Abstract
Feingold syndrome (FS) is an autosomal dominant disorder characterized by microcephaly, short stature, digital anomalies, esophageal/duodenal atresia, facial dysmorphism, and various learning disabilities. Heterozygous deletion of the miR-17–92 cluster is responsible for a subset of FS (Feingold syndrome type 2, FS2), and the developmental abnormalities that characterize this disorder are partially recapitulated in mice that harbor a heterozygous deletion of this cluster (miR-17–92Δ/+ mice). Although Feingold patients develop a wide array of learning disabilities, no scientific description of learning/cognitive disabilities, intellectual deficiency, and brain alterations have been described in humans and animal models of FS2. The aim of this study was to draw a behavioral profile, during development and in adulthood, of miR-17–92Δ/+ mice, a genetic mouse model of FS2. Moreover, dopamine, norepinephrine and serotonin tissue levels in the medial prefrontal cortex (mpFC), and Hippocampus (Hip) of miR-17–92Δ/+ mice were analyzed. Our data showed decreased body growth and reduced vocalization during development. Moreover, selective deficits in spatial ability, social novelty recognition and memory span were evident in adult miR-17–92Δ/+ mice compared with healthy controls (WT). Finally, we found altered dopamine as well as serotonin tissue levels, in the mpFC and Hip, respectively, of miR-17–92Δ/+ in comparison with WT mice, thus suggesting a possible link between cognitive deficits and altered brain neurotransmission.
Keywords: Animal model, Feingold 2 syndrome, Behavior, Cognitive deficit
Introduction
MicroRNAs (miRNAs) are small noncoding RNAs, approximately 21 nucleotides in length, that regulate gene expression at the post-transcriptional level by inducing mRNA destabilization and translational inhibition of target mRNAs (Baek et al. 2008; Bartel 2004; Selbach et al. 2008). Initially described as modulators of developmental timing in Caenorhabditis elegans (Lee et al. 1993; Wightman et al. 1993), miRNAs are an abundant and conserved class of regulatory molecules, recently emerged as modulators of nearly every cellular processes, from normal development to pathogenesis (Lemons et al. 2013). More than 2000 miRNAs have been identified in humans (Kozomara and Griffiths-Jones 2011). MiRNAs are believed to modulate the expression of a significant proportion of the transcriptome (Friedman et al. 2009) and thus control many processes, such as proliferation, survival, apoptosis and differentiation (De Pietri Tonelli et al. 2008; Kanellopoulou et al. 2005; Mogilyansky and Rigoutson 2013). Thus, deregulation of miRNAs has been associated with human diseases (Borkhardt et al. 2006; Calin et al. 2005; Hayashita et al. 2005; Mencía et al. 2009). Human and animal studies indicated that members of the miR-34 family of miRNAs are involved in several psychopathological phenotypes (Bavamian et al. 2015; Bocchio-Chiavetto et al. 2013; Dickson et al. 2013; Dias et al. 2014; Papaioannou et al. 2014; Haramati et al. 2011; Parsons et al. 2008; Zhou et al. 2009; Zovoilis et al. 2011).
FS is an autosomal dominant disorder characterized by microcephaly, short stature, digital anomalies (i.e., brachymesophalangy of the second and fifth fingers and brachysyndactyly of the toes), facial dysmorphism (i.e. short palpebral fissures, hypertelorism, epicanthic folds), esophageal/duodenal atresia, and various learning disabilities (Celli et al. 2000; 2003; Blaumeiser et al. 2008; Feingold et al. 1997; Marcelis and De Brouwer 2009; Cognet et al. 2011).
In particular, digital abnormalities and mild-to-moderate microcephaly form the core phenotype. Intestinal atresia and other malformations of internal organs occur frequently. Many patients have hypoplastic thumbs, or flexion, limitation, or hyperextensibility of the thumbs. Camptodactyly of one or more fingers, cubitus valgus, or limitation of elbow extension may all be present. Most patients have syndactyly of the toes, both second and third, or more characteristically of the fourth and fifth toes. Sensorineural deafness and microcephaly are both recurrent features of Feingold syndrome. Approximately, 85 % of reported cases have congenital microcephaly, which in some cases became more pronounced after the neonatal period. Microcephaly reflects reduced growth and development of the dorsal telencephalon (see Celli et al. 2003 for review), and learning disability has been reported in about half of those with microcephaly. Cerebral and cerebellar white matter abnormalities have also been reported (Lehman et al. 2009).
Two forms of FS have been described: FS1, due to a heterozygous mutation in MYCN gene on chromosome 2, and FS2 (FGLDS2), due to a heterozygous microdeletion of miRNA 17–92 cluster on chromosome 13 (De Pontual et al. 2011).
Several of the key features observed in FS2 patients (Tassano et al. 2013; Ganjavi et al. 2014) carrying a heterozygous deletion for miR-17–92 are also evident in mice with a targeted deletion of a single miR-17–92 allele (miR-17–92/+) (De Pontual et al. 2011). miR-17–92 cluster is essential for vertebrate development, as universal disruption of Mirn17 in mice, results in smaller embryos and immediate postnatal death. miR-17–92 cluster has been reported to target many proteins regulating cell cycle, proliferation and apoptosis. Heterozygous deletion of miR-17–92 inhibited osteoblast proliferation and differentiation in vitro, and caused osteopenia phenotype in vivo. Finally, Zhou and colleagues recently showed that the miR-17–92 cluster is essential for normal skeletal development and maturation (Zhou et al. 2012). These results demonstrated that miRNAs impact human development and identified miR-17–92, a well-described human oncogene (He et al. 2005; Mu et al. 2009; Olive et al. 2009), as an important cause of FS.
Feingold patients develop a wide range of learning disabilities (Herman and Siegel 2004; Lehman et al. 2009; Marcelis and De Brouwer 2009), nevertheless specific learning/cognitive disabilities, intellectual impairments, and brain alterations have not been described in humans and animal models of FS. This study examined the behavioral profiles of infant and adult miR-17–92/+ mice. We monitored the development of physical landmarks, reflex, ultrasonic vocalizations and locomotors activity in pups. Moreover, motor, cognitive, spatial, memory and emotional deficits were investigated in adult miR-17–92Δ/+ in comparison with their control (WT) mice. Finally, dopamine (DA), norepinephrine (NE) and serotonin (5-HT) tissue levels in the medial prefrontal cortex (mpFC) and Hippocampus (Hip) of miR-17–92Δ/+ mice were also analyzed.
Materials and methods
Animals
Control (mixed C57/B6 and 129SvJae), and miR-17–92Δ/+ mice (Ventura et al. 2008) were initially provided by Dr. Ventura and both genotypes were following crossed with C57BL/6 mice for 3–4 generations. For genotyping, genomic DNA was isolated from tail using the Easy DNA Kit (Invitrogen, Carlsbad, CA, USA). PCRs were performed using PCR Master Mix (Fermentas, Glen Bur-nie, MD) with primer sequences for floxed miR-17–92 gene relative to wild type miR-17–92 (Zhou et al. 2012). Age matched wild type mice (miR-17–92+/+ (WT)) from the same littermate were used as control.
Ten days after being paired for breeding, females were housed individually and inspected daily for pregnancy and delivery. The day of birth was considered postnatal day 0 (P0).
On postnatal day 28, 24 sex-matched animals were housed per standard breeding cage with food and water ad libitum on a 12:12 h dark-light cycle (lights on from 7 a.m. to 7 p.m.).
Development was recorded when the animals reached 4 days of age (P4). Each litter contained between 6 and 9 pups. Pups were marked individually on P4. Adult experiments began at 3 months of age (P90). Behavioral testing was conducted between 2 and 6 p.m. Mice were housed in the appropriate animal facilities, and every effort was made to minimize animal discomfort. Adult mice evaluated in behavioral tests were not the same tested in the acquisition of developmental milestones (pups).
