Abstract
Rationale
Sardinian alcohol-preferring (sP) rats displayed high sensitivity to time schedule and consumed intoxicating amounts of alcohol during the last portion of the dark phase of the light/dark cycle when exposed to daily drinking sessions of one hour, with concurrent availability of multiple alcohol concentrations, and unpredictability of time of alcohol access.
Objectives
The present study investigated whether sensitivity of sP rats to time schedule extended to operant procedures of alcohol self-administration.
Methods
In Experiment 1, three different alcohol solutions (10%, 20%, and 30%, v/v) were concurrently available under a Fixed Ratio 4 schedule of reinforcement and with unpredictable time schedule; water was available uncontingently. Experiments 2 and 3 assessed the sensitivity of the motivational properties of alcohol to time schedule; rats were exposed to (a) self-administration sessions under the Progressive Ratio (PR) schedule of reinforcement and (b) sessions of alcohol seeking under the extinction responding (ER) schedule.
Results
In Experiment 1, number of lever-responses and amount of self-administered alcohol were positively correlated with time of alcohol access during the dark phase. When the self-administration session occurred at the first and latest hours of the dark phase, the amount of self-administered alcohol averaged 0.95–1.0 and 1.55–1.65 g/kg, respectively. In Experiments 2 and 3, values of breakpoint and ER for alcohol were approximately 50% higher when the sessions occurred at the last than first hour of the dark phase.
Conclusions
The reinforcing and motivational properties of alcohol were sensitive to time schedule and stronger at the end of the dark phase.
Keywords: Unpredictability of alcohol access, Alcohol self-administration, Fixed Ratio, Progressive Ratio, Extinction responding, Reinforcing properties, Motivational properties, Binge-like drinking, Sardinian alcohol-preferring rats
Introduction
This laboratory has recently proposed a new rodent model of binge-like drinking: when exposed to daily drinking sessions of one hour, with concurrent availability of multiple alcohol concentrations (0%, 10%, 20%, and 30%, v/v), and – most importantly – unpredictability of time of access to alcohol, selectively bred Sardinian alcohol-preferring (sP) rats consumed up to intoxicating amounts of alcohol and displayed high sensitivity to time schedule (Colombo et al. 2014; 2015). Specifically, it was found that – as the time of alcohol access moved over the dark phase of the 12:12 hour light/dark cycle – alcohol intake increased progressively; when the drinking session occurred at the end of the dark phase, alcohol intake averaged ≥2 g/kg, resulted in blood alcohol levels (BALs) of approximately 100 mg% [meeting the criterion posed for binge drinking in humans (NIAAA 2004)], and produced severe motor-incoordination (Colombo et al. 2014).
The present study was designed to assess whether sensitivity to time schedule of alcohol access and escalation to exceptionally high levels of alcohol consumption generalizes to procedures of operant, oral alcohol self-administration. In comparison to the homecage alcohol-drinking procedure, operant procedures of alcohol self-administration provides the remarkable advantage of measuring – besides the mere amount of alcohol consumed – the strength of the reinforcing and motivational properties of alcohol.
To this end, in the present study sP rats were initially exposed to 12 consecutive daily sessions of alcohol self-administration under the Fixed Ratio (FR) 4 (FR4) schedule of reinforcement: each fourth response on the lever [i.e., the response requirement (RR)] was reinforced by the concurrent presentation, for a time period of 3 s, of 3 different alcohol concentrations (10%, 20%, and 30%, v/v). Time of the self-administration session was changed every day in a semi-random order, so that rats experienced all 12-hour time periods of the dark phase as periods of access to alcohol. This first experiment evaluated the sensitivity of the reinforcing properties of alcohol to time schedule.
Rats were then exposed, at the 1st and 12th hour of the dark phase, to sessions of (a) alcohol self-administration under the within-session Progressive Ratio (PR) schedule of reinforcement and (b) alcohol-seeking under the within-session extinction responding (ER) procedure. Under the PR procedure, RR is progressively increased after the delivery of each single reinforcer; under the ER procedure, lever-responding is never reinforced, irrespective of the number of lever-responses. These two experiments evaluated the strengths of the motivational properties of alcohol at the two ends of the dark phase: the lowest ratio not completed (named breakpoint) in the PR procedure and the highest number of lever-responses (named ER) in the ER procedure are indeed conventionally taken as measures of the rats’ motivation to “work” for alcohol (see Markou et al. 1993; Samson et al. 2001).
The working hypothesis of the present study was that lever-responding for alcohol and amount of self-administered alcohol positively correlated with time of alcohol access during the dark phase, leading to increased reinforcing properties of alcohol and large intakes of alcohol when the self-administration sessions occurred in the latest hours of the dark phase. Additionally, it was expected that values of breakpoint and ER for alcohol were higher when the sessions occurred at the 12th rather than 1st hour of the dark phase.
Materials and Methods
All experimental procedures employed in the present study were in accordance with the Italian law on the “Protection of animals used for scientific reasons”.
Animals
Male sP rats (see Colombo et al. 2006) from the 87th and 88th generation and 50-days-old at the start of the study, were utilized. Rats were alcohol-naive before the start of the study. Rats were singly housed in standard plastic cages with wood chip bedding; single housing started at the age of approximately 40 days. The animal facility was under an inverted 12:12-hour light-dark cycle (lights on at 7:00 p.m.), at a constant temperature of 22±2°C and relative humidity of approximately 60%. Food pellets (Harlan, San Pietro al Natisone, Italy) and water were always available in the homecage, except as noted.
The total number of rats used was n=24; this sample size equated to that used in the first drinking study (Experiment 1; Colombo et al. 2014). As only 8 operant chambers were available for self-administration sessions (see below), rats were divided into 3 separate groups of n=8, exposed sequentially to the same identical experimental procedure. Data from each single set of rats were then aggregated.
