Abstract
In this mini-review we focus on the use of time-lapse light microscopy to study membrane remodeling during protein secretion in live animals. In particular, we highlight how subcellular intravital microscopy has enabled imaging the dynamics of both individual secretory vesicles and the plasma membrane, during different steps in the exocytic process. This powerful approach has provided us with the unique opportunity to unravel the role of the actin cytoskeleton in regulating this process under physiological conditions, and to overcome the shortcomings of more reductionist model systems.
Introduction
Membrane remodeling encompasses a series of changes, in shape and molecular composition of the cellular membrane, that enable the cell to perform its basic functions [1]. Membrane trafficking is a perfect example of a process in which membranes must constantly modify their geometry to ensure the transport of molecules throughout the cell. Membranes undergo dramatic changes in curvature, which drive basic processes such as fusion and fission during the biogenesis, transport, and delivery of transport intermediates [1, 2]. Remodeling is the result of complex coordination among various cellular machineries that modify both protein and lipid composition of the membranes, and exert precise and timely mechanical forces. These machineries have been studied in reductionist model systems ranging from model membranes to isolated sub-cellular compartments, cell cultures, and explanted organs. Although several aspects of the regulation of membrane remodeling have been unraveled, little has been done to determine whether the main conclusions derived from in vitro and ex vivo studies are valid in intact organs in vivo. The development of subcellular intravital microscopy (IVM) [3–7] has provided the opportunity to address this issue for the first time. Specifically, recent studies have been focusing on dissecting the role that the cytoskeleton plays in controlling membrane remodeling during regulated exocytosis in the main exocrine organs of live rodents.
Regulated Exocytosis and the actin cytoskeleton
Exocytosis is a fundamental biological process that occurs in every cell and is used to deliver proteins, lipids, and other molecules to the plasma membrane (PM) and the extracellular space. Molecules destined to secretion are sorted into membranous carriers such as vesicles and tubules, which are transported from the trans Golgi network (TGN) to the cell surface where they undergo fusion with the PM. Exocytosis is termed either constitutive or regulated based on whether the fusion step requires a specific extracellular trigger. Constitutive exocytosis occurs in every cell and it is utilized to maintain the homeostasis of the PM, to regulate cell polarity, and to release components of the extracellular matrix [8, 9]. Regulated exocytosis occurs in specialized secretory cells, where it is triggered by the extracellular stimulation of PM receptors. Regulated exocytosis is fundamental for several physiological functions, such as neurotransmission, respiration, digestion, reproduction, and the immune response [8, 9]. There are four main types of secretory cells: neuronal, endocrine, exocrine, and hematopoietic cells. Their respective secretory vesicles differ in size and in the nature of their cargo, and the types vary in the overall duration of the exocytic process [8, 10, 11]. For example, neuronal cells contain small synaptic vesicles (50–100 nm in diameter), which transport small neurotransmitters such as acetylcholine or epinephrine, and undergo exocytosis within few milliseconds. On the other hand, exocrine cells contain large secretory vesicles (termed secretory granules, 1–1.5 μm in diameter), which transport large macromolecules, such as zymogens and glycosylated mucins, and undergo exocytosis in a matter of minutes [3, 10].
Secretory vesicles undergo two modes of regulated exocytosis, kiss-and-run and full fusion, which are defined by their behavior at the PM. During kiss-and-run, the fusion pore closes after a portion of the cargo molecules is released, leading to the detachment of the secretory vesicle from the PM. The detached vesicles are either transported to an intracellular compartment to replenish their content or re-utilized in another secretion event [12]. During full fusion, which is the type observed in most secretory cells, the fusion pore is progressively expanded in order to completely release the vesicle’s content and allow the integration of the granular membrane into the PM. Notably, in order to maintain PM homeostasis, the excess of membranes delivered by exocytosis is rapidly retrieved through compensatory endocytosis, a fundamental cellular process whose molecular basis has not yet been fully elucidated [12–14].