Developmental assay
Body measurements
Body weights and lengths of pups were measured on P5, 8, 11, 14, 17, 21, 24, and 28 and before the sacrifice at P90. Body weight and length data were analyzed by repeated measures ANOVA (genotype, 2 levels: WT, miR-17–92Δ/+ as the between factor; days, 8 levels: 5, 8, 11, 14, 17, 21, 24, and 28 as within factor; n = 20 for group). Weight and length in adulthood were analyzed by one-way ANOVA (genotype, 2 levels: WT, miR-17–92Δ/+) (n = 8 for group).
Ultrasonic vocalization (USV) in separated pups
Photographs of the USV apparatus are shown in Fig. 1f. After 1 h of acclimatization in the test room, each pup, at P4, 6, 8 and 10, was separated from its mother and placed in a glass beaker that contained clean bedding for the USV. The test started after 5 min of separation, during which the animals were maintained on a heated plate (36 °C), and USV was recorded for 5 min. At the end of the recording session, each pup was returned to its nest. No more than 5–6 animals per litter were tested, in order to avoid too long separation (Moles et al. 2004).
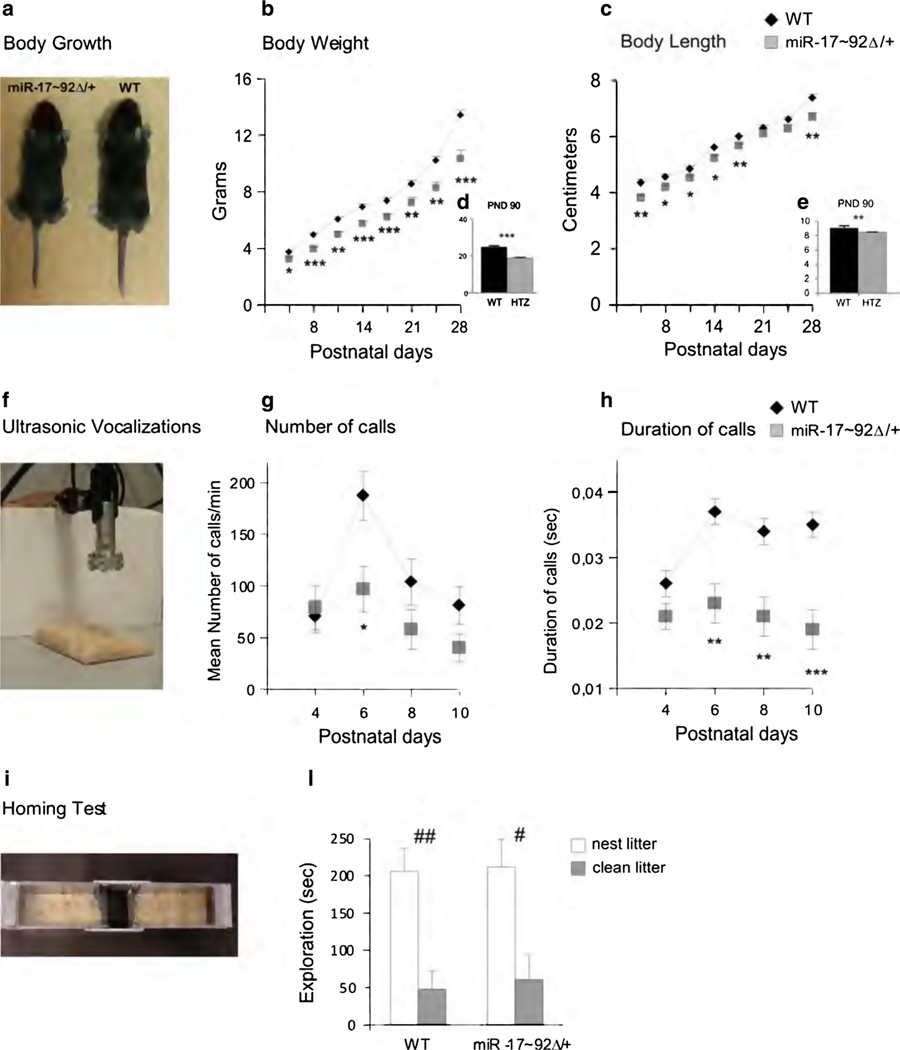
Fig. 1.
Developmental assay. Body growth and behavioral profile of infant and adult miR-17–92/+ and WT mice. Body Growth a Representative photograph of body size differences between miR-17–92Δ/+ (left) and WT (right) pups on P15. Analysis of body weight (b) and length (c) in miR-17–92Δ/+ and WT pups (from P5 to P28). Analysis of body weight (d) and length (e) in miR-17–92/+ and WT adult mice (on P90). Ultrasonic vocalization (USV) f Photograph of USV apparatus. Analysis of number (g) and duration (h) of USVs on P4, 6, 8, and 10 in response to social separation during a 5-min session in miR-17–92Δ/+ and WT mice. Homing test i Photograph of homing test apparatus. l Analysis of exploration of the area containing nest or clean litter by miR-17–92Δ/+ and WT mice. All data are expressed as mean ± SEM. *, **, *** p < 0.05, 0.01, and 0.001 for miR-17–92Δ/+ versus WT. #,## p < 0.05 and 0.01 for nest versus clean litter
An UltraSoundGate Condenser Microphone (CM16, Avisoft Bioacoustics, Berlin, Germany), sensitive to frequencies of 15180 kHz, with a flat frequency response (± 6 dB) between 25,140 kHz, was placed 1 cm above the beaker and connected to a computer that recorded data as 250,000 Hz in 16-bit format wav files. Sound files were transferred to SASLab Pro (version 5.2; Avisoft Bioacoutics) for sonographic analysis, and fast Fourier transformation was conducted (512 FFT-length, 100 % frame, Hamming window, and 75 % time window overlap). Spectrograms were produced at 488 Hz frequency resolution and 0.512 ms time resolution. To detect USVs, an automatic threshold-based algorithm and a hold time mechanism (hold time 20 ms) were used. Signals below 40 kHz were truncated to reduce background noise to 0 dB (Crawley 2007). Inaccurate detections were adjusted manually by an experienced user before the automatic parameter analysis was run (D’Amato et al. 2011).
USV parameters were analyzed by repeated measures ANOVA (genotype, 2 levels: WT, miR-17–92Δ/+ as between factor; days, 4 levels: 4, 6, 8, 10 as within factor (n = 13 for group)).
Developmental milestones
From P5 to P17, the age of appearance of developmental milestones was recorded and compared between the groups. The age of appearance of different reflexes (righting, screen test, vertical screen test, air startle, auditory startle, cliff avoidance, grasp reflex, visual placing response) was recorded in WT (n = 14) and miR-17–92Δ/+ mice (n = 13) (Heyser 2004; Scattoni et al. 2008). All tests were performed from 12 a.m. to 2 p.m.
Development of reflexes was analyzed by repeated measures ANOVA (genotype, 2 levels: WT, miR-17–92Δ/+ as between factor; days, 5 levels: 5, 8, 11, 14, 17 for each reflex, as within factor (n = 13–14 for group)).
Homing test (HT)
A photograph of the HT apparatus is shown in Fig. 1i. On P10, pups were separated from their mother and placed on a heated plate (36 °C) to maintain normal body temperature. Pups were transferred individually to a Plexiglas apparatus (31 cm × 4 cm with walls 6 cm in height), comprising a central arena and 2 lateral arenas that were covered with home cage bedding or clean sawdust. The pup was placed in the center for 1 min of habituation and then tested for 5 min. HT was scored as latency to reach, the time spent, and the entries into the arena containing the nesting litter (Ognibene et al. 2007; Scattoni et al. 2008).