Apparatus
Self-administration sessions were conducted in 8 modular chambers (Med Associates, St. Albans, VT, USA) located in sound-attenuated cubicles, with fans for ventilation and background white noise. Chambers were arranged to replicate the 4-bottle choice procedure of the previous drinking studies (Colombo et al. 2014; 2015). To this end, the front panel of each chamber was equipped with (a) one retractable response-lever, (b) the retractable spouts of 3 sipper bottles (250-ml capacity) located outside the chamber, and (c) 3 stimulus lights (yellow, green, and light blue) mounted above the holes through which sipper spouts were made available. The portion of the panel surrounding each spout hole was painted the same color (yellow, green, or light blue) of the corresponding stimulus light. Sipper bottles were filled with 10%, 20%, and 30% (v/v) alcohol, respectively, except as noted; each alcohol concentration was associated to a fixed stimulus-light color throughout the study. The 3 sipper bottles were mounted so that their position combination differed among the 8 chambers as much as possible. On each daily self-administration session, rats were allocated to a chamber on a random basis, with the intent of preventing development of bottle-position preference or habit. The back panel of each chamber was equipped with (a) the non-retractable spout of a sipper bottle (filled with water) and (b) a white house light (centered at the top of the panel).
Start of the self-administration session was signaled by illumination of the house light and insertion of the lever. Achievement of RR (see below) resulted in (a) concurrent exposure of the 3 sipper spouts and (b) concurrent illumination of the corresponding stimulus lights; both events lasted 3 s. End of the self-administration session was associated to retraction of the lever and switch off of the house light.
Experimental procedure
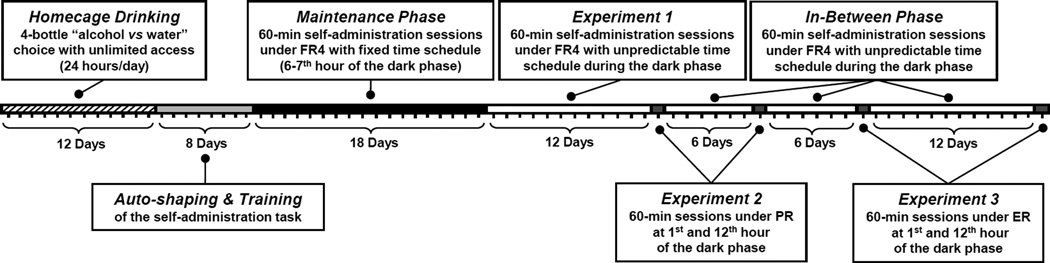
Schematic representation of the different experimental phases of this study is given in Fig. 1.
Figure 1.
Schematic representation of the experimental design.
Homecage, 4-bottle “alcohol vs water” choice drinking
At the age of approximately 50 days, rats were exposed to the homecage, 4-bottle choice regimen between water and 10%, 20%, and 30% (v/v) alcohol with unlimited access (24 hours/day) for 12 consecutive days. This initial phase was conducted to allow the rats to become accustomed to the taste of the 3 alcohol solutions and start to experience the pharmacological effects of alcohol, in order to possibly shorten the subsequent auto-shaping phase once rats are introduced into the operant chambers. On a daily basis, bottles were refilled with fresh solution and their positions changed randomly to avoid development of position preference. Alcohol and water intake was monitored by weighing the bottles (0.01-g accuracy) every day immediately before the start of the dark phase. Possible fluid spillage was calculated by using multiple bottles filled with the different alcohol concentrations and positioned in empty cages interspersed in the cage racks; mean spilt volumes were subtracted before data analysis.
Auto-shaping, training, and maintenance phases of alcohol self-administration
Immediately after the homecage, 4-bottle choice regimen, rats were introduced into the operant chambers and trained to lever-respond for alcohol. Over these initial phases, sessions were conducted 5 days per week (Monday to Friday) during the 6–7th hour of the dark phase of the daily light/dark cycle. Sessions lasted 60 min. Rats were deprived of water during the 12 hours preceding the first 3 sessions in the operant chamber. In the first session, all 3 sipper bottles were filled with water and sipper spouts were made available uncontingently via a variable time 120-s schedule (VT20 s); this session was conducted to allow the rats to become accustomed to the noise generated by the sipper-bottle apparatus. In the second session, all 3 sipper bottles were filled with water; water was available under an FR1 schedule of reinforcement. Starting from the third session, the 3 alcohol solutions were made concurrently available; this third session completed the auto-shaping phase. FR was increased from FR1 to FR4 over 6 consecutive sessions; in the third session, which was the first one with alcohol available, the number of reinforcer presentations was limited to 40 in order to avoid rats, made thirsty by water deprivation, experiencing alcohol overdoses. After these initial 6 sessions with alcohol available (that completed the training phase), rats were exposed to 18 consecutive sessions with the 3 alcohol solutions concurrently available under the FR4 schedule of reinforcement and the water sipper-bottle freely available (maintenance phase).
Bottles were refilled with fresh solution on a daily basis. Alcohol and water intake was monitored by weighing the bottles with a 0.01-g accuracy immediately before and after each daily self-administration session. Possible fluid spillage was calculated using a custom-made apparatus composed of fennel and reservoir positioned beneath each single sipper spout; mean spilt volumes were subtracted before data analysis.
One rat never learned to lever-respond and was excluded from the study; its alcohol and water intake during the homecage drinking phase has been excluded from data analysis. From here onwards, the sample size was n=23.
Experiment 1: Alcohol self-administration sessions with unpredictable time schedule
Starting from the day after the end of the maintenance phase, rats were exposed to 12 consecutive daily self-administration sessions (60-min duration; no weekend interruption), with an FR4 schedule of reinforcement and the 3 alcohol solutions; water was always freely available. The time of each daily self-administration session was established semi-randomly so that, over 12 consecutive days, all 12 hours of the dark phase were tested (sequence used: 9, 1, 6, 8, 5, 11, 2, 10, 3, 7, 4, and 12). Alcohol and water intake was monitored as described above.
