Abstract
Hepatic lipase (HL) is an important enzyme in the clearance of triacylglycerol (TAG) from the circulation, and has been proposed to have pro-atherogenic as well as anti-atherogenic properties. It hydrolyzes both phospholipids and TAG of lipoproteins, and its activity is negatively correlated with HDL levels. Although it is known that HL acts preferentially on HDL lipids, the basis for this specificity is not known, since it does not require any specific apoprotein for activity. In this study, we tested the hypothesis that sphingomyelin (SM), whose concentration is much higher in VLDL and LDL compared to HDL, is an inhibitor of HL, and that this could explain the lipoprotein specificity of the enzyme. The results presented show that the depletion of SM from normal lipoproteins activated the HL roughly in proportion to their SM content. SM depletion stimulated the hydrolysis of both phosphatidylcholine (PC) and TAG, although the PC hydrolysis was stimulated more. In the native lipoproteins, HL showed specificity for PC species containing polyunsaturated fatty acids at sn-2 position, and produced more unsaturated lyso PC species. The enzyme also showed preferential hydrolysis of certain TAG species over others. SM depletion affected the specificity of the enzyme towards PC and TAG species modestly. These results show that SM is a physiological inhibitor of HL activity in lipoproteins and that the specificity of the enzyme towards HDL is at least partly due to its low SM content.
Keywords: Hepatic lipase, sphingomyelin, PC species, TAG species, substrate specificity, enzyme inhibition
1. Introduction
Hepatic lipase (HL) is a lipolytic enzyme that plays a critical role in the clearance of triacylglycerol (TAG) from the plasma and in the metabolism of HDL as well as other lipoproteins [1–3]. It hydrolyzes both TAG and phospholipids of the lipoproteins, its substrate preference being intermediate between that of lipoprotein lipase (LPL) which is specific for TAG, and of endothelial lipase which is specific for phospholipids. Quantitatively, however, HL hydrolyzes more TAG than phospholipids in plasma [4]. HL is a major determinant of LDL subfraction distribution [5]. Together with LPL, it is responsible for the clearance of TAG from the circulation. However, unlike LPL which is important for clearance of both diet-derived (chylomicron) and endogenous (VLDL) TAG, HL is more important for the clearance of endogenous TAG from VLDL, LDL and HDL, and in re-modeling of these lipoproteins. Since the activity of HL is known to be inversely correlated with the plasma HDL levels [2] the study of factors which regulate HL activity is important in understanding the regulation of HDL metabolism. Whereas the regulation of HL transcription, synthesis and secretion from the liver have been well studied [1,6–8], the physiological factors that regulate its activity in the plasma compartment are not well understood. Furthermore, while it is known that HL acts preferentially on HDL compared to VLDL and LDL [4], the reason for this specificity is unknown, since it does not require an apoprotein cofactor for its activity. Our previous studies showed that sphingomyelin, the most abundant sphingolipid in the plasma lipoproteins, is an inhibitor of several lipolytic enzymes, including LCAT [9], secretory phospholipases II, V, and X [10,11], as well as endothelial lipase [12]. Since SM/PC ratio is lower in HDL than in VLDL or LDL [9], it is possible that SM also inhibits HL activity on lipoproteins, and this could explain its differential effects on lipoproteins. We tested this hypothesis in this study and the results presented here show that depletion of SM from the lipoproteins activated the hydrolysis of both TAG and PC in the lipoproteins and that the extent of activation is correlated with the initial concentration of SM in the lipoproteins. In addition, the specificity of the enzyme for various molecular species of PC and TAG was influenced by the presence of SM. These results show that SM plays an important physiological role in the regulation of HL activity and specificity, and thus indirectly influences HDL metabolism, and the subfraction distribution of LD and HDL.
2. Materials and Methods
2.1 Chemicals, reagents and substrates
Egg PC, egg SM, 1, 2-diheptadecanoyl-sn-glycero-3-phosphocholine (PC 17:0) and 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 17:0) were purchased from Avanti polar lipids Inc. (Alabaster, AL). Tripentadecanoin (TAG 15:0) was purchased from Nu-Chek Prep, Inc (Elysian, MN). Di-palmitoyl [14C] PC (55 mCi/mmole) and triolein (9, 10-3H-oleoyl) (60 Ci/mmol) were purchased from American Radiochemicals Inc (St. Louis, MO). Sphingomyelinase (EC 3.1.4.12) from B. cereus (100 units/mg) was obtained from Sigma-Aldrich (St. Louis, MO). Methanol and chloroform are LC/MS grade from Fisher Chemical (Pittsburgh, PA). All other reagent used here are analytical grade.
2.2 Lipoproteins and HL
Pooled normal human plasma (never frozen) was obtained from a local blood bank. VLDL, LDL and HDL were isolated sequential ultracentrifugation according to published method [9]. The lipoproteins were dialyzed extensively against 10 mM Tris-0.15 M NaCl-1mM EDTA pH 7.4, and stored at 4°C. Protein content was quantified by Coomassie Brilliant Blue assay, using bovine serum albumin (BioRad) as standard. The concentrations of SM and PC in the lipoproteins were determined from the estimation of lipid phosphorus, following the separation of the phospholipids by TLC. The SM/PC ratios of the lipoproteins were: 0.20 ± 0.01 for VLDL, 0.39 ± 0.036 for LDL, and 0.156 ± 0.003 for HDL. The lipoproteins were used for enzyme treatment within three weeks after preparation. Chinese Hamster Ovary cells stably transfected with human HL [13] were kindly provided by by Dr. John S. Hill, University of British Columbia. The cells were grown to confluence in 10% fetal calf serum in DMEM, and the medium was changed to DMEM containing 1% Nutridoma and 10 units/ml heparin. The medium was collected and replaced every day for 7 days, and the pooled medium was cleared of cell debris by centrifugation and concentrated by ultrafiltration using Centriflo filters. The concentrated medium was stored in aliquots of 10 ml each at −80 °C until use. Each tube was thawed only once before use.
2.3 Labeling of lipoproteins
VLDL, LDL, and HDL, freshly isolated from pooled human plasma were labeled with di[14C] PC 16:0 or [3H] triolein in order to trace the hydrolysis of lipoprotein PC or TAG by HL. To 1 ml of the lipoprotein containing 2 mg protein, 50 μl of ethanolic solution of di-[14C] palmitoyl PC (0.2 μCi) was added, and the solvent was evaporated under nitrogen at room temperature. The labeled lipoproteins were further incubated under nitrogen for 30 min at 37°C, and stored at 4 °C until use. The labeled lipoproteins were used for the HL assay within one week. Under these conditions, more than 95% of the label is associated with the lipoproteins after ultracentrifugation at the respective isolation densities of the lipoproteins.
