effects of cholinergic dysregulation induced by nicotine withdrawal (Wilkinson et al. 2013).
We therefore evaluated the effects of guanfacine in male and female mice in well characterized assays of antidepressant and anxiolytic efficacy. We also determined whether guanfacine could reverse the effects of increased cholinergic signaling, a validated rodent model of depression induced by hypercholinergic tone (Mineur et al, 2013). Next, we evaluated the electrophysiological responses of PFC neurons following guanfacine treatment to determine whether this might correlate with changes in behavioral tasks. Finally, we used c-fos immunoreactivity as a marker of neuronal activity to identify any brain areas that could be responsible for NE-ACh interactions in female and male mice.
METHODS
Animals
Male and female C57BL/6J mice (10–12 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). Upon arrival, mice were housed 5/cage under standard conditions (21± 2°C, 12:12 light-dark cycle, lights on at 7.00 a.m.). Food and water were available ad libitum. Mice were acclimated to the facility for at least 7 days before the beginning of any experiments. Mice were randomly assigned to one treatment group per cage (10 mice/group at the beginning of experiments). A marker was used to color the tail of each mouse the day before the start of drug administration. For chronic treatments, mice were earmarked. All behavioral tests were performed during the light period to avoid re-entrainment when animals were transferred to the procedure room. Since this procedure results in robust antidepressant-like effects, we maintained the testing schedule for consistency with previously published studies. Mice were allowed to habituate to the procedure room for 30 min before any behavioral testing took place.
All tests took place between 9.00 a.m. and 5 p.m. If animals were tested in more than one paradigm, at least 48 hr was allowed between each test. All studies were carried out in strict accordance with the standards and procedures for animal care and use established by the National Institutes of Health and were approved by the Yale University Animal Care and Use Committee.
Drug treatments
Guanfacine, mecamylamine and physostigmine were purchased from Sigma Aldrich. Drugs were dissolved in PBS (0.1 M, pH = 7.4) and stock solutions were frozen until use. For injections, drugs were diluted such that the final solution was injected at 10 ml/kg of body weight. For acute experiments, drugs were injected 30 min before behavioral testing. For chronic administration, mice receive one injection once a day for 15 days. No injection was given on the day of behavioral testing.
Behavioral assays
Details and timeline of behavioral experiments are summarized in Table 1.
Table 1.
| Experiment | Treatment | Dose(s) of guanfacine | Regimen | Assay performed | Other assays |
|---|---|---|---|---|---|
| 1 | Guanfacine | 0 to 0.3 mg/kg | Acute (30 min before test) | Forced swim | Body temperature |
| 2 | Guanfacine | 0 to 0.3 mg/kg | Chronic (once daily, 15 days) | Forced swim | Locomotion |
| 3 | Guanfacine + Physostigmine | 0.15 mg/kg | Acute, separate injections | Light/Dark, Forced swim | c-fos immunoreactivity |
| 4 | Guanfacine | 0.15 mg/kg | Chronic (once daily, 15 days) | Electrophysiological recording of PFC neurons |
Light/dark test of anxiety-like behavior: testing took place in a two-compartment box connected by an opening, with one covered dark compartment and a second light compartment illuminated by a 60W bulb. Mice were placed into the light compartment facing the wall and the time to cross into the dark compartment was recorded. After the first cross, time in the dark side and the number of transitions between compartments was recorded for 6 min. Time in each compartment was scored with a stop watch in real-time by the experimenter.
Forced swim test: Each mouse was placed in a 4-liter beaker filled with water maintained at room temperature for 15 min. 24 hours later, the same procedure was repeated for 5 min. The time spent immobile was recorded over the 5 min of the second test. Mice were tested one cage at a time and all behavior was filmed. Videos were later scored manually by a trained observer. Some videos were analyzed twice to ensure test-retest reliability of scoring. The depth of the water was 15 cm. Water was changed between subjects to remove any animal waste.
Locomotor activity: mice were placed into a clean cage (48 × 22 × 18 cm) with no bedding for 30 min in a brightly lit room. Beam breaks (Optomax) were used as an index of locomotor activity.
Body temperature: Body temperature was measured using an anal probe 30 min following an acute injection of guanfacine after all behavioral assays were completed.
