Abstract
There are many reports of the anti-inflammatory, anti-cancer, and anti-atherosclerotic activities of conjugated linolenic acids (cLNA). They constitute a small percentage of fatty acids in the typical human diet, although up to 80% of the fatty acids in certain fruits such as pomegranate. In the course of studying a bacterial fatty acid dioxygenase (Nostoc linoleate 10S-DOX, an ancient relative of mammalian cyclooxygenases), we detected strong inhibitory activity in a commercial sample of linoleic acid. We identified two cLNA isomers, β-eleostearic (9E,11E,13E-18:3) and β-calendic acid (8E,10E,12E-18:3), as responsible for that striking inhibition with a Ki of ~49 nM and ~125 nM, respectively, the most potent among eight cLNA tested. We also examined the effects of all eight cLNA on the activity of COX-1 and COX-2. Jacaric acid (8Z,10E,12Z-18:3) and its 12E isomer, 8Z,10E,12E-18:3, strongly inhibit the activity of COX-1 with a Ki of ~1.7 and ~1.1 μM, respectively. By contrast, COX-2 was ≤ 30% inhibited at 10μM concentrations of the cLNA. Identifying the activities of the naturally occurring fatty acids is of interest in terms of understanding their interaction with the enzymes, and for explaining the mechanistic basis of their biological effects. The study also highlights the potential presence of inhibitory fatty acids in commercial lipids prepared from natural sources. Analysis of seven commercial samples of linoleic acid by HPLC and UV spectroscopy is illustrated as supplementary data.
Keywords: fatty acid dioxygenase, cyclooxygenase, linoleic acid, conjugated fatty acid, 10S-DOX, Nostoc punctiforme
1. Introduction
Homologues of the animal prostaglandin synthases or cyclooxygenase (COX)1 enzymes are well studied in plants (α-dioxygenases, α-DOX) and fungi (linoleate-DOX/P450 fusion proteins and α-DOX). The one bacterial enzyme characterized so far is a cyclooxygenase homologue in the cyanobacterium Nostoc punctiforme PCC 73102 that we identified as a linoleate (and oleate) 10S-dioxygenase (10S-DOX), while the existence of other bacterial homologues is documented in silico [1, 2]. The Nostoc enzyme has relatively low sequence identity with mammalian cyclooxygenases (~27%), yet it retains the key catalytic residues of the COX enzymes. Some sequence near the N-terminus is missing (mostly involving the EGF and membrane-binding domains) as are a few well studied amino acids in COX-1 and COX-2 (Arg-120, Ser-530), but the critical amino acids on the distal and proximal heme site are mostly conserved, along with the catalytic Tyr in the fatty acid binding channel. The neighboring Nostoc gene to the 10S-DOX encodes a catalase-related hemoprotein that acts as a specific 10S-hydroperoxide lyase, cleaving the linoleate 10S-hydroperoxide (10S-HPODE) to C10 aldehyde acid and C8 alcohol fragments. These reactions match a long-known fungal pathway of linoleic acid metabolism that occurs prominently in mushrooms (the enzyme(s) of which have yet to be identified) although the physiological role in Nostoc remains an open issue.
In the course of studying the Nostoc 10S-DOX using oxygen uptake assays, we observed unexpectedly weak activity with a newly prepared batch of linoleic acid. The anomaly was confirmed by comparison with other samples of linoleate and further investigation revealed trace impurities of conjugated linolenic acids (cLNA) that exhibited profound inhibition of the Nostoc dioxygenase. Earlier studies have reported the inhibitory effects of certain conjugated diene and conjugated triene-containing fatty acids on cyclooxygenase activities [3, 4]. Herein we describe isolation and identification of the Nostoc 10S-DOX inhibitors. The findings prompted us to test other conjugated fatty acid isomers for their inhibitory properties on the 10S-DOX and to extend the study to include the effects of these fatty acids on COX-1 and COX-2.
2. Materials and Methods
2.1. Materials
Linoleic acid was purchased from Nu-Chek Prep Inc. (Elysian, MN), Cayman Chemical (Ann Arbor, MI), and Sigma. The pure linoleic acid used as substrate herein was prepared from a batch that contained no significant impurities near the linoleic acid as monitored on RP-HPLC (Nu-Chek Prep, lot no. U59A-S24-S, Supplementary Fig. S1, S2, S4); it was collected from RP-HPLC and quantified by reaction of an aliquot with soybean lipoxygenase (ε = 25,000 at 235 nm). Five conjugated triene isomers of linolenic acid listed in Table 1 were purchased from Larodan (Sweden). Purified ovine COX-1 was kindly provided by Dr. Lawrence J. Marnett at Vanderbilt University and used for the detailed kinetic study of the most potent isomers [5]. Ram seminal vesicle microsomes (RSVM) were used as the source of ovine COX-1 for initial screening of the conjugated cLNA. Human COX-2 [6] and Nostoc 10S-DOX [7] were prepared as explained previously.
Table 1. The C18:3 conjugated trienes used in this study.
The trivial names and natural source of fatty acids are given if available. Most of these fatty acids were purchased from Larodan and the rest were prepared in this study.