Full fusion exocytosis requires an extensive and complex remodeling of both granular membranes and PM. This remodeling relies on the precise temporal and spatial coordination of several factors, which include signaling complexes, lipid modifying enzymes, fusion machinery (e.g. SNAREs), and the cytoskeleton [2, 8, 10, 12, 15, 16]. In particular, the actin cytoskeleton has been shown to regulate several steps of regulated exocytosis in almost every secretory system. In neuron, endocrine, neuroendocrine cells, and immune cells, F-actin and various myosin motors have been proposed to work primarily at the level of the PM, by either acting as a functional barrier or modulating the fusion pore. On the other hand, the actin cytoskeleton has been observed to be recruited on large secretory granules in secretory systems such as: 1) acinar cells in the main exocrine organs (i.e. pancreas, salivary and lacrimal glands) [3, 17–21], 2) alveolar epithelial type II cells [22–24], 3) endothelial cells (Weibel–Palade bodies) [25], and 4) Xenopus eggs during fertilization [26–28]. In these systems, F-actin has been proposed to control the fate of the granules by 1) providing a scaffold that regulates the PM integration of the granular membranes, 2) facilitating the expulsion of cargo molecules through the generation of a compressing force on the granular membranes, and 3) regulating the dynamics of the fusion pore [10, 11, 17–29]. However, several details regarding the integration of signaling and the mechanism regulating the assembly and disassembly of the actin cytoskeleton have not been elucidated yet.
Time-lapse imaging to investigate the mechanism controlling regulated exocytosis
The two most common strategies to study regulated exocytosis have been either measuring the amount of molecules secreted in the extracellular space or visualizing the secretory vesicles within the cells during stimulated secretion. The former approach relies on biochemical, immunological, and electrochemical methods, whereas the latter is based on either electron microscopy or indirect immunofluorescence [8, 15, 16]. Although these powerful tools have been instrumental in identifying several key molecules controlling regulated exocytosis, their main limitation is the fact that they do not provide any spatio-temporal information about the process. In this respect, time-lapse light microscopy complements these methods by enabling visualization of the dynamics of individual vesicles during secretion. The advent of GFP technology has made it possible to image several components of the exocytic machinery, including cargo molecules, membranes of the secretory vesicles, and the target membranes. Among the various light microscopy-based techniques, total internal re ection (TIRF) microscopy has permitted the observation of exocytic events close to the PM, thus providing novel information on tethering, docking, and fusion of the secretory vesicles [30]. Moreover, its high temporal resolution makes TIRF suitable to image very rapid events such as neurotransmission or insulin secretion [31, 32]. However, TIRF cannot image exocytic vesicles that are removed from the PM, and its use is restricted to adherent cultured cells. Confocal and two-photon microscopy, which provide high spatial resolution, have been also been widely used to study the dynamics of regulated exocytosis, and have allowed tracking of exocytic vesicles from the TGN to the PM [3, 33]. However, temporal resolution of these methods is rather slow, and they are more suited to study large vesicular structures that undergo slower exocytosis. For example, individual exocytic events have been visualized in explanted and cultured lacrimal glands [19], in Xenopus eggs during fertilization [26–28], in isolated pancreatic acini [20, 21, 34–36], in primary-cultured type II pneumocytes cells [22–24], and in the Weibel-Palade bodies in endothelial cells [25]. A combination of pharmacological and genetic approaches has revealed ground-breaking information about the basic machinery regulating secretion, ranging from the role of specific components of the actin cytoskeleton, to the characterization of the signaling pathways, to the role of specific lipids [17, 19, 22–25, 27, 28, 34, 37].
Subcellular intravital microscopy and regulated exocytosis in live rodents
Both cell cultures and explanted organs have been useful models to study regulated exocytosis. Their main advantages are the ability 1) to tightly control the experimental conditions, 2) to perform genetic manipulation by gene transfection, siRNA and shRNA technology, and 3) to perform pharmacological treatments. Nonetheless, a crucial question is whether these reductionist model systems faithfully recapitulate the modality and regulation of exocytosis in the tissue in vivo. Indeed, both cell cultures and isolated organs lack the contribution of those unique combination of factors provided by the vasculature and the central nervous system [14]. In addition, cell cultures lack three-dimensional organization of the tissues and interactions with the surrounding cells. The salivary gland, which undergoes rapid de-differentiation upon explantation, is a prime example of this loss of biological context. In tissue explants, the number of secretory granules, secretory capacity, and cell polarity are altered with respect to the in vivo characteristics [38]. Therefore, imaging analysis for exocrine cells has hitherto been restricted to freshly isolated cells. In addition, cultured primary cells and organ cultures are prepared using procedures based on the mechanical or enzymatic disruption of the tissue, whose effects on the biology of the system are largely unknown.
