Abstract
Background
Cardiovascular disease and obesity are now becoming leading causes of morbidity and mortality in low- and middle-income countries (LMICs). We investigated the relationship between prevalent heart disease (HD) and current anthropometric indices and body size perception over time from adolescence to adulthood in Iran.
Methods
We present a cross-sectional analysis of baseline data from a prospective study of adults in Golestan Province, Iran. Demographics, cardiac history, and current anthropometric indices—body mass index (BMI), waist circumference (WC), and waist to hip ratio (WHR)—were recorded. Body size perception for age 15, age 30, and at the time of interview was assessed via pictograms. Associations of these factors and temporal change in perceived body size with HD were evaluated using multivariable logistic regression models.
Results
Complete data were available for 50,044 participants; 6.1% reported having HD. Higher BMI, WC, and WHR were associated with HD (p < 0.001). Men had a U-shaped relationship between HD and body size perception at younger ages. For change in body size perception, men and women demonstrated a U-shaped relationship with prevalent HD from adolescence to early adulthood, but a J-shaped pattern from early to late adulthood.
Conclusions
HD was associated with anthropometric indices and change in body size perception over time for men and women in Iran. Due to the increasing prevalence of overweight and obesity in LMICs, interventions focused on decreasing the cumulative burden of risk factors throughout the life course may be an important component of cardiovascular risk reduction.
Keywords: body size perception, body mass index, heart disease, prevalence, middle-income country
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality throughout the world, with 80% of CVD deaths occurring in low- and middle-income countries (LMICs) [1–5]. This number is projected to increase significantly in the future [6,7]. The impact of CVD in the Middle East is particularly apparent, where more than 35% of all deaths are attributable to CVD, more than half of which are due to ischemic heart disease (IHD) [1,8,9].
Obesity, once thought to pervade only high-income countries, is now becoming an imminent public health threat in LMICs as well [10,11]. The age-adjusted prevalence of overweight and obese individuals in Golestan, Iran, is as high as in the United States [12]. Such rates of obesity are concerning due to the correspondingly increased prevalence of CVD [13,14]. Recent investigations have elucidated this relationship further, demonstrating that individuals at both ends of the spectrum for body mass index (BMI) have an increased risk of CVD and overall mortality. However, there may be regional differences in this relationship [15–18]. In addition, an increasing number of studies are investigating the relationship between life course trends in BMI and the development of CVD at older ages [19–24]. However, data are relatively lacking regarding this relationship between life course trends in BMI and CVD in LMICs.
In this cross-sectional analysis of baseline data from the Golestan Cohort Study (GCS) in Iran, a middle-income country [25], we present an investigation of the relationship between prevalent heart disease (HD) and current anthropometric indices such as body mass index (BMI), waist circumference (WC), and waist to hip ratio (WHR). In addition, we evaluate the relationship between HD and change in self-reported body size from adolescence through adulthood.
Methods
The design of the GCS has been previously described [25]. GCS is a prospective population-based cohort study initially designed to investigate risk factors for upper gastrointestinal cancers in Golestan, Iran. The study enrolled 50,045 adults (40 to 75 years old) between 2004 and 2008; complete data for the present analysis were available for 50,044 participants. Both urban and rural community dwellers were represented including participants from Gonbad City, the main urban center in eastern Golestan, and participants from 326 surrounding rural villages (Figure 1). Eligibility criteria included willingness to participate in the study, being a permanent resident in Golestan, and a negative personal history of cancer. Written informed consent was obtained from all participants.
Figure 1.
Map of Golestan, Iran; Golestan Province highlighted in green.
A baseline face-to-face interview using a structured questionnaire was conducted by trained nurses or physicians to record basic demographics including age, sex, education, ethnicity, place of residence (rural/urban), ownership of household appliances, and level of physical activity. Smoking history was obtained by recording the starting and stopping ages and daily amount of cigarette use in different time periods, which captured changes in use over time. Past medical history including hypertension, diabetes, and HD, as well as current medication use, was documented. Indicators of socioeconomic status were assessed by education (highest level attained) and ownership of household appliances [26]. Physical activity at work was assessed using two questions: if the person worked every month throughout the year, and if intense physical activity was a part of daily work. Three levels were defined based on the responses—intense, non-intense but regular, and non-intense irregular.