| cLNA isomers | Trivial name | Source/Supplier | Example of natural source | Conc. (%) | Ref. |
|---|---|---|---|---|---|
| 8Z,10E,12Z | Jacaric acid | from Jacaranda seeds | Jacaranda seeds | ||
| 8Z,10E,12E | by isomerization | (occurrence not described) | |||
| 8E,10E,12Z | α-Calendic acid | Larodan AB | Pot marigold seed | 62.2 | [9] |
| 8E,10E,12E | β-Calendic acid | Larodan AB | Pot marigold seed | 4.7 | [10] |
| 9Z,11E,13Z | Punicic acid | Larodan AB | Pomegranate seed | 83 | [9] |
| 9Z,11E,13E | α-Eleostearic acid | Larodan AB | Tung seed | 67.7 | [9] |
| 9E,11E,13Z | Catalpic acid | Larodan AB | Catalpa seed | 42.3 | [9] |
| 9E,11E,13E | β-Eleostearic acid | by isomerization | Tung oil | 11.3 | [9] |
2.2. Isolation of jacaric acid
Jacaric acid (8Z,10E,12Z-octadecatrienoic acid) was isolated from Jacaranda mimosifolia seeds [8]. Briefly, jacaranda seeds (2 g) obtained from a commercial source (via eBay) were homogenized in CHCl3/MeOH (2:1) in two consecutive extractions of 50 ml using a Polytron. The combined extract was centrifuged and half of the solvent taken to dryness using a stream of nitrogen. The residue was suspended in 1 ml CHCl3 and 20 ml of freshly-prepared 1M methanolic KOH and 2 ml of water were added and the sample heated at 60 °C under nitrogen for 1 h, followed by additional water (4 ml) and a further 30 min at 60 °C. After evaporation to half volume, addition of water and acidification, the free fatty acids were extracted into 20% EtOAc in hexane, washed twice with water and analyzed by UV spectroscopy, indicating a total of ~75 mg of fatty acid conjugated trienes (25 ml of 3.1 mg/ml, assuming ε = 47,900 [4]. Four 1.5 ml aliquots of the extract were reconstituted in RP-HPLC column solvent (methanol/water/glacial acetic acid, 85:15:0.01 by volume) and purified using a semi-preparative Beckman 5 μ ODS column (25 × 1 cm) with a flow rate of 2 ml/min and diode array UV detection monitoring 205, 220, 235 and 270 nm; the major conjugated triene (jacaric acid, λmax 274 nm) eluted at 38 min, a minor conjugated triene impurity at 42 min (~5%, λmax 270 nm), and linoleic acid at 46 min. The jacaric acid fraction was evaporated to one third volume under a stream of nitrogen, diluted with an equal volume of water and extracted with hexane: UV analysis indicated an almost complete absence of jacaric acid in the aqueous phase. The hexane was evaporated to dryness and the jacaric acid stored in ethanol (3 ml of 5.2 mg/ml, ε = 47,900 [4]) at −20 °C under argon.
2.3. Preparation of 9E,11E,13E-octadecatrienoic acid (β-eleostearic acid) and 8Z,10E,12E-octadecatrienoic acid
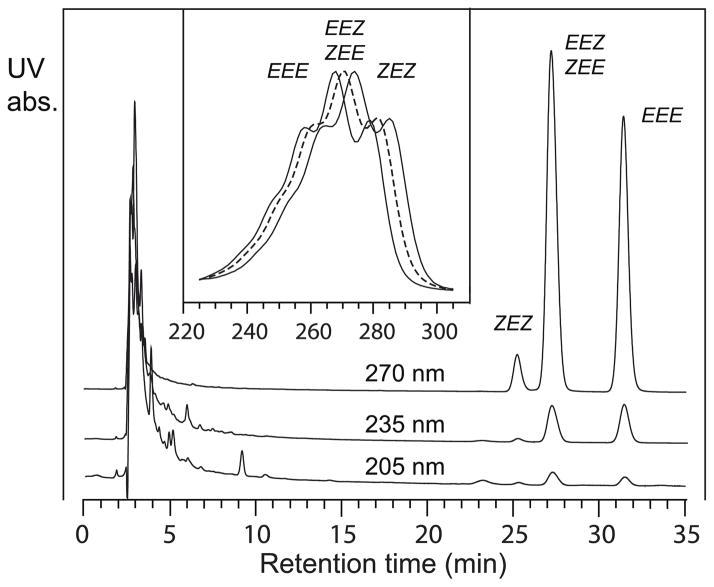
9Z,11E,13E-Octadecatrienoic acid (α-eleostearic acid) and jacaric acid were isomerized to 9E,11E,13E-octadecatrienoic acid (β-eleostearic acid) and 8Z,10E,12E-octadecatrienoic acid, respectively, by treatment of 1 mg of fatty acid in 100 μl toluene in a 1 ml Reactivial with 0.25 μg iodine (1.25 μl of a 0.2 mg/ml solution in petroleum ether) [4]. The reaction takes place in daylight and appeared complete within 2 min at room temperature. Sodium sulfite (50 μl of 2 mg/ml in water) was mixed vigorously with the sample prior to fatty acid extraction to remove the iodine. The solution was acidified to pH 4 by addition of 25 μl 1 M KH2PO4 and the fatty acids extracted into 0.5 ml hexane. In the case of α-eleostearic acid isomerization, the fatty acid isomers were subsequently resolved by RP-HPLC using a Waters Symmetry C18 column (25 × 0.46 cm) and a solvent of methanol/water/glacial acetic acid (85:15:0.01 by volume) at a flow rate of 1 ml/min. Complete resolution is achieved of newly formed 9Z,11E,13Z-18:3 (~3%, at 25 min), from the mixture of 9Z,11E,13E (starting material) and 9E,11E,13Z isomers (~40%, 27 min), and the all-trans derivative 9E,11E,13E-18:3 (~57%, 31.5 min); the RP-HPLC chromatogram is illustrated as Figure 2 under Results.
Figure 2. RP-HPLC analysis of isomerized α-eleostearic acid.
The sample was chromatographed on a Waters Symmetry C18 column (25 × 0.46 cm) using a solvent of methanol/water/glacial acetic acid (85:15:0.01 by volume) with a flow rate of 1 ml/min with UV detection at 205, 235 and 270 nm (with all channels on the same sensitivity). The compounds are identified as 9Z,11E,13Z-C18:3 (ZEZ), followed by a mixture of 9Z,11E,13E-C18:3 (ZEE, α-eleostearic acid, starting material) and 9E,11E,13Z-C18:3 (EEZ), and lastly all trans isomer 9E,11E,13E-C18:3 (EEE). Inset: the UV spectrum of the three individual peaks shown with maximum absorbance of 274, 270, and 268 nm for ZEZ, ZEE/EEZ, and EEE isomers, respectively.
Isomerized jacaric acid was chromatographed initially by argentation HPLC using a Sepralyte SCX 5-μm, 25 × 0.46 cm cation exchange column in the silver form [11, 12] with a solvent of hexane/isopropyl alcohol/glacial acetic acid in the proportions 100:1:0.1 (by volume). The early-eluting 8E,10E,12E-18:3 isomer (at 15 ml) separated easily from a partially resolved mixture of the 8E,10E,12Z and 8Z,10E,12E isomers (eluting in that order at 22 and 23 ml) followed by jacaric acid starting material at 30 ml. The desired isomer (ZEE) and α-calendic acid (EEZ, available as an authentic standard) were completely resolved by SP-HPLC using a Beckman 5 μ silica column (25 × 0.46 cm) with a solvent of hexane/isopropyl alcohol/acetic acid (100:0.05:0.01, by volume) run at 1 ml/min with retention times of 64 min and 68 min, respectively. The ZEE isomer was collected from several injections and quantified by UV spectroscopy (ε = 49,100) [4].