Experiment 2: Alcohol self-administration sessions under the Progressive Ratio schedule of reinforcement
The day after the completion of 12-day period of self-administration sessions with unpredictable time schedule, rats were divided into 2 groups of n=11–12, matched for lever-responding, amount of self-administered alcohol, and schedule sensitivity over the previous phase, and exposed to a self-administration session – under the PR schedule of reinforcement and with the 3 alcohol solutions – occurring during the 1st or 12th hour of the dark phase; water was always freely available. This experiment was conducted according to a crossover design, so that – one week after the first PR session – rats were exposed to an additional PR session with opposite time schedule (i.e., rats exposed to the first PR session at the 1st hour of the dark phase were exposed to the second PR session at the 12th hour of the dark phase; vice versa, rats exposed to the first PR session at the 12th hour of the dark phase were exposed to the second PR session at the 1st hour of the dark phase). Six consecutive daily self-administration sessions (no weekend interruption) under FR4 schedule of reinforcement were interposed between the two PR sessions (named in-between phase in Fig. 1); the time of these 6 self-administration sessions was changed daily and established semi-randomly (sequence used: 6, 3, 8, 10, 5, and 2). During the PR sessions (60-min duration), RR was increased progressively over the session according to the procedure described by Richardson and Roberts (1996) with some slight modifications in the first RRs; namely, RR was increased as follows: 4, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, etc. Alcohol and water intake was monitored as described above.
Experiment 3: Alcohol-seeking behavior (extinction responding)
After the second self-administration session under the PR schedule of reinforcement, rats were exposed to 6 consecutive daily self-administration sessions (no weekend interruption) under FR4 schedule of reinforcement and unpredictable time schedule (sequence used: 9, 3, 11, 2, 8, and 5) (in-between phase in Fig. 1). On the 7th day, rats were divided into 2 groups of n=11–12, matched for lever-responding, amount of self-administered alcohol, and schedule sensitivity over the last 6 self-administration sessions, and exposed to a session of ER occurring during the 1st or 12th hour of the dark phase. This experiment was conducted according to a crossover design, so that – two weeks after the first ER session – rats were exposed to an additional ER session with opposite time schedule (i.e., rats exposed to the first ER session at the 1st hour of the dark phase were exposed to the second ER session at the 12th hour of the dark phase; vice versa, rats exposed to the first ER session at the 12th hour of the dark phase were exposed to the second ER session at the 1st hour of the dark phase). Twelve consecutive daily self-administration sessions (no weekend interruption) under FR4 schedule were interposed between the two ER sessions (in-between phase in Fig. 1); the time of these 12 self-administration sessions was changed daily and established semi-randomly (sequence used: 8, 1, 10, 5, 12, 7, 4, 11, 3, 9, 2, and 6). During the ER sessions (60-min duration), lever-responding was never reinforced; water was not available.
Measured variables and data analysis
In the 12-day period of the homecage drinking, measured variables were as follows: (a) daily alcohol intake from each single bottle (expressed in g/kg pure alcohol); (b) daily total alcohol intake (i.e., sum of the amount consumed from each single sipper bottle; g/kg); (c) daily water intake (ml/kg).
In all self-administration sessions under the FR4 schedule of reinforcement (Experiment 1 and in-between phases), measured variables were as follows: (a) number of lever-responses; (b) amount of self-administered alcohol from each single sipper bottle (g/kg); (c) total amount of self-administered alcohol (i.e., sum of the amount consumed from each single sipper bottle; g/kg); (d) amount of consumed water (ml/kg); (e) latency (s) to the first lever-response; (f) time (s) of the last reinforcer. In the self-administration sessions under the PR schedule of reinforcement (Experiment 2), measured variables were breakpoint for alcohol (defined as the lowest RR not achieved) along with all those of the self-administration sessions under the FR4 schedule of reinforcement (see above). In the ER sessions (Experiment 3), measured variables were (a) number of lever-responses (defined ER) and (b) latency (s) to the first lever-response.
In all experiments, data were initially tested for normality using the D’Agostino and Pearson test. When normally distributed, data were analyzed by paired Student t test or ANOVA; ANOVA was followed by Tukey’s test for post hoc comparisons. When not normally distributed, data were analyzed by Wilcoxon test or Friedman test.
In Experiment 1, data on number of lever-responses, total amount of self-administered alcohol, and water intake in the 12 self-administration sessions were also exposed to regression analysis (mean number of lever-responses, mean amount of self-administered alcohol, or mean water intake vs time of the self-administration session) and calculation of the Pearson correlation coefficient.
All statistical analyses were conducted using GraphPad Prism (Version 6.05) software package (GraphPad Software Inc., La Jolla, CA, USA).
Results
Homecage, 4-bottle “alcohol vs water” choice drinking
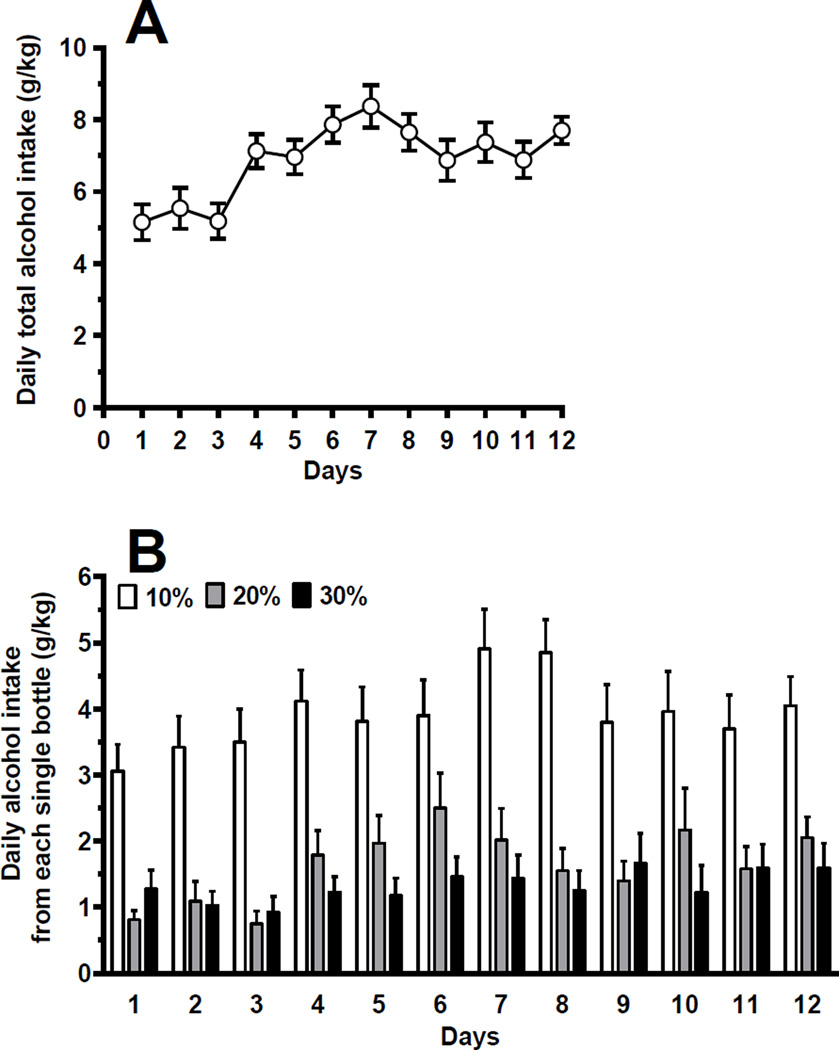
Friedman test indicated highly significant differences in daily total alcohol intake during the 12 days of homecage drinking [χ2=59.70, df=11, P<0.0001]. Rats rapidly acquired alcohol drinking: their mean daily total alcohol intake exceeded 5 g/kg already on the first 3 days and then rose to and stabilized at 7–8 g/kg (Fig. 2a). Two-way ANOVA with repeated measures on the factor day indicated a highly significant effect of alcohol concentration [F(2,44)=29.16, P<0.0001] and day [F(11,242)=6.40, P<0.0001], but no significant interaction [F(22,484)=0.84, P>0.05], on daily alcohol intake from the 3 different bottles. On each day, 10% was the preferred alcohol concentration (approximately 60% of total alcohol intake over the entire 12-day phase), with similar intakes (approximately 20%) from the bottles containing 20% and 30% alcohol concentrations (Fig. 2b).