Subcellular (IVM) has made it possible to overcome these issues. IVM encompasses a range of light-microscopy based techniques, such as confocal, two-photon, and spinning disk microscopy, which are applied to the surgically-exposed organ of an anesthetized animal, while the organ is maintained under proper physiological conditions. Although this approach was first developed in 1839 [39], only in the last decade has it been implemented to visualize subcellular structures. After the pioneering work of Dunn, Molitoris and colleagues, which successfully imaged endocytic events in the kidney of live mice and rats, our group has further developed this technique by using rodent salivary glands as a main model [3–7, 10, 33, 40–45]. Submandibular salivary glands offer several advantages to perform subcellular IVM when compared with other organs. First, in rodents the glands are located in the neck area, which is easily accessible and partially shielded from motion artifacts of heartbeat and respiration. This ensures an adequate stability of the exposed organ and therefore the ability to image small structures in the subcellular range. Second, exposure of the glands does not affect the secretory process or any other cellular function [33]. Finally, the submandibular glands can be easily and selectively manipulated both pharmacologically and genetically. Indeed, drugs and fluorescent probes can be delivered to the epithelium of the glands by retro-injection through the main excretory duct, which is accessible through the oral cavity. The same route can be used to effectively deliver genes via either non-viral or viral mediated approaches [5, 6, 42, 43].
These combinations of methods have enabled the investigation and characterization of both the dynamics and the properties of organelles in the sub-micron range. For example, various aspects of endocytosis, such as the trafficking of molecules throughout the endo-lysosomal system were investigated, revealing that the kinetics of the endocytic pathways in vivo differ from what was previously reported in cultured cells [5, 44]. Moreover, a novel modality of basal mitochondrial metabolism that occurs in vivo in the salivary epithelium was recently described, and for the first time, the coordination of mitochondria, and its regulation through gap junctions was unraveled [45].
Step-wise imaging of individual secretory granules in vivo: what we have learned and what we can still learn
a. Degranulation and individual granules
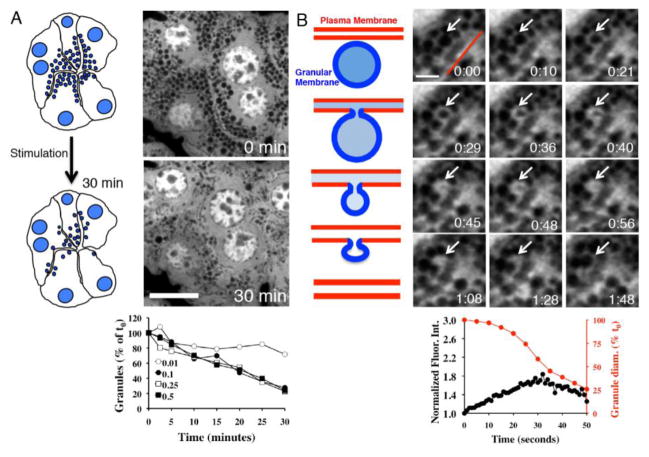
In order to image the dynamics of secretory granules during regulated exocytosis, our group has taken advantage of a transgenic mouse strain that expresses GFP in the cytosol [3, 33, 37]. Since the fluorescent molecule is not sorted into the secretory pathway, the secretory granules appear as discrete vesicles, devoid of GFP, and clustered in close proximity to the APM [3] (Fig. 1A). Notably, granules that undergo fusion are highlighted by an enrichment of the levels of the GFP around their membranes, making it possible to selectively follow fused granules [3] (Fig. 1B, arrows). This model is very robust and can be used to characterize the mechanisms of degranulation in vivo, and to accurately measure the kinetics of individual exocytic