Abstract
Studies have demonstrated that episodic memory (EM) is often preferentially disrupted in schizophrenia. The neural substrates that mediate EM impairment in this illness are not fully understood. Several functional magnetic resonance imaging (fMRI) studies have employed EM probe tasks to elucidate the neural underpinnings of impairment, though results have been inconsistent. The majority of EM imaging studies have been conducted in chronic forms of schizophrenia with relatively few studies in early phase patients. Early phase schizophrenia studies are important because they may provide information regarding when EM deficits occur and address potential confounds more frequently observed in chronic populations. In this study, we assessed brain activation during the performance of visual scene encoding and recognition fMRI tasks in patients with earlyphase psychosis (n=35) and age, sex, and race matched healthy control subjects (n = 20). Patients demonstrated significantly lower activation than controls in the right hippocampus and left fusiform gyrus during scene encoding and lower activation in the posterior cingulate, precuneus, and left middle temporal cortex during recognition of target scenes. Symptom levels were not related to the imaging findings, though better cognitive performance in patients was associated with greater right hippocampal activation during encoding. These results provide evidence of altered function in neuroanatomical circuitry subserving EM early in the course of psychotic illness, which may have implications for pathophysiological models of this illness.
Keywords: Episodic memory, Encoding, Recognition, Early phase psychosis, fMRI, Cognition
Background
Cognitive dysfunction is a core, pathophysiologic dimension of schizophrenia that is present in more than 70 % of patients (Palmer et al. 1997). Cognitive deficits have been observed in multiple domains, including abstraction, attention, language, and memory (Saykin et al. 1991), and these deficits have been associated with impaired vocational and social functioning (Friedman et al. 2001; Harvey and McGurk 2000). Cognitive impairment is evident at the time of illness onset and persists throughout the course of illness (Green 1996; Green et al. 2000; Pantelis et al. 2003; Saykin et al. 1994; Sponheim et al. 2010).
Episodic memory (EM) is an important cognitive domain that has been shown to be impaired in schizophrenia (Achim and Lepage 2003; Aleman et al. 1999; Danion et al. 2007; Gold et al. 1992; Leavitt and Goldberg 2009; Snitz et al. 2006). EM combines event-specific autobiographical experiences and information regarding the context in which events took place (Danion et al. 2007), aiding in making decisions and guiding actions in the present. Normal EM engages pre-frontal, medial temporal, and parietal regions (Burgess et al. 2002; Cohen et al. 1999; Shallice et al. 1994; Tulving 1972), which have all been implicated in the pathophysiology of schizophrenia (Fallon et al. 2003; Kelsoe et al. 1988) suggesting that EM may be a useful probe of key circuits underlying this illness. Studies indicate that patients with schizophrenia may exhibit broad impairments in EM (Tracy et al. 2001), with deficits occurring during encoding and retrieval with both visual (Heinrichs and Zakzanis 1998) and verbal (Flor-Henry 1990; Taylor and Abrams 1984) tasks.
The mechanisms underlying impaired EM in schizophrenia are unclear. Several imaging studies have investigated the neural correlates of EM dysfunction in schizophrenia and have reported alterations in prefrontal (Barch et al. 2002; Kubicki et al. 2003; Ragland et al. 2001, 2004, 2009), medial temporal (Achim and Lepage 2005a; Hofer et al. 2003; Leube et al. 2003; Ragland et al. 2001, 2004), and parietal (Hofer et al. 2003; Lepage et al. 2010; Wiser et al. 1998) cortices. However, the EM functional magnetic resonance imaging (fMRI) literature contains a number of inconsistencies. For example, while the prefrontal cortices mediate normal EM performance, EM fMRI studies report prefrontal hypo-activation (Achim and Lepage 2005b; Allen et al. 2011; Eyler Zorrilla et al. 2003; Heckers et al. 1998; Hofer et al. 2003) and hyper-activation (Bonner-Jackson et al. 2005; Stolz et al. 2012) in patients with schizophrenia. These inconsistencies are not well accounted for but may be related to task differences, small sample sizes, confounding effects of psychotic symptomatology and poor attention, and differences in patient characteristics.
The vast majority of EM fMRI studies have been conducted in patients with chronic forms of schizophrenia, with a relative paucity of studies in early phase patients (Allen et al. 2011; Stolz et al. 2012). It is important to study early phase schizophrenia because these studies may be informative to determine the onset of neural changes that underlie EM impairment in schizophrenia. In addition, early phase patients, compared to chronic patients, tend to have fewer and less severe comorbidities, shorter durations of antipsychotic treatment, and lower severity of illness, all of which have the potential for impacting data interpretation.
In this study, we assessed brain activation patterns during performance of visual scene encoding and recognition fMRI tasks in patients with early phase psychosis (EPP) (n=35) and matched healthy control subjects (n=20). Patients had symptom assessments to address potential confounding issues related to this illness. We hypothesized that patients would demonstrate decreased activation during encoding and recognition in the main areas that mediate EM function, namely the hippocampus, prefrontal, and parietal cortices.