2.4. Isolation of impurities in linoleic acid
Linoleic acid (Nu-Chek Prep Inc, U-59A, lot number 3A-W, total of 3 mg) was chromatographed on RP-HPLC using a semi preparative Beckman ODS column (25 × 1 cm) and a solvent of methanol/water/glacial acetic acid (90:10:0.01 by volume) at a flow rate of 2 ml/min. Peaks at elution times of ~10, 19, 20.5, 22, 23, and 28 min were collected. Peak 5 (a conjugated triene at ~23 min retention time) was separated from the co-eluting linoleic acid using the silver column described above and a solvent of hexane/isopropanol/glacial acetic acid (100:2:0.1 by volume) at a flow rate of 0.5 ml/min; the broad chromatographic peak of conjugated triene eluted from the silver column was further resolved into two components designated 5a and 5b (~66 and 70 min, respectively) using a Thomson Instrument 5μ silica column (25 × 0.46 cm) and a solvent of hexane/isopropanol/glacial acetic acid (100:0.1:0.1 by volume) at a flow rate of 0.5 ml/min.
2.5. Derivatization and GC-MS analysis
Dimethyloxazoline (DMOX) derivatization was accomplished by reacting the conjugated triene-containing fatty acid with 30 μL of 2-amino-2-methyl-1-propanol in a nitrogen atmosphere at 180 °C overnight [13]. Derivatized fatty acids were extracted into 2 ml of chloroform and washed with 2 ml of water 3 times followed by washing with 2 ml of brine. The extract was applied to a SP-HPLC silica column (25 × 0.46 cm) using a solvent of hexane/isopropanol (100:1 by volume) at a flow rate of 0.5 ml/min with monitoring of the conjugated triene at 270 nm. The collected DMOX derivative (~18 min retention time) were subsequently dissolved in hexane for GC-MS and analyzed using a ThermoFinnigan DSQ mass spectrometer in the positive ion electron impact mode (70 eV). The initial oven temperature was set for 160 °C, held for 1 min and then increased to 320 °C at 20 °C/min increment and held at 320 °C for 3 min.
2.6. NMR analysis
1H NMR spectra were recorded on a Bruker DXR 600 MHz spectrometer at 298 K. Analysis of 5a and 5b was conducted at 298 K in CDCl3. The ppm values were calibrated using residual non-deuterated solvent (δ = 7.26 ppm for CHCl3).
2.7. Assay of Nostoc 10S-dioxygenase activity
The enzyme activity was measured by oxygen uptake in a 2 ml cell at 25 °C using a YSI model 5300 Biological Oxygen Monitor. As noted earlier, the pure linoleic acid used as substrate in these experiments was from a batch that contained no detectable impurities near the linoleic acid on RP-HPLC (Fig. S4). Initial evaluation of the inhibitory activity of linoleic acid impurities (peaks 1 – 5) was performed in 50 mM Tris buffer pH 7.5, 150 mM NaCl, in the presence of 200 μM linoleic acid and 10 % of each RP-HPLC-isolated component. Reaction was started by addition of the Nostoc 10S-DOX at a final concentration of 75 nM. The inhibitory activity of the pure conjugated triene fatty acids listed in Table 1 was evaluated in the same way using 10 μM of each conjugated triene. The inhibition constants for 8E,10E,12E-C18:3 and 9E,11E,13E-C18:3 were determined using several concentrations of linoleic acid (25, 50, 100, and 200 μM) in the presence of different concentrations of either 8E,10E,12E-C18:3 (0, 68, 136, 272, 544 nM) or 9E,11E,13E-C18:3 (0, 50, 100, 200, 400 nM).
2.8. Analyzing the effect of cLNA on COX-1 and COX-2
The inhibitory effects on RSVM and purified human COX-2 were measured by oxygen consumption at 30 °C in the 2 ml cell containing 0.1 M potassium phosphate pH 7.2, 1 mM phenol, 1 μM hematin, using 100 μM arachidonic acid substrate. After adding 10 μM of conjugated triene (Table 1) in 5 μl ethanol, reaction was started by addition of COX-2 (310 nM) or 10 μl of RSVM. To further evaluate the inhibitory effect on COX-1 of 8Z,10E,12Z-C18:3 (jacaric acid) and 8Z,10E,12E-C18:3, the assays were performed using purified ovine COX-1 with several concentrations of arachidonic acid (25, 50, and 100 μM) and conjugated triene (0, 1, 2.5, 5 μM) with reaction initiated using 66 nM enzyme.
3. Results
3.1. A trace impurity in linoleic acid strongly inhibits 10S-DOX
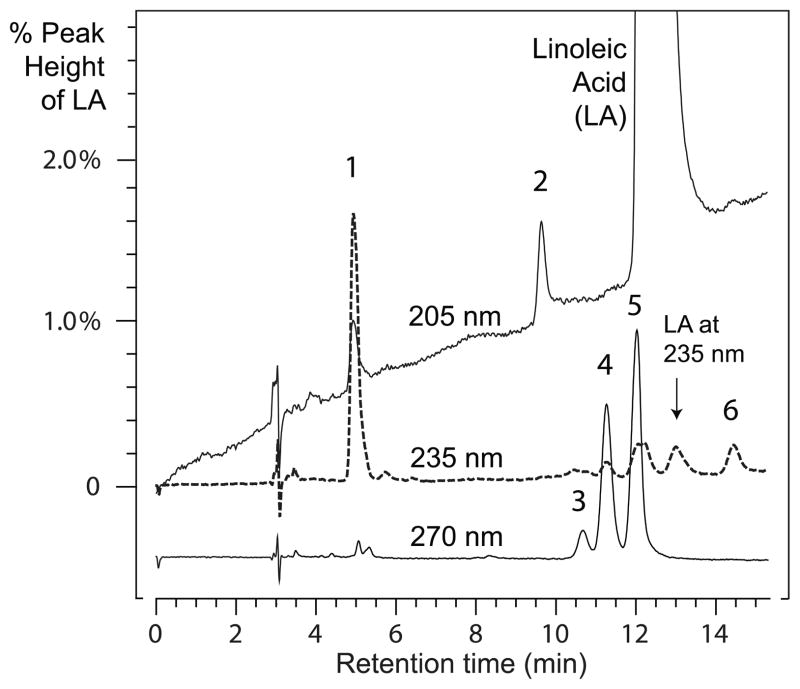
In the course of analyzing the Nostoc 10S-DOX using an oxygen uptake assay, we noted a batch of linoleic acid that gave unexpectedly weak activity with the enzyme. Side-by-side comparison with highly purified linoleic acid confirmed the anomaly (Supplementary data, Fig. S1). A careful RP-HPLC analysis of the suspect LA revealed several minor impurities, designated 1 – 6 (Fig. 1), and these were collected following injection of larger quantities of the linoleate. Peak 1 was oxidized linoleic acid (a mixture of HPODEs), and peak 2 is likely a trace of α-linolenic acid due to isolation of the linoleate from plant sources. Peaks 3, 4, and 5 had conjugated triene chromophores and the very minor peak 6 exhibited a weak conjugated diene. Because Peak 5 co-eluted with the beginning of the linoleic acid peak, the two were resolved using a silver-loaded HPLC column, and peak 5 further resolved by SP-HPLC into two compounds, designated 5a and 5b (see Methods, 2.4).