A video-based EthoVision system (Noldus, The Netherlands) was used to record and analyze the data. HT data were analyzed by repeated-measures ANOVA (genotype, 2 levels: WT, miR-17–92Δ/+ as between factor; arena, 2 levels: clean and nest as within factor (n = 11–16 for group)).
Open field (OF)
The OF apparatus was a Plexiglas square (30 cm × 30 cm, height 16 cm) with grey walls and a white floor that was divided into 10 sectors (Bignami 1996; Scattoni et al. 2010). We decided to use this apparatus due to the small size of the animals at this age (P18). Although several different shapes have been used as rodent open field arenas, the most common design for mice is a square chamber. Rodents will typically spend a significantly greater amount of time exploring the periphery of the arena, usually in contact with the walls (thigmotaxis: time spent near the walls (Branchi and Alleva 2006; Carola et al. 2002), than the unprotected center area. Mice that spend significantly more time exploring the unprotected center area demonstrate anxiolytic-like baseline behavior (Bailey and Crawley 2009).
Therefore, the time spent in the periphery of the squared apparatus can provide and index of the anxiety levels. Mice were tested individually for 5 min on P18, and distance moved (cm), velocity (cm/s) and time spent in the periphery of the arena were recorded (Scattoni et al. 2008). The EthoVision system (Noldus, The Netherlands) was used to record data.
One-way ANOVA was used to analyze the effects of genotype (WT and miR-17–92Δ/+ (n = 20 for group) on all parameters.
Adult behavioral assay
Elevated plus maze (EPM)
Mice were tested individually in a single 5-min session of the EPM test. The percentage of entries into the open arms (open entries/open + closed × 100) and the percentage of time spent in the open arms (time in open/open + closed × 100) were recorded.
One-way ANOVA was used to analyze the effects of genotype (WT and miR-17–92Δ/+) on all parameters (n = 14–16 for group).
Object recognition test (ORT)
A schematic representation of the ORT apparatus is shown in Fig. 2a. Each mouse (n = 12–16 for group) was subjected individually to 3 successive habituation sessions (used as open field test), pretest, and test. At the end of each session, the subject was returned to its home cage for 3 min, and the apparatus was cleaned with a solution of ethanol at 5 % in order to eliminate olfactory cues. In the open field, the velocity and the distance moved have been analyzed by one-way ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+). During the pretest session (6 min), the total time spent exploring the objects was analyzed by one-way ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+). Object recognition was evaluated in the test session (6 min) by comparing the new versus the familiar object exploration. The total time spent exploring each object in the test session was evaluated by repeated measures ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; object: 2 levels, novel and familiar, as within factor). The “object” factor was also analyzed within each group.
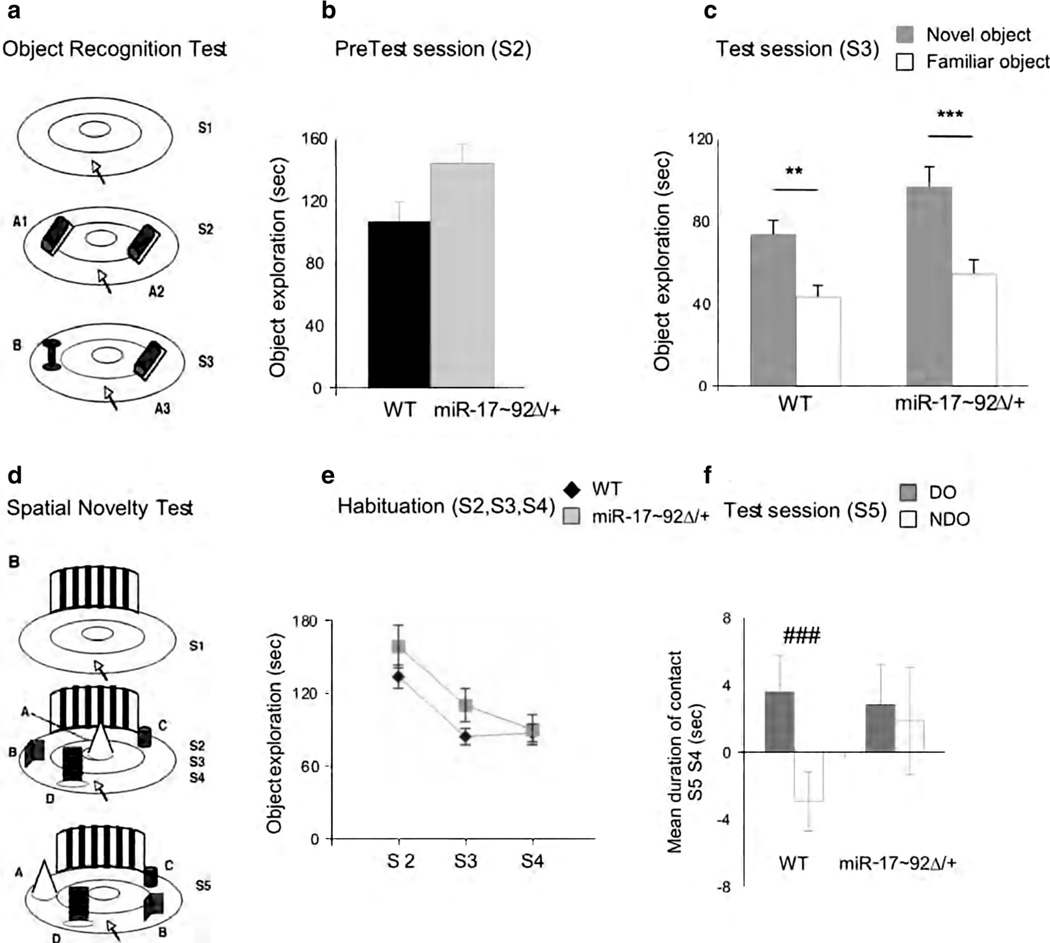
Fig. 2.
Adult behavioral assay. Object recognition test a Schematic representation of object recognition apparatus. Results are expressed as mean time spent exploring the 2 identical objects during the pretest session (S2) (b) and time spent exploring the novel or familiar object during the test session (S3) (c). Spatial novelty test d Schematic representation of spatial novelty apparatus. Results are expressed as mean time spent exploring all objects during S2, S3, and S4 (e) and as changes in the time spent exploring the displaced objects (DOs) and nondisplaced objects (NDOs) between the last habituation session (S4) and test session (S5) (f)
Spatial novelty test (SpNT)
A schematic representation of the SpNT apparatus is shown in Fig. 2d. The apparatus was the same as in the ORT test, except for a black-and-white-striped pattern, 30 × 20 cm, attached to the wall of the field as local cue. Each mouse (n = 14–16 for group) was subjected individually to 5 successive 6-min sessions. At the end of each session, the subject was returned to its home cage for 3 min.
During the first session (S1), the mouse was introduced into a specific sector of the open field and left free to explore the empty apparatus. During sessions 2, 3, and 4 (S2, S3, and S4), the mouse was introduced into the same sector of the open field, containing 4 objects, to allow the mouse to learn the configuration: a gray wooden pyramid (10 cm in height and 9 cm in diameter); 2 gray iron perforated squares (8 × 3.5 cm), forming a right angle; a white plastic circle on a squared base (5.4 cm × 5.4 cm base and 8 cm in diameter); and a black plastic cylinder (7 cm in height and 4 cm in diameter) on a squared base (5.4 cm × 5.4 cm). Object exploration was recorded as the time (s) spent in contact with an object. The apparatus and objects were cleaned between subjects with a solution of ethanol at 5 %.