Methods
Participants
Subjects were recruited through the Indiana University Psychotic Disorders Program, within the Indiana University School of Medicine. After receiving an explanation of study procedures, subjects gave their written informed consent prior to enrollment. Thirty-five patients, ages 18–35, were enrolled, all within the first 5 years of psychotic illness onset, and with diagnoses of schizophrenia (n=26), schizophreniform disorder (n=4), or schizoaffective disorder (n=5, 3 bipolar type and 2 depressed type) as determined by the Structured Clinical Interview for DSM-IV (SCID)(First et al. 2002). One patient with schizoaffective disorder was noted to have had clinically significant depressive symptoms within the month prior to enrollment in the study, while all others were without significant affective symptoms for at least one month prior to the time of study entry. All patients were on antipsychotic medication, including aripiprazole (n= 1), clozapine (n =1), quetiapine (n=3), paliperidone (n=11), risperidone (n=12), olanzapine (n=5), lurasidone (n=2), and ziprasidone (n=1). Seven patients were on antidepressant medication while one patient was on a mood stabilizer (valproate). Two patients were taking benztropine, two were prescribed clonazepam, and one was prescribed dextroamphetamine/amphetamine mixed salt. These patients were instructed to withhold these medications for 24 h prior to the MRI and cognitive tasks due to potential confounding effects. Patients were determined to be clinically stable at study entry by research psychiatrist (MMF, EL, AB) evaluation. Controls were recruited from the community via word of mouth or print media and were determined to be without history of psychiatric disorder as determined by SCID interview. Controls were group matched with patients on age, sex, and race.
Procedures
At baseline, demographic information and medical history were reviewed. Patient and control groups were administered the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) and the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al. 2008) by raters who were extensively trained and consistently achieved inter-rater reliability (intraclass correlation) scores on the PANSS between .83 and .95. These measures were used to determine if symptom levels and global cognitive function, respectively, were related to activation patterns. Subjects performed visual scene encoding and recognition EM tasks during fMRI (Detre et al. 1998; Killgore et al. 1999). Before the scan, subjects were instructed on each task, and practice trials were provided to ensure that directions were understood. Instructions were repeated to subjects immediately prior to each task (i.e., in the scanner). Images were presented and button responses during recognition recorded using Presentation software (each task lasted 5:15).
During the encoding task, subjects viewed complex neutral scenes one at a time and were instructed to remember as much as they could about each image for later recognition testing. Images were shown in a block design with interleaved 36-s image and control blocks. Image blocks consisted of nine separate consecutive images, each displayed for 3.5 s, with an inter-stimulus interval (ISI) of 500 milliseconds (ms). A total of 36 scene images were shown. Control blocks consisted of a retiled image, repeatedly displayed at the same rate. Subjects were instructed not to press any buttons during the encoding phase.
The recognition task was a separate event-related fMRI paradigm administered immediately after the encoding phase. During the recognition task, subjects were shown the 36 scenes from the encoding phase (targets) intermixed with 36 new (foil) scenes. These images were displayed consecutively in a pseudorandomized manner, each for 3.5 s with an ISI of 500 ms. Subjects were instructed to indicate, via button press, whether each displayed image was previously seen (target) or new (foil). Button-press responses were recorded to assess reaction time and accuracy.
MRI data acquisition
This study was conducted on a Siemens 3 T Tim Trio scanner. First, high-resolution magnetization prepared rapid acquisition gradient-echo (MPRAGE) scans were acquired, comprised of 160 sagittal slices with voxel dimensions 1×1×1.2 mm (mm). During functional activation scans, blood-oxygen level-dependent (BOLD) signal was measured using a T2*-weighted gradient echo-planar imaging (EPI) sequence, with 39 interleaved axial slices, TR/TE of 2250/29 ms, flip angle of 79°, field-of-view 220×220 mm, and a voxel size of 2.5×2.5×3.5 mm. In addition, a field-mapping scan with slice characteristics identical to the functional scans was made to correct for potential inhomogeneities in the magnetic field.
Data analysis
Behavioral analysis
Scoring distinguished among correct target responses, correct foil responses, incorrect target responses, and incorrect foil responses. Independent group t-tests were used to analyze reaction time and accuracy to target and foil scenes during the recognition task.
MRI analysis
Each functional BOLD time-series underwent standard preprocessing and was analyzed using AFNI software (Cox 1996). Preprocessing included correction for magnetic inhomogeneities with a dewarping method via FSL software (http://www.fmrib.ox.ac.uk/fsl), correction for motion by aligning each functional measurement to the initial time point, smoothing with a 6 mm full-width at half-maximum (FWHM) Gaussian kernel, and utilizing a “despiking” algorithm to smooth time-series outliers.
For each task, a general linear model was employed to quantify the BOLD response to each condition. Regression coefficients for conditions of interest were measured and contrasted within each individual. For encoding, block designs were convolved with a hemodynamic response function (HRF) to quantify the time-series for scene and control conditions, with scene vs. control contrasts the primary measure of interest. For recognition, an event-related design was employed, with target and non-target images separately modeled for correct and incorrect responses (creating four distinct conditions) and convolved with the HRF. For recognition we were able to utilize the event-related design to separate correct and incorrect responses to target and non-target scenes. Correct target vs. correct non-target coefficients were then contrasted for each participant. By analyzing correct responses only we were able to ensure activation differences were not due to factors unrelated to recognition processes such as poor attention to task.
For group analyses, individual results were transformed to a standardized brain (Talairach and Tournoux 1988), and group t-tests of regression contrasts were performed. An individual voxel value of p<.01 indicated significance, with Monte Carlo analyses indicating that clusters of 110 voxels corrected for multiple comparisons at p<.05 across the entire brain. Planned correlations were performed within the patient population between mean activity in significantly different clusters and PANSS total, positive, and negative symptom subscale scores and BACS composite t-scores.