Figure 1. RP-HPLC separation of fatty acid impurities in linoleic acid.
The sample was chromatographed on a Beckman semi-preparative 5μ ODS column (25 × 1 cm) using a solvent of methanol/water/glacial acetic acid (90:10:0.01 by volume) with a flow rate of 4 ml/min with UV detection at 205, 235 and 270 nm (with all channels on the same sensitivity). The graph is expanded to illustrate the minor components with 0 – 2% of the maximal absorbance of the linoleic acid. The 270 nm trace is lowered from the origin for clarity.
To test for potential inhibitory activity, 10% of each isolated peak was co-incubated with the 10S-DOX in the presence of 50 μM linoleic acid. By far the most substantial inhibition (76%) occurred using peak 5 (at this stage unresolved into 5a and 5b), followed by 41% inhibition using peak 4. The other components gave weak or negligible inhibition (6%, 13%, and 5% for peaks 1, 2, and 3, respectively) with too little available peak 6 for testing. Subsequent assays using synthetic linoleate conjugated dienes (9Z,11E and 10E,12Z), up to 4.5 μM in concentration, resulted in only weak inhibition of the Nostoc 10S-DOX.
3.2. Structural analysis of the most potent inhibitor, peak 5
Peak 5 was resolved into its components 5a and 5b by SP-HPLC and the location of the double bonds identified by GC-MS of the DMOX derivative [14]. Electron impact ionization of the fatty acid DMOX derivative produces a series of fragments that increase in mass by 14 a.m.u. along the saturated portion of the carbon chain, with a change to 12 a.m.u. at the position of the first double bond. In 5a, the characteristic fragmentation ions are observed at m/z 182 (C7), 196 (C8), 208 (C9), 222 (C10), 234 (C11), 248 (C12), 260 (C13), 274 (C14), 288 (C15), 302 (C16), 316 (C17) and finally 331 (the molecular ion, identifying an octadecatrienoic fatty acid). Cleavage of the C9-C10 bond creates the m/z 208 ion, the first 12 a.m.u. mass difference, and defining the location of the first double bond at C9, and defining 5a as a 9,11,13-octadecatrienoic acid. Indeed, the mass spectrum of 5a matched the reported spectra of the DMOX derivative of three geometric isomers of 9,11,13-18:3 [15].
The corresponding analysis of 5b as the DMOX derivative produced fragmentation ions at m/z 182 (C7), 194 (C8), 208 (C9), 220 (C10), 234 (C11), 246 (C12), 260 (C13), 274 (C14), 288 (C15), 302 (C16), 316 (C17) and 331. In this case the C8-C9 cleavage creates m/z 194 ion, the first 12 a.m.u. mass difference, and defining the location of the first double bond at C8, and defining 5b as a 8,10,12-octadecenoic acid. The mass spectrum matched the reported spectrum of the DMOX derivative of a conjugated 8,10,12-octadecatrienoic acid [16].
The 1H NMR spectra of both 5a and 5b in CDCl3 were very similar to that of β-eleostearic acid previously reported [15], although the overlapping signals in the symmetrical all-trans conjugated triene systems obscure the individual coupling constants. Instead, we identified the 5a and 5b isomers by UV and chromatographic comparison with authentic standards. The UV absorbances of compounds 5a and 5b exhibit a λmax at 268 nm with well-defined shoulders at 258 and 279 nm, and precisely matching the spectra of the all-trans conjugated triene standards, β-eleostearic and β-calendic acids. Also 5a co-chromatographed identically with authentic β-eleostearic acid (prepared in this work) and 5b with βcalendic acid (Larodan). Thus, we identified 5a as β-eleostearic acid (9E,11E,13E-18:3) and 5b as βcalendic acid (8E,10E,12E-18:3).
3.3. Preparation of β-eleostearic acid and 8Z,10E,12E-octadecatrienoic acid for biological testing
These fatty acids were unavailable commercially and were prepared by iodine-induced isomerization [4] of α-eleostearic acid (9Z,11E,13E-18:3) and jacaric acid (8Z,10E,12Z-18:3), respectively, as described in detail in Materials and Methods (2.3). RP-HPLC separation of isomerized α-eleostearic acid gave peaks corresponding to ZEZ, EEZ/ZEE, and EEE conjugated trienes, the last one being the desired β-eleostearic acid, Figure 2. The UV spectra are both distinctive and definitive of the different conjugated triene systems (Fig. 2, inset). Production of pure 8Z,10E,12E-octadecatrienoic acid from iodine-isomerized jacaric acid was more challenging as the EEZ/ZEE mixture (which fails to resolve by RP-HPLC, cf. Figure 2) was only partially resolved using argentation chromatography and a final SP-HPLC at 50 min retention times was required to give the pure 8Z,10E,12E isomer (Materials and Methods, 2.3).
3.4. Screening the cLNA for inhibition of 10S-DOX, RSVM (COX-1), and COX-2
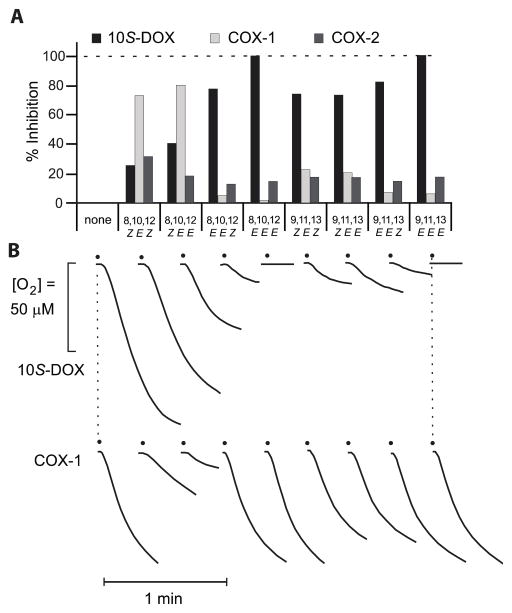
The percent inhibition by a 10 μM concentration of eight conjugated linolenic acid isomers, listed in Table 1, on the activity of purified Nostoc 10S-DOX, RSVM and human COX-2 is summarized in Figure 3A. Linoleic acid (100 μM) was used as substrate for the Nostoc 10S-DOX and arachidonic acid (100 μM) for RSVM and COX-2 and the reactions were monitored as O2 consumption using an oxygen electrode.