The duration of object exploration in sessions S2, S3, and S4 was measured and used to determine the rate of habituation to experimental stimuli. These data were analyzed by repeated-measures ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; session, 3 levels: S2, S3, S4 as within factor), followed by post hoc planned comparisons, where appropriate.
In the test session (S5), object A was moved to object B’s position, and object B was placed in a new location, altering the initial spatial configuration. Discrimination of spatial novelty was measured as the increase or decrease in exploration time of displaced objects (DOs) and non-displaced objects (NDOs), expressed as the mean time in contact with the objects in S5 minus the mean time spent in contact with the same object category in S4. The data were analyzed by repeated-measures ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; object category, 2 levels: DO, NDO as within factor). Simple effects analysis of the factor “object” was performed within each group.
Social novelty test (SoNT)
A schematic representation of the SoNT apparatus is shown in Fig. 3a. The apparatus comprised a Plexiglas box that was divided into 3 chambers (60 cm × 40 cm) by walls (23 cm in height) with removable openings, containing 2 perforated Plexiglas cylinders in lateral chambers (Nadler et al. 2004). After 10-min habituation with the apparatus, the animal was confined into the center chamber while an unfamiliar sex- and age-matched adult intruder (subject) or a falcon (object) was placed inside of the cylinders (sociability test). The locations of the subject and object were alternated between mice.
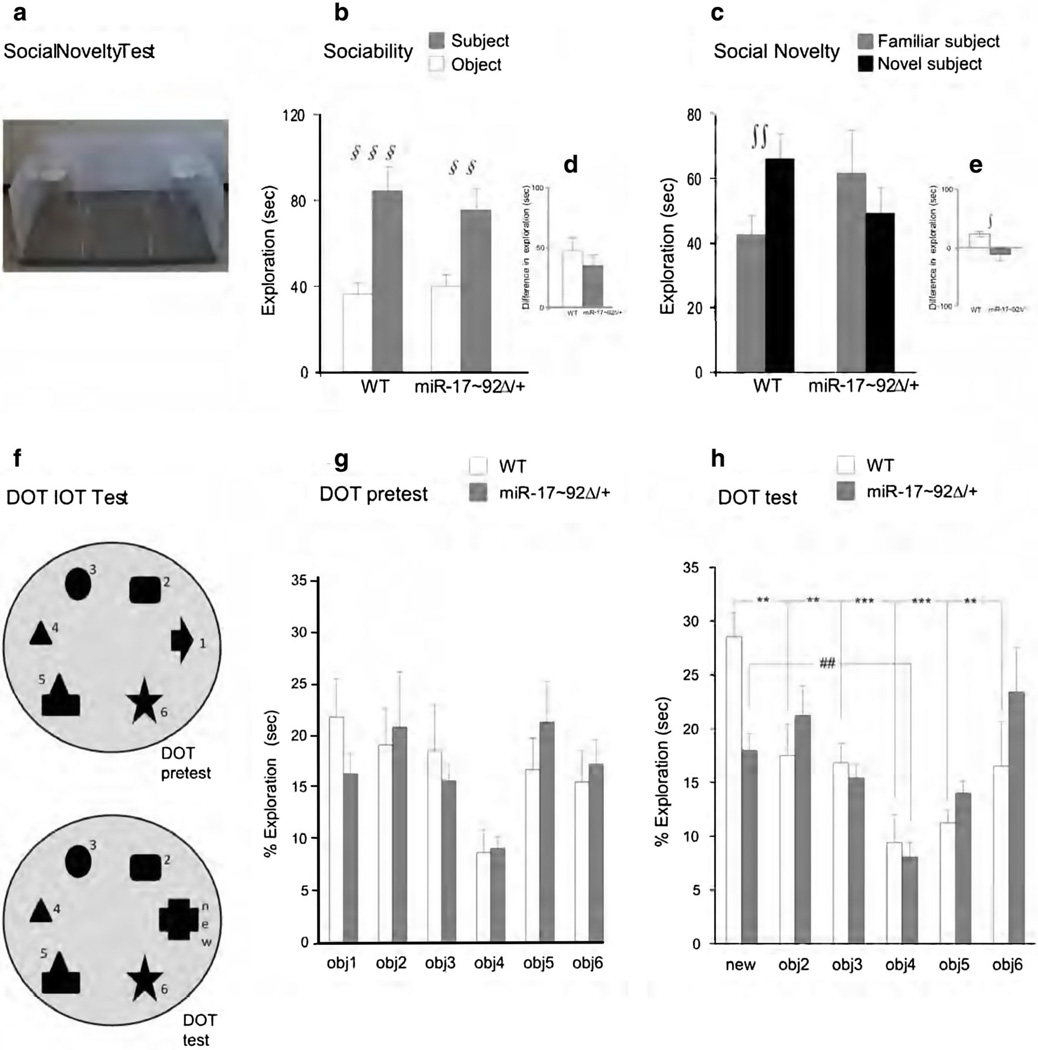
Fig. 3.
Adult behavioral assay. Social novelty test a Photograph of social novelty apparatus. Results are expressed as mean time spent sniffing object and subject (b) and as mean time sniffing the familiar subject and the new subject (c). In d, e data are presented as difference between the time spent sniffing the subject minus the time spent sniffing the object (d) or the novel subject minus the familiar subject (e). DOT IOT test f Representation of DOT-IOT apparatus. Results are expressed as mean percentage of time spent exploring the six objects (g) and the new objects versus the familiar ones (h). All data are expressed ad mean + SEM. §§,§§§ p < 0.01 and 0.001 for subject versus object. ++ p < 0.01 for familiar versus novel subject. **, *** p < 0.01 and 0.001 for new versus familiar object for WT. ## p < 0.01 for new versus familiar object for miR-17–92Δ/+
Following the sociability test, mice were administered a 10 min test for social novelty preference. A second unfamiliar mouse was placed in lieu of the object. The mouse then had to choose between the new, unfamiliar mouse and the previously explored, familiar animal (social novelty test). During all sessions, the mouse (n = 13–14) was allowed to move freely in the 3 chambers for 10 min. Time spent in each room and the number of entries were recorded using an EthoVision system (Noldus, The Netherlands). Time spent sniffing each cylinder was scored manually by a trained observer. During habituation, the total time spent exploring cylinders was analyzed by one-way ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+).
The total time spent exploring each cylinder in the sociability session was analyzed by repeated-measures ANOVA (genotype: 2 levels: WT and miR-17–92Δ/+ as between factor; object category: 2 levels, subject and object, as within factor). The total time spent exploring each cylinder in the social novelty session was evaluated by repeated-measures ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; subject category, 2 levels: familiar subject and novel subject, as within factor). Simple effects analysis of “object or subject category” was also performed within each group.
Object memory span task (DOT-IOT)
A schematic representation of DOT-IOT apparatus is shown in Fig. 3f. After 15 min of isolation, animals were individually submitted to 10 min habituation in the empty Open Field. The study phase followed 1 min in the waiting cage. During the study phase, animals could explore the objects. They were exposed to six different objects in the DOT and to six identical objects in the IOT. The DOT and IOT were similar (same number of items, same spatial arrangements) except for the amount of information to be stored (different vs identical objects) (Sannino et al. 2012). The first phase was the DOT. Animal had 210 s (35 s for each object) to explore the six different objects in the apparatus (Pre Test). After 1 min in the waiting cage, the animal was exposed to identical copies of the familiar objects and to one new object (Test). The position of the novel object was changed across animals in a random order.