events [37] (Fig. 1A and 1B). Indeed, we showed that secretion in vivo is elicited exclusively by β-adrenergic signaling, and that the secretory granules undergo full fusion [3]. This finding clearly highlights the differences between in vivo and ex vivo model systems, since in the latter: 1) muscarinic signaling was reported to stimulate exocytosis [46], and 2) compound exocytosis (e.g. the sequential fusion of secretory vesicles) was described as the main modality of secretion [12, 34]. This model has been complemented with another transgenic mouse, which ubiquitously expresses a membrane targeted peptide, the myristoylated alanine-rich C-kinase substrate (MARCKS), fused with the fluorescent protein, tandem-tomato (mTomato, [47]). In the salivary glands, mTomato is expressed both at the apical and basolateral membranes, whereas it is excluded from the secretory granules and all other intracellular membranes. After the granules fuse with the APM, the mTomato probe diffuses into the granular membranes within 2–3 seconds, thus enabling the visualization of their fate (Fig. 2A and 2B-II). The diffusion of membranes from the APM seems to be a largely general phenomenon, as it has also been shown in pancreatic acinar cells and Xenopus eggs [27, 28, 35]. This mouse model provides a reliable assay to accurately measure the kinetics of membrane integration under a variety of experimental conditions. Although the resolution of light microscopy does not allow resolution of the ultrastructural features of the granules during their integration, it is still possible to argue that their size decreases as the integration progresses, thus leading to an increase in their curvature and membrane tension. Neither the bioenergetic coordination of this process nor the application of force to the granules during fusion is understood. Correlative electron microscopy may provide ultrastructural information on the various stages of exocytosis, thus providing the means to model this process [48]. In addition, we envision that the mTomato mouse can be used in combination with Fluorescence Recovery After Photobleaching (FRAP)–[49] to measure membrane mobility both at the APM and the granular membranes, thus enabling the unique opportunity to determine biophysical parameters during regulated exocytosis in vivo.
Figure 1.
GFP-mouse model to study the dynamics of regulated exocytosis in vivo. Cytoplasmic GFP is excluded from the secretory granules in salivary acinar cells, which appear as dark circles. Secretory granules were imaged upon subcutaneous injection of isoproterenol (Iso) by time-lapse IVM. (A) Snapshots were taken at 0 min (upper panel) or 30 min (lower panel) after Iso injection. Scale bar: 5 μm. Lower graph: degranulation expressed as percentage of granules with respect to time 0 in response to various doses of Iso: 0.01 mg/kg (○), 0.1 mg/kg (●), 0.25 mg/kg (□), and 0.5 mg/kg (■). (B) Time-lapse IVM image series of a single granule (white arrows) fusing with the APM (red line). Scale bar:3 μm. Lower graph: quantitation of both the GFP fluorescence intensity around an individual secretory granule (black circles) and its diameter (red circles) during exocytosis. Pictures and graphs are adapted from [3].
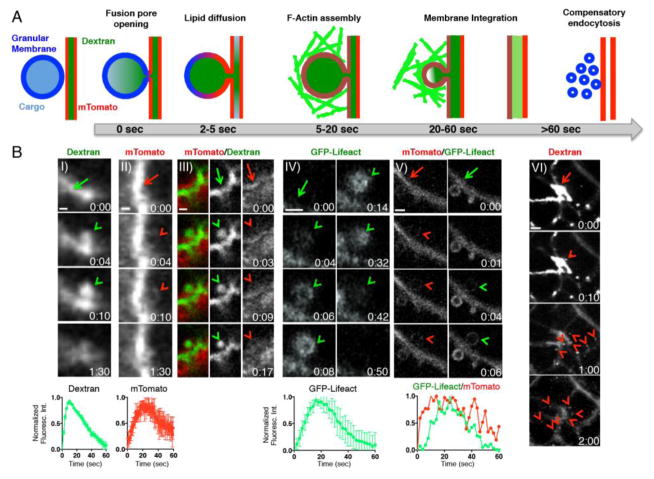
Figure 2.
