Abstract
In addition to their fermentable dietary fiber and the soluble β-glucan fiber, oats have unique avenanthramides that have anti-inflammatory and antioxidant properties that reduce coronary heart disease in human clinical trials. We hypothesized that oat consumption will increase insulin sensitivity, reduce body fat, and improve health span in Caenorhabditis elegans through a mechanism involving the daf-2 gene, which codes for the insulin/insulin-like growth factor-1–like receptor, and that hyperglycemia will attenuate these changes. Caenorhabditis elegans wild type (N2) and the null strains sir-2.1, daf-16, and daf-16/daf-2 were fed Escherichia coli (OP50) and oat flakes (0.5%, 1.0%, or 3%) with and without 2% glucose. Oat feeding decreased intestinal fat deposition in N2, daf-16, or daf-16/daf-2 strains (P < .05); and glucose did not affect intestinal fat deposition response. The N2, daf-16, or sir-2.1 mutant increased the pharyngeal pumping rate (P < .05), a surrogate marker of life span, following oat consumption. Oat consumption increased ckr-1, gcy-8, cpt-1, and cpt-2 mRNA expression in both the N2 and the sir-2.1 mutant, with significantly higher expression in sir-2.1 than in N2 (P < .01). Additional glucose further increased expression 1.5-fold of the 4 genes in N2 (P < .01), decreased the expression of all except cpt-1 in the daf-16 mutant, and reduced mRNA expression of the 4 genes in the daf-16/daf-2 mutant (P < .01). These data suggest that oat consumption reduced fat storage and increased ckr-1, gcy-8, cpt-1, or cpt-2 through the sir-2.1 genetic pathway. Oat consumption may be a beneficial dietary intervention for reducing fat accumulation, augmenting health span, and improving hyperglycemia-impaired lipid metabolism.
Keywords: Oats, C elegans model, Insulin sensitivity, Lipid metabolism, Health span
1. Introduction
Modern lifestyle-related chronic diseases, such as obesity, insulin resistance, and type 2 diabetes mellitus, are major health challenges of the 21st century [1]. Changes in dietary habits and lifestyle are among the primary causes for this undesirable development, which is already being seen in the younger population.
An inverse connection of longevity and fat mass is characteristic of humans above 25% ± 5% body fat when accompanied by obesity-related disease [2]. Both obesity and aging decrease insulin sensitivity, impair the immune response, increase inflammation, weaken the gut-bloodstream barrier, and decrease physical mobility [2]. Promotion of dietary intervention, exercise, and lifestyle modification, including second-line obesity treatments, to sustain weight loss has been elusive [3,4]. Even though a direct relationship between calorie restriction and health span has been observed in animal models [5,6], controlling food intake in humans is difficult, mainly transient, and often unsustainable because it involves multiple factors including psychological factors.
Caenorhabditis elegans is well suited to obesity studies. Lipid oxidation pathways are present in the C elegans model, such as cluster differentiation transport protein, carnitine palmitoyltranferase–1, acetyl coenzyme A carboxylase, and acetyl CoA synthetase. Resistant starch and short-chain fatty acids reduce intestinal fat deposition (IFD) in C elegans, demonstrating that the model can be used to evaluate bioactive components from gut fermentation [7–9]. Caenorhabditis elegans is a small, free-living soil nematode, a multicellular eukaryotic organism, distributed widely around the world. Caenorhabditis elegans is the first animal to have its genome completely sequenced and conserves 65% of the genes associated with human disease [8]. Caenorhabditis elegans deposits fat for energy storage along its intestinal tract of its transparent body [10]. Thus, lipid-staining dyes such as Nile red can be visualized directly and quantitated photometrically in the intact C elegans [11]. Food intake and transport in C elegans are regulated by pharyngeal movements and relaxation in the terminal bulb of the pharynx. The pharyngeal pumping rate (PPR) declines with age because of sarcopenia, and PPR is a surrogate marker of aging [12].
Many dietary interventions with enriched nondigestible but fermentable carbohydrates may reinforce optimal nutrition, enhance physiological function, reduce obesity, improve metabolic dysfunction, limit disease [4], and produce anticancer effects in humans [13,14]. The mechanisms of these interventions have been reported to be due to increased mRNA expression of the GLP-1 precursor, proglucagon and peptide YY in rodents [15], improved glucose tolerance [16,17], increased phase I xenobiotic metabolizing enzymes that are a hallmark of long-lived mice [18], promotion of mitochondrial fatty acid oxidation, and increased energy expenditure in Drosophila or adult rodents at ages analogous to 56 to 65 years in humans [17,19,20]. Consequently, fermentable dietary fiber reduces body fat accumulation [19,21], plasma cholesterol and triglycerides [22], and insulin resistance [23] in rodents and humans.
In addition to resistant starch, oats (Avena sativa) contain the soluble fermentable dietary β-glucan fiber and the unique phytoalexins avenanthramides (avns). Avenanthramides provide safe, antioxidant, anti-inflammatory, and antiangiogenic properties [24–26]. Consuming oats introduces a hypocaloric intervention through caloric dilution [27,28]; reduces low-density lipoprotein cholesterol [29], body fat [30], and coronary heart disease risk factors in human clinical trials [31,32]; increases fasting peptide YY, GLP-1, postmeal satiety, and insulin sensitivity [33]; and elevates endothelium nitric oxide production [34,35]. Like resistant starch, β-glucans are fermentable dietary fibers resistant to digestion and high-temperature cooking. An association was detected between total fermentable phenolics, a reduction in triacylglycerols, and fewer aorta inflammatory lesions in mice [36].