Brain slice and electrophysiology
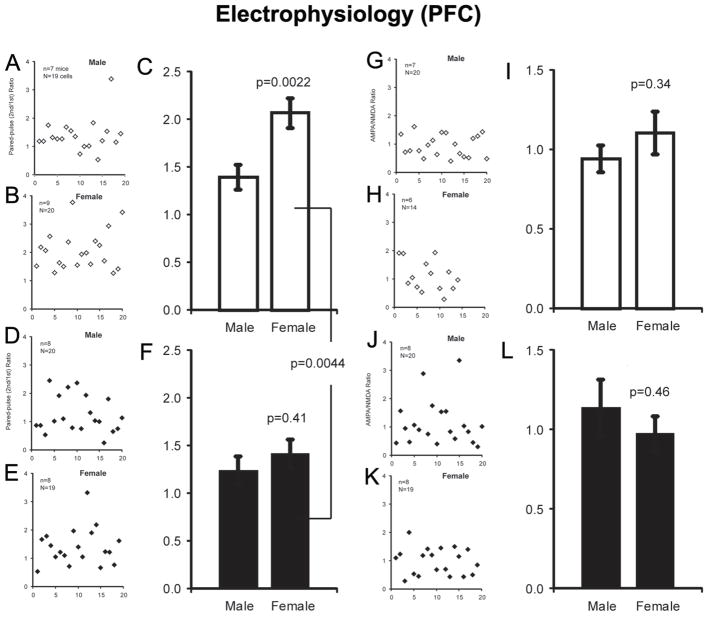
Mouse PFC brain slices were obtained as described previously (Zhou and Antic 2012). Briefly, brains were harvested following rapid decapitation and immediately immersed in ice cold, oxygenated artificial cerebrospinal fluid (ACSF). ACSF contained (in mM): 125 NaCl, 26 NaHCO3, 10 glucose, 2.3 KCl, 1.26 KH2PO4, 2 CaCl2 and 1 MgSO4, pH 7.4. Coronal slices (300 μm) were cut from frontal lobes on a vibratome. Whole-cell recordings were made from visually identified Layer 5 pyramidal neurons within the ventral medial PFC, including prelimbic and infralimbic cortex, in the presence of picrotoxin (100 μM) and Tetraethylammonium chloride (5 mM), at room temperature. Micropipettes with a tip resistance of 4–7 MΩ were made of borosilicate glass using a Sutter micropipette puller (P-97) and back filled with an intracellular solution containing (in mM): 135 Cs-gluconate, 2 MgCl2, 10 Hepes, 1.1 EGTA, 2 Mg-ATP, 10 Na2-phosphocreatine, 0.3 Na2-GTP (pH 7.3). Electrical signals were amplified with Multiclamp 700B and digitized with Digidata 1440A (Molecular Devices, Sunnyvale, CA). Both input resistance and series resistance were monitored throughout the experiments. Only those recordings with a stable series resistance (≤30 MΩ) and input resistance were accepted. To generate evoked EPSCs, a bipolar electrode was placed laterally 50–100 μm to the recorded cell for stimulating presynaptic axon terminals. All data were sampled at 5 KHz and filtered at 500 Hz for analysis using Clampfit 10.3. The amplitudes of AMPA currents were measured at peak at −70 mV; the amplitudes of NMDA currents were measured at 50 ms after peak at +40 mV, as shown in Fig. 4H,I. For drug treatment, two groups of mice, male/female, at age of 25 day, were treated with either guanfacine (0.15 mg/kg i.p. daily) or PBS for 14 days prior to electrophysiology experiments.
Histology and c-fos immunohistochemistry
Subjects selected for immunostaining were chosen randomly, independent of behavioral performance. Following behavioral testing, mice were irreversibly anesthetized with 15% pentobarbital and were perfused intracardially with PBS, followed by 4% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed for 24 hr in 4% PFA at 4°C, then transferred to 30% sucrose for cryoprotection and stored at 4°C. Fixed brain tissue was sliced at 40 μm using a sliding microtome and brain slices were stored in PBS with 0.2% sodium azide.
Brain slices were transferred to a 12–well plate, washed 5 times with PBS and placed in blocking solution (3% normal goat serum/0.3% Triton/PBS). Slices were incubated with a rabbit polyclonal anti-c-fos antibody (1:500; sc-52, Santa Cruz Biotechnology, Santa Cruz, CA) for 48 hr at 4 °C, washed again with PBS and incubated with a goat secondary anti-rabbit antibody conjugated to a red fluorophore (1:2000; Alexa Fluor594, Abcam, Cambridge, MA). Slices were then mounted onto Superfrost microscope slides and coverslipped.
Micrographs were taken with an inverted fluorescent microscope (Apotome, Zeiss) and positive cells were counted in brain areas defined in Image J (NIH). Data are expressed as the number of counted cells per unit area, standardized to the measured surface.
Statistical analyses
In dose-response experiments, comparison of mean values between treatment groups was evaluated using two-way analysis of variance (ANOVA) with “dose” and “sex” as between-subject factors. In experiments using physostigmine and guanfacine, three-way ANOVA was used with “physostigmine”, “guanfacine”, and “sex” as between-subject factors. Post-hoc analyses were used when appropriate with Bonferonni corrections. P was set at 0.05. Electrophysisological data were analyzed between sex for each treatment group, or between treatment for each sex, by Student’s t-test.