IVM imaging of the dynamics of individual secretory vesicles and the plasma membrane during different steps in the exocytic process. (A) Diagram of the different steps in the exocytic process. After injection of Iso, the secretory granules (blue) fuse with the APM (red), and fusion pore is opened, releasing their content (light blue) and allowing the access of the dextran (dark green) loaded in the acinar canaliculi. The PM diffuses into the granular membranes followed by F-actin (green) assembly onto the fused-granules. Secretory granules gradually collapse and are integrated into the APM, and the excess of membranes delivered by exocytosis is retrieved through compensatory endocytosis (blue vesicles). (B) Fusion pore opening was visualized by loading of FITC–dextran (70 kDa) into the Wharton’s duct of either wt (I, arrow) or mTomato mice (III, arrows). Iso was injected subcutaneously into the mouse and the exocytic granules fused with APM (arrow) and filled with dextran (green arrowheads). Lower graph: quantitation of FITC–dextran fluorescence intensity during exocytosis. Lipid diffusion from APM (arrow) into granular membranes was visualized by using the mTomato mouse (II and III arrowheads). When FITC-dextran was present in the duct, opening of the fusion pore and lipid diffusion occured simultaneously (III, arrrowheads). IV) F-actin recruitment onto the exocytic granules fused with APM (arrow) was visualized by using transgenic mice expressing either GFP-Lifeact alone (IV green arrowheads) or with the mTomato probe (V, red arrowhead). Lower graph: quantitation of GFP-Lifeact and mTomato fluorescence intensities around a fused-granule during exocytosis. VI) Compensatory endocytosis was visualized by retro-injection of Alexa 647-dextran (10 kDa) in the rat Wharton’s duct. Iso was injected subcutaneously to stimulate compensatory endocytosis, and small vesicles were formed from the canaliculi (arrows). Pictures in panel 2B-III are adapted from [3]. Scale bar, 1 μm.
b. Fusion pore
To determine the temporal correlation between the diffusion of the membranes and the opening of the fusion pore, we developed an in vivo assay based on signal from small fluorescent tracers, which are introduced to the duct initially, and can diffuse into the lumen of accessible secretory granules. [3, 6]. The probes, such as low molecular weight dextrans, are delivered to the acinar canaliculi through the salivary duct in either wt or mTomato mice, and their signal pattern indicates that after stimulation of exocytosis, the opening of the pore and the diffusion of the membranes occur at the same time (at least within the temporal resolution of our microscope ≈ 1 sec [3]) (Fig. 2B-I and 2B-III). This approach can potentially provide valuable data on the kinetics of the expansion of the fusion pore that could be measured by using retro-ductal delivery of a mixture of dextrans of different molecular weights. Indeed, these molecules are expected to enter the lumen of the granules sequentially, in order of their Stokes radii, as shown in isolated pancreatic acini [36].
c. Actin Cytoskeleton
The actin cytoskeleton is an important regulator of exocytosis of the secretory granules [3, 10, 17, 20, 22–29]. To investigate the role F-actin during secretion, we took advantage of fluorescently labeled Lifeact, a 17-amino-acid peptide derived from the yeast binding protein Abp140 that selectively binds F-actin without affecting its dynamics [50, 51]. We took two approaches. The first is based on the transient non viral-mediated transfection of plasmids encoding for Lifeact tagged with either GFP or RFP [3, 4, 33, 37, 42]. The second is based on the use of transgenic mice expressing these probes [33, 37] (Fig. 2B-IV). We showed that F-actin is recruited onto the membranes of secretory granules after the opening of the fusion pore, and more specifically, 2–5 seconds after the diffusion of the membrane probes from the APM [3] (Fig. 2B-V). Similar observations have been made in primary-cultured chromaffin cells [52] and in Xenopus eggs expressing farnesylated-GFP as a marker for the PM and Utr1–261–mRFP as a marker for F-actin [27]. Pharmacological disruption of the actin cytoskeleton assembly has revealed that the F-actin coat plays a fundamental role in controlling the membrane integration of the secretory granules. Indeed, F-actin provides a scaffold that is required for 1) the recruitment of myosin motors, which provide the contractile activity [3, 10, 17, 19, 23–25], 2) the stabilization of the granules to counteract the forces generated by the intra-luminal pressure due to simultaneous fluid secretion, and 3) preventing compound exocytosis [3, 10, 13, 53]. Notably, the role of the actin cytoskeleton in stabilizing the granules could only be revealed in vivo, where the ductal system is fully preserved and allows for the generation of a luminal hydrostatic pressure [3, 10, 13, 53]. Moreover, our data on the role of F-actin may suggest that the difference between in vivo and ex vivo model systems (e.g. full fusion vs. compound exocytosis) is due to the impairment of the kinetics of assembly of the actin cytoskeleton onto the secretory granules. This may be the result of either the procedures used to isolate the lobules and the acini, or an alteration of the cellular metabolism that provides the energy required to sustain actin polymerization. The latter is consistent with our recent observations that mitochondrial metabolism is altered when salivary glands are explanted [45].