Decreased daf-2 signaling, the only homolog of the human insulin and insulin-like growth factor (IGF) receptor gene in C elegans, increases C elegans’ life span in a DAF-16/FOXO–dependent manner [37–39], which is reversed by hyperglycemia [40]. Furthermore, doubling the C elegans sir-2.1 gene number increases life span by 50% in a daf-16–dependent manner [41,42]. We hypothesized that oat consumption would reduce IFD, improve insulin resistance created by added glucose (2%) to the C elegans feeding media, improve hyperglycemia-impaired lipid metabolism, and increase health span through daf-16 and/or sir-2.1 pathway(s). A number of genes in C elegans have been proven to promote fat storage and mediate satiety, for example, guanylyl cyclases and the cholecystokinin (CCK) receptor. The objectives of this study were to quantify IFD by the fluorescence intensity of Nile red staining, to evaluate health span by counting PPR, and to determine the genetic pathway(s) using daf-16– and/or sir-2.1–deficient mutants.
2. Methods and materials
Caenorhabditis elegans strains and their standard laboratory food source Escherichia coli (OP50, uracil auxotroph) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN). The C elegans model does not require Institutional Animal Care and Use Committees regulation or approval [43].
2.1. Culture of E coli (OP50)
OP50 were cultured by the standard method described elsewhere [7]. Briefly, approximately 10 μL of stock E coli solution was added to media and incubated at 37°C for 24 hours. The OP50 were then subcultured in Petrifilm (3M Corporate, St Paul, MN) at 37°C for 24 hours, the colonies were confirmed to be at a density of 5 × 108 to 5 × 1011 cfu/mL, and the C elegans were allowed to eat ad libitum.
2.2. Culture of C elegans
Caenorhabditis elegans wild type (N2) and null mutants sir-2.1(ok434)IV, daf-16(mgDf50)I, and daf-16(mgDf50)I;daf-2(m65)III were used in this study (Table 1). They were grown on Nematode Growth Media agar plates (∅35 mm) and stored in a low-temperature incubator (Revco Tech, Nashville, NC) at 20°C or 15°C (daf-16(mgDf50)I;daf-2(m65)III). Caenorhabditis elegans were age synchronized. Mature gravid C elegans were transferred individually onto the agar plates and treated with a NaOH (1 mol/L) and sodium hypochlorite solution (5.25%, 5:2 ratio) to dissolve the body and release viable eggs [7]. One day before the experiment, 200 μL of a feeding media containing E coli was added to the agar dish.
Table 1.
Information on C elegans strains
| Strains | Gene deficient | Human homologs | Functions | References |
|---|---|---|---|---|
| Wild type (N2) | – | – | – | [7,59] |
| daf-16(mgDf50)I | daf-16 | Forkhead box protein O (FOXO) | Caenorhabditis elegans daf-16 has a central role in mediating the downstream insulin signaling pathways and is the major target of the daf-2 pathway. | [8] |
| daf-16(mgDf50)I; daf-2(m65)III | daf-16 and daf-2 | FOXO and insulin/IGF-1 receptor | The C elegans daf-2 gene encodes the homolog of the mammalian insulin receptor, which has preserved ligand-binding and tyrosine kinase domains | [8] |
| sir-2.1(ok434)IV | sir-2.1 | NAD-dependent protein deacetylase sirtuin-1 | The sir-2.1 transgene functions upstream of daf-16 in the insulin-like signaling pathway; regulates lifespan independently. | [42] |
2.3. Diet composition
Oats (Quaker Oats instant flakes, PepsiCo Inc, Chicago, IL) (Table 2) were powdered using a centrifugal mill with a 0.75-mm sieve (ZM 200; Retsch, Haan, Germany), autoclaved at 121°C, and suspended in distilled deionized water (5% w/v).
Table 2.
Composition of oats (Quaker® Oats)
| Content | Quantity (%) | Content | Quantity (ppm) |
|---|---|---|---|
| β-Glucan | 4.6 | Ave 2c | 5.4 |
| Insoluble dietary fiber | 7.3 | Ave 2f | 8.8 |
| Starch | 60.9 | Ave 2p | 5.3 |
| Protein | 13 | Ave 5p | 1.2 |
| Lipids | 6.6 | Ave total | 20.8 |
| Moisture | 10.3 |
Control C elegans were fed with OP50 only. The experimental groups were fed OP50 supplemented with 0.5%, 1.0%, or 3.0% oats at the larval stage L2 (Table 3). Two percent glucose was added to an additional group of each strain with the oats. The dietary nutrient composition is listed in Table 4 [44]. Caenorhabditis elegans were transferred to fresh dishes every other day receiving treatment (50 μL).
Table 3.
Protocol of experiment
| Treatments (50 μL) | Dosage (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Without glucose | Without glucose | |||||||
| 0 | 0.5 | 1 | 3 | 0 | 0.5 | 1 | 3 | |
| OP50 (2 × 109 cfu/mL, μL) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Oats (5%, μL) | 0 | 5 | 10 | 30 | 0 | 5 | 10 | 30 |
| Glucose (50%, μL) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 |
Table 4.
Diet ingredient composition
| Nutrients | Oat diet (mg/mL) | OP50 (mg/mL) | Oat treatment (mg/plate)
|
|||
|---|---|---|---|---|---|---|
| 0 | 0.5% | 1% | 3% | |||
| β-Glucan | 0.046 | – | 0.000 | 0.012 | 0.023 | 0.069 |
| Insoluble dietary fiber | 0.073 | – | 0.000 | 0.018 | 0.037 | 0.110 |
| carbohydrate | 0.609 | 7.5 | 0.375 | 0.527 | 0.680 | 1.289 |
| Protein | 0.130 | 37.5 | 1.875 | 1.908 | 1.940 | 2.070 |
| Lipids | 0.066 | 5.0 | 0.250 | 0.267 | 0.283 | 0.349 |
2.4. Pharyngeal movement (PPR)
Caenorhabditis elegans were examined periodically using a stereomicroscope (SMZ1500; Nikon, Melville, NY) with transmitted light. The PPR was recorded manually by independent observers then were returned to the incubators [9,45].