The control task, the IOT, was performed 1 week after the DOT and consisted in exposing the mice to the same number of identical objects showed in the DOT during the Pre Test, and substitute one of them with a new object in the test phase. The video were recorded and analyzed by a trained observer using EthoVision system (Noldus, The Netherlands).
In the Pre Test (for both DOT and IOT), the percentage of time spent exploring the objects was analyzed by repeated-measure ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; object, 2 levels: novel and familiar, as within factor). In the Test session (for both DOT and IOT), the percentage of time spent in contact with the objects was evaluated by repeated measures ANOVA (genotype, 2 levels: WT and miR-17–92Δ/+ as between factor; object, 2 levels: novel and familiar, as within factor). The “object” factor was also analyzed within each group.
Sannino et al. (2012) distinguished between different degrees of discrimination/impairment: (1) new object discrimination/no impairment: when the new object was explored significantly more than all the other objects; (2) new object discrimination impairment: when the new object was explored significantly more than the same objects but similarly to other objects; and (3) complete lack of new object discrimination: when the new object was explored similarly to all the other objects.
Neurotransmitters tissue levels analysis
Frozen brains were sliced and punches of the medial prefrontal cortex (mpFC) and hippocampus (Hip) were obtained as previously described (Puglisi-Allegra et al. 2000). Punches were obtained from brain slices (coronal sections) no thicker than 300 µm. Stainless steel tubes of 0.8, 1.0, or 1.5 mm inside diameter were used. The coordinates were measured according to the atlas of Franklin and Paxinos (1997) (coronal sections), as follows: medial prefrontal cortex (mpFC) two slices from sections 80 to 130 (1.5 mm tube); hippocampus (HIP) 3 slices from sections 301 to 350 (0.8 and 1.0 mm tube; including CA1, CA2 and CA3 fields). The punches were stored in liquid nitrogen until the day of the analysis.
Levels of DA, NE, 5-HT and their metabolites
On the day of the analysis, frozen samples of mpFC and Hip were weighed and homogenized in 0.05 M HClO4. The homogenates were centrifuged at 14,000 rpm for 20 min at 4 °C. Tissue levels of 5-HT, DA, NE and their metabolites [5 hydroxyindoleacetic acid (5-HIAA), 3–4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 3-methoxy-4-hydroxyphenylethyleneglycol (MHPG)] were simultaneously assessed using HPLC system as previously described (Puglisi-Allegra et al. 2000). Briefly, the HPLC system consisted of an Alliance (Waters) system and a coulometric detector (ESA Model 5200A Coulochem II) provided with a 5011 high sensitivity analytical cell and a 5021 conditioning cell, the potential being set at .450 mV and 0.100 mV, respectively. The column, a Nova-Pack Phenyl column (3.93150 mm) and a Sentry Guard Nova-Pack pre-column (3.9 × 20 mm) were purchased from Waters Assoc. The flow rate was 1 ml/min. The mobile Phase consisted of 3 % methanol in 0.1 M Na-phosphate buffer pH 3, 0.1 mM, Na2 EDTA and 0.5 mM 1-octane sulphonic acid Na salt. Statistical analysis was performed by one-way ANOVA (genotype, two levels: WT, miR-17–92Δ/+).
Results
Developmental assay
Body measurements
Representative photographs of body size of miR-17–92Δ/+ and WT pups at P15 are shown in Fig. 1a.
Statistical analysis revealed a significant interaction between genotype and day for the weight (F(7,266) = 7.42; p < 0.001) and a significant genotype effect for the length (F(1,38) = 10.37; p < 0.005). Post hoc analyses revealed a significant effect of genotype for all days for weight (Fig. 1b) and for all days, except P21 and P24, for length (Fig. 1c).
Based on growth data in embryos (Ventura et al. 2008), miR-17–92Δ/+ grew less than WT mice.
This difference is also evident at P90; WT weight significantly more than miR-17–92/+ (F(1,14) = 65.673; p < 0.001), and are longer than miR-17–92Δ/+ (F(1,14) = 9.333; p < 0.01) (Fig. 1d, e).
USV
A representative photograph of the USV apparatus is shown in Fig. 1f. Although no significant interaction between genotype and postnatal day was observed for number (F(3,72) = 2.37; ns) or duration (F(3,72) = 2.35; ns) of USV, single comparisons revealed that miR-17–92Δ/+ emitted significantly fewer calls on P6 (Fig. 1g) and significantly shorter calls on P6, 8, and 10 (Fig. 1h) than WT mice.
Developmental milestones
No significant difference between WT and miR-17–92Δ/+ mice was evident regarding the acquisition of developmental milestones (Table 1).
Table 1.
Age of appearance of adult-like reflex
| Reflex | Day of appearance WT |
Day of appearance miR-17–92Δ/+ |
|---|---|---|
| Righting | 11 | 11 |
| Screen test | 14 | 14 |
| Vertical screen test | 17 | 17 |
| Air startle | 14 | 14 |
| Auditory startle | 17 | 17 |
| Cliff avoidance | 11 | 11 |
| Grasp reflex | 11 | 11 |
| Visual placing response | 14 | 14 |
Data are expressed as age of appearance of adult-like reflex in WT (n = 14) and miR-17–92Δ/+ mice (n = 13 for group). Reflex development scoring is based on the Likert (Heyser 2004) scale: 0 = no response; 1 = weak response; 2 = reflex present but incomplete; and 3 = adult-like response. A reflex is considered as acquired on the day when the mean score was >2.5 ± SEM
HT
A representative photograph of the HT apparatus is shown in Fig. 1i. No significant interaction between genotype and arena was evident (F(1,25) = 0.01; ns). WT and miR-17–92Δ/+ mice spent more time in the nest than the clean litter arena, showing similar social olfactory discrimination and maternal bound (Fig. 1l).
OF
No significant difference between WT and miR-17–92Δ/+ mice for velocity (F(1,38) = 0.002; ns), distance moved (F(1,38) = 0.593; ns) or time spent in the periphery (F(1,38) = 0.250; ns) in the OF (data not shown) was evident, thus demonstrating normal locomotor activity and anxiety levels in miR-17–92Δ/+ mice.
Adult behavioral assay
EPM
The percentage of entries (F(1,28) = 0.231; ns) and time spent (F(1,28) = 0.59; ns) in the open arms of the EPM did not differ between WT and miR-17–92/+ mice (Table 2), thus demonstrating that miR-17–92Δ/+ mice did not show alterations in emotional reactivity.
Table 2.
Elevated Plus Maze and Open Field in adult mice
| WT | miR-17–92Δ/+ | |
|---|---|---|
| Elevated plus maze | ||
| % time open arms | 6892 ± 1.52 | 6417 ± 1.17 |
| % entries open arms | 17.03 ± 1.41 | 15.99 ± 1.65 |
| Open field | ||
| Distance moved | 2290.67 ± 113.63 | 2799.21 ± 155.99* |
| Velocity | 6.41 ± 0.32 | 7.78 ± 0.43* |
Values are expressed as mean ± SEM
p < 0.05;
p < 0.01;
p < 0.001 WT versus miR-17–92Δ/+ Elevated plus maze data are expressed as percentage of time spent in the open arms (time in open/open + closed × 100), entries are expressed as percentage of entries in the open arms (open entries/open + closed × 100). (n WT = 16; miR-17–92Δ/+ = 14)
Open field Distance moved is expressed in cm, velocity is expressed in cm/s. (n WT = 15; miR-17–92Δ/+ = 14)
OF
The analysis of adult OF data revealed significant difference in distance moved (F(1,27) = 7.082; p < 0.05) and velocity (F(1,28) = 6.577; p < 0.05) between miR-17–92/+ mice and WT mice, thus indicating that miR-17–92Δ/+ mice are more active than WT (Table 2).