d. Lipid diffusion from the plasma membrane
The diffusion of components of the APM into the granular membranes plays a fundamental role in the fate of secretory granules after fusion. Indeed, in Xenopus eggs it has been proposed that lipids such as diacylglcerol (DAG) or phosphatidylinositol 4,5-bisphosphate (PIP2) initiate the assembly of F-actin onto the granules through the activation of actin nucleation promoting factors [27, 28, 52]. This activation is possibly mediated by various small GTPases, such as Cdc42 and Rho, as proposed for pancreas, Type II pneumocytes, and Xenopus eggs [24, 26, 34]. Other studies have proposed an intriguing two-compartment mixing model, where lipid-modifying enzymes, such as the PI4 kinase, are localized on the secretory granules whereas their substrates are localized at the PM [54, 55]. These crucial aspects of the regulation of exocytosis have not been addressed in vivo yet. However, the generation of transgenic mice expressing reporters for these lipids (e.g. fluorescently labeled PH or C2 domains) and conditional knock-out models for the enzymes which synthetize or modify them will provide powerful tools to dissect their roles in membrane remodeling during secretion.
e. Compensatory endocytosis
The integration of granular membranes into the APM causes an increase in its surface, which results in the expansion of the acinar canaliculi [3, 13]. Previous work has established that in each acinar cell, 150–200 granules undergo exocytosis upon β-adrenergic stimulation, and that 50–75 vesicles of 100–200 nm in diameter would be required to retrieve the membranes delivered by a single granule [6, 13, 29]. Therefore, in order to maintain the homeostasis of the APM, exocytosis must be coupled to retrieval of the excess membranes by compensatory endocytosis [12–14]. Surprisingly, in using the mTomato mouse we do not observe any massive internalization of membranes [3] (Fig. 2B-II). However, when we retro-injected a small dextran into the salivary duct, we observed a series of small vesicles in close proximity of the APM. This observation suggests that compensatory endocytosis is not a bulk process, but instead exhibits an exquisite selectivity for specific membranes. However, the main caveat of this approach is that the internalization can be followed only for the first 2–3 minutes after the stimulation. Indeed, stimulation of exocytosis elicits secretion of small volumes of fluids, which leads to the expulsion of the dextran from the ducts (Fig. 2B-VI). Although the machinery regulating compensatory endocytosis is not completely understood, our previous work has ruled out the role of clathrin-mediated pathways, a conclusion mainly based on the lack of co-localization of clathrin and the APM [43]. Moreover, vesicles internalized from the APM did not co-localize with any of the classical endocytic markers, suggesting that this process has unique features. The further dissection of the internalization step and the identification of a specific marker for the compensatory vesicles will be essential to understand the coordination between exocytic and endocytic process under physiological conditions.
Conclusion
In summary, subcellular IVM is a powerful tool to investigate the details and machinery of membrane remodeling during trafficking events in vivo. Indeed, we have developed a series of techniques that enable one to visualize and dissect the sequential steps of exocytosis of large secretory granules in rodent salivary glands. Our studies provide a clear example of the advantages and limitations to this approach. The main benefit of IVM is the possibility to image a subcellular process in its own physiological environment. In addition, it permits an understanding of how intracellular events are integrated at the tissue level, and how they contribute to organ physiology. The recent introduction of confocal and two-photon microscopes equipped with resonant scanners [56], which increase the acquisition speed to more than 30 frames per seconds, will allow increased temporal resolution of the exocytic processes and surely enable discovery of novel aspects of their machinery. This breakthrough in combination with 1) transgenic mice expressing reporter of various signaling and lipid pathways, 2) knock-in mice expressing fluorescently tagged component of the actin cytoskeleton, and 3) conditional knock-outs for selected molecules will make it possible to garner a fully comprehensive appreciation of membrane remodeling in vivo.