2.5. Fluorescence microscopy
Lipophilic dye Nile red was used to stain for IFD [7]. S-basal solution was added to the dish to wash the C elegans. The solution containing the C elegans was centrifuged for 20 seconds at 805g, and this procedure was repeated twice. Caenorhabditis elegans were then fixed with 4% paraformaldehyde over 2 hours at 4°C and washed with phosphate-buffered saline for 5 minutes × 3. Nile red (50 μL) was applied to the specimens for 10 minutes. Ten microliters of Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL) was applied to a glass slide followed by 20 μL of the medium containing Nile red–stained C elegans. A cover glass was mounted on the glass slide, and the slides were viewed with an epifluorescence microscope (Nikon Eclipse, Ti) equipped with a Texas red filter. Fluorescent micrographs were taken with a digital camera (Andor, DU-885k) and were analyzed using Nikon-Elements (version 3.22.11). Optical densities (arbitrary units, % of control) of Nile red–labeled IFD were determined for adult C elegans (larvae stage 4).
2.6. Quantitative real-time reverse transcription polymerase chain reaction reagents
Trizol reagent (T9424), chloroform (C2432), and isopropanol (I9516) were purchased from Sigma-Aldrich (St Louis, MO). Taqman PCR core kit (N8080228), MuLV Reverse Transcriptase (N8080018), and Ribonuclease (RNase) Inhibitor (N8080119) were obtained from Life Technologies (Grand Island, NY). RNase free micro tubes and pipette tips were used in RNA isolation and quantitative real-time reverse transcription polymerase chain reaction (Q-RT-PCR).
2.7. RNA isolation
Total RNA was extracted using the Trizol reagent as described elsewhere [23]. Caenorhabditis elegans samples were homogenized in 1 mL Trizol reagent per 50 to 100 mg of tissue. Samples went through 5 freeze-thaw cycles in which they were frozen in liquid nitrogen and thawed in a 37°C water bath. The homogenized samples were vortexed at room temperature to allow the complete dissociation of nucleoprotein complexes. Two hundred microliters of cold pure chloroform was added, and the samples were vortexed for 15 seconds and incubated at room temperature for 5 minutes. The samples were centrifuged at 15,616g for 10 minutes at 4°C, and the top aqueous phase was transferred to a fresh tube. A 600-μL cold isopropanol was used for the initial homogenization; and the samples were incubated at room temperature for 10 minutes, vortexed for 15 seconds, and centrifuged at 15,616g for 10 minutes at 4°C. The supernatant was removed, and the RNA pellet was washed once with 1 mL of cold 75% ethanol. The sample was vortexed and centrifuged at 6100g for 5 minutes at 4°C. The supernatant was removed completely, and the RNA pellet was briefly air-dried. The RNA pellet was resuspended in 0.1% diethyl pyrocarbonate–treated water and stored at −80°C. The RNA concentration was analyzed with a Nanodrop ND-1000 spectrophotometer (Wilmington, DE).
2.8. Quantitative RT-PCR
The mRNA levels of ckr-1, gcy-8, cpt-1, and cpt-2 were determined using Taqman Q-RT-PCR. The following components were added in 1-step RT-PCR: 2.2 μL H2O, 1 μL 10× Taqman buffer A, 2.2 μL 25 mmol/L MgCl2 solution, 0.3 μL 10 mmol/L dATP, 0.3 μL 10 mmol/L dCTP, 0.3 μL 10 mmol/L dGTP, 0.3 μL 10 mmol/L dUTP, 0.05 μL 20 U/μL RNase inhibitor, 0.05 μL 50 U/μL MuLV reverse transcriptase, 0.05 μL 5 U/μL Amplitaq gold, 0.25 μL Taqman probe and primers, and 3 μL 5 ng/μL RNA. The PCR was conducted in triplicate using a Taqman probe and primer set (Life Technologies, Grand Island, NY) for cholecystokinin receptor homolog (ckr-1, Ce02408606_m1), guanylyl cyclase–8 (gcy-8, Ce02456184_g1), carnitine palmitoyltranferase–1 (cpt-1, Ce02440434_m1), and carnitine palmitoyltranferase–2 (cpt-2, Ce02459919_g1). The mRNA signal was normalized over a eukaryotic 18S rRNA (Hs99999901-s1) internal control. The reaction was conducted using a 7900 HT Fast real-time PCR system (Life Technologies). Reverse transcription was added at 48°C for 30 minutes, and AmpliTaq gold activation (denaturation) was performed at 95°C for 10 minutes. Amplification of the DNA involved 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Data were analyzed using the Sequence Detector Software (Life Technologies, Carlsbad, CA). The relative quantification of gene expression (2−ΔΔCt) was calculated.
2.9. Statistical analyses
All data were presented as means ± SEM. Student t tests and analysis of variance were used for IFD, and analysis of covariance was used to compare the slopes of PPR data (SAS 9.4, Cary, NC). Statistical significance was set at P ≤ .05. Principle component analysis (PCA) was performed to cluster the factors of the oats results (SAS 9.4). Power analyses were performed to predict the sample size to achieve 80% power for PPR (minimum n = 7) and IFD (n = 5) with statistical significance as .05.