RESULTS
Acute guanfacine administration results in antidepressant-like effects in female and male mice in the forced swim test
There was an overall decrease in immobility in the forced swim test following guanfacine (Mineur et al. 2007) (p<0.0001; Fig. 1A). Further analyses also identified a main effect of sex in the forced swim test (F1, 128=30.23, p<0.0001), although there was no interaction between dose and sex, indicating that guanfacine treatment had a similar antidepressant-like effect in female and male mice. Post-hoc analysis showed that only 0.15 mg/kg guanfacine decreased time spent immobile significantly in female (p=0.016) and male (p=0.006) mice following acute treatment in the forced swim test.
Figure 1.
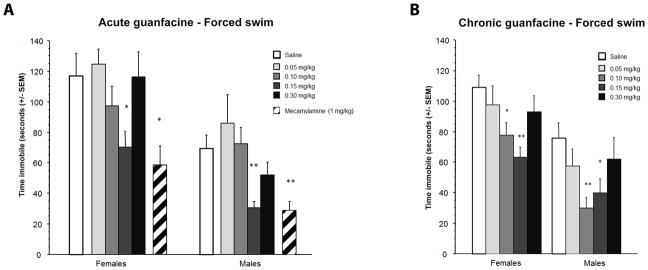
Effect of acute (A) and chronic (B) guanfacine administration in the forced swim test of antidepressant efficacy. Data represent time spent immobile and are expressed in seconds +/− SEM. N = 10 per treatment group. * p < 0.05; ** p < 0.01 compared to the Saline control group.
Mecamylamine, a non-selective nicotinic antagonist that has validated antidepressant-like effects in mice (Mineur et al. 2007; Rabenstein et al. 2006) was used as a positive control in this paradigm and significantly decreased time spent immobile in female (p=0.003) and male (p=0.004) mice.
Chronic guanfacine administration has antidepressant-like effects in female and male mice in the forced swim test
As observed with acute guanfacine treatment, there was an overall decrease in time spent immobile in the forced swim test induced by chronic guanfacine administration, with a significant dose (F4, 89=6.07, p=0.0002) and sex (F1, 89=30.58, p<0.0001) effect, but no interaction between dose and sex in time spent immobile (Fig. 1B). Posthoc analyses showed that both 0.1 mg/kg (female mice, p=0.022; male mice, p=0.003) and 0.15 mg/kg (female mice p=0.016; male mice, p=0.019) guanfacine significantly reduced immobility following chronic administration.
Effect of acute guanfacine administration on locomotor activity and body temperature
There was a significant dose-dependent effect of acute guanfacine administration on locomotor activity in both male and female mice that was inversely correlated with the dose of guanfacine (F(4, 70)=6.44, p=0.0002; Fig. 2A). This effect was contrary to the decrease in immobility at these doses in the forced swim test.
Figure 2.
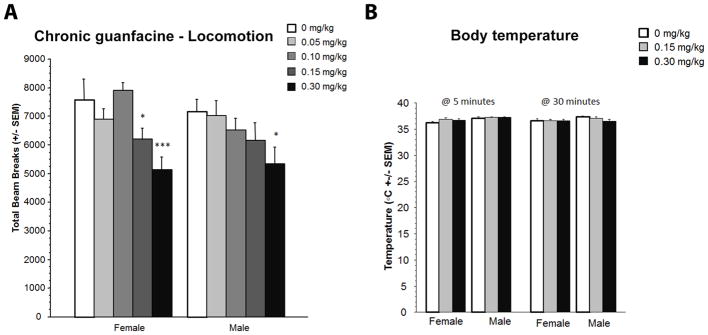
Effects of chronic guanfacine administration on locomotor activity (A). Data are expressed as number of beam breaks +/− SEM. (B) Body temperature 5 and 30 min following guanfacine injection. N = 10 per treatment group. * p < 0.05; *** p < 0.001 compared to the Saline control group.
In the current study, guanfacine had no effects on body temperature 5 min or 30 min after administration of the 0.15 or 0.3 mg/kg dose (all p>0.05, Fig. 2B), which is consistent with published studies suggesting that changes in body temperature only occur at later time points and higher doses of the drug (greater than 0.5 mg/kg; Sillence et al, 1992).
Acute guanfacine administration can reverse the behavioral effects of physostigmine in assays of anxiety- and depression-like behavior
Administration of the acetylcholinesterase antagonist physostigmine can induce anxiety- and depression-like behaviors in mice (Mineur et al, 2013) and healthy humans (Risch et al, 1981), that mimic a hypercholinergic state observed in depressed human subjects (Saricicek et al, 2012). To determine whether NE-ACh interactions can alter stress-related behaviors in a sex-dependent manner, we determined whether guanfacine could reverse the effects of physostigmine administration in male and female mice in a dual-injection paradigm. In the light/dark test of anxiety, two-way ANOVA demonstrated an interaction between physostigmine and guanfacine treatment (F(1, 72)=6.646, p=0.0120; Fig. 3A). There was no overall sex difference, nor a sex by treatment interaction. As predicted, post-hoc analyses showed that physostigmine significantly increased time spent in the dark compartment (p=0.0005), consistent with an increase in anxiety-like behavior. Acute guanfacine administration completely reversed the effects of physostigmine, suggesting that altering NE signaling could reverse the effects of cholinergic hyperactivity.