2.4. SMase treatment
Freshly prepared lipoproteins (400 μg protein each), labeled with 14C-PC 16:0 or 3H-triolein were incubated with 0.1 units of B. cereus SMase at 37°C for 2h, and then heated at 65°C for 15 min to inactivate the SMase. The extent of SM degradation under these conditions was assessed by LC/MS after adding an internal standard of 12:0 SM, and in all cases over 95% of SM was found be degraded with no effect on PC (see Figs 1–3, Supplemental Data) or TAG (not shown). The unlabeled lipoproteins were similarly depleted of SM by treatment with SMase.
2.5. HL activity on labeled lipoproteins
Control or SMase-treated HDL, LDL or VLDL (400 μg protein each) were incubated with 500 μl of recombinant human HL containing 32 μg protein for the indicated periods of time at 37 °C and the reaction was stopped by adding 2 mL of methanol, and the total lipids were extracted by Bligh and Dyer [14] procedure. The total lipids were separated by silica gel TLC using the solvent system of chloroform: methanol: water 65:25:4 (v/v) for the PC-labeled lipoproteins, and the solvent system of hexane-diethyl ether-acetic acid 70:30:1 (v/v) in the case of TAG-labeled lipoproteins. In the case of PC-labeled substrates, the spots corresponding to LPC, PC, and free fatty acids were scraped and their radioactivity determined in a liquid scintillation counter after addition of 0.5 ml water to each vial to maximize elution of the lipids from the silica gel. In the case of TAG-labeled substrates the spots corresponding to TAG, MAG, and free fatty acids were scraped and their radioactivity determined in the scintillation counter after adding 0.5 ml water.
2.6. Molecular species analysis by LC/MS of unlabeled lipoproteins treated with HL
Control and SM-depleted HDL, LDL, and VLDL were treated with 0.5 ml of recombinant HL containing 32 μg protein for 4 h at 37 C. The enzyme reactions were stopped by the addition of methanol containing 1.0 μg each of the following internal standards: di 17:0 PC, 17:0 LPC, tri-15:0 TAG. The total lipids were extracted by Bligh and Dyer procedure [14] and the molecular species of various lipids were analyzed by LC/MS using an AB Sciex QTRAP 6500 mass spectrometer coupled with Agilent 2600 UPLC system. The samples were diluted with Solvent A (80/19.5/0.5 chloroform/methanol/water). A normal phase silica column (Supelco Ascentis Si 3μ, 10cm×2.1mm) was used for LC separation. Mobile phase A consisted of 80/19.5/0.5 (v/v) chloroform/methanol/water and mobile phase B consisted of 60/34.5/5.5 chloroform/methanol/water (v/v). Both A and B were supplemented with 0.1% of formic acid and 0.1% ammonium hydroxide. Chromatographic separations were accomplished with 100% solvent A for 5 min, then a linear gradient of 100% solvent A to 100% solvent B from 5 to 30 min, and 100% solvent B from 30 to 35 min. The column temperature was held at 25°C. The flow rate was set to 350μL min−1. ESI-MS was performed in both positive and negative multiple reaction monitoring (MRM) mode for the quantitative and qualitative analysis of PC and TAG species (see Table-1 and Table-2, Supplemental Data, for the MRM values used). The spray voltage was 4.5kV, the source temperature was set at 450°C. Mass spectra were acquired and recorded by Analyst software (AB Sciex). The major PC species known to be present in the plasma lipoproteins were analyzed in MRM mode, with the precursor and product ion pairs as shown in Table-1, Supplemental Data. The position of the acyl groups in each PC as shown (conventionally sn-1 acyl group, followed by sn-2 acyl group) was assumed based on the known preponderance of saturated acyl groups at the sn-1 position, and the unsaturated acyl groups at sn-2. The TAG species were analyzed in neutral loss mode with the ammoniated precursor ion (Q1) and the product ion derived from the loss of individual ammoniated fatty acids (Q3) as shown in Table-2 of Supplemental Data. The position of the individual acyl groups was not determined for the TAG species. Quantitation of individual molecular species PC, LPC, and TAG was performed from the relative intensities of the various species and the corresponding internal standards (17:0–17:0 PC, 17:0 LPC, 15:0/15:0/15:0 TAG respectively).
3. Results
3.1. Effect of SM on the hydrolysis of labeled PC in lipoproteins
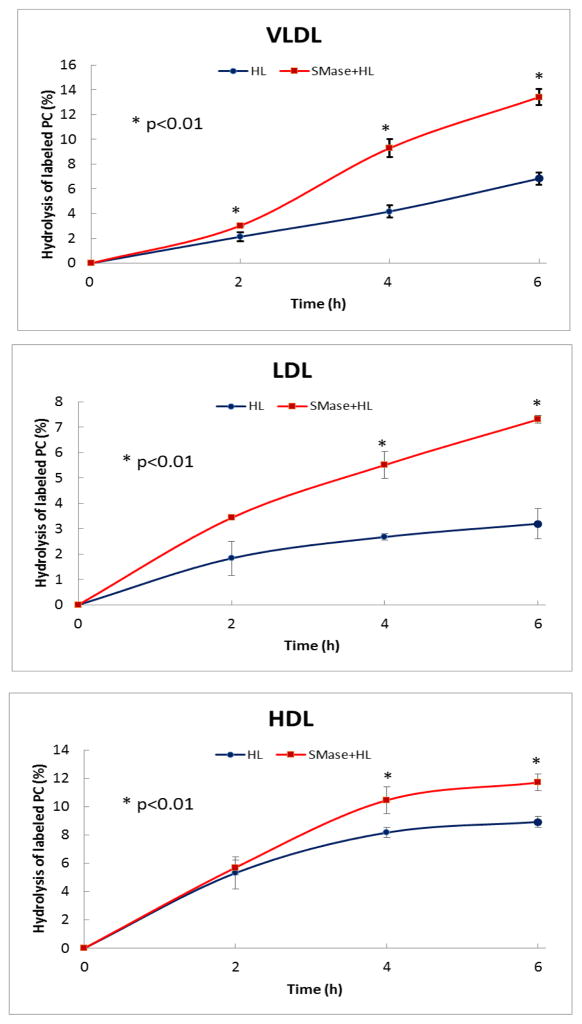
VLDL, LDL, and HDL isolated from normal human plasma were labeled with trace amounts of di [14C]-palmitoyl PC, and aliquots of labeled lipoproteins were treated with bacterial SMase for 2 h at 37 °C. The SMase was then inactivated by heating the reaction mixture at 60 °C for 15 min. More than 95% of the SM was hydrolyzed under these conditions, as measured by LC/MS (see Figs 1–3, Supplemental Data). The SM-depleted and control lipoproteins (400 μg protein) were then incubated with recombinant HL (32 μg protein) for varying periods of time, and the radioactivity in PC, LPC and free fatty acids was determined after the TLC separation of lipids. The HL activity was expressed as % of PC hydrolyzed. As shown in Fig. 1, the hydrolysis of labeled PC was stimulated in all lipoproteins by the depletion of SM. The extent of stimulation (on average 87% for VLDL, 129% for LDL, and 22% for HDL) was positively correlated with the SM/PC ratio of the native lipoprotein, suggesting that the concentration of SM is a major determinant of the HL activity. There was no hydrolysis of PC by SMase alone, in the absence of HL(see Figs 1–3, Supplemental Data).