3. Results
3.1. Intestinal fat deposition
Oat feeding reduced IFD by 30% (0.5% and 1.0%) in the N2 strain (P < .05), and the addition of glucose to the oats increased IFD. Intestinal fat deposition decreased by 17% in response to 0.5% oats and 37% in response to 1.0% oats in the daf-16(mgDf50)I and increased with glucose treatment compared with the group that did not receive glucose (P < .05). The daf-16(mgDf50)I;daf-2(m65)III showed a similar dose-dependent reduction in IFD in response to oat feeding as well as in the presence of glucose (P < .05). Intestinal fat deposition was mildly lower following oat (0.5% and 1.0%) consumption in the sir-2.1(ok434)IV (P > .05) (Fig. 1).
Fig. 1.
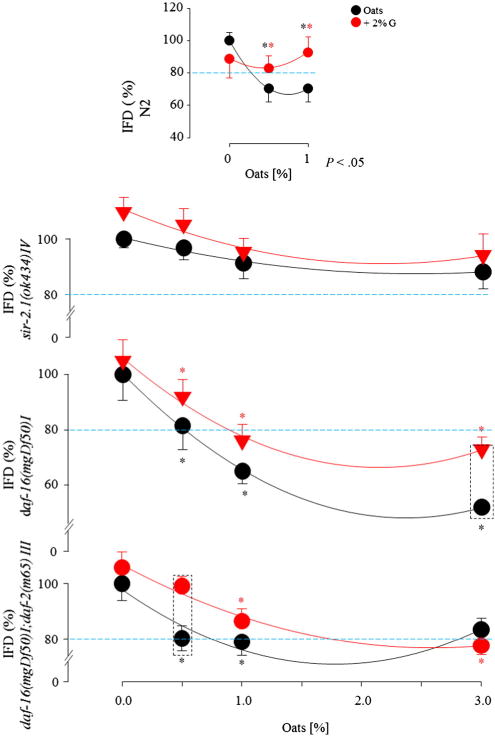
Nile Red staining of the IFD in C elegans after oat feeding in the absence or presence of 2% glucose.
3.2. Pharyngeal pumping rate
The PPRs declined in all groups as the C elegans aged. The oat treatment (0.5%, 1.0%, or 3%) increased the PPR in N2, daf-16, and sir-2.1 mutants (n = 24 C elegans/3 dishes, P < .001–.05). The PPR in N2 was increased in the oat group (0.5%, 1.0%, or 3%) in the presence of glucose (P < .005–.03). This increase persisted in the presence of glucose at a low dose in daf-16 or daf-16/daf-2 mutant (P < .05) (Fig. 2).
Fig. 2.
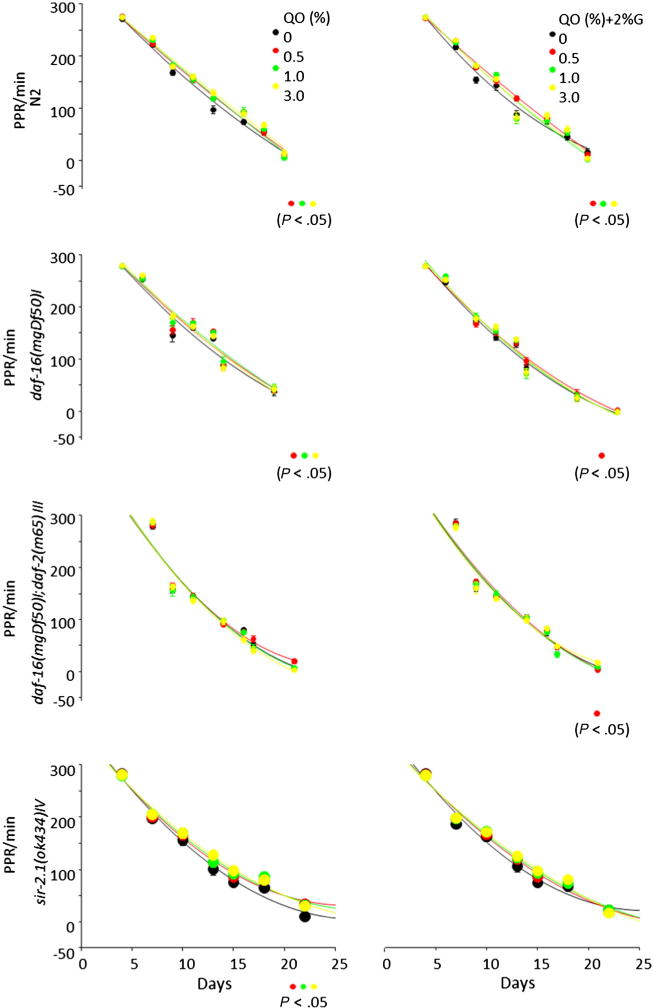
Oat feeding affected PPR, a surrogate marker of life span in C elegans.
3.3. Lipid metabolism gene expression
Oat feeding (0.5% and 1%) increased the mRNA expression of ckr-1, gcy-8, cpt-1, and cpt-2 in the C elegans N2, and sir-2.1– and daf-16–deficient strains (P < .01). Additional glucose further increased these mRNA expressions in N2 and sir-2.1. In the daf-16–deficient strain, ckr-1 and gcy-8 were increased after oat feeding (P < .01). All 4 genes tested were elevated in response to glucose treatment alone, and oat consumption in the presence of glucose significantly reduced mRNA expression of the 4 genes (P < .01). Oat feeding with glucose reduced mRNA expression of all 4 genes in daf-16/daf-2–deficient strains (P < .01). Oats (0.5%) plus glucose increased cpt-1 and cpt-2 (P < .01, Fig. 3).
Fig. 3.