Acknowledgments
Work in the author’(s) laboratory is supported by the Intramural Research Program of the NIH, NIDCR. We would like to thank Drs. Amornphimoltham and Ms. Olivia Harding for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 2.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annual review of biochemistry. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 3.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochemistry and cell biology. 2010;133:481–491. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic (Copenhagen Denmark) 2008;9:1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. American journal of physiology Cell physiology. 2009;297:C1347–1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigert R, Porat-Shliom N, Amornphimoltham P. Imaging cell biology in live animals: ready for prime time. The Journal of cell biology. 2013;201:969–979. doi: 10.1083/jcb.201212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiological reviews. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 9.Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annual review of cell biology. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- 10.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cellular and molecular life sciences: CMLS. 2013;70:2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felmy F. Modulation of cargo release from dense core granules by size and actin network. Traffic. 2007;8:983–997. doi: 10.1111/j.1600-0854.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annual review of physiology. 2014;76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masedunskas A, Sramkova M, Weigert R. Homeostasis of the apical plasma membrane during regulated exocytosis in the salivary glands of live rodents. BioArchitecture. 2011;1:225–229. doi: 10.4161/bioa.18405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigert R. Imaging the dynamics of endocytosis in live mammalian tissues. Cold Spring Harbor perspectives in biology. 2014 doi: 10.1101/cshperspect.a017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annual review of biochemistry. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 16.Söllner TH. Regulated exocytosis and SNARE function (Review) Molecular membrane biology. 2003;20:209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- 17.Akihisa Segawa S. Roles of Microfilaments in Exocytosis: A New Hypothesis. Cell Stracture and function. 1989;544:531–544. doi: 10.1247/csf.14.531. [DOI] [PubMed] [Google Scholar]
- 18.Valentijn K, Valentijn JA, Jamieson JD. Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance. Biochem Biophys Res Commun. 1999;266:652–661. doi: 10.1006/bbrc.1999.1883. [DOI] [PubMed] [Google Scholar]
- 19.Jerdeva GV, Wu K, Yarber Fa, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. Journal of cell science. 2005;118:4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang Y, Soekmadji C, Mitchell JM, Thomas WG, Thorn P. Real-time measurement of F-actin remodelling during exocytosis using Lifeact-EGFP transgenic animals. PloS one. 2012;7:e39815. doi: 10.1371/journal.pone.0039815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nature cell biology. 2001;3:253–258. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 22.Miklavc P, Wittekindt OH, Felder E, Dietl P. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: A prerequisite for content release or kiss-and-run. Annals of the New York Academy of Sciences. 2009 doi: 10.1111/j.1749-6632.2008.03989.x. [DOI] [PubMed] [Google Scholar]
- 23.Miklavc P, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C, Frick M. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion--a role for Rho, formins and myosin II. Journal of cell science. 2012;125:2765–2774. doi: 10.1242/jcs.105262. [DOI] [PubMed] [Google Scholar]
- 24.Miklavc P, Ehinger K, Sultan A, Felder T, Paul P, Gottschalk KE, Frick M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. Journal of cell science. 2015;128:1193–1203. doi: 10.1242/jcs.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. The Journal of cell biology. 2011;194:613–629. doi: 10.1083/jcb.201011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nature cell biology. 2003 doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 27.Yu HYE, Bement WM. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nature cell biology. 2007;9:149–159. doi: 10.1038/ncb1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bement AMSaWM. Kiss-and-Coat and Compartment Mixing: Coupling Exocytosis to Signal Generation and Local Actin Assembly. Molecular biology of the cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masedunskas A, Porat-shliom N, Weigert R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Commun Integr Biol. 2012;5:84–87. doi: 10.4161/cib.18258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oheim M, Loerke D, Stuhmer W, Chow RH. The last few milliseconds in the life of a secretory granule. Docking, dynamics and fusion visualized by total internal reflection fluorescence microscopy (TIRFM) European biophysics journal: EBJ. 1998;27:83–98. doi: 10.1007/s002490050114. [DOI] [PubMed] [Google Scholar]