Figure 2.
Daily total alcohol intake (a) and daily alcohol intake from each single bottle (b) in Sardinian alcohol-preferring (sP) rats exposed, for 12 consecutive days, to the homecage, 4-bottle “alcohol (10%, 20%, and 30%, v/v) vs water” choice regimen with unlimited access (24 hours/day). Each point or bar is the mean ± SEM of n=23 rats.
One-way ANOVA with repeated measures indicated no significant difference in daily water intake during the 12 days of homecage drinking [F(11,242)=1.34, P>0.05]. Mean daily water intake varied between 70 and 90 ml/kg (data not shown).
Experiment 1: Alcohol self-administration sessions with unpredictable time schedule
Friedman test indicated highly significant differences in number of lever-responses [χ2=35.59, df=11, P<0.0005] and total amount of self-administered alcohol [χ2=76.22, df=11, P<0.0001] during the 12 consecutive alcohol self-administration sessions with unpredictable time schedule. Specifically, mean number of lever-responses varied from approximately 90, when the self-administration session occurred during one of the first 2 hours of the dark phase, to values equal to or higher than 130, when the self-administration session occurred during one of the last 4 hours of the dark phase (Fig. 3a). Mean total amount of self-administered alcohol varied from 0.95–1.0 g/kg, when the self-administration session occurred during one of the first 3 hours of the dark phase, to 1.55–1.65 g/kg, when the self-administration session occurred during one of the last 4 hours of the dark phase (Fig. 3b). Both mean number of lever-responses and total amount of self-administered alcohol resulted as being positively correlated with time of access to alcohol (number of lever-responses: r=0.875, slope=4.091, intercept=88.240, P<0.0005; total amount of self-administered alcohol: r=0.950, slope=0.064, intercept=0.873, P<0.0001; n=23) (Fig. 3a and 3b). When limiting the comparison to data from the sessions occurring at the 1st and 12th hour of the dark phase, mean number of lever-responses was approximately 45% higher when the self-administration session occurred at the 12th rather than 1st hour (P<0.005, paired Student t test); mean total amount of self-administered alcohol was approximately 65% higher when the self-administration session occurred at the 12th rather than 1st hour (P<0.0005, Wilcoxon test).
Figure 3.
Number of lever-responses (a), total amount of self-administered alcohol (b), amount of self-administered alcohol from each single sipper bottle (c), latency to the first lever-response (d), and cumulative number of lever-responses (e) in Sardinian alcohol-preferring (sP) rats exposed to 12 consecutive daily 1-hour self-administration sessions of alcohol (10%, 20%, and 30%, v/v; concurrently available) under the Fixed Ratio 4 schedule of reinforcement; water was available uncontingently. Self-administration sessions occurred during one of the 12 hours of the dark phase of the daily light/dark cycle; time of the self-administration session was changed daily in a semi-random order and was unpredictable to rats. Arrows in panel e indicate the time of the last reinforcer. Each point or bar is the mean ± SEM of n=23 rats. ★: P<0.05 (Wilcoxon test).
Two-way ANOVA with repeated measures on the factor time indicated a significant effect of alcohol concentration [F(2,44)=5.01, P<0.05] and time of self-administration session [F(11,242)=9.55, P<0.0001], but no significant interaction [F(22,484)=0.49, P>0.05], on amount of self-administered alcohol from the 3 different sipper bottles. At each time of the self-administration session, 20% concentration was the preferred alcohol concentration (approximately 50% of total amount of self-administered alcohol over the entire 12-day phase), followed by 30% (approximately 30%), and 10% (approximately 20%) with relatively modest differences among the 12 hours of the dark phase (Fig. 3c). When limiting the comparison to data from the sessions occurring at the 1st and 12th hour of the dark phase, 2-way ANOVA with repeated measures on the factor time indicated a significant effect of alcohol concentration [F(2,44)=4.00, P<0.05] and time of self-administration session [F(1,22)=24.25, P<0.0001], but no significant interaction [F(2,44)=1.11, P>0.05], on amount of self-administered alcohol from the 3 different sipper bottles. Post hoc analysis indicated that, when the session occurred at the 12th hour of the dark phase, amount of self-administered alcohol from the 20% alcohol solution reached statistical significance in comparison to that from both 10% and 30% alcohol solutions (P<0.05, Tukey’s test).
Mean latency to the first lever-response was approximately 45% shorter when the self-administration session occurred at the 12th rather than 1st hour of the dark phase (P<0.05, Wilcoxon test) (Fig. 3d).