Fig. 1. Effect of SM depletion on the hydrolysis of labeled PC incorporated into lipoproteins.
The lipoproteins were pre-labeled with dipalmitoyl [14C] PC, and an aliquot of the labeled lipoprotein was subjected to SMase treatment as described in the text. The SMase-treated and untreated lipoproteins were then incubated with recombinant HL (500 μl, 32 μg protein) for the indicated periods of time at 37 °C. The lipids were extracted, and the radioactivity in LPC, PC, and FFA was determined following their TLC separation. The results show the decrease in PC counts (%) compared to the control (incubated with no HL), and are mean ± SEM of 4 experiments. * p < 0.01 HL vs HL + SMase.
3.2. Effect of SM on the hydrolysis of labeled TAG in the lipoproteins
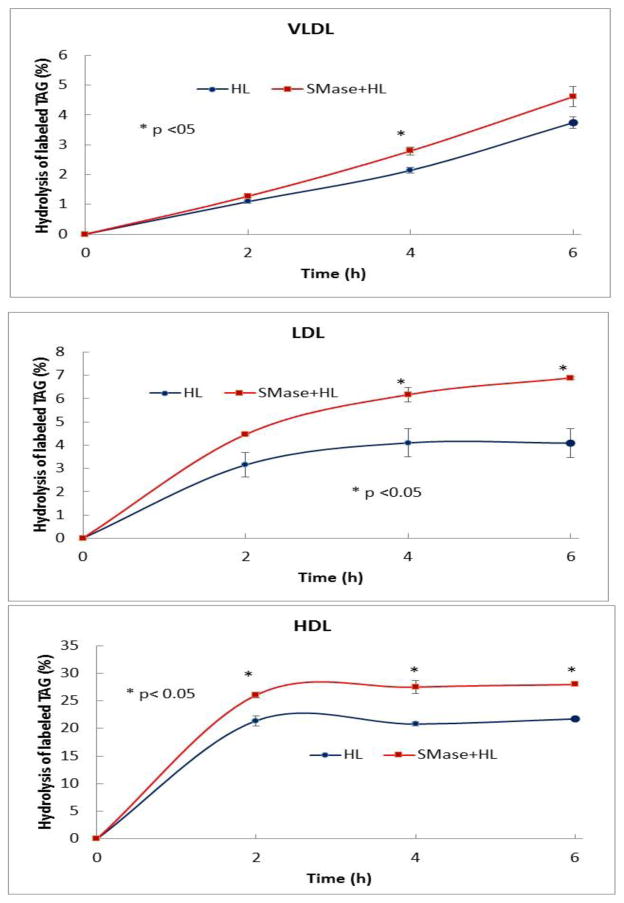
VLDL, LDL, and HDL were labeled with trace amounts of 3H-triolein (labeled in all 3 acyl groups) and the SM was depleted by SMase treatment as described above for the PC-labeled lipoproteins. The SM-depleted and control lipoproteins (400 μg protein) were treated with recombinant HL (32 μg protein) for various periods of time and the radioactivity in TAG and free fatty acids was determined after TLC separation of the lipids. The hydrolysis of TAG (expressed as % TAG hydrolyzed) was linear for 6 h in the case of VLDL but reached a plateau earlier in HDL and LDL (Fig. 2), possibly due to limited amount of endogenous TAG. In all cases the hydrolysis was stimulated by the depletion of SM. The average stimulation was about 23% in the case of HDL, 45% for LDL, and 18% for VLDL. Again the extent of stimulation appeared to correlate with the SM concentration in the native lipoprotein, as in the case of PC hydrolysis, suggesting an important role for SM in the hydrolysis of TAG by HL.
Fig. 2. Effect of SM depletion on the hydrolysis of labeled TAG incorporated into lipoproteins.
The lipoproteins were labeled with 3H triolein, and subjected to SMase treatment as described in the text. The SMase-treated and untreated lipoproteins were reacted with recombinant HL (32 μg protein) at 37 °C for the indicated periods of time. The lipids were extracted and the radioactivity in TAG and FFA was determined after TLC separation. The results shown are % decrease in TAG radioactivity from the control (no HL), and are mean ± SEM of 4 experiments. * p,< 0.05 SMase-treated vs untreated samples.
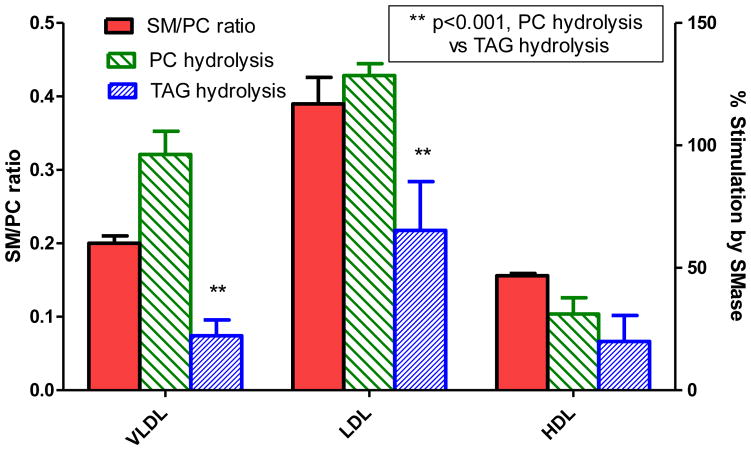
Fig 3 shows the comparative effects of SM depletion on PC and TAG hydrolysis by HL in the three lipoproteins (6 h incubation), as well as the SM/PC ratios in native lipoproteins. Depletion of SM stimulated the hydrolysis of PC more than that of TAG in all lipoproteins. However the differences were more marked in VLDL and LDL which are richer in SM. These results suggest that SM may have a greater inhibitory effect on PC hydrolysis than on TAG hydrolysis in the native lipoproteins.
Fig. 3. SM/PC ratios of the lipoproteins, and the effect of SM depletion on the hydrolysis of labeled PC and TAG incorporated into lipoproteins.
SM and PC values were determined from the estimation of lipid phosphorus following the separation of the lipids by TLC. The stimulation of PC or TAG hydrolysis by SM depletion was calculated from the samples of 6 h incubation with HL (Fig 1 and Fig 2). The values shown are mean ± SEM of 4 experiments.