Figure 3.
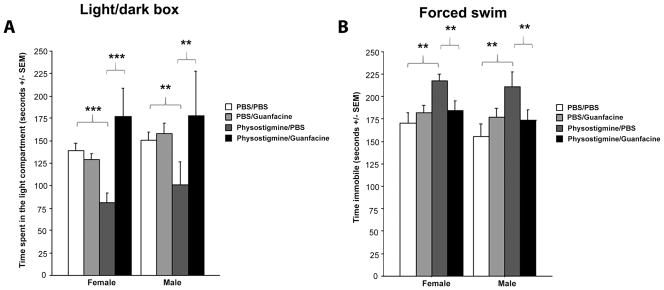
Effect of guanfacine in the light/dark test of anxiety-like behavior following pre-treatment with physostigmine (A) Data represent time spent in the lit side. Time spent immobile in male and female mice in the forced swim test (B). Data are expressed in seconds +/− SEM. N = 10 per treatment group. ** p < 0.01; *** p < 0.001.
In the forced swim test, there was a similar interaction between physostigmine and guanfacine administration (F(1, 72)=9.675, p=0.0027) with no significant effect of sex or sex by treatment interactions. Post-hoc analyses identified a significant decrease in time spent immobile following guanfacine administration in physostigmine-treated mice (p=0.004). In this experiment guanfacine did not induce a significant behavioral effect on its own, likely because the double injection paradigm decreased baseline immobility and appeared to lead to a floor effect, as we have observed previously with the antidepressant fluoxetine (Mineur et al. 2013).
Prefrontal cortical Layer 5 pyramidal neurons display a higher paired-pulse ratio in slices from female mice that is normalized following chronic guanfacine treatiment
To determine whether effects of guanfacine on PFC activity might underlie its anxiolytic or antidepressant-like properties, we evaluated whether chronic, in vivo guanfacine administration could alter the electrophysiological properties of principle neurons in PFC. Paired-pulse facilitation is a form of short-term synaptic plasticity, which reflects the release probability of the presynaptic cell. We began by measuring baseline PPF in PFC Layer 5 pyramidal neurons in slices from male and female mice. We successively evoked excitatory post-synaptic currents (EPSC) at a 50 msec inter-pulse interval, and calculated the paired-pulse ratio in postsynaptic medial PFC Layer 5 pyramidal neurons. Surprisingly, in naïve, untreated animals, neurons in brain slices from female mice displayed a significantly larger paired-pulse ratio than neurons in brain slices from male mice (p=0.0022; Fig. 4A–C). Following 14 days of guanfacine administration in vivo, the paired-pulse ratio was significantly reduced in slices from female mice compared to vehicle treatment (p=0.0044). No change in the paired–pulse ratio was observed in PFC neurons is slices from male mice treated chronically with guanfacine, thereby eliminating the sex difference in paired-pulse ratio in PFC Layer 5 pyramidal neurons observed at baseline (p=0.41; Fig. 4D–F). We also calculated AMPA/NMDA ratio from the same population of cells. No significant sex differences or effects of chronic guanfacine administration were observed in the AMPA/NMDA ratio (Fig. 4G–L), suggesting that guanfacine had presynaptic, rather than postsynaptic, effects on synaptic physiology in Layer 5 pyramidal neurons from female mice.
Figure 4.
Effects of guanfacine treatment on paired-pulse ratio. (A and B), individual paired-pulse ratios calculated in neurons recorded in PFC slices from PBS-treated male and female mice. (C), mean values of paired-pulse ratio in neurons recorded in PFC slices from PBS-treated male and female mice. (D and E), individual paired-pulse ratios calculated following chronic guanfacine (14 days, 0.15 mg/kg) administration in neurons recorded in PFC slices from male and female mice. (F), mean values of paired-pulse ratio in neurons recorded in PFC slices from PBS-treated male and female mice. (G and H), individual AMPA/NMDA ratios calculated in neurons recorded in PFC slices from PBS-treated male and female mice. (I), mean values of AMPA/NMDA ratio in neurons recorded in PFC slices from PBS-treated male and female mice. (J and K), individual AMPA/NMDA ratios calculated in neurons recorded in PFC slices from guanfacine-treated male and female mice. (L), mean values of AMPA/NMDA ratio in neurons recorded in PFC slices from guanfacine-treated male and female mice.