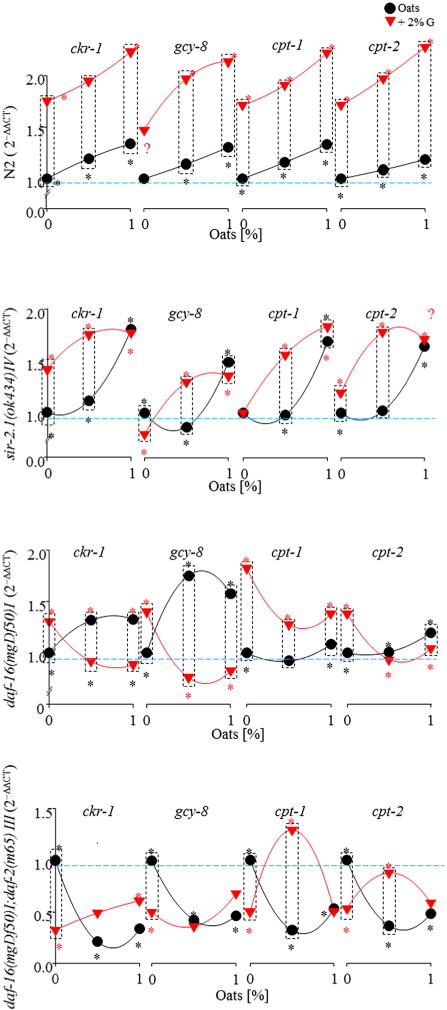
Oat feeding altered gene expression of ckr-1, gcy-8, cpt-1, and cpt-2 by Q-RT-PCR in C elegans.
3.4. Principle component analysis
Principle component analyses revealed 2 theoretical strong factors that played important roles in the relationship of the components that were tested in this study (Fig. 4, Table 5). Inverse relationship between the IFD and the PPR was detected in N2, daf-16/daf-2, and sir-2.1 mutants. The PPRs were strongly and positively related to the 2 factors in the 3 strains. By contrast, the IFDs were strongly and positively related to the factor 1 in the N2 and the daf-16/daf-2 double mutant, which was negatively associated in the sir-2.1 mutant. The IFD or PPR seemed weakly related to the ckr-1, cpt-1, cpt-2, and gcy-8. The ckr-1, cpt-1, cpt-2, and gcy-8 had strong positive relationships with factor 1 N2, daf-16/daf-2, and sir-2.1 mutants. A loose but positive relationship of the 4 genes with the factor 2 was seen in the daf-16 mutant, whereas the IFD was negatively related to the factor 1 and the PPR did not have any association with either the factors or the 4 genes.
Fig. 4.
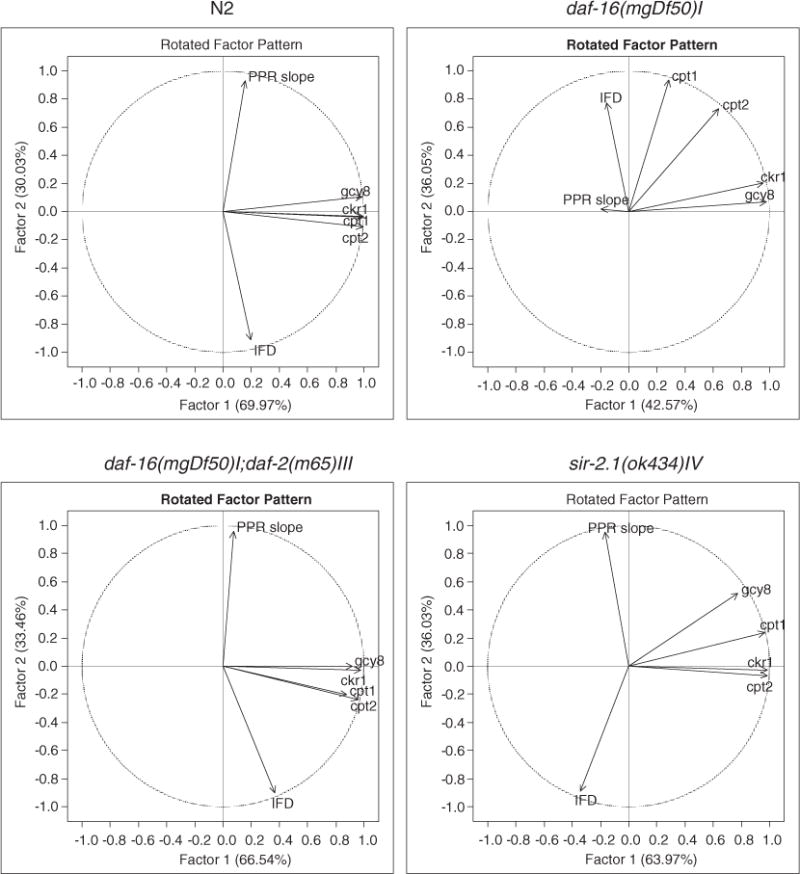
Scatter plot of PCA showing the 2 strong factors in relationship with ckr-1, cpt-1, cpt-2, gcy-8, PPR slope, and IFD.
Table 5.