A variety of quality control measurements were employed. First, functional time points with motion >3.5 mm displacement were censored, along with the previous and two subsequent time points. Subjects with >10 % of time points censored were excluded from analysis. In addition, we quantified the average time-series variance across the entire brain to identify potential outliers that indicated abnormally low signal-to-noise ratios, representative of artifact due to participant motion or scanner noise. Finally, any subject with ≥25 % non-responses during recognition task performance was excluded from analysis due to failure to attend.
Results
Thirty-five patients with EPP (26 with schizophrenia, 4 with schizophreniform disorder, and 5 with schizoaffective disorder), who had a mean (SD) duration of illness of 19.4 (18.7) months, and 20 healthy controls participated in the study. The groups were matched for age, sex and race but the controls had higher parental socio-economic status (SES) (Table 1). Because of failure to meet the quality control methods noted above, seven patients and three controls were excluded from each analysis. However, the patients excluded from analysis of each task were not the same, so the final patient samples for each task (supplement Tables 1 and 2) were not identical: data from four patients were excluded from both analyses, and data from six patients were excluded from only one analysis (three from each). The control sample in each task was identical.
Table 1. Demographic and diagnostic information.
| Patients n=35 | Controls n=20 | Analysis | |
|---|---|---|---|
| Age | 22.7 (4.7) | 23.1 (3.4) | t(53) = −0.3, p=0.13 |
| Sex (M:F) | 26:9 | 14:6 | X2 (1) = 0.1, p=0.73 |
| Race, n (%) | X2 (1) = 2.0, p=0.15 | ||
| AA | 21 (60.0 %) | 8 (45.0 %) | |
| C | 14 (42.5 %) | 12 (55.0 %) | |
| Parental SES (Hollingshead) | 2.7 (1.1) | 3.3 (0.8) | X2 (4) = 11.4, p<0.05 |
| Antipsychotic drug exposure (CPZ equivalents in g) | 102.5 (154.7) | ||
| PANSS score (total) | 55.1 (14.67) | 33.1 (2.9) | t(51) = 7.12, p<0.001 |
| PANSS positive | 13.9 (6.3) | 7.4 (0.9) | t(51) = 4.6, p<0.0001 |
| PANSS negative | 14.9 (5.6) | 8.15 (1.0) | t(51) = 5.3, p<0.0001 |
| BACS composite score | 28.7 (14.8) | 49.4 (11.5) | t(51) = 5.3, p<0.0001 |
SES Socioeconomic Scale; PANSS Positive and Negative Syndrome Scale; CPZ chlorpromazine; CGI-S Global Impression – Severity Score; BACS Brief Assessment of Cognition in Schizophrenia
Nonetheless, the groups for each task were largely overlapping and remained matched on age, sex, and race. As expected, patients had significantly higher PANSS scores and significantly lower BACS composite scores than controls (Table 1). There were no significant differences between subjects included and excluded from encoding analysis in any demographic variables. There was a higher proportion of males in the included versus excluded group during recognition (χ2=2.0, p= 0.003). There was also a significant difference in PANSS score between patients included and excluded in recognition analysis, with excluded patients having a greater overall PANSS score (t=2.1, p=0.05).
Behavioral performance
Performance during the recognition task was examined via repeated-measures ANCOVA tests (group by condition) with sex and SES scores considered as covariates (with only patients included in fMRI analysis) (see Table 2 for performance data). Patients were less accurate in their responses overall (Main effect of group: F(1, 41) = 11.62, p=.001), with a trend for poorer accuracy to target images (Group × condition interaction: F(1, 41) = 3.19, p=.08). In addition, patient reaction time was significantly slower during responses (Main effect of group: F(1, 41) = 13.61, p=.001), particularly during foil images (Group × condition interaction: F(1, 41) = 6.22, p=.02) compared to controls. Examining only patients included in the encoding task yielded results identical in nature.
Table 2. Performance during scene recognition task.
| Patients | Controls | |
|---|---|---|
| Overall accuracy, % (SD) | 78.3 (16.6) | 92.5 (5.0) |
| Target accuracy, % (SD) | 66.3 (24.0) | 87.0 (10.3) |
| Foil accuracy, % (SD) | 90.3 (15.8) | 97.9 (4.2) |
| Target RT, ms (SD) | 1253 (25) | 1033 (14) |
| Foil RT, ms (SD) | 1310 (27) | 978 (13) |
SD standard deviation; RT reaction time; ms milliseconds
fMRI results
Scene encoding
For both groups, activation (scene > control) during the encoding task was consistent with a well-established network of structures associated with visual processing and EM, including the bilateral visual cortex, prefrontal, and hippocampal regions (Table 3; Fig. 1). Activation was also observed in supplementary motor, precuneus, posterior cingulate, left inferior parietal, and cerebellar areas in both groups. The patient group also demonstrated significant right inferior parietal and right insula activation.