Analysis of cumulative response patterns of the self-administration sessions conducted at the 1st and 12th hour of the dark phase suggests that frequency in lever-responding during the initial 20-min portion of the session – i.e., the time period during which lever-responding was more intense – was higher when the self-administration session occurred at the 12th rather than 1st hour (Fig. 3e). After the initial 20-min period, lever-responding (a) continued throughout the entire session – although with reduced frequency – when the self-administration session occurred at the 12th hour of the dark phase, while (b) virtually stopped when the self-administration session occurred at the 1st hour of the dark phase (Fig. 3e). Accordingly, mean time of the last reinforcer was approximately 50% longer when the self-administration session occurred at the 12th rather than 1st hour of the dark phase (P<0.05, Wilcoxon test) (see arrows in Fig. 3e).
Water intake was negligible (averaging <1 ml/kg in each self-administration session) and not correlated with time of the alcohol self-administration session (r=0.006, slope=0.0003, intercept=0.735, P>0.05) (data not shown).
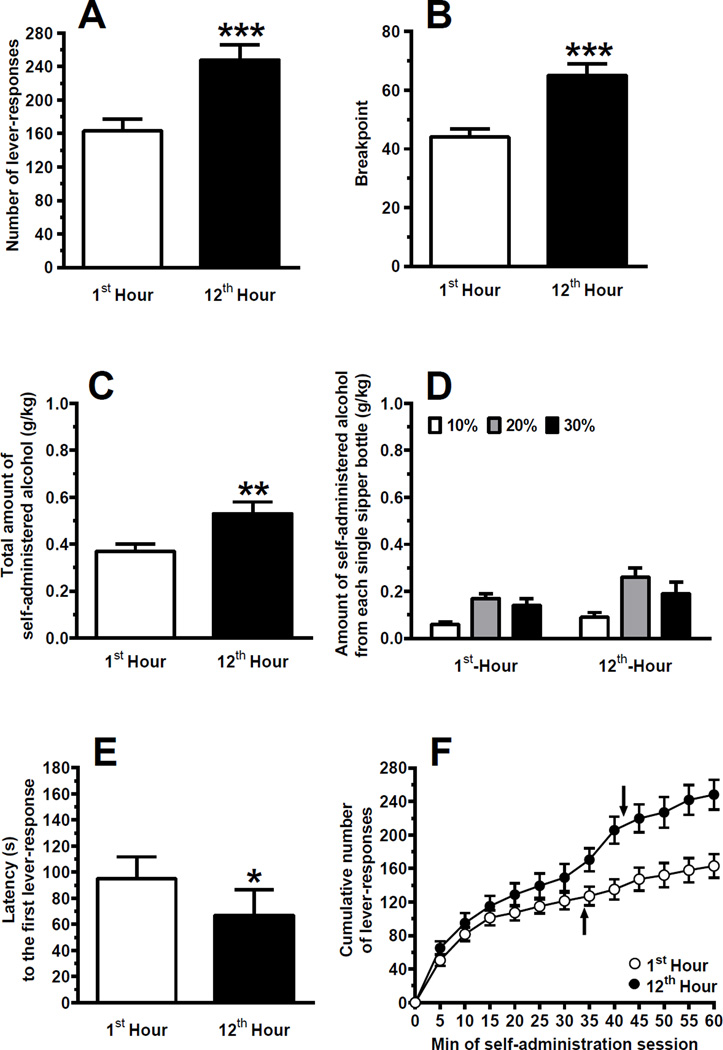
Experiment 2: Alcohol self-administration sessions under the Progressive Ratio schedule of reinforcement
In the self-administration sessions under the PR schedule of reinforcement, mean number of lever-responses and value of breakpoint for alcohol were approximately 50% higher when the self-administration sessions occurred at the 12th rather than 1st hour of the dark phase [number of lever-responses: P<0.001, paired Student t test (Fig. 4a); breakpoint value: P<0.0001, paired Student t test (Fig. 4b)].
Figure 4.
Number of lever-responses (a), value of breakpoint (b), total amount of self-administered alcohol (c), amount of self-administered alcohol from each single sipper bottle (d), latency to the first lever-response (e), and cumulative number of lever-responses (f) in Sardinian alcohol-preferring (sP) rats initially exposed to 12 consecutive daily 1-hour self-administration sessions of alcohol (10%, 20%, and 30%, v/v; concurrently available) under the Fixed Ratio 4 schedule of reinforcement and with unpredictable time schedule and then exposed to two, 1-hour self-administration sessions under the Progressive Ratio (PR) schedule of reinforcement occurring at the 1st and 12th hour of the dark phase. This experiment was conducted according to a crossover design, so that rats were divided into 2 groups and exposed to both PR sessions (i.e., rats exposed to the first PR session at the 1st hour were exposed to the second PR session at the 12th hour; vice versa, rats exposed to the first PR session at the 12th hour were exposed to the second PR session at the 1st hour). Six self-administration sessions with unpredictable time schedule elapsed between the two PR sessions. In all sessions, water was available uncontingently. Arrows in panel f indicate the time of the last reinforcer. Each point or bar is the mean ± SEM of n=23 rats. ★: P<0.05, ★★: P<0.005, and ★★★: P<0.001 (paired Student t or Wilxocon test).
Mean total amount of self-administered alcohol was approximately 45% higher when the self-administration sessions occurred at the 12th rather than 1st hour of the dark phase (P<0.005, Wilcoxon test) (Fig. 4c). Two-way ANOVA with repeated measures on the factor time indicated a significant effect of alcohol concentration [F(2,44)=7.24, P<0.005] and time of self-administration session [F(1,22)=11.68, P<0.005], and no significant interaction [F(2,44)=0.69, P>0.05], on amount of self-administered alcohol from the 3 different sipper bottles. In both self-administration sessions, 20% concentration was the preferred alcohol concentration (approximately 50% of total amount of self-administered alcohol over the entire 12-day phase), followed by 30% (approximately 35%), and 10% (approximately 15%) (Fig. 4d); post hoc analysis indicated that amount of self-administered alcohol from the 20% alcohol solution reached statistical significance in comparison to that from the 10% alcohol solution (P<0.05, Tukey’s test).
Mean latency to the first lever-response was approximately 30% shorter when the self-administration session occurred at the 12th rather than 1st hour of the dark phase (P<0.05, Wilcoxon test) (Fig. 4e).