- 31.Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Lifshitz LM, Jones C, Bellve KD, Standley C, Fonseca S, Corvera S, Fogarty KE, Czech MP. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Molecular and cellular biology. 2007;27:3456–3469. doi: 10.1128/MCB.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masedunskas A, Milberg O, Porat-shliom N, Sramkova M, Wigand T, Amornphimoltham P, Weigert R. Intravital microscopy: A practical guide on imaging intracellular structures in live animals. Bioarchitecture. 2012;2:143–157. doi: 10.4161/bioa.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. The Journal of biological chemistry. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 35.Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280:1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- 36.Olga Larina PB, BSL, Pickett James A, Amit Shah WAK, Michael Edwardson aPTJ. Dynamic Regulation of the Large Exocytotic Fusion Pore in Pancreatic Acinar Cells. Molecular biology of the cell. 2008;19:308–317. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milberg O, Tora M, Shitara A, Takuma T, Masedunskas A, Weigert R. Probing the Role of the Actin Cytoskeleton During Regulated Exocytosis by Intravital Microscopy. 2014;1174:407–421. doi: 10.1007/978-1-4939-0944-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. Journal of dental research. 2005;84:500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner R. Erla uterungstafeln zur Physiologie und Entwicklungsgeschichte. Leopold Voss; Leipzig: 1839. [Google Scholar]
- 40.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. American journal of physiology Cell physiology. 2002;283:C905–916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 41.Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. American journal of physiology Cell physiology. 2004;287:C517–526. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 42.Sramkova M, Parente L, Wigand T, Aye MP, Shitara A, Weigert R. Polyethylenimine-mediated expression of transgenes in the acinar cells of rats salivary glands in vivo. Frontiers in Cell and Developmental Biology. 2015;2:1–8. doi: 10.3389/fcell.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sramkova M, Masedunskas A, Weigert R. Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals. Histochemistry and cell biology. 2012;138:201–213. doi: 10.1007/s00418-012-0959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masedunskas A, Porat-Shliom N, Rechache K, Aye MP, Weigert R. Intravital Microscopy Reveals Differences in the Kinetics of Endocytic Pathways between Cell Cultures and Live Animals. Cells. 2012;1:1121–1132. doi: 10.3390/cells1041121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porat-Shliom N, Chen Y, Tora M, Shitara A, Masedunskas A, Weigert R. In vivo tissue-wide synchronization of mitochondrial metabolic oscillations. Cell reports. 2014;9:514–521. doi: 10.1016/j.celrep.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segawa A, Terakawa S, Yamashina S, Hopkins CR. Exocytosis in living salivary glands: direct visualization by video-enhanced microscopy and confocal laser microscopy. European journal of cell biology. 1991;54:322–330. [PubMed] [Google Scholar]
- 47.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis (New York, NY: 2000) 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 48.Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. The Journal of cell biology. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unni VK, Weissman TA, Rockenstein E, Masliah E, McLean PJ, Hyman BT. In vivo imaging of alpha-synuclein in mouse cortex demonstrates stable expression and differential subcellular compartment mobility. PloS one. 2010;5:e10589. doi: 10.1371/journal.pone.0010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riedl J, Flynn KC, Raducanu A, Gärtner F, Beck G, Bösl M, Bradke F, Massberg S, Aszodi A, Sixt M, Wedlich-Söldner R. Lifeact mice for studying F-actin dynamics. Nature methods. 2010;7:168–169. doi: 10.1038/nmeth0310-168. [DOI] [PubMed] [Google Scholar]
- 51.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Bista M, Bradke F, Jenne D, Holak Ta, Werb Z, Sixt M, Wedlich-soldner R, Klopferspitz A, Avenue P, Francisco S. Lifeact: a versatile marker to visualize F-actin. Nature methods. 2008;5:1–8. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gormal RS, Nguyen TH, Martin S, Papadopulos a, Meunier Fa. An Acto-Myosin II Constricting Ring Initiates the Fission of Activity-Dependent Bulk Endosomes in Neurosecretory Cells. Journal of Neuroscience. 2015;35:1380–1389. doi: 10.1523/JNEUROSCI.3228-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masedunskas A, Porat-Shliom N, Weigert R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Communicative & integrative biology. 2012;5:84–87. doi: 10.4161/cib.18258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. The EMBO journal. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 55.Gasman S, Chasserot-golaz S, Hubert P, Aunis D, Bader M-f. Identification of a Potential Effector Pathway for the Trimeric Go Protein Associated with Secretory Granules. The Journal of biological chemistry. 1998;273:16913–16920. doi: 10.1074/jbc.273.27.16913. [DOI] [PubMed] [Google Scholar]
- 56.Kirkpatrick ND, Chung E, Cook DC, Han X, Gruionu G, Liao S, Munn LL, Padera TP, Fukumura D, Jain RK. Video-rate resonant scanning multiphoton microscopy: An emerging technique for intravital imaging of the tumor microenvironment. Intravital. 2012;1 doi: 10.4161/intv.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]