3.3. Effect of SM on the hydrolysis of molecular species of PC in lipoproteins
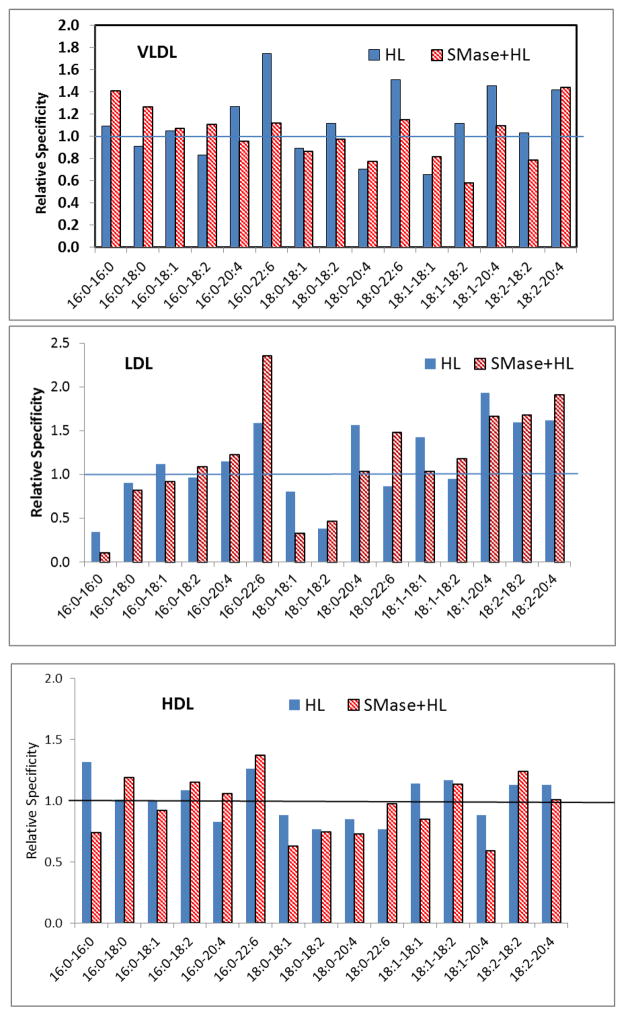
Although the results with labeled PC showed that its hydrolysis is stimulated by SM depletion, it is important to study the effect of SM in native lipoproteins in order to determine its physiological relevance and its potential effect on the specificity towards molecular species of PC. For this purpose, we incubated isolated unlabeled lipoproteins before and after SM depletion with recombinant HL for 4 h at 37 °C, and determined the molecular species composition of PC by LC/MS/MS. In addition, the species of LPC generated were measured. From these results, the decrease in total PC as well as individual PC species was determined. Since the lipoproteins contain various molecular species of PC in unequal and varying amounts, the specificity of the enzyme was calculated by dividing the % contribution of individual species to the total decrease in PC by their initial concentration, as described previously for endothelial lipase [12]. By this criterion, a relative specificity value of greater than 1.0 indicates that the given species is preferred over the “average” PC, whereas a value lower than 1.0 indicates a lower preference than “average” PC.
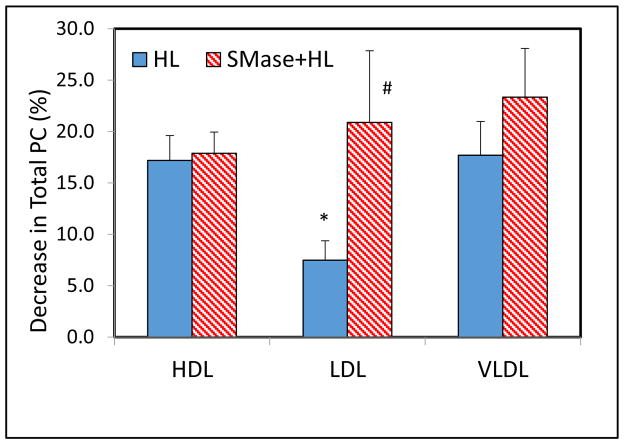
As shown in Fig 4, the hydrolysis of total PC was lower in native LDL and VLDL compared to HDL, although the only difference between HDL and LDL was statistically significant. SMase treatment increased the hydrolysis of PC in in all lipoproteins, although only the effect on LDL was statistically significant. These results support the results with labeled lipoproteins and show that LDL, which has the highest SM/PC ratio is affected most by SM depletion. The hydrolysis of individual molecular species of PC is shown in Fig 5. In all lipoproteins, quantitatively the major PC species hydrolyzed were 16:0–18:2, 16:0–18:1, 16:0–20:4 and 18:0–18:2, which are also the most abundant. SM depletion stimulated the hydrolysis of all species. However the hydrolysis of some of the species reached statistical significance (compared to control with no HL) only after SM depletion suggesting that SM may protect certain PC species more than others from degradation by the lipase. When the relative specificity (selectivity) of HL was calculated as described above, the differences between the lipoproteins, as well as the effect of SM depletion, are more apparent (Fig. 6). Thus in native LDL, which has the highest SM/PC ratio, HL appears to preferentially hydrolyze the PC species containing polyunsaturated fatty acids at sn-2 position (16:0–22:6, 18:0–20:4, 18:1–20:4, 18:2–20:4). In HDL, which has the lowest SM/PC ratio, the specificity towards these species is significantly lost. SM depletion in LDL reduced the specificity factor for many, although not all of these PC species.
Fig. 4. Hydrolysis of PC in unlabeled lipoproteins.
Unlabeled lipoproteins (400 μg each) were first treated with SMase, followed by heat-inactivation of SMase, as described in the text. The SM-depleted and intact lipoproteins were then incubated with recombinant HL (32 μg protein) for 4 h at 37 °C.The lipids were extracted after adding an internal standard (17:0–17:0 PC) and the PC composition was analyzed by LC/MS/MS as described in the text, by MRM. The decrease in PC amount was calculated from the difference in the total PC between control (no HL) and experimental (with HL) samples. Values shown are mean ±SEM of 6 analyses (HDL) or 4 analyses (for VLDL and LDL), but were all obtained from the same batch of the enzyme..
* p< 0.05 compared to HDL; # p< 0.05 HL vs SMase + HL.
Fig. 5. Hydrolysis of individual molecular species of PC in the lipoproteins.
The SMase-treated and native lipoproteins (400 μg protein) were incubated with 32 μg recombinant human HL for 4 h at 37 °C (Fig. 4), and the composition of molecular species of PC was analyzed by LC/MS/MS, with 17:0 PC as the internal standard. The decrease in individual PC species from the control (no HL) values was calculated. Values shown are mean ± SEM of 4 separate analyses, performed with a single batch of enzyme and 2 different batches of lipoproteins. * p< 0.05 compared to the control (no HL) values (not shown).
Fig. 6. Selectivity of HL for the molecular species of PC in native lipoproteins.
The relative specificity values were calculated by dividing the % contribution of each PC species to the total decrease in PC by the % concentration of the species in the native lipoprotein (incubated with no HL). The PC species with a value of > 1.0 are those preferred by the enzyme, whereas the PC species with values < 1.0 are poorer substrates than “average” PC.