Figure 3. Inhibition of Nostoc 10S-DOX, ovine COX-1, and human COX-2 by conjugated triene isomers of linolenic acid.
Activity was measured using the steepest part of the O2 uptake curve. A) Percentage inhibition of Nostoc 10S-DOX (black), ovine COX-1 (RSVM, light gray), and human COX-2 (dark gray) is shown in the presence of eight cLNA (10 μM). This experiment was performed using 100 μM substrate (LA for 10S-DOX and AA for COX-1 and -2) and 10 μM of each conjugated triene. B) Representative records of O2 uptake in each reaction of 10S-DOX and COX-1. The data show single determinations using 10 μM cLNA following preliminary tests using 0.2 μM (10S-DOX), 2 μM (all three enzymes), and 10 μM (tested one time with 10S-DOX and COX-2, three times with RSVM or COX-1). Detailed examination of the most potent inhibitors of 10S-DOX and COX-1 is reported in Figures 4 and 5, respectively.
Complete inhibition of Nostoc 10S-DOX is achieved in the presence of either β-calendic acid (8E,10E,12E-C18:3) or β-eleostearic acid (9E,11E,13E-C18:3), Fig. 3A, 3B. Indeed, six of the eight cLNA isomers gave greater than 50% inhibition of the 10S-DOX at the 10 μM concentration tested. On the other hand, testing COX-1, only two of the eight produced 50% or more inhibition; jacaric acid (8Z,10E,12Z-C18:3) and 8Z,10E,12E-C18:3 produced the strongest inhibition of approximately 73% and 80%, respectively. In contrast, these conjugated fatty acids have comparatively weak inhibitory effect on the COX-2 activity with the most pronounced inhibition, 30%, in the case of jacaric acid (8Z,10E,12Z-C18:3). The percent inhibition in these tests as plotted in Fig. 3A was assessed as the change in slope of the oxygen consumption graph. Notably, however, these conjugated trienes decrease not only the rate of reaction (the slope of the O2 uptake), they also severely limit the duration of the activity and bring the reactions to halt through enzyme inactivation, Figure 3B.
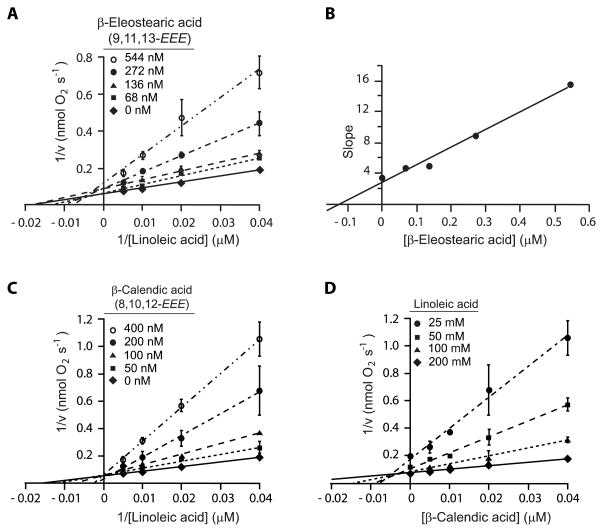
3.5. Inhibition of 10S-DOX by β-eleostearic acid and β-calendic acid
To investigate the nature of the 10S-DOX inhibition a more complete kinetic analysis was carried out using β-eleostearic and β-calendic acids. Each reaction was started by addition of 10S-DOX at the final concentration of 75 nM and the activity was monitored as a function of the rate of O2 consumption. The Lineweaver-Burk plots are used to obtain the Km and Vmax of the 10S-DOX with linoleic acid and the Kmapp and Vmaxapp and the effects of β-eleostearic and β-calendic acids (Table 2). The plots of 1/v (nmol O2−1 s) versus 1/[LA] (μM−1) in the presence of different concentrations of the conjugated trienes are used to illustrate the type of inhibition (Fig. 4, A and C). In the case of β-eleostearic acid all lines intersect neither on the x-nor y-axis, signifying a mixed inhibition (Fig. 4A). In the presence of β-calendic acid all the lines intersect at one point on the x-axis indicative of a competitive inhibitor. To determine the inhibition constant (Ki) of β-eleostearic acid as a mixed inhibitor, the slopes of the lines in panel A of Fig. 4 are plotted versus the concentration of β-eleostearic acid (Fig. 4B); the intersection on the x-axis gives the negative value of the Ki, computed from these data as 125 nM [17]. To obtain the Ki of β-calendic acid as a competitive inhibitor, 1/v (nmol O2−1 s) is plotted against the concentration of β-calendic acid (Fig. 4D); the x coordinate of the point that all the lines intersect is equal to – Ki, computed as 46 nM.
Table 2.
Kinetic parameters of Nostoc 10S-DOX (75 nM) with linoleic acid in the absence or presence of several concentrations of β-eleostearic acid and β-calendic acid.
| Inhibitor | Inhibitor (μM) | Km (μM) | Vmax (nmol O2 s−1) |
|---|---|---|---|
| None | 0 | 60.3 | 17.9 |
| β-eleostearic acid | 68 | 76.3 | 16 |
| 136 | 58.2 | 11.8 | |
| 272 | 104 | 11.7 | |
| 544 | 133 | 8.6 | |
| β-calendic acid | 50 | 77.8 | 15.4 |
| 100 | 136.3 | 17.6 | |
| 200 | 450.9 | 28.5 | |
| 400 | 419.3 | 16.8 |
Figure 4. Inhibition of Nostoc linoleate 10S-DOX (75 nM) by β-eleostearic acid (β-EA) and βcalendic acid (β-CA).