Analysis of cumulative response patterns suggests that frequency in lever-responding during the initial 15 min was relatively similar in the self-administration sessions occurring at the 1st and 12th hour of the dark phase (Fig. 4f). Subsequently, frequency in lever-responding markedly diverged: after the initial 15-min period, lever-responding tended to slow when the self-administration session occurred at the 1st hour of the dark phase, while it was maintained with sustained frequency when the self-administration session occurred at the 12th hour of the dark phase (Fig. 4f). Time of the last reinforcer was longer when the self-administration session occurred at the 12th rather than 1st hour of the dark phase, although this difference reached statistical significance (P<0.05) only when the 1-tailed, but not 2-tailed, Wilcoxon test was used (see arrows in Fig. 4f).
Although water intake was relatively low, it resulted to be slightly higher at the 12th rather than 1st hour of the dark phase (0.72 ± 0.20 and 0.89 ± 0.10 ml/kg, respectively; P<0.05, Wilcoxon test).
Experiment 3: Alcohol-seeking behavior (extinction responding)
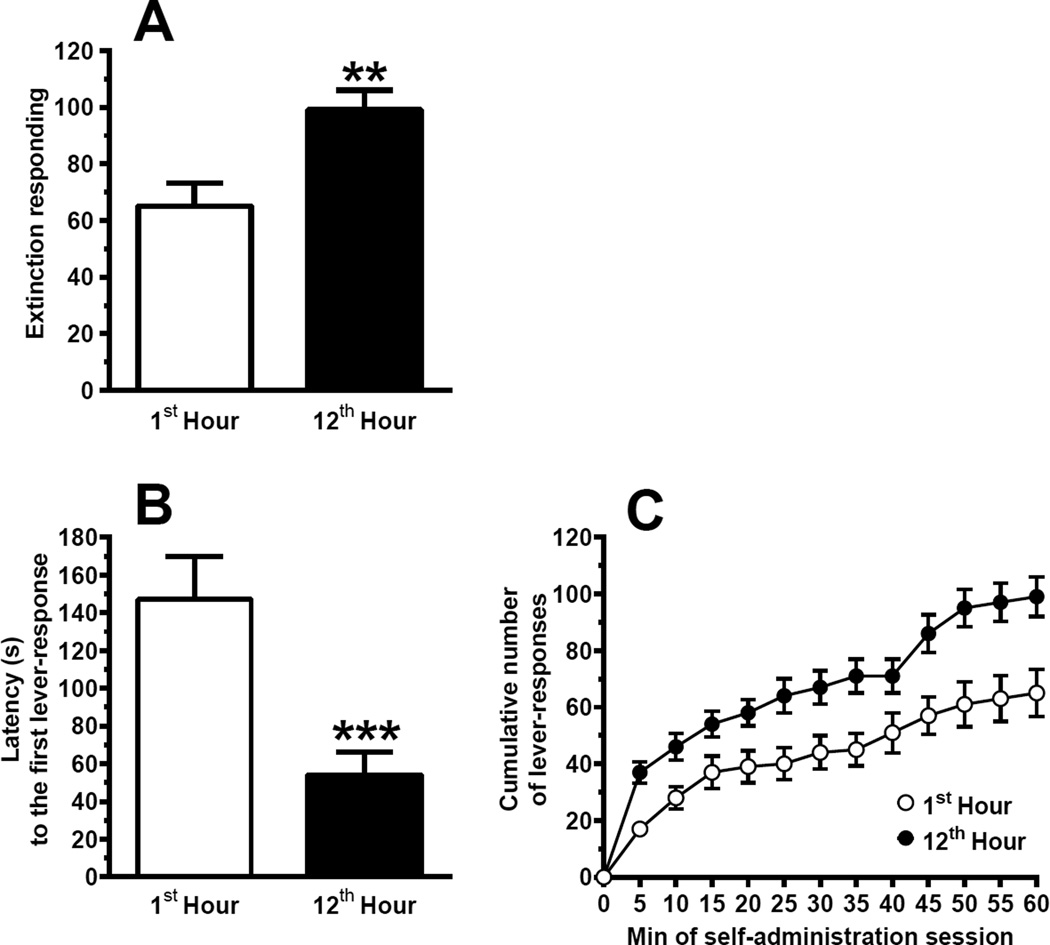
In the alcohol-seeking sessions under the ER procedure, mean value of ER for alcohol was approximately 50% higher when the self-administration sessions occurred at the 12th rather than 1st hour of the dark phase (P<0.005, Wilcoxon test) (Fig. 5a). Mean latency to the first lever-response was approximately 65% shorter when the self-administration session took place at the 12th rather than 1st hour of the dark phase (P<0.001, Wilcoxon test) (Fig. 5b).
Figure 5.
Values of extinction responding (ER) (a), latency to the first lever-response (b), and cumulative number of lever-responses (c) in Sardinian alcohol-preferring (sP) rats initially exposed to several daily 1-hour self-administration sessions of alcohol (10%, 20%, and 30%, v/v; concurrently available) under the Fixed Ratio 4 schedule of reinforcement and with unpredictable time schedule and then exposed to two, 1-hour sessions of ER occurring at the 1st and 12th hour of the dark phase. This experiment was conducted according to a crossover design, so that rats were divided into 2 groups and exposed to both ER sessions (i.e., rats exposed to the first ER session at the 1st hour were exposed to the second ER session at the 12th hour; vice versa, rats exposed to the first ER session at the 12th hour were exposed to the second ER session at the 1st hour). Twelve self-administration sessions with unpredictable time schedule elapsed between the two ER sessions. Only in the ER sessions, water was not available. Each point or bar is the mean ± SEM of n=23 rats. ★★: P<0.005 and ★★★: P<0.001 (Wilcoxon test).
Cumulative response patterns differed widely between the two self-administration sessions: (a) over the first time interval (0–5 min), the cumulative number of lever-responses in the self-administration session occurring at the 12th hour of the dark phase was more than double than that recorded in the self-administration session occurring at the 1st hour of the dark phase [37.2 ± 3.8 and 17.0 ± 2.6 (mean ± SEM), respectively]; (b) large differences in lever-responding frequency were kept throughout the two self-administration sessions, with a further separation over the last 20-min period (Fig. 5c).