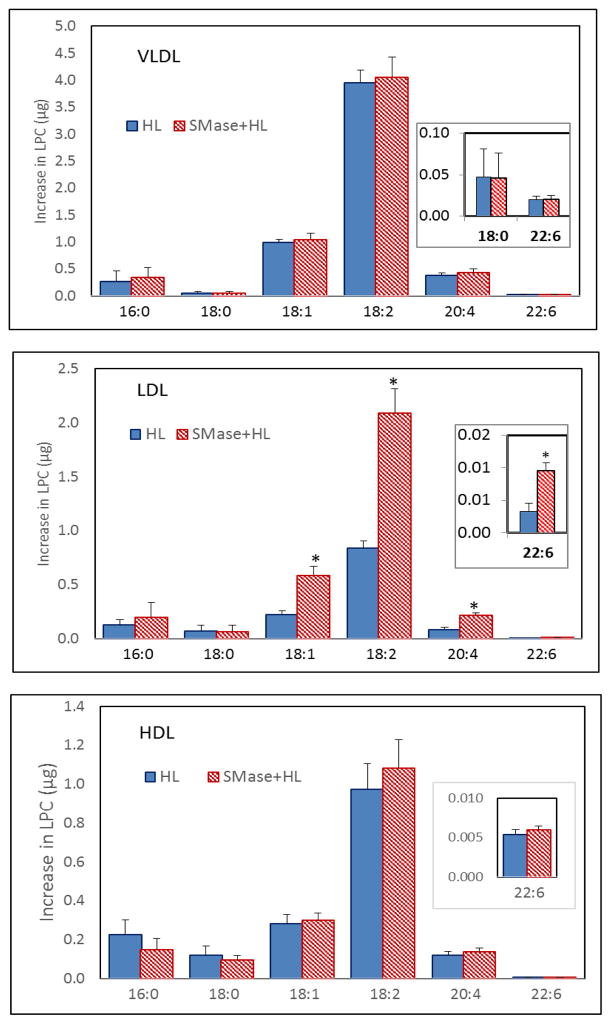
3.4. LPC molecular species
Fig. 7 shows the increase in various species of LPC in the three lipoproteins following HL treatment before and after SM depletion. As expected from the results on PC species, and the known positional specificity of HL for the sn-1 acyl group, the most abundant LPC generated in all lipoproteins was 18:2 LPC, followed by 18:1 and 20:4 species. Treatment with SMase stimulated the formation of all unsaturated LPC species, including 22:6 LPC, although statistically significant effect of SM depletion was seen only in LDL. These results show that similar to endothelial lipase [12], the effect of HL is to increase unsaturated LPC species, whereas LCAT increases mainly the saturated LPC [15]. It may be noted that since the SMase was inactivated by heat before the addition of HL, the hydrolysis of LPC by SMase as observed in the endothelial lipase studies [12] is avoided. However it is possible that some hydrolysis of LPC has occurred by HL or SMase or other enzymes in lipoproteins because the increase in LPC is lower than the decrease in corresponding PC species (Fig. 5), especially in VLDL.
Fig. 7. LPC species generated by HL in lipoproteins.
The SM-depleted and native lipoproteins were reacted with HL for 4 h (Fig. 5) and the molecular species of LPC were analyzed by LC/MS/MS. The values shown are mean ± SEM of 4 experiments, performed with a single batch of the enzyme and 2 different batches of lipoproteins. * p < 0.05 HL vs SMase + HL
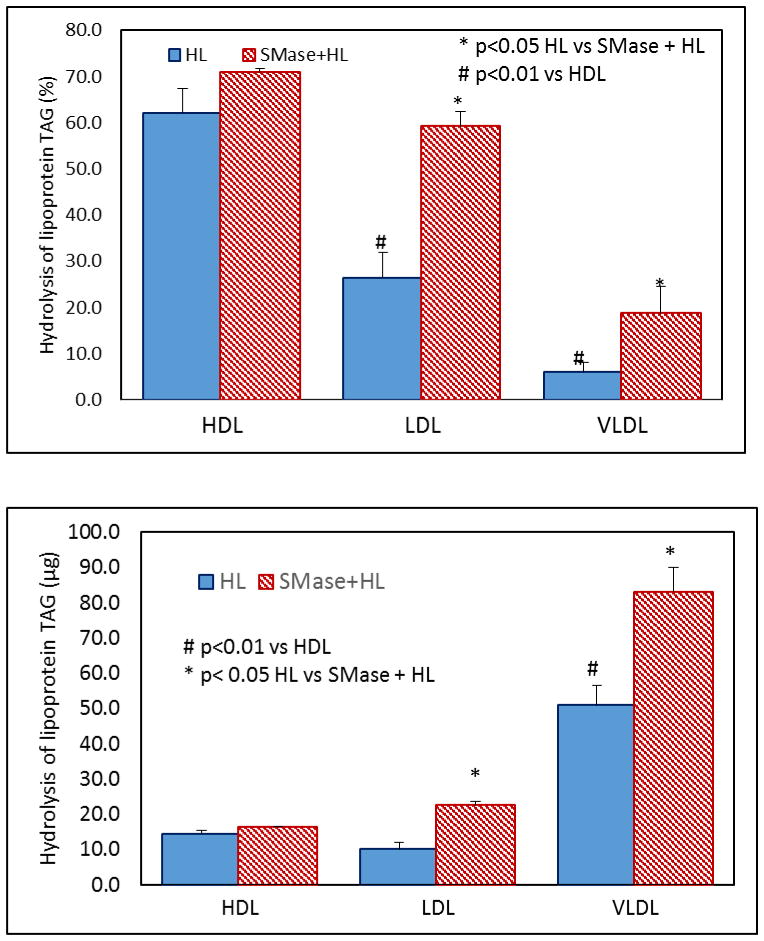
3.5. Hydrolysis of lipoprotein TAG by HL
Fig 8 shows the hydrolysis of lipoprotein TAG by HL before and after SM depletion. In terms of percentage of TAG hydrolyzed, HL hydrolyzed the TAG of HDL more efficiently than the TAG of LDL or VLDL. This is similar to the hydrolysis of PC in the three lipoproteins (Fig. 4). However in terms of the total TAG hydrolyzed, much greater amount of TAG was hydrolyzed in VLDL because of the high concentration of TAG in this lipoprotein. SM depletion activated the hydrolysis of TAG in all lipoproteins, but only the stimulation in LDL and VLDL was statistically significant. These results show that the hydrolysis of TAG by HL is also regulated by the SM content of the lipoprotein.
Fig. 8. Effect of SM depletion on TAG hydrolysis in lipoproteins.