A) Lineweaver-Burk plot of 1/v (nmol O2−1 s) vs 1/[LA] (μM−1) in the presence of several concentrations of β-EA (0, 68, 136, 272, 544 nM). B) The slope of each line from the Lineweaver-Burk graph of part A is plotted vs [β-EA] (μM). The computed fitted line with equation of y = 22.743 x + 2.8535 (R2 = 0.984) provides the inhibition constant of β-EA (y = 0 ⇒ x = - Ki). C) The Lineweaver-Burk plot of 1/v (nmol O2−1 s) vs 1/[LA] (μM−1) in the presence of different concentrations of β-CA (0, 50, 100, 200, 400 nM). D) The Dixon plot of 1/v (nmol O2−1 s) vs [β-CA] (μM) in the presence of several concentrations of substrate, LA, (25, 50, 100, 200 μM). The x value of the point of intersection of all four fitted lines provides the inhibition constant of β-calendic acid (x = - Ki). Error bars represent the S.E. from three independent experiments.
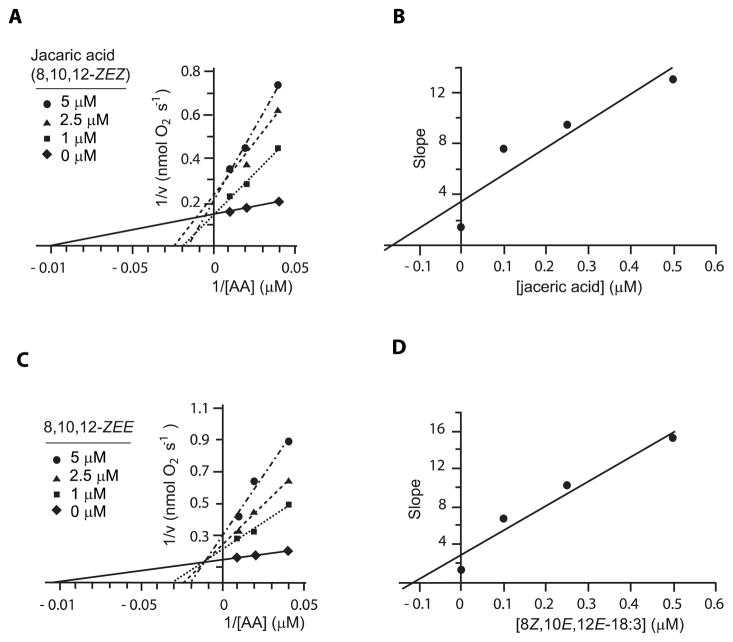
3.6. Inhibition of ovine COX-1 by jacaric acid and 8Z,10E,12E-18:3
To examine the nature of the inhibition by the two most potent cLNA, jacaric acid and 8Z,10E,12E-18:3 (Figure 2), the oxygen uptake assays were started by addition of purified COX-1 at the final concentration of ~66 μM. The Lineweaver-Burk plots of 1/v (nmol O2−1 s) versus 1/[AA] (μM−1) show complex inhibition characteristics for jacaric acid, with no distinct point of intersection of the extrapolated lines (Fig. 5A). The 8Z,10E,12E-18:3 isomer shows clean mixed inhibition behavior with a distinct point of intersection (Fig. 5C). The Ki constants are determined from the plot of the slope of the lines in panels A and C versus the concentration of the cLNA (Fig. 5B and D), giving values of ~1.7 and ~1.1 μM for jacaric and 8Z,10E,12E-18:3, respectively.
Figure 5. Inhibition of human COX-1 (66 nM) in presence of either jacaric acid (JA) or 8Z,10E,12E-18:3.
A) The Lineweaver-Burk plot of 1/v (nmol O2−1 s) vs 1/[AA] (μM−1) in presence of jacaric acid (0, 1, 2.5, and 5 μM). B) The slope of each line from Lineweaver-Burk graph of part A is plotted vs [JA] (μM). The computed fitted line with equation of y = 2.0878 x + 3.4423 (R2 = 0.87) provides the inhibition constant of jacaric acid (y = 0 ⇒ x = - Ki). C) The Lineweaver-Burk plot of 1/v (nmol O2−1 s) vs 1/[AA] (μM−1) in presence of 8Z,10E,12E-18:3 (0, 1, 2.5, and 5 μM). D) The slope of each line from Lineweaver-Burk graph of part B is plotted vs [8Z,10E,12E-18:3] (μM). The computed fitted line with equation of y = 2.6304 x + 2.9129 (R2 = 0.95) provides the inhibition constant of 8Z,10E,12E-18:3 (y = 0 ⇒ x = - Ki). Each data point is average of two independent experiments.
4. Discussion
4.1. LA impurities and their effects on 10S-DOX activity
Activity of the Nostoc 10S-DOX was inhibited profoundly by trace impurities in a freshly opened linoleic acid ampule. Analysis of the linoleic acid sample revealed the presence of small amounts of oxidized linoleic acid, linolenic acid, linolenic acid conjugated triene isomers, and linoleic acid conjugated diene isomers (Fig. 1). Since linoleic acid is isolated from natural sources, there is the possibility of contamination with other naturally occurring fatty acids [16]. Further investigation of each isolated impurity from the sample on 10S-DOX activity showed strong inhibition by linolenic acid conjugated triene isomers characterized as 8E,10E,12E-18:3 (β-calendic acid) and 9E,11E,13E-18:3 (β-eleostearic acid). This finding prompted us to analyze the effect of eight conjugated triene isomers of linolenic acid (listed in Table 1) on the 10S-DOX activity. At the concentration tested (10 μM), βcalendic acid and β-eleostearic acid inactivated the enzyme completely. Analyzing the inhibitory effects of β-eleostearic acid and β-calendic acid in more detail showed mixed and competitive inhibition behavior with a Ki of ~49 and ~125 nM, respectively. In our original testing of the contaminated batch of linoleic acid at 50 μM concentration, the concentration of the cLNA impurities (at very approximately 0.2 % relative abundance to the linoleate) would be ~100 nM, around the computed Ki of the two contaminating isomers.
4.2. Nature of 10S-DOX inhibition by cLNA isomers
Monitoring the inhibition of 10S-DOX by conjugated trienes shows a reduced rate of reaction along with irreversible inactivation of the enzyme (Fig. 3B, Fig. S1). The irreversible inactivation is not reflected in determination of the inhibitor kinetics, which are computed from the slope of the curve, not the extent of reaction. The inactivation appears similar in character to the suicide-like inactivation of cyclooxygenase and lipoxygenases by acetylenic fatty acids [18, 19]; for both classes of enzyme this involves a time-dependent and oxygen-dependent transformation of the inhibitory fatty acid [20]. Detailed studies of the interaction of octadecadiynoate (ODYA) with soybean lipoxygenase implicate a hydroperoxide product of the acetylenic fatty acid as the inactivating inhibitor [21, 22]. Further investigation is required to establish the mechanism of inhibition of the conjugated trienes on the Nostoc 10S-DOX.