Guanfacine administration leads to sex differences in c-fos expression in brain areas involved in stress-related behaviors
Since synaptic properties of PFC neurons were not reflective of behavioral effects of guanfacine, we evaluated c-fos immunoreactivity in several brain regions related to stress reactivity in a subset of female and male mice following vehicle, physostigmine and/or guanfacine administration to identify other brain areas that might be involved in the anxiolytic- and antidepressant-like effects of guanfacine and it ability to attenuate the effects of cholinergic hyperactivity. Surprisingly, there were significant sex differences in c-fos reactivity in some of these brain areas, despite similar behavioral responses to these drug treatments. For example, there was an overall effect of physostigmine administration on c-fos immunoreactivity in the basolateral amygdala (BLA; F(1, 38)=13.23, p=0.0008) with a trend for an effect of sex (F(1, 38)=3.58, p=0.06), and a sex*physostigmine*guanfacine interaction (F(1, 38)=17.98, p=0.0001). These data show that the effects of guanfacine plus physostigmine on neuronal activity in the BLA was significantly different between male and female mice (Fig. 5). Post-hoc analysis indicated that guanfacine had no effect on its own in female mice, while physostigmine decreased c-fos expression by nearly 50% (p<0.001). The effect of physostigmine on c-fos immunoreactivity in female BLA was reversed to baseline by guanfacine administration, mirroring the behavioral effects in the forced swim test. Conversely, in male mice, the combination of physostigmine and guanfacine induced a significant decrease in c-fos immunoreactivity (p<0.001), which was not concordant with the behavioral effects of these drugs in the forced swim test in male mice. In contrast, there were no sex differences in c-fos immunoreactivity in the lateral septum, where there was an overall increase in c-fos expression in response to guanfacine injection (F(1, 24)=11.17, p=0.0027), but no interaction with physostigmine pre-treatment and/or sex (Fig. 5), suggesting that increased ACh activity in the lateral septum could underlie the depression-like effects of physostigmine, and the ability of guanfacine to decrease neuronal activity in this brain could be critical for its anxiolytic- and antidepressant-like effects.
Figure 5.
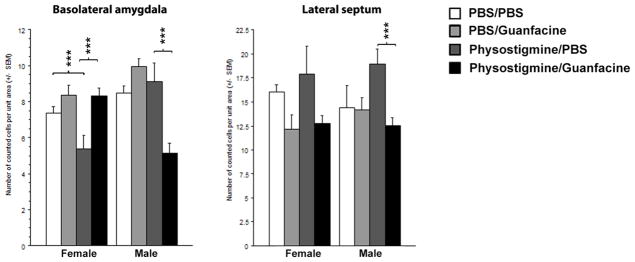
Total number of c-fos positive cells counted in brain sections from male and female mice treated with physostigmine and/or guanfacine. Data represent the total number of counted cells normalized to area unit +/− SEM, in the basolateral amygdala (BLA) and lateral septum (LTS). Inset represents an example of c-fos immunostaining in BLA from a male mouse treated with Saline (A) or Guanfacine (0.15 mg/kg) (B). N = 4 to 6 animals per treatment group.
We also examined c-fos immunoreactivity in a number of other brain areas involved in stress-related behaviors (not shown). There were no significant effects of treatment or sex on c-fos immunoreactivity in the medial septum. In the PFC, there was an overall sex effect (F(1, 14)=6.48, p=0.048) and a marginal sex*physostigmine*guanfacine interaction (F(1, 14=4.07, p=0.06). The overall sex effect is mostly due to lower c-fos expression in the PFC across treatment groups in male compared to female mice, which could be consistent with the sex difference observed in the paired-pulse ratio in PFC. Post-hoc analyses did not yield significance. There was an overall increase of c-fos expression in the paraventricular nucleus of the hypothalamus (PVN) following physostigmine administration (F(1, 30)=13.08, p=0.001). Guanfacine had no effect on its own (F<1), although a sex difference was observed (F(1, 30)=5.08, p=0.03). Post-hoc analyses indicated that only female mice treated with physostigmine in combination with guanfacine had a significant increase in c-fos expression in the PVN (p<0.0001) compared to the other treatment groups. Taken together, these data do not provide a single, clear target for the anxiolytic- and antidepressant-like effects of guanfacine, although they point to the lateral septum as a potential site for ACh-NE interactions in these behaviors, and identify sex differences in brain activity that are not reflected in the behaviors measured here.