ORT
A schematic representation of the ORT apparatus is shown in Fig. 2a. One-way ANOVA showed no significant difference between the genotypes in time spent exploring the objects during the PreTest session (F(1,26) = 4.115; ns), thus suggesting that all mice had normal reactivity to the presentation of stimuli (Fig. 2b). No significant interaction between genotype and “object” was evident with regard to time spent exploring the new versus the familiar object in the Test session (F(1,26) = 0.819; ns) (Fig. 2c). Both genotypes showed preferences for the novel stimuli.
SpNT
A schematic representation of the SpNT apparatus is shown in Fig. 2d. Time spent exploring the objects during S2, S3, and S4 did not differ between the groups (F(1,28) = 1.634; ns) (Fig. 2e). Although no significant genotype × object interaction was observed (F(1,28) = 2.06; ns), single comparisons revealed significant difference in time spent exploring DO versus NDO in WT mice but not in HTZ animals (Fig. 2f), thus suggesting that miR-17–92Δ/+ mice are less able to recognize spatial novelty.
SoNT
A schematic representation of the SoNT apparatus is shown in Fig. 3a. For each session, the time spent in the center of the arena was not included in the analyses.
Statistical analysis revealed no significant interaction between genotype and “object category” (F(1,26) = 0.81; ns). Both WT and miR-17–92Δ/+ mice spent more time exploring the subject than the object (Fig. 3b, d).
Repeated-measures ANOVA for the social novelty session revealed a significant genotype × “subject category” interaction (F(1,26) = 6.60 p < 0.05). Only WT mice spent significantly more time exploring novel versus familiar subject (Fig. 3c, e), thus suggesting that miR-17–92Δ/+ mice were less able to recognize social novelty.
DOT-IOT
Concerning the PreTest session for DOT-IOT task, repeated-measures ANOVA did not reveal a significant interaction between genotypes × object (DOT: F(5,140) = 1.09; ns; IOT: F(5,65) = 0.72; ns) (Fig. 3g). Both WT and miR-17–92Δ/+ mice showed normal reactivity to the presentation of stimuli. Concerning Test session, no significant interaction between genotype and object was evident for the IOT (F(5,65) = 1.32; ns). However, a significant interaction was observed for DOT test (F(5,65) = 2.44; p < 0.05). Single comparisons revealed that only WT mice spent more time exploring the new object than the known ones (Fig. 3h). These results suggest that miR-17–92Δ/+ mice have a shorter memory span than WT animals that depends on the amount of information to be retained.
Neurotransmitters tissue levels analysis
In Table 3 are shown the results of neurotransmitters brain tissue levels. One-way ANOVA showed a significant difference between WT and miR-17–92Δ/+ mice for DA and HVA/DA ratio in the mpFC (DA: F (1,15) = 6.56; p < 0.05; HVA/DA: F(1,15) = 9.11; p < 0.01) (Table 3). miR-17–92Δ/+ mice showed lower DA levels and higher HVA/DA ratio in comparison with WT mice. Moreover, miR-17–92Δ/+ mice showed lower 5-HT as well as HIAA/5-HT ratio in Hip in comparison with WT mice.
Table 3.
DA, NE, 5-HT and their metabolites tissue levels (ng/g wet weight) in the medial prefrontal cortex (mpFC) and hippocampus (HIP) of WT and miR-17–92Δ/+ mice
| mpFC |
HIPP |
|||
|---|---|---|---|---|
| WT | miR-17–92Δ/+ | WT | miR-17–92Δ/+ | |
| NE | 615 ± 24 | 588 ± 35 | 781 ± 267 | 698 ± 69 |
| DA | 93 ± 11 | 62 ± 6* | 121 ± 40 | 148 ± 49 |
| 5-HT | 718 ± 39 | 674 ± 34 | 1081 ± 273 | 905 ± 79 |
| MOPEG | 80 ± 1 | 78 ± 1 | 130 ± 29 | 93 ± 32 |
| DOPAC | 60 ± 6 | 35 ± 8* | 14 ± 3 | 24 ± 6 |
| HVA | 54 ± 5 | 54 ± 7 | 43 ± 8 | 45 ± 5 |
| HIIA | 353 ± 13 | 309 ± 26 | 112 ± 23 | 64 ± 6* |
| MOPEG/NE | 0.13 ± 0.004 | 0.13 ± 0.007 | 0.154 ± 0.02 | 0.112 ± 0.03 |
| HVA/DA | 0.63 ± 0.05 | 0.86 ± 0.07* | 0.47 ± 0.7 | 0.63 ± 0.16 |
| DOPAC/DA | 0.774 ± 0.16 | 0.515 ± 0.1 | 0.13 ± 0.01 | 0.26 ± 0.07 |
| HIIA/5-HT | 0.499 ± 0.021 | 0.468 ± 0.042 | 0.11 ± 0.009 | 0.07 ± 0.005** |
p < 0.05;
p < 0.01
Discussion
FS is characterized by various combinations of microcephaly, limb malformation, and learning disabilities, the latter occurring in 52 % to 90 % of FS patients (Herman and Siegel 2004; Marcelis and De Brouwer 2009).
Two forms of FS have been described: FS1, due to a heterozygous mutation in MYCN gene on chromosome 2 (van Bokhoven et al. 2005) and FS2, due to a heterozygous microdeletion of miRNA 17–92 cluster on chromosome 13 (De Pontual et al. 2011). FS2 shares features with FS1, including microcephaly, mild growth retardation and skeletal findings, digital abnormalities, gastrointestinal abnormalities, and short palpebral fissures.
In approximately 70 % of families, FS is caused by germline loss-of-function mutations involving the MYCN gene (MIM 164840) at 2p24 (Marcelis et al. 2008; van Bokhoven et al. 2005; Tészás et al. 2006). However, De Pontual et al. (2011) reported that a subset of patients affected by Feingold Syndrome, carry heterozygous loss of the entire miR-17–92 cluster (De Pontual et al. 2011). Several of the key features observed in FS patients carrying a heterozygous deletion for miR-17–92 were also evident in miR-17–92Δ/+ mice (de Pontual et al. 2011), thus demonstrating the regulatory role of miR-17–92 in growth and skeletal development (de Pontual et al. 2011). Although FS patients have a wide range of learning disabilities, specific learning/cognitive disabilities, intellectual disabilities, and specific brain alterations have not been reported in humans and animal models of FS. Moreover, no behavioral characterization of a genetic mouse model of FS2 has been performed. Thus, the goal of this study was to draw the behavioral profile of miR-17–92Δ/+ mice during development and in adulthood. Because measures of sensory and motor development in rodents may be considered as a model of development in human newborns (see Le Roy et al. 2001 for a review), we evaluated these early functions in miR-17–92Δ/+ and WT mice.
Consistent with the function of the miR-17–92 cluster in normal development (Mogilyansky and Rigoutson 2013) and with embryonic growth data (Ventura et al. 2008), body weight and length were reduced in miR-17–92Δ/+ in comparison with WT mice, both during development and in adulthood. Moreover, miR-17–92Δ/+ mice showed alterations in ultrasonic vocalization; particularly fewer calls and decreased calls duration when separated from their mothers and siblings were evident in miR-17–92Δ/+ mice compared to WT mice. Although, we can not exclude that reductions in USV calls are due to reduced growth in miR-17–92Δ/+ mice, they could also be precursors of following deficits evident in adult mice.
Conversely, during development, miR-17–92Δ/+ mice had similar locomotor activity and age of appearance of adult-like reflex as WT mice.