4.3. Effects of cLNA isomers on COX-1 and COX-2
The similarity of Nostoc 10S-DOX to the animal COX enzymes prompted us to evaluate the effects of the cLNA isomers on the activity of COX-1 (ovine) and COX-2 (human). In 1987, with COX-1 being the only isozyme recognized, Nugteren and Hazelhof tested several cLNA on RSVM activity and identified jacaric acid and 8Z,10E,12E-18:3 as potent inhibitors [4]. Here we extended the screening to include eight cLNA isomers, and with COX-1 our results confirmed the identification of jacaric acid and the 8Z,10E,12E-18:3 isomer as the most potent inhibitors, with mixed inhibition behavior for both with a Ki of ~1.7 and ~1.1 μM, respectively. By contrast, in the case of COX-2 our results indicate that none of the fatty acids tested had appreciable inhibitory activity on COX-2, except for jacaric acid, which had only a modest effect (30% inhibition at 10 μM). As selective COX-1 inhibitors are already well characterized [23], the cLNA selectivity for COX-1 versus COX-2 is probably insufficient for practical use in research. Nonetheless the selectivity should be considered in evaluating the bioactions of cLNA and their potential to inhibit prostaglandin biosynthesis.
5. Concluding remarks
There are a large number of investigations on the bio-activity and effect of the conjugated fatty acids on health and disease and their anti-inflammatory, anti-cancer, and anti-atherosclerotic activities [24, 25]. The conjugated linolenic acids (cLNA) constitute a small percentage of fatty acids in the typical human diet, although up to 80% of the fatty acids in certain fruits such as pomegranate [9]. Since cyclooxygenases play an important role in inflammation, hemostasis and other diseases such as cancer, controlling their activities is of therapeutic importance. In addition to the non-steroidal anti-inflammatory drugs, inhibition of COX enzymes by fatty acids, especially polyunsaturated fatty acids have been studied before in the early years of establishing COX catalysis [26, 27] and more recently with the interest in interactions between the two monomers of the COX homodimer [28, 29]. Identifying the naturally occurring fatty acids that affect the activity of these enzymes is of interest, since dietary manipulation can change the fatty acid composition of the cell which will affect the inflammatory process. Based on our results, out of eight cLNA tested, jacaric acid and its 12E isomer strongly inhibit COX-1 but not COX-2. There are studies on cancer prevention, anti-tumor, and cancer-apoptosis inducing affects of jacaric acid [30, 31]. Further studies are required to investigate the mechanism of action and also in vivo effects of these two cLNA on COX-1 activity.
Finally, our results highlight the possibility of samples of polyunsaturated fatty acids containing hard-to-remove impurities that may have a profound effect on catalytic activity. Particularly when small changes in activity are to be interpreted as of mechanistic significance (such as secondary kinetic isotope effects that change activity by a very few percent), then the use of scrupulously purified fatty acid substrates is essential. Our analysis of seven commercial samples of linoleic acid by UV spectroscopy and HPLC is illustrated as supplementary data.
Supplementary Material
Highlights.
Fatty acid impurities in linoleic acid inhibited Nostoc linoleate 10S-dioxygenase
We identified the inhibitors as specific conjugated linolenic acids (cLNA)
Of cLNA tested, β-calendic and β-eleostearic acids inhibit with a Ki ~ 0.1 μM
Jacaric acid and its 12E-isomer inhibited COX-1 (Ki 1–2 μM) but not COX-2
Naturally occurring cLNA should be considered as fatty acid dioxygenase inhibitors
Acknowledgments
This work was supported by NIH grants GM-074888 and GM-15431 to A.R.B. We thank Dr. Don F. Stec for assistance with the NMR, and Dr. Wade Calcutt for assistance with the mass spectrometry.
Footnotes
Abbreviations footnote: COX, cyclooxygenase; DOX, dioxygenase; RSVM, ram seminal vesicle microsomes; RP-HPLC, reversed-phase high pressure liquid chromatography; SP-HPLC, straight-phase high pressure liquid chromatography; DMOX, dimethyloxazoline; GC-MS, gas chromatography-mass spectroscopy; HPODE, hydroperoxyoctadecadienoic acid; AA, arachidonic acid; LA, linoleic acid; cLNA, conjugated linolenic acid; α-CA, α-calendic acid; β-CA, β-calendic acid; β-EA, β-eleostearic acid.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee DS, Nioche P, Hamberg M, Raman CS. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature. 2008;455:363–368. doi: 10.1038/nature07307. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Selinsky BS. Bacterial and algal orthologs of prostaglandin H(2)synthase: novel insights into the evolution of an integral membrane protein. Biochim Biophys Acta. 2015;1848:83–94. doi: 10.1016/j.bbamem.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Nugteren DH. Inhibition of prostaglandin biosynthesis by 8cis, 12trans, 14cis-eicosatrienoic acid and 5cis, 8cis, 12trans, 14cis-eicosatetraenoic acid. Biochim Biophys Acta. 1970;210:171–176. doi: 10.1016/0005-2760(70)90072-x. [DOI] [PubMed] [Google Scholar]
- 4.Nugteren DH, Christ-Hazelhof E. Naturally occurring conjugated octadecatrienoic acids are strong inhibitors of prostaglandin biosynthesis. Prostaglandins. 1987;33:403–417. doi: 10.1016/0090-6980(87)90022-0. [DOI] [PubMed] [Google Scholar]
- 5.Marnett LJ, Siedlik PH, Ochs RC, Pagels WR, Das M, Honn KV, Warnock RH, Tainer BE, Eling TE. Mechanism of the stimulation of prostaglandin H synthase and prostacyclin synthase by the antithrombotic and antimetastatic agent, nafazatrom. Mol Pharmacol. 1984;26:328–335. [PubMed] [Google Scholar]
- 6.Schneider C, Boeglin WE, Brash AR. Identification of two cyclooxygenase active site residues, Leucine 384 and Glycine 526, that control carbon ring cyclization in prostaglandin biosynthesis. J Biol Chem. 2004;279:4404–4414. doi: 10.1074/jbc.M307431200. [DOI] [PubMed] [Google Scholar]