PCA description: factor 1 and factor 2
| Relation to factors | Factor 1
|
Factor 2
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPR | IFD | ckr-1 | gcy-8 | cpt-1 | cpt-2 | PPR | IFD | ckr-1 | gcy-8 | cpt-1 | cpt-2 | |
| N2 | 11 | 24 | 99 | 98 | 100 | 100 | 94 | −90 | 1 | 15 | 2 | −6 |
| daf-16(mgDf50)I | −20 | −15 | 95 | 98 | 29 | 64 | 2 | 77 | 20 | 7 | 93 | 73 |
| daf-16(mgDf50)I;daf-2(m65)III | −24 | 64 | 93 | 87 | 90 | 99 | 93 | −73 | 29 | 30 | 9 | 8 |
| sir-2.1(ok434)IV | −17 | −34 | 98 | 78 | 96 | 98 | 95 | −89 | −3 | 52 | 24 | −7 |
4. Discussion
The effect of oat consumption paired with or without 2% glucose was evaluated in the C elegans model by observing changes in IFD determined by the fluorescent intensity of Nile red, alterations in health span indicated by PPR, and variations in selected mRNA expression indicated by PCR in this study. The IFD was reduced in N2, and the daf-16– and daf-16/daf-2–deficient mutants. Apart from natural age-related decline of the PPR in all groups, oat treatment sustained the PPR in N2 with or without glucose. Oat treatment also sustained the PPR in daf-16 and sir-2.1 mutants without glucose. Low-dose oat treatment (0.5%) increased the PPR in the presence of glucose. Oat consumption increased mRNA expressions of ckr-1, gcy-8, cpt-1, and cpt-2 genes, which were greater in N2 than in the sir-2.1 mutant; and additional glucose further increased the expression by 1.5-fold in both strains. The effects of oats on health span and IFD in N2 was mediated by daf-2 and partially depended on daf-16, with and without the presence of hyperglycemia.
Hyperglycemia often coexists with hyperlipidemia. In C elegans, inactivation of more than 300 genes has been shown to reduce IFD; and inactivation of more than 100 genes, to increase fat storage [46]. Oat consumption significantly reduced IFD in the N2, and daf-16– and daf-16/daf-2–deficient mutants, whereas the presence of glucose increased IFD. However, oats reduced IFD even when there was insulin resistance, as seen in the daf-16/daf-2–deficient group and other groups with additional glucose. Glucose did not attenuate the oat-induced IFD reduction in the daf-16/daf-2–deficient mutant, suggesting an indirect improvement in insulin sensitivity through lipid metabolism activating other pathway(s), such as the serotonin pathway. In rodent diabetes, hyperglycemia is associated with an increase in peroxisome proliferator activator alpha (PPARα) activity associated with insulin resistance [10]. In a C elegans model, deletion of the nuclear hormone receptor–49, a homologue of PPARα, prevents hyperlipidemia and restores insulin sensitivity in a manner similar to PPARα-null mice [47]. The reduced IFD following oat consumption in the daf-16/daf-2–deficient mutant suggests that oats may improve hyperglycemia-impaired lipid metabolism.
Reduced food intake, reduced lipid metabolism, and a reduction in metabolic turnover in N2 are associated with an extension of life span [41]. Unlike the N2, or daf-16/daf-2– and daf-16–deficient mutants, the IFD was only mildly reduced by the same amount of oat consumption in the sir-2.1–deficient mutant with increased mRNA expression of the 4 genes similar to N2, suggesting increased lipid metabolism. The ability of both the wild-type and mutant nematodes to sustain the PPR indicates that oat consumption improved health span independent of the sir-2.1 pathway, which relates to the co-regulator/genes in the aging process in C elegans [48,49]. Our data are in agreement with the literature that shows that involvement of sir-2.1 in lipid metabolism has multiple factors similar to life span [41], and either a higher dose is required in the absence of the sir-2.1 gene pathway or the sir-2.1 pathway was not critically involved in the IFD reduction.
Hyperglycemia reduced the C elegans life span [50] and completely suppressed the long life span of daf-2(−) insulin/IGF-1 receptor mutants in C elegans [4]. The daf-16 gene plays a central role in activating the downstream insulin signaling pathways and is the major target of the daf-2 that negatively regulates daf-16 [50,51]. In the present study, oat feeding increased PPR and decreased IFD, which were mediated by the daf-16/daf-2 pathways. The benefit from oats’ may be attributed to its being high in fibers, β-glucan, proteins, and unique avns.
Although activation of the CCK receptor does not change circulating triacylglycerol or adipose tissue, it increases glucose tolerance and insulin sensitivity and decreases liver triacylglycerol in mice [52,53]. Similarly, in humans, activation of CCK pathway does not appear to play a central role in long-term energy balance but signals postprandial satiety [53,54]. However, ckr-1, the analogous gene in the C elegans species, increased in response to oat consumption that decreased IFD in the presence of glucose and preserved PPR, suggesting that ckr-1 has a potential role in the lipid metabolism. The gcy-8 is exclusively expressed in AFD neurons and contributes to thermotaxic behavior [55]. Increased expression of gcy-8 may indicate an increase in metabolic rate. In the daf-16–deficient mutant, the mRNA expression of ckr-1 and gcy-8 was increased in response to oat feeding. This effect was reversed in presence of glucose that initially elevated cpt-1 and cpt-2 followed by a reduction when oats were added.
An elevated cpt-1 or cpt-2 suggests an augmented lipid β-oxidation and turnover. Expression of all 4 genes was reduced in the daf-16/daf-2–deficient mutant when treated with oats, and cpt-1 and cpt-2 expression increased nearly 2-fold with additional glucose. The reduced cpt-1 or cpt-2 in daf-16/daf-2–deficient mutant suggests that the C elegans were either unable to use/metabolize the nutrients from the food as seen in daf-16–deficient mutant or were unable to adequately downregulate metabolic turnover as observed in the daf-16/daf-2–deficient mutant, thus reducing IFD. In the 3 strains, N2, daf-16/daf-2, and sir-2.1 mutants, the factor 2 of the PCA was closely related to health span, which is inversely related to IFD; and the factor 1 had strong positive relationships with cpt-2 and gcy-8. The PCA data showed an inverse relationship of the daf-16 to the IFD, cpt-1, and cpt-2.