To investigate cognitive performance in adult mice, we used 3 non-associative tests: the object recognition test, the spatial novelty test and the DOT-IOT test (Figs. 2a, d, 3d) that do not require reinforcement and that exploit rodents’ spontaneous preference for novelty. ORT, SNT and DOT-IOT tests are behavioral paradigms that measure different aspects of memory. The object recognition test for rodents is a variant of the delayed non-match to sample task, whereas the spatial novelty test measures the ability of rodents to encode spatial relationships; both tasks involve the prefrontal cortical areas. Finally, the DOT-IOT test has been developed to investigate the memory load in rodents and required normal hippocampal functions (Sannino et al. 2012).
In the ORT and in the SNT, miR-17–92Δ/+ mice were normally interested in the object, showed normal levels of object exploration and habituation, and were perfectly capable of object discrimination, as evidenced by increased exploration of the novel object by both groups in the ORT. Nonetheless, miR-17–92Δ/+ mice lacked increased exploration of the displaced objects, spending similar times exploring DOs and NDOs in the spatial novelty test. These results indicate that miR-17–92Δ/+ mice are not able to recognize whether a spatial change has occurred in the familiar environment, but they recognize whether a stimulus has been encountered before, thus suggesting that miR-17–92Δ/+ mice show difficulties in processing spatial information, likely due to a loss of behavioral flexibility, attentional set-shifting or reduced memory load. To investigate this possibility, we evaluated the memory span of miR-17–92Δ/+ mice and WT mice using a task requiring an elevated memory load, the DOT-IOT test. Because in the IOT test the objects were all identical, this should not increase the memory load, while in the DOT test, where the objects were all different from each other, this should increase the memory load. No significant difference between miR-17–92Δ/+ and WT mice was evident in the IOT test; both groups were able to recognize the new object. However, when the memory load increased, in the DOT test, only WT mice explored the new object significantly more than all the familiar objects, thus supporting the hypothesis that miR-17–92Δ/+ mice have a reduced memory span.
MiR-17–92Δ/+ mice showed normal emotional reactivity in the elevated plus maze and normal sociability in the social test. However, in the social novelty test, they did not display the typical preference for the new subject that was observed in WT mice. In the first session, both WT and miR-17–92Δ/+ mice explored more the subject than the object. However, in the second session, they explored more the known subject than the new subject. These data support the hypothesis of a specific deficit in behavioral flexibility in mutant mice, because this inclination likely reflects the inability to develop a flexible acting pattern in response to changing environmental conditions (Floresco and Magyar 2006). However, it is also possible that miR-17–92Δ/+ mice show a selective social deficit, as suggested by lack of preference for the new subject in comparison with the familiar subject. Further experiments are needed to investigate this hypothesis.
Finally, we found altered DA as well as 5-HT transmission in the mpFC and Hip, respectively, of miR-17–92Δ/+ mice in comparison with WT animals, thus suggesting a possible link between selective cognitive deficits and altered neurotransmission in specific brain areas involved in some executive functions. However, further experiments are needed to elucidate the mechanism by which altered neurotransmission in the mpFC and Hip, as well as in other brain regions, could induce behavioral deficits.
Because many rare diseases share pathophysiological mechanisms, advances in the knowledge of one disease can contribute to knowledge of many other diseases. Deregulation of miRNAs provides an example of shared patho-physiological mechanisms. In fact, alterations of miRNAs functions may produce abnormalities of brain development and impair cognitive functions in different rare neurodevelopmental disorders, thus suggesting that the post-transcriptional activity of miRNAs could be one of the common mechanisms involved in the development of several rare syndromes (see Roubertoux and de Vries 2011 for a comprehensive discussion). Brain and behavioral investigation of rare diseases in animal models may help to understand behavioral features as well as brain systems involved not only in the rare disease themselves, but also in many different disorders and/or normal behavior (Roubertoux and de Vries 2011).
The selective deficits observed in miR-17–92Δ/+ mice can provide more detailed information about the some cognitive deficits sometimes described in humans. A deeper knowledge of these deficits might clarify the frequent learning disabilities in Feingold patients throughout school and adulthood (Herman and Siegel 2004; Marcelis and de Brouwer 2009; Lehman et al. 2009) and, eventually, provide targeted supportive therapy and prevention interventions to counteract negative consequences in FS patients.
However, although extremely useful, animal models should also be used carefully when studying particular behavioral phenotypes such as cognitive functions, because ‘intellectual disability’ in humans does not have a real equivalent in animal models (Roubertoux and de Vries 2011). Great efforts have to get in order to obtain an exhaustive explanation and understanding of cognitive function in human diseases also using animal models.
Conclusion
Although the physical symptoms of FS have been extensively characterized (Feingold et al. 1997; Celli et al. 2003; Blaumeiser et al. 2008), the cognitive and neuropsychological functions in developing and adult patients have not been as well described.
Our data, showing selective cognitive deficits as well as neurotransmission alterations in miR-17–92Δ/+ mice suggest an important role of this cluster in modulating these alterations. However, although the role miR-17–92/+ can be investigated to clarify the brain mechanisms underlying selective cognitive disabilities in FS patients, many detailed studies are still needed to draw a comprehensive picture. The exploration of this model could improve the knowledge of behavioral aspects of this rare disease, but experiments are only beginning.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10519-015-9724-8) contains supplementary material, which is available to authorized users.
Conflict of Interest E. Fiori, L. Babicola, D. Andolina, A. Coassin, T. Pascucci, L. Patella, Y.-C. Han, A. Ventura, R. Ventura declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human participants.