Unlabeled lipoproteins (400 μg protein) were first depleted of SM by SMase treatment as described in the text. The SMase-treated and native lipoproteins were incubated with recombinant human HL (32 μg) for 4 h, and the TAG composition was analyzed by MRM using 15:0/15:0/15:0 TAG as internal standard. The decrease in TAG from the controls (incubated with no HL) was calculated, and expressed either as % decrease (top) or as decrease in mass (bottom). The values shown are mean ± SEM of 4 analyses (two analyses performed in duplicate, with a single batch of enzyme). # p<0.05 compared to HDL; * p< 0.05 HL alone vs SMase+ HL.
3.6. Specificity of HL towards TAG species
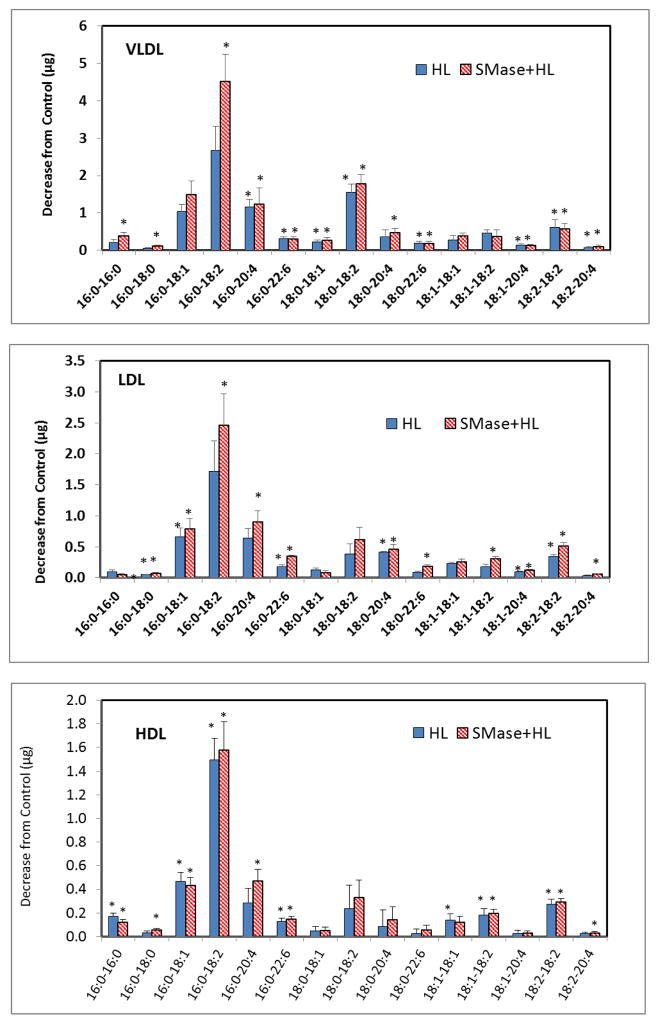
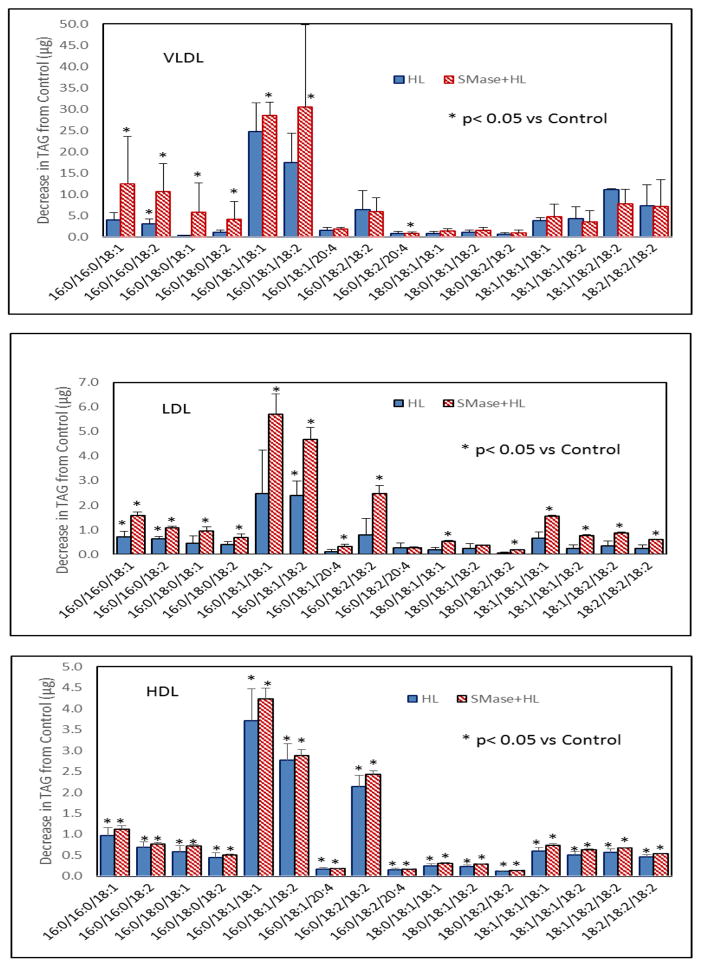
Fig. 9 shows the decrease in various species of TAG following HL treatment in yhe native and SM-depleted lipoproteins. In all of the lipoproteins, the major TAG species hydrolyzed were 16:0/18:1/18:1, and 16:0/18:1/18:2. SM depletion stimulated the hydrolysis of most of the TAG species. However, while the decrease in the amount (from the control with no HL) of all TAG specie reached statistical significance in HDL before depletion of SM, it did not reach statistical significance in VLDL and LDL for many species until after SM depletion. This shows that SM may preferentially inhibit the hydrolysis of some TAG species (including the major species 16:0/18:1/18:1, and 16:0/18:1/18:2).
Fig. 9. Hydrolysis of molecular species of TAG by HL.
Unlabeled lipoproteins were first depleted of SM by SMase treatment as described in the text. The SM-depleted and native lipoproteins (400 μg) were then incubated with recombinant HL (32 μg) for 4 h and the TAG species were analyzed by MRM, using 15:0/15:0/15:0 TAG as the internal standard. The decrease in each species compared to the control (samples incubated without HL) was calculated. The values shown are mean ± SEM of 4 analyses, as in Fig. 8.. * p< 0.05 compared to control.