Table 3. Activation during scene encoding.
| Region | BA | Talairach peak | Peak t | Cluster size |
|---|---|---|---|---|
| Controls | ||||
| Scene > Control | ||||
| R/L fus Gyrus/Lat occipital cortex/Hippocampus | 18/19/20 | (33, −39, −18) | 16.2 | 20,522 |
| L inferior frontal gyrus | 9 | (−39, 7, 26) | 5.7 | 2087 |
| Supplementary motor area | 6 | (−7, 9, 58) | 6.1 | 963 |
| R inferior frontal gyrus | 9 | (43, 7, 24) | 5.8 | 387 |
| Cerebellum | – | (11, −69, −34) | 5.1 | 380 |
| R anterior insula | 13 | (29, 23, 4) | 4.0 | 245 |
| R Inf/Middle frontal gyrus | 46 | (51, 31, 10) | 4.4 | 142 |
| Cerebellum | – | (−1, −47, −32) | 4.4 | 113 |
| Patients | ||||
| Scene > Control | ||||
| R/L fus gyrus/Lat occipital cortex | 18/19/20 | (29, −37, −18) | 13.8 | 26,064 |
| L inferior/Middle frontal gyrus | 9/45 | (−39, 5, 28) | 6.3 | 1916 |
| Supplementary motor area | 6 | (−1, 15, 42) | 5.4 | 834 |
| R inferior frontal gyrus | 6/9 | (39, 1, 32) | 4.8 | 525 |
| Controls > Patients | ||||
| Scene > Control | ||||
| L fusiform gyrus | 37 | (−33, −59, −20) | 4.3 | 138 |
| R hippocampus | – | (17, −7, 16) | 3.7 | 117 |
Regions, along with Talairach coordinates and value of peak t-value, of significant differences in activation for the specified contrasts are displayed
Individual voxel significance was set at p<.01, with a minimum cluster size threshold of 110 voxels for a cluster-level significance of p<.05. Cluster size reports voxel counts (2×2×2 mm voxels)
Abbreviations: BA Brodmann Area; Fus Fusiform; Lat, Lateral; Inf Inferior; Post Posterior; Sup Superior
Fig 1.
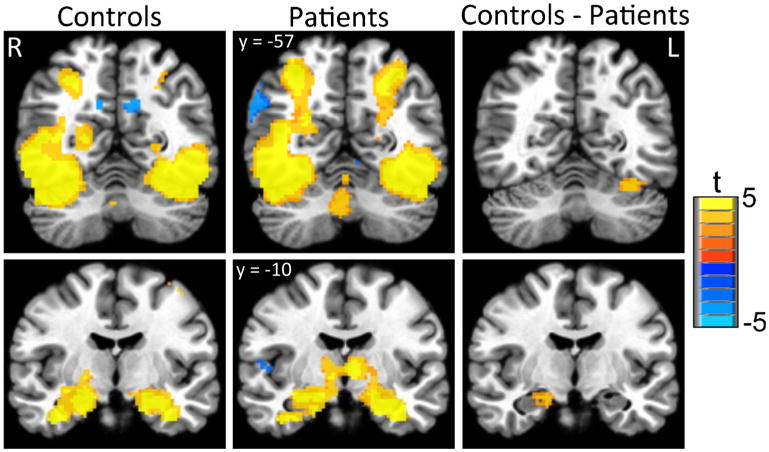
Activation during encoding task. The control sample had significantly greater activity (scene–control) than the patient sample in left fusiform gyrus and right hippocampus. Images depict voxels significant at p<.01 in contiguous clusters of at least 110 voxels, correcting for multiple comparisons at p<.05
Comparing groups, patients demonstrated significantly lower BOLD response when encoding images in the right hippocampus and left fusiform gyrus (Table 3; Fig. 1). There was no region where significantly greater activation was observed in the patient group compared to controls. Inclusion of overall recognition accuracy as a covariate did not significantly change the pattern of findings.
Within the patient group, BACS composite t-scores were positively correlated with activity in the hippocampal cluster during encoding (r(27) = .47, p=.01; Fig. 2), though no significant relationship was present in the control group. In other words, patients with better cognitive performance had activity more like controls in these regions. No relationship was found between illness duration, overall PANSS score or positive/negative subscale scores and activity in either of the significantly different clusters, indicating that duration or severity of symptomatology was unlikely to influence activation during EM encoding.
Fig 2.
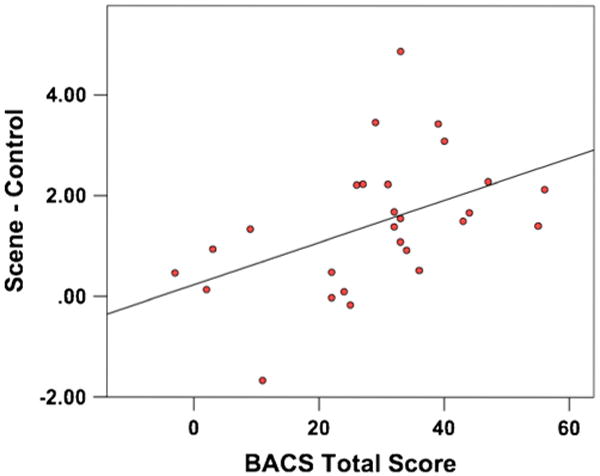
Association of hippocampus activity with Brief Assessment of Cognition in Schizophrenia (BACS) score in patients. Mean activity during encoding (Scene – Control) extracted from the significant hippocampus cluster was significantly correlated with patients' total score from the BACS
Scene recognition
During the recognition task, the control group showed greater activation to correct target relative to non-target images in the precuneus, posterior cingulate cortex (PCC), and medial and lateral prefrontal cortex. Greater activation to target versus non-target images was also demonstrated in the visual cortex, cerebellum, and insula. The patient group demonstrated a similar pattern of activation in these regions for targets relative to non-targets, albeit with smaller clusters of activation (Table 4; Fig. 3). A direct contrast between groups (controls (correct target > correct foil) vs. patients (correct target > correct foil)) revealed that patients had a significantly attenuated cortical response in two clusters during correct recognition of target scenes: the PCC and precuneus and the left middle temporal cortex. There was no region where greater activation was observed in the patient group. Within the patient group, activity in these clusters was not correlated with illness duration, PANSS or BACS scores. There were no associations between reaction time or accuracy and activation in significant clusters in either patient or control groups during recognition task performance.