The exclusive and unique avns in oats are fermentable polyphenols that also resist digestion and provide health benefits. A high β-glucan concentration increases viscosity in the gastrointestinal tract, delays nutrient absorption, decreases the glycemic peak by 50%, lowers low-density lipoprotein cholesterol, reduces body fat in humans and rodents [56], counteracts obesity-related inflammation [25], and enhances immunity in C elegans by activating the dectin-1 receptor and DAF-2/insulin-like receptor pathways [57,58]. These avns, along with the β-glucan content of oats, make oats a unique food that may aid in lowering body fat and controlling body weight. Future studies will investigate possible synergistic effects of avns and β-glucan, as well as the role of the serotonin pathway in improving hyperglycemia-induced hyperlipidemia and increasing health span.
This study had limitations as well. Caenorhabditis elegans have two-thirds of genes that are related to human diseases. Thus, C elegans is used as a stage for use in high-throughput screening to search for nutritional interventions for health before confirmatory studies in higher animal models and human clinical trials. Compensatory pathways that exist in higher animals may be not discovered by the present study using C elegans. Active components of the oats extract will be further tested in followup studies using higher animal species.
In conclusion, our data indicate that oats have unique properties that induce an improvement in insulin sensitivity, reduce IFD, and extend health span in the C elegans model organism, giving support to our study hypothesis. As part of a healthy diet, oats are an appropriate food to improve public health. Avenanthramides and β-glucan are functional food components of oats that can be incorporated into the daily diet in various ways. Caenorhabditis elegans is an attractive in vivo animal model for initial studies of nutrition interventions before confirmation in higher animal species.
Acknowledgments
The nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. Drs. Burton and Johnson are supported by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center.
Abbreviations
- Avns
avenanthramides
- IFD
intestinal fat deposition
- IGF
insulin-like growth factor
- PPARα
peroxisome proliferator activator alpha
- PPR
pharyngeal pumping rate
- Q-RT-PCR
quantitative real-time reverse transcription polymerase chain reaction
Footnotes
Conflict of interest statement
This research was supported by a nonrestricted donation from PepsiCo Inc. Oats used in this study were a gift of PepsiCo Inc. Y. Chu is an employee of PepsiCo, Inc, which manufactures oatmeal products under the brand name Quaker Oats. The views expressed in this article are those of the author and do not necessarily reflect the opinion or policies of PepsiCo, Inc.
References
- 1.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 2.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:E1059–66. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kress AM, Hartzell MC, Peterson MR, Williams TV, Fagan NK. Status of U.S. military retirees and their spouses toward achieving Healthy People 2010 objectives. Am J Health Promot. 2006;20:334–41. doi: 10.4278/0890-1171-20.5.334. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–8. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 6.Lee GD, Wilson MA, Zhu M, Wolkow CA, de CR, Ingram DK, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–24. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J, Enright F, Keenan M, Finley J, Zhou J, Ye J, et al. Resistant starch, fermented resistant starch, and short-chain fatty acids reduce intestinal fat deposition in Caenorhabditis elegans. J Agric Food Chem. 2010;58:4744–8. doi: 10.1021/jf904583b. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Greenway FL. Caenorhabditis elegans as a model for obesity research. Int J Obes (Lond) 2012;36:186–94. doi: 10.1038/ijo.2011.93. [DOI] [PubMed] [Google Scholar]
- 9.Finley JW, Sandlin C, Holiday DL, Keenan MJ, Prinyawiwatkul W, Zheng J. Legumes reduced intestinal fat deposition in the Caenorhabditis elegans model system. J Funct Foods. 2013;5:1487–93. [Google Scholar]
- 10.Hostetler HA, Huang H, Kier AB, Schroeder F. Glucose directly links to lipid metabolism through high affinity interaction with peroxisome proliferator-activated receptor alpha. J Biol Chem. 2008;283:2246–54. doi: 10.1074/jbc.M705138200. [DOI] [PubMed] [Google Scholar]
- 11.Fei YJ, Liu JC, Inoue K, Zhuang L, Miyake K, Miyauchi S, et al. Relevance of NAC-2, an Na+-coupled citrate transporter, to life span, body size and fat content in Caenorhabditis elegans. Biochem J. 2004;379:191–8. doi: 10.1042/BJ20031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow DK, Glenn CF, Johnston JL, Goldberg IG, Wolkow CA. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp Gerontol. 2006;41:252–60. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994:937–42. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87:761–8. [PubMed] [Google Scholar]
- 15.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, Xi X, Raggio AM, et al. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity (Silver Spring) 2006;14:683–9. doi: 10.1038/oby.2006.77. [DOI] [PubMed] [Google Scholar]
- 16.Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A, et al. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics. 2012;5:26–44. doi: 10.1159/000335319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch up-regulates total GLP-1 and PYY in a sustained daylong manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–6. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–95. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 2006;14:1523–34. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol Nutr Food Res. 2011;55:1499–508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L, Keenan MJ, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–5. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Deckere EA, Kloots WJ, van Amelsvoort JM. Resistant starch decreases serum total cholesterol and triacylglycerol concentrations in rats. J Nutr. 1993;123:2142–51. doi: 10.1093/jn/123.12.2142. [DOI] [PubMed] [Google Scholar]