4. Discussion
The importance of HL in lipoprotein metabolism, in particular its effect on plasma HDL levels and function, is well established [1,2]. HL activity is negatively correlated with levels of plasma HDL cholesterol, as well as with small dense LDL particles, two of the most important but opposite markers of coronary heart disease, and consequently HL has been attributed to have both pro-atherogenic and anti-atherogenic effects [1]. The activity of the enzyme varies by up to 8-fold among normal individuals, but less than half of this variability is attributable to genetic polymorphism in the HL gene [1], indicating the activity is regulated by other factors in the circulation. The preference of HL for the hydrolysis of phospholipids and TAG of HDL was reported by several studies [4,16], but the basis for the specificity for HDL is not clear, since the enzyme does not require any specific apoprotein for its activity. The present studies show that the SM concentration of the substrate lipoprotein may be an important modulator of its activity. This property of HL is similar to that of several other lipolytic enzymes in the plasma, namely LCAT [9], secretory phospholipases [10,11], and endothelial lipase [12], all of which have been shown to be inhibited by SM. A common feature of these enzymes is that they all utilize PC as substrate, and since SM has several physicochemical characteristics similar to that of PC, but is resistant to hydrolysis by lipolytic enzymes, it could act as a competitive inhibitor of their activities [9]. In addition, SM may affect the surface properties of the lipoproteins and alter the binding of lipolytic enzymes to their substrate lipoproteins [17]. In contrast to the other enzymes inhibited by SM, HL hydrolyzes several plasma lipids including TAG, PC, PE, monoacylglycerol, and acyl CoA thioesters [4]. We also found that SM inhibited the hydrolysis of monoacylglycerol and PE in addition to that of PC and TAG (results not shown) supporting the hypothesis that SM interacts non-productively with the active site of the enzyme and thus acts as a competitive inhibitor.
It has been shown that HL hydrolyzes quantitatively more TAG than PC in the lipoproteins [4], and therefore the inhibition of TAG hydrolysis by SM could be physiologically significant in the clearance of TAG from the plasma. Previous studies [18,19] have in deed reported that incorporation of SM into TAG emulsions inhibited the clearance of those emulsions from the circulation, but this was attributed to an inhibition of LPL activity, and the possible contribution of HL for TAG clearance was not investigated in these studies. SM is the second most abundant phospholipid in the plasma, but its physiological role in lipoprotein metabolism in plasma is not well understood. The SM/PC ratio is increased in abetalipoproteinemia [20,21] as well as in several hyperlipidemias [22–24], but the impact of this increase on the overall lipoprotein metabolism is not clear. Interestingly, the HL activity has been shown to be decreased in abetalipoprteinemia [25], as well as in Type I [26] and Type III [27] hyperlipoproteinemias, although the possible role of increased SM levels cannot be ascertained. While some previous studies in humans and mice suggested a pro-atherogenic role for SM [28–30], the recent multi-ethnic epidemiologic study in a large population indicated that SM has a modestly protective effect [31]. Since the atherogenic effects of HL also appear to be dependent on the concurrent presence of other lipoprotein abnormalities [1], it is possible that the inhibition of HL by SM in hyperlipidemic subjects may play a role in this variability.
The substrate specificity of HL towards the molecular species of PC was previously investigated by Duong et al [32] employing recombinant HDL containing only a single PC species at a time. These studies showed the order of specificity as 16:0–18:1>16:0–18:2>16:0–22:6>16:0–20:4. However this order of preference may not be applicable to the physiological conditions where the PC species not only occur in varying concentrations, but also compete with each other. In another study [33], the molecular species composition was investigated in HL deficient mice, the rationale being that the species preferred by HL would accumulate in the absence of the enzyme. These studies revealed that 36:4 PC (presumably16:0–20:4 and 18:2–18:2), and 36:2 PC (presumably 18:0–18:2 and 18:1–18:1) accumulated in the HL-deficient mice, suggesting preference of the enzyme for these PC species. However this may not necessarily represent the specificity of HL, since these PCs are also quantitatively major species in the lipoproteins. Furthermore other phospholipase activities in plasma (LCAT, endothelial lipase sPLA2) could alter the PC species composition in the absence of HL. Our studies are performed in the absence of other lipolytic activities, and with the physiological substrates (native lipoproteins) which not only contain several PC species in varying concentrations, but also all other lipids and apoproteins that could influence the specificity of the enzyme. Whereas the major species hydrolyzed in terms of mass were 16:0–18:2 and 16:0–18:1 because of their higher concentrations, the selectivity, as calculated from the relative concentrations of the PC species, showed the enzyme preferring certain minor PC species, especially those containing polyunsaturated fatty acids at sn-2 position, such as 16:0–22:6, 18:0–20:4, and 18:1–18:20:4. This is similar to the specificity exhibited by endothelial lipase, although endothelial lipase exhibited stronger preference for the polyunsaturated species compared to HL. Furthermore, the depletion of SM altered the specificity more modestly than observed for endothelial lipase.
Unlike the specificity towards PC species, the substrate specificity of HL towards the molecular species of TAG has not been investigated previously. Our results show that the major TAG species hydrolyzed in all the lipoproteins were 16:0/18:1/18:1, and 16:0/18:1/:18:2. The relative specificity values show the enzyme preferring the more abundant species in VLDL. However in LDL the enzyme showed preference for the less abundant 16:0/16:0/18:2 and 16:0/18:0/18:2, before SM depletion, but showed loss of this specificity after SM depletion. In HDL there was no clear specificity for any TAG species.
5. Conclusions
These studies show that SM modulates the hydrolysis of both PC and TAG by HL in plasma lipoproteins, and also affects the substrate specificity of the enzyme towards individual molecular species of TAG and PC. This property of SM may be important in the clearance of TAG from the circulation, and in the remodeling of lipoproteins.
Supplementary Material
Highlights.
Hepatic lipase is activated by sphingomyelin depletion from lipoprotein substrates
The stimulation was greater in LDL and VLDL, compared to HDL
Hydrolysis of phosphatidylcholine was stimulated more than that of triacylglycerol
Hepatic lipase showed specificity for polyunsaturated phosphatidylcholines
Sphingomyelin is a physiological regulator of hepatic lipase activity
Acknowledgments
These studies were supported by grants from NIH HL-68585, and AT-008457, and in part by a Merit Review award I01 BX001090 from US Department of Veterans Affairs (to PVS). Research reported in this publication was supported by the Office of The Director, National Institutes of Health under Award Number S10OD010660 (LC/MS equipment). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or US Department of Veterans Affairs.