- 7.Brash AR, Niraula NP, Boeglin WE, Mashhadi Z. An ancient relative of cyclooxygenase in cyanobacteria is a linoleate 10S-dioxygenase that works in tandem with a catalase-related protein with specific 10S-hydroperoxide lyase activity. J Biol Chem. 2014;289:13101–13111. doi: 10.1074/jbc.M114.555904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisholm MJ, Hopkins CY. Isolation and Structure of a New Conjugated Triene Fatty Acid. J Org Chem. 1962;27:3. [Google Scholar]
- 9.Takagi T, Itabashi Y. Occurrence of Mixtures of Geometrical-Isomers of Conjugated Octadecatrienoic Acids in Some Seed Oils - Analysis by Open-Tubular Gas-Liquid-Chromatography. Lipids. 1981;16:546–551. [Google Scholar]
- 10.Nagao K, Yanagita T. Conjugated fatty acids in food and their health benefits. J Biosci Bioeng. 2005;100:152–157. doi: 10.1263/jbb.100.152. [DOI] [PubMed] [Google Scholar]
- 11.Powell WS. Argentation High-Pressure Liquid-Chromatography of Prostaglandins and Monohydroxyeicosenoic Acids. Methods Enzymol. 1982;86:530–543. [Google Scholar]
- 12.Cao Y, Gao HL, Chen JN, Chen ZY, Yang L. Identification and characterization of conjugated linolenic acid isomers by Ag+-HPLC and NMR. J Agric Food Chem. 2006;54:9004–9009. doi: 10.1021/jf0616199. [DOI] [PubMed] [Google Scholar]
- 13.Fay L, Richli U. Location of Double-Bonds in Polyunsaturated Fatty-Acids by Gas-Chromatography Mass-Spectrometry after 4,4-Dimethyloxazoline Derivatization. J Chromatogr. 1991;541:89–98. [Google Scholar]
- 14.Spitzer V. Structure analysis of fatty acids by gas chromatography--low resolution electron impact mass spectrometry of their 4,4-dimethyloxazoline derivatives--a review. Prog Lipid Res. 1996;35:387–408. doi: 10.1016/s0163-7827(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Yang L, Gao HL, Chen JN, Chen ZY, Ren QS. Re-characterization of three conjugated linolenic acid isomers by GC-MS and NMR. Chem Phys Lipids. 2007;145:128–133. doi: 10.1016/j.chemphyslip.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Kinami T, Horii N, Narayan B, Arato S, Hosokawa M, Miyashita K, Negishi H, Ikuina J, Noda R, Shirasawa S. Occurrence of conjugated linolenic acids in purified soybean oil. J Am Oil Chem Soc. 2007;84:23–29. [Google Scholar]
- 17.Copeland RA. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. 2. Wiley-VCH; 2000. Place Published. [Google Scholar]
- 18.Sun FF, McGuire JC, Morton DR, Pike JE, Sprecher H, Kunau WH. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds. Prostaglandins. 1981;21:333–343. doi: 10.1016/0090-6980(81)90151-9. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwenhuizen WF, Schilstra MJ, van der Kerk-Van Hoof A, Brandsma L, Veldink GA, Vliegenthart JF. Fe(III)-lipoxygenase converts its suicide-type inhibitor octadeca-9,12-diynoic acid into 11-oxooctadeca-9,12-diynoic acid. Biochemistry. 1995;34:10538–10545. doi: 10.1021/bi00033a028. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn H, Holzhutter HG, Schewe T, Hiebsch C, Rapoport SM. The mechanism of inactivation of lipoxygenases by acetylenic fatty acids. Eur J Biochem. 1984;139:577–583. doi: 10.1111/j.1432-1033.1984.tb08044.x. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwenhuizen WF, Van der Kerk-Van Hoof A, van Lenthe JH, Van Schaik RC, Versluis K, Veldink GA, Vliegenthart JF. Lipoxygenase is irreversibly inactivated by the hydroperoxides formed from the enynoic analogues of linoleic acid. Biochemistry. 1997;36:4480–4488. doi: 10.1021/bi962956l. [DOI] [PubMed] [Google Scholar]
- 22.Vanderhoek JY, Lands WE. Acetylenic inhibitors of sheep vesicular gland oxygenase. Biochimica et biophysica acta. 1973;296:374–381. doi: 10.1016/0005-2760(73)90095-7. [DOI] [PubMed] [Google Scholar]
- 23.Perrone MG, Scilimati A, Simone L, Vitale P. Selective COX-1 inhibition: A therapeutic target to be reconsidered. Curr Med Chem. 2010;17:3769–3805. doi: 10.2174/092986710793205408. [DOI] [PubMed] [Google Scholar]
- 24.Wahle KW, Heys SD, Rotondo D. Conjugated linoleic acids: are they beneficial or detrimental to health? Prog Lipid Res. 2004;43:553–587. doi: 10.1016/j.plipres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi M, Miyazawa T. Preparation and fractionation of conjugated trienes from alpha-linolenic acid and their growth-inhibitory effects on human tumor cells and fibroblasts. Lipids. 2005;40:109–113. doi: 10.1007/s11745-005-1365-5. [DOI] [PubMed] [Google Scholar]
- 26.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 27.Corey EJ, Shih C, Cashman JR. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc Natl Acad Sci U S A. 1983;80:3581–3584. doi: 10.1073/pnas.80.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duggan KC, Hermanson DJ, Musee J, Prusakiewicz JJ, Scheib JL, Carter BD, Banerjee S, Oates JA, Marnett LJ. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat Chem Biol. 2011;7:803–809. doi: 10.1038/nchembio.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan C, Sidhu RS, Kuklev DV, Kado Y, Wada M, Song I, Smith WL. Cyclooxygenase Allosterism, Fatty Acid-mediated Cross-talk between Monomers of Cyclooxygenase Homodimers. J Biol Chem. 2009;284:10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinohara N, Tsuduki T, Ito J, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Nishiyama K, Nakagawa K, Miyazawa T, Ikeda I. Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. Biochim Biophys Acta. 2012;1821:980–988. doi: 10.1016/j.bbalip.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Gasmi J, Thomas Sanderson J. Jacaric acid and its octadecatrienoic acid geoisomers induce apoptosis selectively in cancerous human prostate cells: a mechanistic and 3-D structure-activity study. Phytomedicine. 2013;20:734–742. doi: 10.1016/j.phymed.2013.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.