Table 4. Activation during scene recognition.
| Region | BA | Talairach peak | Peak t | Cluster size |
|---|---|---|---|---|
| Controls | ||||
| Target > Foil | ||||
| Precuneus/Inf parietal lobule/Occip cortex | 7/18/31 | (−9, −61, 24) | 12.0 | 21,179 |
| L precentral gyrus | 4 | (−39, −17, 40) | 5.3 | 1443 |
| R mid frontal gyrus | 9 | (47, 23, 30) | 5.2 | 517 |
| L insula | 13/47 | (−33, 17, −4) | 5.5 | 305 |
| L mid temporal gyrus | 21 | (−57, −33, −8) | 5.6 | 181 |
| R sup temporal gyrus | 22 | (57, −47, 18) | 4.0 | 125 |
| Patients | ||||
| Target > Foil | ||||
| Precuneus/Inf parietal lobule | 7/31 | (−7, −71, 30) | 9.3 | 18,781 |
| R inf frontal gyrus/Insula | 13 | (31, 23, −2) | 5.9 | 3112 |
| Dorsal anterior cingulate cortex | 6/32 | (−1, 23, 40) | 6.6 | 2109 |
| L inf frontal gyrus/Insula | 13 | (−29, 19, −6) | 4.7 | 550 |
| L mid frontal gyrus | 10 | (−37, 49, 10) | 5.5 | 463 |
| R precentral gyrus | 6 | (33, −1, 52) | 5.3 | 397 |
| Midbrain | – | (−3, −11, −16) | 4.9 | 316 |
| R mid frontal gyrus | 10 | (25, 55, 2) | 5.0 | 218 |
| L mid temporal gyrus | 22 | (−59, −45, −8) | 4.7 | 174 |
| Controls > Patients | ||||
| Target > Foil | ||||
| Precuneus/Post cingulate cortex | 18/31 | (−9, −57, 24) | 4.59 | 1091 |
| L mid temporal gyrus | 38 | (−37, 13, −26) | 5.0 | 124 |
Regions, along with Talairach coordinates and value of peak t-value, of significant differences in activation for the specified contrasts are displayed
Individual voxel significance was set at p<.01, with a minimum cluster size threshold of 110 voxels for a cluster-level significance of p<.05. Cluster size reports voxel counts (2×2×2 mm voxels)
Abbreviations: BA Brodmann Area; Inf Inferior; Mid Middle; Post Posterior
Fig. 3.
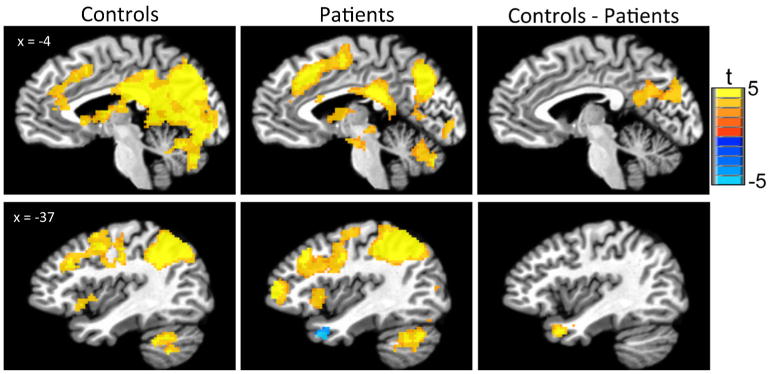
Activation during Recognition task. The control sample had significantly greater activity (target–foil) than the patient sample in precuneus, posterior cingulate, and left inferior parietal lobe. Images depict voxels significant at p<.01 in contiguous clusters of at least 110 voxels, correcting for multiple comparisons at p<.05
Discussion
The current study employed a visual EM task during fMRI to examine the neural correlates of encoding and retrieval in patients with EPP compared to matched healthy controls. Both patients and controls demonstrated activation of frontal, temporal, and parietal regions that are known to subserve EM function (Spaniol et al. 2009; Tulving 1972; Tulving et al. 1994). While encoding visual scenes, patients exhibited significantly lower activation in the hippocampus and fusiform gyrus than controls. During recognition of target scenes, patients demonstrated significantly lower activation in the precuneus and PCC as well as the left middle temporal cortex. These findings demonstrate a pattern of alteration in hippocampal, parietal, and temporal activity during EM processes in patients with EPP. Contrary to our study hypothesis, we failed to find altered prefrontal activation effects in patients with EPP when compared to controls.
Similar to our results, several previous EM studies have reported decreased hippocampal activation during encoding, as noted in a recent meta-analysis (Achim and Lepage 2005a). Other studies have failed to find decreased hippocampal activity (Allen et al. 2011; Ragland et al. 2001; Stolz et al. 2012). This discrepancy may be related to differences in sample characteristics and design issues, such as level of task difficulty and visual versus verbal EM.