Discussion
The results of Experiment 1 indicate that lever-responding for alcohol and amount of self-administered alcohol were highly sensitive to time schedule and positively correlated with time of alcohol access in alcohol-preferring sP rats exposed to daily, 1-hour self-administration sessions with unpredictable and concurrent availability of multiple alcohol concentrations. Specifically, lever-responding for alcohol and amount of self-administered alcohol increased progressively as the self-administration session moved from the earliest to the latest hours of the dark phase; when the self-administration session occurred during one of the latest hours of the dark phase (9th to 12th hour), number of lever-responses for alcohol and amount of self-administered alcohol were approximately 50% higher than those recorded when the self-administration session occurred during the first hours (1st to 3rd hour) of the dark phase, with intermediate values over the in-between hours. These data suggest that the reinforcing properties of alcohol were stronger at the end, rather than the beginning, of the dark phase. Additional support to this conclusion is provided by data on latency to the first lever-response: it was markedly shorter when the self-administration session occurred at the 12th rather than 1st hour of the dark phase, indicative of a stronger urge to access alcohol at the end than beginning of the dark phase.
Additional, relevant information is provided by comparison of the cumulative response patterns of lever-responding for alcohol when the self-administration session occurred at the 1st and 12th hour of the dark phase: when the self-administration session occurred at the 12th hour, rats (a) displayed a steeper curve over the first 10–15 min of the session (indicative of higher frequency in lever-responding for alcohol during the session period when rats’ activity was more intense) and (b) apparently never reached a plateau value, as rats kept on lever-responding throughout the session, often gaining the last reinforcer at the end of the session. Conversely, when the self-administration session occurred at the 1st hour of the dark phase, rats displayed minimal lever-responding activity after the first 20–25 min of the session.
Irrespective of the time of the alcohol self-administration session, rats consumed the largest amounts of alcohol from the sipper bottle containing the 20% alcohol concentration. We may reasonably rule out that this preference was due to the position of the sipper bottles, as they were mounted differently in the 8 operant chambers and rat allocation to the operant chamber was established daily on a random basis. Conversely, it is likely that rats selected the 20% alcohol solution as it represented an optimal balance between the strength of the reinforcer (a relatively highly concentrated solution, providing a substantial amount of alcohol, however not being as aversive, in terms of taste, as the 30% concentration likely was) and the short period of time (3 s) during which the reinforcer was available. This hypothesis is supported by data on the preferred alcohol concentration during the initial homecage drinking phase: having unlimited access to the 3 alcohol bottles, and – therefore – not being forced to consume alcohol in a short period of time, rats clearly preferred the 10% alcohol solution (likely, the less aversive in terms of taste).
Mean amounts of self-administered alcohol as high as those recorded in the present study when self-administration sessions were conducted during the last portion of the dark phase had never been monitored in sP rats exposed to more conventional FR procedures with a single alcohol concentration (15%, v/v) and daily self-administration sessions always occurring during the first 4–6 hours of the dark phase (e.g.: Maccioni et al. 2012; 2015). These comparisons suggest how powerfully the experimental procedure used in the present study produced an escalation in the reinforcing properties of alcohol in sP rats.
The results of the present study confirm, and extend to an operant procedure of alcohol self-administration, the results of two recent studies assessing alcohol intake in sP rats exposed, in their homecage, to daily drinking sessions of one hour with concurrent availability of multiple alcohol concentrations (0%, 10%, 20%, and 30%, v/v), unpredictable time schedule, and over the dark phase of the daily light/dark cycle (Colombo et al. 2014; 2015). Alcohol intake was indeed highly sensitive to time schedule, as it rose progressively from 0.6–0.8 g/kg, when alcohol was made available during one of the first hours (1st and 2nd hour) of the dark phase, to ≥2 g/kg, when alcohol was made available during one of the latest hours of the dark phase (11th and 12th hour) (Colombo et al. 2014; 2015). Alcohol intake at the 12th hour of the dark phase was associated to mean BALs of 100 mg% and signs of intoxication, leading us to propose that experimental procedure as a rodent model of binge drinking (Colombo et al. 2014).
The experimental design of the present study did not allow us to assess separately the contribution of two relevant aspects of this experimental procedure: exposure to alcohol over the last portion of the dark phase and unpredictability of alcohol exposure. In one of the previous drinking (“nonoperant”) studies (Colombo et al. 2014), both factors clearly contributed to those exceptionally high intakes of alcohol: (a) rats constantly exposed to 1-hour alcohol drinking sessions at the 12th hour of the dark phase consumed an average of 1.5 g/kg alcohol (approximately 50% higher than that recorded when the drinking session occurred at the 1st hour of the dark phase, irrespective of whether or not it was under the unpredictable time schedule); (b) rats exposed to alcohol at the 12th hour of the dark phase with unpredictable time schedule consumed approximately 2 g/kg (approximately 35% more than the intake recorded in the rat group constantly exposed to alcohol at the 12th hour of the dark phase). The close similarity between the data of the present, “operant” study with those of the previous “non-operant” study allows to hypothesize that also the reinforcing properties of alcohol (present study), as well as alcohol drinking (Colombo et al. 2014), were raised additively by both factors.
The present study also investigated whether the motivational properties of alcohol were sensitive to, and eventually increasable by, time of alcohol availability and unpredictability of time schedule. To this end, rats were also exposed to (a) self-administration sessions under the PR schedule of reinforcement (Experiment 2) and (b) sesions of alcohol seeking under the ER procedure (Experiment 3), i.e. two experimental procedures validated for measurements of rats’ motivation to search, or “work”, for alcohol (see Markou et al. 1993; Samson et al. 2001). In these two experiments, comparison was limited to the two extreme time periods of the dark phase (1st and 12th hour). Data collected from these two experiments were highly consonant: (a) values of breakpoint and ER were approximately 50% higher at the 12th rather than 1st hour of the dark phase; (b) latency to the first lever-response was shorter at the 12th rather than 1st hour of the dark phase; (c) cumulative response patterns displayed steeper curves and longer times to gain the last reinforcer (applicable to the PR experiment only) at the 12th rather than 1st hour of the dark phase. Together, these results suggest that the motivational properties of alcohol – similarly to the reinforcing properties – were stronger at the end than at the beginning of the dark phase.