- 23.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CY, Milbury PE, Collins FW, Blumberg JB. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J Nutr. 2007;137:1375–82. doi: 10.1093/jn/137.6.1375. [DOI] [PubMed] [Google Scholar]
- 25.Meydani M. Potential health benefits of avenanthramides of oats. Nutr Rev. 2009;67:731–5. doi: 10.1111/j.1753-4887.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 26.Chu YF, Wise ML, Gulvady AA, Chang T, Kendra DF, Jan-Willem van KB, et al. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013;139:426–31. doi: 10.1016/j.foodchem.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 27.Greenway F, O’Neil CE, Stewart L, Rood J, Keenan M, Martin R. Fourteen weeks of treatment with Viscofiber increased fasting levels of glucagon-like peptide-1 and peptide-YY. J Med Food. 2007;10:720–4. doi: 10.1089/jmf.2007.405. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ, et al. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. J Nutr. 2001;131:1465–70. doi: 10.1093/jn/131.5.1465. [DOI] [PubMed] [Google Scholar]
- 29.Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. Am J Clin Nutr. 2002;76:351–8. doi: 10.1093/ajcn/76.2.351. [DOI] [PubMed] [Google Scholar]
- 30.Weickert MO, Mohlig M, Schofl C, Arafat AM, Otto B, Viehoff H, et al. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–80. doi: 10.2337/diacare.29.04.06.dc05-2374. [DOI] [PubMed] [Google Scholar]
- 31.Chang HC, Huang CN, Yeh DM, Wang SJ, Peng CH, Wang CJ. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum Nutr. 2013;68:18–23. doi: 10.1007/s11130-013-0336-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Li L, Song P, Wang C, Man Q, Meng L, et al. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutr J. 2012;11:54. doi: 10.1186/1475-2891-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang XF, Yu Y, Beck EJ, South T, Li Y, Batterham MJ, et al. Diet high in oat beta-glucan activates the gut-hypothalamic (PYY(3)(−)(3)(6)-NPY) axis and increases satiety in diet-induced obesity in mice. Mol Nutr Food Res. 2011;55:1118–21. doi: 10.1002/mnfr.201100095. [DOI] [PubMed] [Google Scholar]
- 34.Andersson KE, Hellstrand P. Dietary oats and modulation of atherogenic pathways. Mol Nutr Food Res. 2012;56:1003–13. doi: 10.1002/mnfr.201100706. [DOI] [PubMed] [Google Scholar]
- 35.Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, Lahteenmaki L, Laaksonen DE, Herzig KH, et al. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr. 2009;139:461–6. doi: 10.3945/jn.108.099945. [DOI] [PubMed] [Google Scholar]
- 36.Brito LF, Queiros LD, Peluzio MC, Ribeiro SM, Matta SL, Queiroz JH. Effect of dry coffee residues fermented with Monascus ruber on the metabolism of Apo E mice. Arq Bras Cardiol. 2012;99:747–54. doi: 10.1590/s0066-782x2012005000068. [DOI] [PubMed] [Google Scholar]
- 37.Hertweck M, Gobel C, Baumeister R. Celegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6:577–88. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- 38.Patel DS, Garza-Garcia A, Nanji M, McElwee JJ, Ackerman D, Driscoll PC, et al. Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics. 2008;178:931–46. doi: 10.1534/genetics.107.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–17. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abate JP, Blackwell TK. Life is short, if sweet. Cell Metab. 2009;10:338–9. doi: 10.1016/j.cmet.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–5. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Barak N, Beck Y, Vasselli JR, King JF, King ML, et al. Caenorhabditis elegans as a model for obesity research. 2013 [Google Scholar]
- 44.Feijo Delgado F, Cermak N, Hecht VC, Son S, Li Y, Knudsen SM, et al. Intracellular water exchange for measuring the dry mass, water mass and changes in chemical composition of living cells. PLoS One. 2013;8:e67590. doi: 10.1371/journal.pone.0067590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng J, Greenway FL, Heymsfield SB, Johnson WD, King JF, King MJ, et al. Effects of three intense sweeteners on fat storage in the C. elegans model. Chem Biol Interact. 2014;215C:1–6. doi: 10.1016/j.cbi.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 47.Atherton HJ, Jones OA, Malik S, Miska EA, Griffin JL. A comparative metabolomic study of NHR-49 in Caenorhabditis elegans and PPAR-alpha in the mouse. FEBS Lett. 2008;582:1661–6. doi: 10.1016/j.febslet.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–54. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 49.Kyrylenko S, Kyrylenko O, Suuronen T, Salminen A. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci. 2003;60:1990–7. doi: 10.1007/s00018-003-3090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–91. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 52.Irwin N, Frizelle P, O’Harte FP, Flatt PR. Metabolic effects of activation of CCK receptor signaling pathways by twice-daily administration of the enzyme-resistant CCK-8 analog, (pGlu-Gln)-CCK-8, in normal mice. J Endocrinol. 2013;216:53–9. doi: 10.1530/JOE-12-0353. [DOI] [PubMed] [Google Scholar]
- 53.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr. 1981;34:154–60. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 54.Jordan J, Greenway FL, Leiter LA, Li Z, Jacobson P, Murphy K, et al. Stimulation of cholecystokinin-A receptors with GI181771X does not cause weight loss in overweight or obese patients. Clin Pharmacol Ther. 2008;83:281–7. doi: 10.1038/sj.clpt.6100272. [DOI] [PubMed] [Google Scholar]
- 55.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–52. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wursch P, Pi-Sunyer FX. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care. 1997;20:1774–80. doi: 10.2337/diacare.20.11.1774. [DOI] [PubMed] [Google Scholar]
- 57.Engelmann I, Pujol N. Innate Immunity in C. elegans. Adv Exp Med Biol. 2011;708:105–21. doi: 10.1007/978-1-4419-8059-5_6. [DOI] [PubMed] [Google Scholar]
- 58.Tsoni SV, Brown GD. beta-Glucans and dectin-1. Ann NY Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 59.Gems D, Riddle DL. Defining wild-type life span in Caenorhabditis elegans. J Gerontol A: Biol Med Sci. 2000;55:B215–9. doi: 10.1093/gerona/55.5.b215. [DOI] [PubMed] [Google Scholar]