The hippocampus plays a key role in memory encoding (Leavitt and Goldberg 2009; Ranganath 2010; Spaniol et al. 2009; Tulving 1972). This structure is believed to participate in the creation of new memory associations to assist with information storage and retrieval (Cohen et al. 1999). Altered hippocampal response in individuals with schizophrenia may reflect dysfunctional binding mechanisms and ultimately less efficient encoding (Achim and Lepage 2005a; Eyler Zorrilla et al. 2003). Interestingly, we observed an association of the BACS composite score with hippocampal activation which may suggest that global cognitive performance deficits could be related to hippocampal dysfunction, as documented in previous work (Heckers et al. 1998; Jessen et al. 2003; Ongur et al. 2006). Notably, the association of BACS composite score with hippocampus activity during encoding was only present in the patient group. The lack of a correlation in the healthy sample may indicate that activity above a certain threshold does not reflect a higher level of cognition. In other words, proper hippocampal function may be necessary for a basic cognitive capacity while enhanced capability may be driven by other regions. Episodic memory encapsulates a facet of cognitive function that is particularly impaired in schizophrenia (Danion et al. 2007; Leavitt and Goldberg 2009) and in which the hippocampus plays a key role. It is possible that impairment in separate aspects of cognition (e.g., working memory, attention) are not driven primarily by this region. We also observed decreased activation of the fusiform gyrus in patients versus controls during encoding. While the fusiform gyrus may play a lesser role in the actual encoding of information, the region has been implicated in the perception and identification of objects, scenes, and faces (Gabrieli et al. 1997; Haxby et al. 1994; Kanwisher et al. 1997). Thus, its function may be important for effective memory encoding and may suggest some significance for this finding, which should be explored in future research.
During recognition we observed decreased activation in the precuneus, PCC, and left middle temporal cortex. Other investigators have found altered precuneus function in cognitive studies in schizophrenia. A positron emission tomography (PET) study examining new object recognition in patients with schizophrenia observed increased precuneus activation (Heckers et al. 2000), while a fMRI study by Ragland and colleagues demonstrated increased right precuneus activation in a group of patients versus controls during a word recognition task (Ragland et al. 2004). More recently, Lepage et al. examined activation during the correct identification of target items and rejection of distractor items, finding similar precuneus activation in patients and controls but higher nearby superior parietal activity in the control group during successful retrieval (Lepage et al. 2010). Cabeza and associates suggested that the precuneus may be involved in the support of retrieval search, monitoring, and verification (Cabeza et al. 2008). The precuneus is active when individuals view familiar information, potentially to help direct resources to the proper internal representations (maintained via hippocampal-prefrontal interactions) (Cavanna and Trimble 2006). The precuneus has also been identified as an important node in modulating default mode and attentional networks (Fransson and Marrelec 2008) and has been shown to have altered activation in default mode network (DMN) studies of schizophrenia (Hulvershorn et al. 2014). Altered function in the precuneus could be related to deficient EM functioning in various ways, including an impaired ability to make accurate assessments of familiarity. Disrupted precuneus function may also impair attentional networks, which could prove problematic for individuals attempting to retrieve previously encoded information.
Decreased PCC activity during EM retrieval in schizophrenia was observed in a recent meta-analysis (Ragland et al. 2009). PCC activation has been associated with retrieval of autobiographical memory retrieval and has been shown to be active during the retrieval of standardized memory stimuli when successful memory retrieval is examined (Maddock et al. 2001, 2003). Altered function of this structure may likely be related to impaired EM in schizophrenia.
We hypothesized that schizophrenia would be related to hypo-function of the prefrontal cortex during EM but failed to find differences between patients and controls. Both the patient and control groups displayed increased prefrontal activation indicating this brain region was engaged during the task. While numerous studies report decreased prefrontal activation during EM performance in schizophrenia (Achim and Lepage 2005a; Ragland et al. 2009), others have not. In fact a number of investigators have reported hyper-activation of the prefrontal cortex compared to controls (Bonner-Jackson et al. 2005; Stolz et al. 2012). Stolz et al. (2012) was one of the few studies enrolling early phase patients with schizophrenia and they found increased frontal activation. It is tempting to speculate that EM related frontal hypo-function may arise later in the illness as the previously reported findings of prefrontal hypo-function come predominately from studies enrolling chronic patients (Achim and Lepage 2005a; Ragland et al. 2004, 2009). Future longitudinal studies with serial scanning are needed to address this issue.
Our study is noteworthy for focusing on early phase patients with a mean duration of illness of 19.4 months. There are relatively few early phase EM fMRI studies in the literature. Allen and colleagues examined verbal EM in individuals with prodromal symptoms of psychosis, finding frontal and parahippocampal, but not hippocampal, activation in patients during encoding (Allen et al. 2011). They did observe altered hippocampal activation during recognition, with increased activation observed in controls and patients during correct recognition and incorrect recognition, respectively. A study by Stolz et al. examined EPP patients, their first-degree relatives, and matched controls and did not detect any activation differences in these groups during visual EM encoding. They did observe increased BOLD response in the prefrontal cortex, the thalamus, and the insula of patients when compared to relatives and controls during recognition (Stolz et al. 2012). Clearly, inconsistencies exist even within early phase populations. There may still be an effect of illness duration, as the average duration of illness in the Stolz study was over 2 years longer than in the present work. Here, we found no association between illness duration and activity in significant different clusters, providing evidence that these abnormalities did not substantially progress with time (although this was not a longitudinal design). Thus, additional work must clarify whether these functional changes arise quickly with the onset of psychosis and then stabilize, or if progressive changes are more apparent beyond the first 5 years of illness.