DISCUSSION
Recent clinical studies have shown that guanfacine, an α2A receptor agonist, can help human subjects quit smoking (McKee et al, 2015). Furthermore, studies have also emphasized the value of targeting the central noradrenergic system as a way to improve the success rate for quitting smoking, particularly through a diminution of withdrawal-induced anxiety (Aston-Jones and Kalivas 2008). In laboratory studies, the effect of guanfacine on stress-induced smoking relapse was observed in female, but not male smokers (McKee et al. 2015; Verplaetse et al. 2015). Animal studies also showed that both stress and drug dependence decrease NE clearance and autoreceptor function in the bed nucleus of stria terminals (BNST) (McElligott et al. 2013): in rats, naloxone-precipitated morphine withdrawal augments NE efflux in the BNST, which is accompanied by anxiety-like behavior. Interestingly, the Lewis stress-sensitive strain of rats also shows a similar stress-induced dysregulation of NE. Similarly, stress and isolation-induced anxiety can alter NE signaling in the BNST and decrease α2A autoreceptor activity in rats (Fox et al. 2015b). Of note, both studies emphasized that baseline stress reactivity of the strains investigated was a critical determinant for the presence or absence of withdrawal-induced NE dysregulation and associated anxiety-like behavior.
We show here that guanfacine is effective in a well-validated rodent model of antidepressant efficacy. These results are consistent with previous work using clonidine, another α2A receptor agonist (Bourin et al. 1991). Acute guanfacine administration had a U-shaped dose-response effect that may suggest that recruitment of NE autoreceptors in different brain regions could have conflicting effects on behavior in the forced swim test. Another possibility could be a change in locomotor activity that may mask an antidepressant-like effect of the highest dose tested (0.3 mg/kg). In contrast, the dose-response function for chronic guanfacine administration was shifted to the left, suggesting an increase in drug efficacy that may be related to a sensitization of the system or cumulative effects of guanfacine given its relatively long half-life (Sorkin and Heel 1986). The maintained antidepressant-like effect of guanfacine following chronic administration indicates that repeated administration of guanfacine does not lead to compensatory mechanisms that could limit its efficacy in long-term regulation of behaviors related to depression. It is also important to note that the behavioral differences observed in were not confounded by changes of overall activity as the decrease in locomotor activity occurred at doses that increased mobility in the forced swim test. Thus, the change in locomotion is unlikely to explain the antidepressant-like effects of guanfacine.
Of the many factors that distinguish people in their successful quit attempts, sex and mood status are critical elements for maintained abstinence (Weinberger et al. 2014). Dysregulation of the cholinergic system can lead to symptoms of depression in human subjects (Janowsky and Overstreet 1990a; Risch et al. 1981) and in mice (Mineur et al, 2013). Further, it has been suggested that nicotine withdrawal during smoking cessation could lead to increased cholinergic signaling due to increased nAChR number due to smoking (Esterlis et al. 2013b; Fenster et al. 1999), greater receptor availability in withdrawal, and an increase in acetylcholine release (Carcoba et al. 2014). We hypothesized that the ability of guanfacine to decrease stress-induced relapse in female smokers and to increase quit rates in a clinical trial could be due to its ability to counteract the increase in depressive symptoms that result from increased cholinergic signaling during withdrawal. We tested this hypothesis in a rodent model of depression-like behavior induced by administration of the cholinesterase blocker physostigmine. In several paradigms related to mood regulation, guanfacine completely reversed the effects induced by physostigmine, independent of sex. Thus, activation of α2a noradrenergic receptors can reverse the negative effects of increased endogenous cholinergic signaling that could occur during nicotine withdrawal.
While the molecular and cellular mechanisms underlying these behavioral effects remain to be elucidated, it is possible that negative feedback induced by NE autoreceptor activation can reduce HPA activation triggered by ACh-induced increases in stress responses. Alternatively, post-synaptic α2A receptor stimulation could decrease neuronal activity through Gi/o-mediated signaling, thereby altering activity in circuits controlling stress reactivity. Future experiments will explore these possibilities. For example, guanfacine could stimulate activity in the PFC resulting in resilience against stress and withdrawal-induced mood dysregulation (Fox et al. 2014) that often leads to compulsive drug use and relapse. One hallmark of stress-induced prefrontal dysregulation is the remodeling and shrinkage of neuronal spines, associated with loss of top-down control of behavior including arousal and emotion (Hains et al. 2009). Animal studies show that guanfacine protects against such stress-induced cortical spine remodeling, and can reverse some of the associated cognitive dysregulation (Hains et al. 2015). In line with this finding, clinical studies have shown that guanfacine promotes attentional shift and inhibitory control during opiate withdrawal (Fox et al. 2015a). Interestingly, our electrophysiological studies indicate that chronic guanfacine administration does not induce a significant effect on PFC physiology in male mice, suggesting that the antidepressant-like effects of guanfacine are not mediated through this brain area. Remarkably, paired-pulse facilitation in Layer 5 pyramidal neurons in the medial PFC was greater at baseline in neurons in slices from female mice compared to those from male mice, suggesting that PFC neurons in slices from female mice respond more robustly to repeated stimuli than neurons from male mice. These findings are in line with recent human imaging studies that describe a sexually dimorphic resting state in amygdala/mPFC connectivity (Alarcon et al. 2015). Chronic guanfacine administration decreased the paired-pulse ratio in neurons from female mice to the level observed in neurons from male mice, defining surprising sex differences in PFC neuronal activity that were not reflected in the behaviors measured here. These differences in synaptic physiology suggest that there may be underlying sex differences in recruitment of brain areas that lead to similar behavioral outcomes. Further anatomical and physiological studies are necessary to determine the various effects that guanfacine may have on brain activity, and how this could contribute to its therapeutic effects in smokers.