References
- Baek KE, Yoon SR, Kim JT, Kim KS, Kang SH, Yang Y, Lim JS, Choi I, Nam MS, Yoon M, Lee HG. Upregulation and secretion of macrophage inhibitory cytokine-1 (MIC-1) in gastric cancers. Clin Chim Acta. 2008;401(1–2):128–133. doi: 10.1016/j.cca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-related behaviors in mice. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. 2nd edn. Boca Raton, FL: CRC Press; 2009. Chap. 5. [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Madison JM, Zhou F, Rueckert EH, Barker D, Perlis RH, Sur M, Haggarty SJ. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015;20(5):573–584. doi: 10.1038/mp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami G. Economical test method for neurobehavioral toxicity. Environ Health Perspect. 1996;104(Suppl 2):285–298. doi: 10.1289/ehp.96104s2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaumeiser B, Oehl-Jaschkowitz B, Borozdin W, Kohlhase J. Feingold syndrome associated with two novel MYCN mutations in sporadic and familial cases including monozygotic twins. Am J Med Genet A. 2008;146A(17):23042307. doi: 10.1002/ajmg.a.32444. [DOI] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur J Neuropsychopharmacol. 2013;23:602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Borkhardt A, Fuchs U, Tuschl T. MicroRNA in chronic lymphocytic leukemia. New Engl J Med. 2006;354(5):524–525. doi: 10.1056/NEJMc053266. [DOI] [PubMed] [Google Scholar]
- Branchi I, Alleva E. Communal nesting, an early social enrichment, increases the adult anxiety-like response and shapes the role of social context in modulating the emotional behavior. Behav Brain Reas. 2006;172:299–306. doi: 10.1016/j.bbr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. New Engl J Med. 2005;353(17):1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Celli J, Van Beusekom E, Hennekam RC, Gallardo ME, Smeets DF, de Córdoba SR, Innis JW, Frydman M, König R, Kingston H, Tolmie J, Govaerts LC, Van Bokhoven H, Brunner HG. Familial syndromic esophageal atresia maps to 2p23-p24. Am J Hum Genet. 2000;66(2):436–444. doi: 10.1086/302779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, Van Bokhoven H, Brunner HG. Feingold syndrome: clinical review and genetic mapping. Am J Med Genet. 2003;122A(4):294300. doi: 10.1002/ajmg.a.20471. [DOI] [PubMed] [Google Scholar]
- Cognet M, Nougayrede A, Malan V, Callier P, Cretolle C, Faivre L, Genevieve D, Goldenberg A, Heron D, Mercier S, Philip N, Sigaudy S, Verloes A, Sarnacki S, Munnich A, Vekemans M, Lyonnet S, Etchevers H, Amiel J, de Pontual L. Dissection of the MYCN locus in Feingold syndrome and isolated oesophageal atresia. Eur J Hum Genet. 2011;19:602–606. doi: 10.1038/ejhg.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assay relevant to the symptoms of autism. Symposium: Neurobiology of autism, International society of neuropathology. Brain pathology. 2007 doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Zanetti C, Lampis V, Coccurello R, Pascucci T, Ventura R, Puglisi-Allegra S, Spatola CA, Pesenti-Gritti P, Oddi D, Moles A, Battaglia M. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS One. 2011;6(4):e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135(23):3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pontual L, Yao E, Callier P, Faivre L, Drouin V, Cariou S, Van Haeringen A, Geneviève D, Goldenberg A, Oufadem M, Manouvrier S, Munnich A, Vidigal JA, Vekemans M, Lyonnet S, Henrion-Caude A, Ventura A, Amiel J. Germline deletion of the miR-17 < 92 cluster causes skeletal and growth defects in humans. Nat Genet. 2011;43(10):1026–1030. doi: 10.1038/ng.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R, LaBonté B, Ribeiro E, Liu X, Kennedy P, Vialou V, Ferguson D, Peña C, Calipari ES, Koo JW, Mouzon E, Ghose S, Tamminga C, Neve R, Shen L, Nestler EJ. β-Catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson JR, Kruse C, Montagna DR, Finsen B, Wolfe MS. Alternative polyadenylation and miR-34 family members regulate tau expression. J Neurochem. 2013;127:739–749. doi: 10.1111/jnc.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold M, Hall BD, Lacassie Y, Martínez-Frías ML. Syndrome of microcephaly, facial and hand abnormalities, tracheoesophageal fistula, duodenal atresia, and developmental delay. Am J Med Genet. 1997;69(3):245–249. [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188(4):567585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci. 2009;106(19):7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjavi H, Siu VM, Speevak M, MacDonald PA. A fourth case of Feingold syndrome type 2: psychiatric presentation and management. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17–92, is over-expressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman TE, Siegel MJ. Feingold syndrome: microcephaly, esophageal atresia, type III laryngeal cleft, malrotation, limb anomalies. J Perinatol. 2004;24(9):568–570. doi: 10.1038/sj.jp.7211144. [DOI] [PubMed] [Google Scholar]
- Heyser CJ. Assessment of developmental milestones in rodents. Curr Protoc Neurosci. 2004;8(18):1–15. doi: 10.1002/0471142301.ns0818s25. [DOI] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19(4):489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(1):152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy I, Carlier M, Roubertoux PL. Sensory and motor development in mice: genes, environment and their interactions. Behav Brain Res. 2001;125(1–2):57–64. doi: 10.1016/s0166-4328(01)00279-0. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lehman VT, Patterson MC, Babovic-Vuksanovic D, Rydberg C. Cerebral and cerebellar white matter abnormalities with magnetic resonance imaging in a child with Feingold syndrome. Am J Med Genet A. 2009;149A(12):2824–2827. doi: 10.1002/ajmg.a.33108. [DOI] [PubMed] [Google Scholar]
- Lemons D, Maurya MR, Subramaniam S, Mercola M. Developing microRNA screening as a functional genomics tool for disease research. Front Physiol. 2013;4:223. doi: 10.3389/fphys.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelis LM, De Brouwer PM. Feingold syndrome 1. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews. 2009. [Google Scholar]
- Marcelis CL, Hol FA, Graham GE, Rieu PN, Kellermayer R, Meijer RP, Lugtenberg D, Scheffer H, van Bokhoven H, Brunner HG, de Brouwer AP. Genotype-phenotype correlations in MYCN-related Feingold syndrome. Hum Mutat. 2008;29(9):1125–1132. doi: 10.1002/humu.20750. [DOI] [PubMed] [Google Scholar]
- Mencía A, Modamio-Høybjør S, Redshaw N, Morín M, Mayo-Merino F, Olavarrieta L, Aguirre LA, del Castillo I, Steel KP, Dalmay T, Moreno F, Moreno-Pelayo MA. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41(5):609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- Mogilyansky E, Rigoutson I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):16031614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Mu P, Han YC, Betel D, Yao E, Squatrito M, Ogrodowski P, De Stanchina E, D’Andrea A, Sander C, Ventura A. Genetic dissection of the miR-17–92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23(24):2806–2811. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Macrì S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behav Brain Res. 2007;177(1):142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of miR-17–92. Genes Dev. 2009;23(24):2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou G, Mirzamohammadi F, Kobayashi T. MicroRNAs involved in bone formation. Cell Mol Life Sci. 2014;71:4747–4761. doi: 10.1007/s00018-014-1700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Grimm CH, Paya-Cano JL, Sugden K, Nietfeld W, Lehrach H, Schalkwyk LC. Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mamm Genome. 2008;19:552–560. doi: 10.1007/s00335-008-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S, Pascucci T, Ventura R, Calì F, Romano V. Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria. Neurochemistry. 2000;1(7):1361–1364. doi: 10.1097/00001756-200004270-00042. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, de Vries PJ. From molecules to behavior: lessons from the study of rare genetic disorders. Behav Genet. 2011;41(3):341–348. doi: 10.1007/s10519-011-9469-y. [DOI] [PubMed] [Google Scholar]
- Sannino S, Russo F, Torromino G, Pendolino V, Calabresi P, De Leonibus E. Role of the dorsal hippocampus in object memory load. Learn Mem. 2012;19:211–218. doi: 10.1101/lm.025213.111. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T + tf/J mouse model of Autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gasparini L, Alleva E, Goedert M, Calamandrei G, Spillantini MG. Early behavioural markers of disease in P301S tau transgenic mice. Behav Brain Res. 2010;208:250–257. doi: 10.1016/j.bbr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Tassano E, Di Rocco M, Signa S, Gimelli G. De novo 13q31.1-q32.1 interstitial deletion encompassing the miR-17–92 cluster in a patient with Feingold syndrome-2. Am J Med Genet A. 2013;161A(4):894–896. doi: 10.1002/ajmg.a.35781. [DOI] [PubMed] [Google Scholar]
- Tészás A, Meijer R, Scheffer H, Gyuris P, Kosztolányi G, van Bokhoven H, Kellermayer R. Expanding the clinical spectrum of MYCN-related Feingold syndorme. Am J Med Genet A. 2006;140A:22542256. doi: 10.1002/ajmg.a.31407. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Celli J, van Reeuwijk J, Rinne T, Glaudemans B, van Beusekom E, Rieu P, Newbury-Ecob RA, Chiang C, Brunner HG. MYCN haplonsufficiency is associated with reduced brain size and intestinal atresias in Feingold syndrome. Nat Genet. 2005;37(5):465–467. doi: 10.1038/ng1546. [DOI] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans . Cell. 1993;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Ma J, Chen S, Chen X, Yu X. MicroRNA-17–92 cluster regulates osteoblast proliferation and differentiation. Endocrine. 2012;45:302–310. doi: 10.1007/s12020-013-9986-y. [DOI] [PubMed] [Google Scholar]
- Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, Bahari-Javan S, Burkhardt S, Sananbenesi F, Fischer A. MicroRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.