It is important to also consider diagnostic heterogeneity in patient populations when comparing these studies. The study by Allen et al. included individuals with prodromal symptoms and it is unclear what proportion of these subjects ultimately went on to develop a psychotic illness (Allen et al. 2011). The study by Stolz and colleagues was similar to the current work in terms of diagnostic breakdown, including individuals with schizophrenia (n=15) or schizoaffective disorder (n=7; subtype unclear) (Stolz et al. 2012). Diagnostic differences could contribute to discrepancies between previous findings and the current work. Certainly, further investigation of EPP populations is warranted.
A number of caveats should be considered when interpreting the findings of this study. First, because no measure of engagement was used during the encoding task, the results reported here could be influenced by in-attentiveness in the patient cohort. However, we observed activation in prefrontal, hippocampal and inferior parietal regions in the patient group during encoding, which suggests that patients were attentive during the encoding task. In addition, although we could not separate correctly or incorrectly encoded images, a limitation of the block design, analyses of BOLD signal change during encoding were reanalyzed covarying for recognition task accuracy (a measure dependent on successful encoding), which did not alter the primary findings. Further, we observed comparable activation of attention-related circuitry in patients and controls. For instance, no differences were present in the primary visual cortex, suggesting that attention to task and primary sensory responses were similar in both groups. Finally, in this study, analyses solely contrasted activity during correct trials, likely indicating that lower precuneus activity in patients was not solely due to poor encoding of these images. Because accuracy rates were so high for control subjects, it was not possible to compare activity during incorrect trials, so we cannot fully separate the contributions of the PCC and precuneus to successful (vs. unsuccessful) recognition and the degree to which abnormalities in these regions contribute to EM deficits. Future investigations can aim to better distinguish neural mechanisms underlying successful encoding from unsuccessful encoding. In this investigation, we opted for the greater signal-to-noise of the block design during the encoding task, at the expense of the inability to distinguish individual trials. In addition, the use of realistic natural images resulted in high accuracy rates that precluded a contrast of successful and unsuccessful trials during recognition. To separate successful and unsuccessful encoding and recognition, it is necessary to use a greater number of images, or images that are more difficult to remember, in an event-related design which would be important studies to conduct in future research.
An additional issue is that though patients were in the early phase of illness, as defined to be within the first 5 years, none were neuroleptic naïve. Though their antipsychotic exposure may be low relative to those with prolonged psychotic illnesses, it is difficult to say if antipsychotic drug exposure impacted the results. Reviews, including one in first-episode psychosis, have found that antipsychotic medication may have potential pro-cognitive effects (Davidson et al. 2009; Keefe et al. 1999). However, further work is needed to add to our understanding of the effect of antipsychotic medication on cognition in those with EPP. The potential impact of non-antipsychotic medication on these results was mitigated by the relatively low numbers of individuals taking anticholinergic medications which are know to impair cognitive performance (Campbell et al. 2009; Spohn and Strauss 1989). Additionally, patients on non-antipsychotic medications with the potential to impact cognitive performance were instructed to hold these medications for 24 h prior to cognitive assessment and MRI. Allen's group (2011) of prodromal patients were neuroleptic naive and Stolz et al. (2012) included first-degree family members who were free of antipsychotic medications, which suggests medication status may not be a key determinant in EM fMRI results. Finally, the patient cohort had lower parental SES than controls, which may have been a factor influencing task performance.
In summary, we found decreased activation in EPP patients versus controls in the hippocampus and fusiform gyrus during EM encoding and in the precuneus, PCC, and left middle temporal cortex during recognition. These findings may be important in establishing what substrates are involved and when neuroanatomical changes associated with cognitive impairment begin. Examining patient populations in all states of disease progression helps characterize the course of cognitive dysfunction in schizophrenia. In particular, continued investigation into early stage illness is necessary to better understand the pathophysiology of these deficits.
Supplementary Material
Acknowledgments
The authors thank Megan Gaunnac, Teresa Kulig, Emmalee Metzler, Heidi Hedrick, John West, Yang Wang, Kelsey Benson, Kami Walters, Katie White, Joan Showalter, and David Spradley for their technical support and recruitment efforts. The authors would also like to thank the Eskenazi Health Midtown Community Mental Health Center for its continued research support.
The authors would like to thank the Stanley Medical Research Institute, grant #10T-002, for providing funding for this study. Additional support was obtained from grant #UH3TR000955, supported by the National Center For Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Stanley Medical Research Institute or the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11682-015-9357-9) contains supplementary material, which is available to authorized users.
Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Disclosures Drs. Francis, Hummer, Vohs, Liffick, Radnovich, McDonald, Saykin, and Breier and Ms. Mehdiyoun and Mr. Yung declare that they have no conflict of interest.
Contributor Information
Michael Matthew Francis, Email: mmfranci@iupui.edu, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA.
Tom A. Hummer, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA; Center for Neuroimaging, Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, USA
Jenifer L. Vohs, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA
Matthew G. Yung, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA
Emily Liffick, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA.