While acute guanfacine administration resulted in an antidepressant-like effect, in the paradigm that involved two injections (saline or physostigmine followed by guanfacine), guanfacine had little effect on its own. We have observed previously that specific injection regimens can lead to decreased immobility time in mouse models of antidepressant-efficacy, likely due to effects on arousal or habituation to handling (Mineur et al. 2013). Previous anatomical and physiological studies have shown that α2A receptors may be expressed on cholinergic neurons of the basal forebrain (Zaborszky et al. 2004). Activation of α2A receptors by guanfacine may therefore exert antidepressant-like effects by decreasing the ACh release in response to stress or physostigmine challenge (Kaufer et al. 1998; Mineur et al. 2013; Shaltiel et al. 2013). This is consistent with studies that show that ACh levels in the hippocampus are directly related to the physiological state of animals under baseline, stress, and recovery conditions (Martinowich et al. 2012). Finally, these results demonstrate that whether or not there is direct cross-talk between the NE and ACh systems in brain areas related to stress response, guanfacine administration appears to be sufficient to reverse the consequences of cholinergic dysregulation on behaviors related to anxiety and depression. The molecular and circuit-level basis for NE-ACh interactions remains to be determined, as the effects of α2A receptor activation depends on the type of cells expressing these receptors.
We used c-fos immunoreactivity as a marker of changes in neuronal activity following guanfacine and physostigmine administration. While we found multiple changes in c-fos expression in brain regions implicated in mood regulation following guanfacine and physostigmine administration, including the BLA, the lateral septum and prefrontal cortex, it was striking that these changes were sex specific. Despite a lack of sex differences across the behavioral experiments performed here, changes in neuronal activity as measured by c-fos expression showed highly sex-specific patterns. For instance, in the BLA, physostigmine treatment decreased c-fos expression while guanfacine reversed this effect to baseline level in female mice. In male mice, physostigmine had no effect on BLA c-fos levels, but in combination with guanfacine, it decreased c-fos expression. Conversely, in the medial septum, the major source of acetylcholine input to the hippocampus and amygdala, male and female mice showed a decrease in c-fos when guanfacine was combined with physostigmine compared to guanfacine alone. Mechanistically, these patterns of neuronal activation strongly suggest that sex-specific recruitment of neuronal pathways in stress-related networks underlie similar behavioral responses to noradrenergic and cholinergic challenge. Understanding the contribution of specific circuits to stress-related behaviors and pharmacological manipulations will help in the development of sex-specific treatments for psychiatric illness and substance abuse.
Acknowledgments
This work was supported by P50 DA033945 (ORWH, NIDA, FDA), MH077681 and MH105824.
Footnotes
The authors declare that there are no conflicts of interest.
References
- Alarcon G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–44. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Kalivas PW. Brain norepinephrine rediscovered in addiction research. Biological Psychiatry. 2008;63:1005–6. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiranvand F, Zlabinger C, Orr-Urtreger A, Ristl R, Huck S, Scholze P. Nicotinic acetylcholine receptors control acetylcholine and noradrenaline release in the rodent habenulo-interpeduncular complex. British Journal of Pharmacology. 2014;171:5209–24. doi: 10.1111/bph.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Colombel MC, Malinge M, Bradwejn J. Clonidine as a sensitizing agent in the forced swimming test for revealing antidepressant activity. J Psychiatry Neurosci. 1991;16:199–203. [PMC free article] [PubMed] [Google Scholar]
- Carcoba LM, Orfila JE, Natividad LA, Torres OV, Pipkin JA, Ferree PL, Castaneda E, Moss DE, O’Dell LE. Cholinergic transmission during nicotine withdrawal is influenced by age and pre-exposure to nicotine: implications for teenage smoking. Developmental Neuroscience. 2014;36:347–55. doi: 10.1159/000360133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier GO, Bishop VS. The interaction of acetycholine and norepinephrine on heart rate. The Journal of Pharmacology and Experimental Therapeutics. 1972;180:31–7. [PubMed] [Google Scholar]
- Dell’Osso B, Palazzo MC, Oldani L, Altamura AC. The noradrenergic action in antidepressant treatments: pharmacological and clinical aspects. CNS Neuroscience & Therapeutics. 2011;17:723–32. doi: 10.1111/j.1755-5949.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Front Behav Neurosci. 2012;6:52. doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilsaver SC. Cholinergic mechanisms in depression. Brain Research. 1986;396:285–316. doi: 10.1016/0165-0173(86)90016-0. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP, Picciotto MR, Laruelle M, Carson RE, Cosgrove KP. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013a;54:78–82. doi: 10.2967/jnumed.112.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, Anticevic A, Carlson J, Niciu MJ, Cosgrove KP, D’Souza DC. In vivo evidence for beta 2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biological Psychiatry. 