Similarly to the FR experiment, PR and ER experiments provided mean values of breakpoint and ER for alcohol that had never been observed in previous studies with sP rats exposed to sessions of alcohol self-administration or alcohol-seeking behavior always occurring during the first 4–6 hours of the dark phase (e.g.: Maccioni et al. 2008; 2012). These comparisons suggest that alcohol availability at the end of the dark phase exacerbated both the reinforcing and motivational properties of alcohol in sP rats with a “history” of repeated, unpredictable exposures to daily, alcohol self-administration sessions.
This lab is currently undertaking a series of experiments to possibly unravel the mechanism underlying the sensitivity to time schedule of alcohol drinking (Colombo et al. 2014; 2015) and alcohol self-administration (present study) in sP rats. A recently collected set of data suggests that expectation of alcohol availability, generated by the unpredictable schedule of alcohol access, likely produces an emotional “distress” that increases progressively as time elapses over the dark phase: indeed, sP rats initially exposed to several, daily drinking sessions with unpredictable time schedule and then to the Social Interaction (SI) test (with no alcohol available on the day of the SI test) displayed higher levels of anxiety-related behaviors at the 12th rather than 1st hour of the dark phase (Colombo et al. 2015). Thus, sP rats appear to use alcohol to cope with the negative affective state generated by the uncertainty of time of alcohol access and expectation of alcohol availability: the increase in alcohol reinforcing and motivational properties, observed in the present study, may be interpreted as an index of the progressively increasing demand for alcohol to cope with this “distress”.
The experimental procedure used in this series of studies with sP rats (Colombo et al. 2014; 2015; present study) combines several different aspects of other paradigms known to successfully induce escalations in alcohol drinking – often up to intoxication – in other lines of selectively bred alcohol-preferring rats, including Indiana P and Alko Alcohol, and alcohol-consuming C57BL/6J mice. These aspects include: daily drinking sessions of limited duration (e.g.: Sinclair et al. 1992; Finn et al., 2005) and occurring during the dark phase of the light/dark cycle (Rhodes et al. 2005; Thiele & Navarro, 2014); concurrent availability of multiple alcohol concentrations (Bell et al. 2006; 2014). The present procedure includes the unpredictability of time of alcohol availability as additional, relevant feature. As a result, when exposed to this combination of different experimental conditions, sP rats (a) consume exceptionally elevated amounts of alcohol, up to intoxicating levels (Colombo et al. 2014), providing convincing evidence on the face validity of this model of human binge alcohol drinking, (b) display increased urge to “work” for alcohol (present study), indicative of an increase in the reinforcing and motivational properties of alcohol, and (c) display an exacerbation of their anxiety-like state (Colombo et al. 2015), suggestive of an emotional “distress” provoked by the uncertainty of time of alcohol access and expectation of alcohol availability.
Once adequately characterized, this experimental procedure may constitute a useful tool for investigations on the neurochemical and behavioral correlates of binge-like drinking, hopefully providing insights on the biological bases of some aspects of such risky and dangerous behavior for a large proportion of the human population (see Courtney & Polich 2009; Petit et al. 2014). Additionally, this experimental procedure may serve to identify drugs potentially effective against binge drinking; notably, alcohol drinking in sP rats exposed to the 4-bottle choice regimen, in a drinking session of 1 hour occurring at the 12th hour of the dark phase, and with unpredictable time of alcohol access, is pharmacologically manipulable, as recently suggested by its suppression after acute treatment with the positive allosteric modulator of the GABAB receptor, GS39783 (Colombo et al. 2015).
Acknowledgements
The authors are grateful to Mrs. Carla Acciaro for animal breeding and care and Ms. Anne Farmer for language editing of the manuscript. Supported by NIAAA-funded “Integrative Neuroscience Initiative on Alcoholism” (INIA-Stress) Consortium.
Footnotes
Conflict of interest:
No author has any conflict of interest.
References
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MAM, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Maccioni P, Acciaro C, Lobina C, Loi B, Zaru A, Carai MAM, Gessa GL. Binge drinking in alcohol-preferring sP rats at the end of the nocturnal period. Alcohol. 2014;48:301–311. doi: 10.1016/j.alcohol.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Maccioni P, Carai MAM, Lorrai I, Zaru A, Contini A, Mugnaini C, Corelli F, Gessa GL. Anxiety-like behaviors at the end of the nocturnal period in sP rats with a “history” of unpredictable, limited access to alcohol. Alcohol. 2015 doi: 10.1016/j.alcohol.2015.04.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Fantini N, Carai MAM, Gessa GL, Colombo G. γ-Hydroxybutyric acid (GHB) suppresses alcohol’s motivational properties in alcohol-preferring rats. Alcohol. 2008;42:107–113. doi: 10.1016/j.alcohol.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Zaru A, Loi B, Lobina C, Carai MAM, Gessa GL, Capra A, Mugnaini C, Pasquini S, Corelli F, Hyytiä P, Lumeng L, Colombo G. Comparison of the effect of the GABAB receptor agonist, baclofen, and the positive allosteric modulator of the GABAB receptor, GS39783, on alcohol self-administration in three different lines of alcohol-preferring rats. Alcohol Clin Exp Res. 2012;36:1748–1766. doi: 10.1111/j.1530-0277.2012.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Vargiolu D, Thomas AW, Malherbe P, Mugnaini C, Corelli F, Leite-Morris KA, Gessa GL, Colombo G. Inhibition of alcohol self-administration by positive allosteric modulators of the GABAB receptor in rats: Lack of tolerance and potentiation of baclofen. Psychopharmacology. 2015;232:1831–1841. doi: 10.1007/s00213-014-3815-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- NIAAA National Advisory Council. NIAAA Council approves definition of binge drinking. NIAAA Newsletter. 2004;3:5. [Google Scholar]
- Petit G, Maurage P, Kornreich C, Verbanck P, Campanella S. Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol Alcohol. 2014;49:198–206. doi: 10.1093/alcalc/agt172. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24:205–209. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Hyytiä P, Nurmi M. The limited access paradigm: description of one method. Alcohol. 1992;9:441–444. doi: 10.1016/0741-8329(92)90045-c. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. "Drinking in the dark" (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]