Nicole F. Mehdiyoun, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA
Alexander J. Radnovich, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA
Brenna C. McDonald, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA; Center for Neuroimaging, Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, USA; Department of Neurology, Indiana University School of Medicine, Indianapolis, IN, USA
Andrew J. Saykin, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA; Center for Neuroimaging, Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, USA; Department of Neurology, Indiana University School of Medicine, Indianapolis, IN, USA
Alan Breier, Indiana University Psychotic Disorders Program, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA.
References
- Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis. Brain and Cognition. 2003;53(2):121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. British Journal of Psychiatry. 2005a;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Neural correlates of memory for items and for associations: an event-related functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2005b;17(4):652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. The American Journal of Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen P, Seal ML, Valli I, Fusar-Poli P, Perlini C, Day F, McGuire PK. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophrenia Bulletin. 2011;37(4):746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? Journal of Abnormal Psychology. 2002;111(3):478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58(1):47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Review Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, Gulati R. The cognitive impact of anticholinergics: a clinical review. Clinical Interventions in Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83∷AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Danion JM, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Canadian Journal of Psychiatry. 2007;52(11):693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, Kahn RS. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) The American Journal of Psychiatry. 2009;166(6):675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D'Esposito M, French JA. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50(4):926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- Eyler Zorrilla LT, Jeste DV, Paulus M, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophrenia Research. 2003;59(2-3):187–198. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Opole IO, Potkin SG. The neuroanatomy of schizophrenia: circuitry and neurotransmitter systems. Clinical Neuroscience Research. 2003;3(1):77–107. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical Interview for DSM-IV-TR Axis I disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Flor-Henry P. Neuropsychology and psychopathology: a progress report. Neuropsychology Review. 1990;1(2):103–123. doi: 10.1007/BF01108713. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, Davis KL. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer's disease and normal aging. The American Journal of Psychiatry. 2001;158(9):1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276(5310):264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. Journal of Abnormal Psychology. 1992;101(3):487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? The American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD, McGurk SR. Cost of schizophrenia: focus on vocational impairment. The Economics of Neuroscience. 2000;2:42–48. [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heckers S, Curran T, Goff D, Rauch SL, Fischman AJ, Alpert NM, Schacter DL. Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biological Psychiatry. 2000;48(7):651–657. doi: 10.1016/s0006-3223(00)00919-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Fleischhacker WW. Neural correlates of episodic encoding and recognition of words in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. The American Journal of Psychiatry. 2003;160(10):1802–1808. doi: 10.1176/appi.ajp.160.10.1802. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen KR, Francis MM, Westlund MK. Developmental resting state functional connectivity for clinicians. Current Behavioral Neuroscience Reports. 2014;1(3):161–169. doi: 10.1007/s40473-014-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. The American Journal of Psychiatry. 2003;160(7):1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bulletin. 1999;25(2):201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophrenia Research. 2008;102(1–3):108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Jr, Cadet JL, Pickar D, Weinberger DR. Quantitative neuroanatomy in schizophrenia. A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1988;45(6):533–541. doi: 10.1001/archpsyc.1988.01800300029003. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Glosser G, Casasanto DJ, French JA, Alsop DC, Detre JA. Functional MRI and the Wada test provide complementary information for predicting post-operative seizure control. Seizure. 1999;8(8):450–455. doi: 10.1053/seiz.1999.0339. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. NeuroImage. 2003;20(4):1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychology Review. 2009;19(3):312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- Lepage M, Pelletier M, Achim A, Montoya A, Menear M, Lal S. Parietal cortex and episodic memory retrieval in schizophrenia. Psychiatry Research. 2010;182(3):191–199. doi: 10.1016/j.pscychresns.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Leube DT, Rapp A, Buchkremer G, Bartels M, Kircher TT, Erb M, Grodd W. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia-an fMRI study. Schizophrenia Research. 2003;64(1):83–85. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Archives of General Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisoonk S, Jesta DV. Is it possible to be schizophrenic and yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. The American Journal of Psychiatry. 2001;158(7):1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. The American Journal of Psychiatry. 2004;161(6):1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. The American Journal of Psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368(6472):633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8-9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spohn HE, Strauss ME. Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. Journal of Abnormal Psychology. 1989;98(4):367–380. doi: 10.1037//0021-843x.98.4.367. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O'Leary DS, Schulz SC. Cognitive deficits in recent-onset and chronic schizophrenia. Journal of Psychiatric Research. 2010;44(7):421–428. doi: 10.1016/j.jpsychires.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz E, Pancholi KM, Goradia DD, Paul S, Keshavan MS, Nimgaonkar VL, Prasad KM. Brain activation patterns during visual episodic memory processing among first-degree relatives of schizophrenia subjects. NeuroImage. 2012;63(3):1154–1161. doi: 10.1016/j.neuroimage.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging 1988 [Google Scholar]
- Taylor MA, Abrams R. Cognitive impairment in schizophrenia. The American Journal of Psychiatry. 1984;141(2):196–201. doi: 10.1176/ajp.141.2.196. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Mattson R, King C, Bundick T, Celenza MA, Glosser G. A comparison of memory for verbal and nonverbal material in schizophrenia. Schizophrenia Research. 2001;50(3):199–211. doi: 10.1016/s0920-9964(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. New York: Academic Press, Inc; 1972. [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(6):2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser AK, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Dysfunctional cortico-cerebellar circuits cause ‘cognitive dysmetria’ in schizophrenia. Neuroreport. 1998;9(8):1895–1899. doi: 10.1097/00001756-199806010-00042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