Abbreviations
- FFA
free fatty acid(s)
- HL
hepatic lipase
- LPC
Lysophosphatidylcholine
- LPL
lipoprotein lipase
- MRM
multiple reaction monitoring
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- SM
sphingomyelin
- TAG
triacylglycerol
Footnotes
Conflicts of Interest
Authors: Peng Yang and P.V. Subbaiah
No conflicts to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zambon A, Deeb S, Pauletto P, Crepaldi G, Brunzell JD. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr Opin Lipidol. 2003;14:179–189. doi: 10.1097/00041433-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Annema W, Tietge U. Role of Hepatic Lipase and Endothelial Lipase in High-Density Lipoprotein Mediated Reverse Cholesterol Transport. Curr Atheroscler Rep. 2011;13:257–265. doi: 10.1007/s11883-011-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon A, Bertocco S, Vitturi N, Polentarutti V, Vianello D, Crepaldi G. Relevance of hepatic lipase to the metabolism of triacylglycerol-rich lipoproteins. Biochem Soc Trans. 2003;31:1070–1074. doi: 10.1042/bst0311070. [DOI] [PubMed] [Google Scholar]
- 4.Ehnholm C, Kuusi T. Preparation, characterization, and measurement of hepatic lipase. Methods Enzymol. 1986;129:716–738. doi: 10.1016/0076-6879(86)29101-6. [DOI] [PubMed] [Google Scholar]
- 5.Watson TD, Caslake MJ, Freeman DJ, Griffin BA, Hinnie J, Packard CJ, Shepherd J. Determinants of LDL subfraction distribution and concentrations in young normolipidemic subjects. Arterioscler Thromb Vasc Biol. 1994;14:902–910. doi: 10.1161/01.atv.14.6.902. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami M, Murase T, Itakura H, Yamada N, Ohsawa N, Takaku F. Lipid metabolism in endotoxic rats: decrease in hepatic triglyceride lipase activity. Microbiol Immunol. 1986;30:849–854. doi: 10.1111/j.1348-0421.1986.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 7.Rufibach LE, Duncan SA, Battle M, Deeb SS. Transcriptional regulation of the human hepatic lipase (LIPC) gene promoter. J Lipid Res. 2006;47:1463–1477. doi: 10.1194/jlr.M600082-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Chellan B, Koroleva EP, Sontag TJ, Tumanov AV, Fu YX, Getz GS, Reardon CA. LIGHT/TNFSR14 Can Regulate Hepatic Lipase Expression by Hepatocytes Independent of T Cells and Kupffer Cells. PLoS ONE. 2013;8:e54719. doi: 10.1371/journal.pone.0054719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbaiah PV, Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin-cholesterol acyltransferase. J Biol Chem. 1993;268:20156–20163. [PubMed] [Google Scholar]
- 10.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 11.Singh DK, Subbaiah PV. Modulation of the activity and arachidonic acid selectivity of group X secretory phospholipase A2 by sphingolipids. J Lipid Res. 2007;48:683–692. doi: 10.1194/jlr.M600421-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Belikova NA, Billheimer J, Rader DJ, Hill JS, Subbaiah PV. Inhibition of endothelial lipase activity by sphingomyelin in the lipoproteins. Lipids. 2014;49:987–996. doi: 10.1007/s11745-014-3944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JS, Davis RC, Yang D, Schotz MC, Wong H. Hepatic Lipase: High-Level Expression and Subunit Structure Determination. In: Byron Rubin EAD, editor. Methods in Enzymology. Lipases, Part A: Biotechnology. Academic Press; 1997. pp. 232–246. [DOI] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Glomset JA. The Plasma lecithin:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 16.Shirai K, Barnhart RL, Jackson RL. Hydrolysis of human plasma high density lipoprotein 2 phospholipids and triglycerides by hepatic lipase. Biochem Biophys Res Commun. 1981;29:591–599. doi: 10.1016/s0006-291x(81)80217-3. [DOI] [PubMed] [Google Scholar]
- 17.Bolin DJ, Jonas A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J Biol Chem. 1996;271:19152–19158. doi: 10.1074/jbc.271.32.19152. [DOI] [PubMed] [Google Scholar]
- 18.Tong MF, Kuksis A. Effect of different neutral phospholipids on apolipoprotein binding by artificial lipid particles in vivo. Biochem Cell Biol. 1986;64:826–835. doi: 10.1139/o86-111. [DOI] [PubMed] [Google Scholar]
- 19.Arimoto I, Saito H, Kawashima Y, Miyajima K, Handa T. Effects of sphingomyelin and cholesterol on lipoprotein lipase-mediated lipolysis in lipid emulsions. J Lipid Res. 1998;39:143–151. [PubMed] [Google Scholar]
- 20.Jones JW, Ways P. Abnormalities of High Density Lipoproteins in Abetalipoproteinemia. The Journal of Clinical Investigation. 1967;46:1151–1161. doi: 10.1172/JCI105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbert PN, Hyams JS, Bernier DN, Berman MM, Saritelli AL, Lynch KM, Nichols AV, Forte TM. Apolipoprotein B-100 deficiency. Intestinal steatosis despite apolipoprotein B-48 synthesis. The Journal of Clinical Investigation. 1985;76:403–412. doi: 10.1172/JCI111986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong TS, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang XC. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto A, ISHIKAWA K, UHARA S, Adachi S, Nishikawa M. Relationship between Serum Lipid Components in Hyperlipemia. The Journal of Biochemistry. 1973;74:59–65. doi: 10.1093/oxfordjournals.jbchem.a130231. [DOI] [PubMed] [Google Scholar]
- 24.Stubiger G, Aldover-Macasaet E, Bicker W, Sobal G, Willfort-Ehringer A, Pock K, Bochkov V, Widhalm K, Belgacem O. Targeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyperlipidemic patients using MALDI-QIT-TOF-MS/MS. Atherosclerosis. 2012;224:177–186. doi: 10.1016/j.atherosclerosis.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Illingworth DR, Alam SS, Alam NA. Lipoprotein lipase and hepatic lipase activity after heparin administration in abetalipoproteinemia and hypobetalipoproteinemia. Metabolism. 1983;32:869–873. doi: 10.1016/0026-0495(83)90199-3. [DOI] [PubMed] [Google Scholar]
- 26.Gamlen TR, Muller DPR. The validation and use of specific methods for the estimation of lipoprotein lipase and hepatic lipase activities in post-heparin plasma of children with hyperlipidaemia. Clin Chim Acta. 1980;106:75–83. doi: 10.1016/0009-8981(80)90376-9. [DOI] [PubMed] [Google Scholar]
- 27.Murase T, Ebara T, Okubo M. Hepatic lipase activity is decreased in Japanese patients with type III hyperlipoproteinemia. Clin Chim Acta. 2012;414:185–187. doi: 10.1016/j.cca.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 29.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of Sphingomyelin Synthesis Reduces Atherogenesis in Apolipoprotein E-Knockout Mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 30.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 31.Yeboah J, McNamara C, Jiang XC, Tabas I, Herrington DM, Burke GL, Shea S. Association of Plasma Sphingomyelin Levels and Incident Coronary Heart Disease Events in an Adult Population: Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:628–633. doi: 10.1161/ATVBAHA.109.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong M, Psaltis M, Rader DJ, Marchadier D, Barter PJ, Rye KA. Evidence that hepatic lipase and endothelial lipase have different substrate specificities for high-density lipoprotein phospholipids. Biochemistry. 2003;42:13778–13785. doi: 10.1021/bi034990n. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Kuwano T, Lagor WR, Albert CJ, Brenton S, Rader DJ, Ford DA, Brown RJ. Lipidomic analyses of female mice lacking hepatic lipase and endothelial lipase indicate selective modulation of plasma lipid species. Lipids. 2014;49:505–515. doi: 10.1007/s11745-014-3907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.