2013b doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–14. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H, Sinha R. The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. Advances in Pharmacology. 2014;69:217–65. doi: 10.1016/B978-0-12-420118-7.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H, Sofuoglu M, Sinha R. Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. Journal of Psychopharmacology. 2015a;29:312–23. doi: 10.1177/0269881114562464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–37. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ME, Studebaker RI, Swofford NJ, Wightman RM. Stress and drug dependence differentially modulate norepinephrine signaling in animals with varied HPA axis function. Neuropsychopharmacology. 2015b;40(7):1752–61. doi: 10.1038/npp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB. Stress and brain noradrenaline: a review. Neurosci Biobehav Rev. 1985;9:233–43. doi: 10.1016/0149-7634(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Gold PW. The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry. 2015;20:32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17957–62. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Yabe Y, Arnsten AF. Chronic stimulation of alpha-2a-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiology of Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, Seibyl JP, McClure-Begley TD, Picciotto MR, Esterlis I. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biological Psychiatry. 2013;74:768–76. doi: 10.1016/j.biopsych.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–5. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH. Cholinergic dysfunction in depression. Pharmacol Toxicol. 1990a;3:100–11. doi: 10.1111/j.1600-0773.1990.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH. Cholinergic dysfunction in depression. Pharmacology & toxicology. 1990b;66(Suppl 3):100–11. doi: 10.1111/j.1600-0773.1990.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:373–7. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS, Greig NH, Manji HK, Lu B. Roles of p75(NTR), long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biological Psychiatry. 2012;71:75–83. doi: 10.1016/j.biopsych.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL, Wightman RM. Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology. 2013;38:1665–73. doi: 10.1038/npp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, Weinberger AH, Ashare R, Sinha R. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. Journal of Psychopharmacology. 2015;29:300–11. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milusheva E, Baranyi M, Zelles T, Mike A, Vizi ES. Release of acetylcholine and noradrenaline from the cholinergic and adrenergic afferents in rat hippocampal CA1, CA3 and dentate gyrus regions. The European Journal of Neuroscience. 1994;6:187–92. doi: 10.1111/j.1460-9568.1994.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3573–8. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–62. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science. 1980;209:1545–6. doi: 10.1126/science.7433977. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC, Murphy DL. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Research. 1981;4:89–94. doi: 10.1016/0165-1781(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. The American Journal of Psychiatry. 2012;169:851–9. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Clerkin SM, Fan J, Halperin JM, Newcorn JH. Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology. 2013;226:261–71. doi: 10.1007/s00213-012-2893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepede G, Corbo M, Fiori F, Martinotti G. Reboxetine in clinical practice: a review. La Clinica terapeutica. 2012;163:e255–62. [PubMed] [Google Scholar]
- Shaltiel G, Hanan M, Wolf Y, Barbash S, Kovalev E, Shoham S, Soreq H. Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Structure & Function. 2013;218:59–72. doi: 10.1007/s00429-011-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence MN, Tudor GD, Matthews ML, Lindsay DB. Effects of the alpha 2-adrenoceptor agonist guanfacine on growth and thermogenesis in mice. J Anim Sci. 1992;70:3429–34. doi: 10.2527/1992.70113429x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addictive Behaviors. 2014;39:428–33. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin EM, Heel RC. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31:301–36. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, Mazure CM, McKee SA. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine Tob Res. 2015;17:486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Kaufman M, McKee SA. Consideration of sex in clinical trials of transdermal nicotine patch: a systematic review. Exp Clin Psychopharmacol. 2014;22:373–83. doi: 10.1037/a0037692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology. 2013;225:201–8. doi: 10.1007/s00213-012-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Rosin DL, Kiss J. Alpha-adrenergic receptor alpha2A is colocalized in basal forebrain cholinergic neurons: a light and electron microscopic double immunolabeling study. Journal of Neurocytology. 2004;33:265–76. doi: 10.1023/B:NEUR.0000044188.67442.9d. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Antic SD. Rapid dopaminergic and GABAergic modulation of calcium and voltage transients in dendrites of prefrontal cortex pyramidal neurons. The Journal of Physiology. 2012;590:3891–911. doi: 10.1113/jphysiol.2011.227157. [DOI] [PMC free article] [PubMed] [Google Scholar]