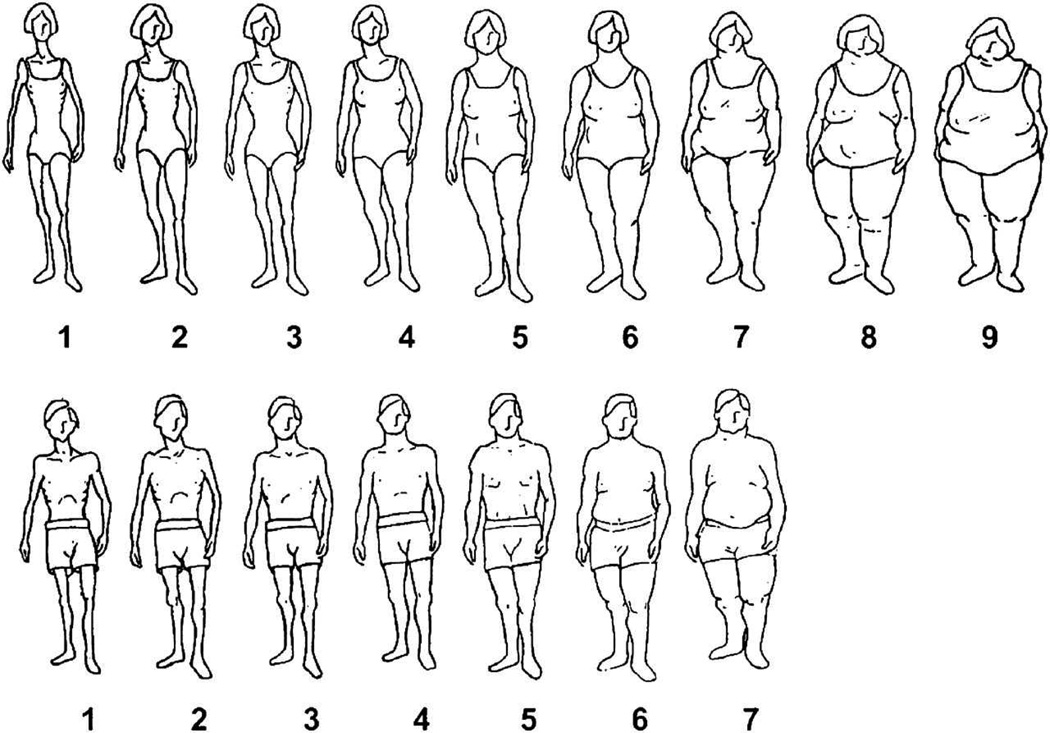
Anthropometric data such as weight, height, BMI, WC, and WHR were measured by trained staff at the time of the baseline survey. Weight and height were recorded to the nearest 0.5 kg and 1 cm, respectively. BMI was calculated by dividing weight (kilograms) by the squared value of height (meters). WC and WHR were categorized by the World Health Organization Criteria with “at risk” defined as ≥ 102.0 cm for men and ≥ 88.0 cm for women and ≥ 0.90 for men and ≥ 0.85 for women, respectively [27]. WC and WHR were divided into quintiles in additional analyses. Individual body size perception at age 15, age 30, and at the time of interview was assessed using a standard pictogram with drawings of men or women ranging from very lean to obese (scores of 1–7 for men, 1–9 for women) (Figure 2) [28]. The pictogram was used as a surrogate for BMI at younger ages [29]. Change in body size perception over time was determined by assessing the change in pictogram identification between age 15 to 30, and age 30 to the time of interview.
Figure 2.
Standard pictogram illustrating spectrum of body size perception for women (top) and men (bottom).
Participants were considered to be hypertensive if they used anti-hypertensive medication or fulfilled the criteria of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (average systolic blood pressure ≥ 140 mmHg, or average diastolic blood pressure ≥ 90 mmHg) [30]. Diabetes mellitus was self-reported based on the following question: “Have you ever been diagnosed by a doctor as having diabetes mellitus?” Duration of self-reported diabetes was categorized as 1–5, 6–10, 11–20, and ≥ 21 years. For HD, participants were asked, “Have you ever been diagnosed by a doctor as having angina, infarction, or heart failure?” Those with a positive reply to this question were considered as having HD.
The Institutional Review Boards of the Digestive Disease Research Center of Tehran University of Medical Sciences, the United States National Cancer Institute (NCI), and the World Health Organization International Agency for Research on Cancer (IARC) approved the study protocol for the GCS.
Statistical Methods
For categorical variables, numbers and percentages were calculated and presented. The primary outcome of interest was prevalent HD, and analyses were stratified by sex. Logistic regression models were used to calculate unadjusted and adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Multivariable models were adjusted for age, ethnicity, place of residence, education level, economic status, cigarette smoking, physical activity, hypertension, and self-reported diabetes. Change in body size perception between age 15 and 30, and age 30 to the time of interview, were additionally adjusted for the individual’s pictogram score at the lower age (age 15 or 30, respectively). Results for the pictogram identified at each age point and for change in body size perception over time were adjusted in a separate analysis for current BMI. P-values for trend were obtained from logistic regression models by assigning consecutive numbers to categories within each categorical variable. P-values < 0.05 were considered as statistically significant. All statistical analyses were performed using STATA, version 11 (Stata Corporation, College Station, TX, USA).
Results
Baseline characteristics of the study population are shown in Table 1. More than half of the participants were women (57.6%), due primarily to a higher participation rate among women than men [25]. The average age for men was 53.2 years old and for women was 51.3 (SD 9.4 and 8.6, respectively). Men reported significantly more work-related physical activity as compared with women, of whom 80.6% reported irregular non-intense activity at work. Women were more likely to be obese, and more likely to have an “at-risk” WC. With regard to body size perception, the proportion of both men and women reporting a heavier body size increased with increasing age. Women were more likely to report both major increase and major decrease in body size from age 15 to 30 and from age 30 to the time of interview.
Table 1.
Characteristics of the population in the Golestan Cohort Study. Figures are the percent of participants.
| Men (N= 21,234) | Women (N=28,810) | P-value# | |
|---|---|---|---|
| Age (years) | |||
| <45 | 21.0 | 25.9 | <0.001 |
| 45–49 | 21.6 | 23.0 | |
| 50–54 | 17.2 | 18.8 | |
| 55–59 | 14.0 | 14.2 | |
| 60–64 | 11.0 | 8.9 | |
| 65+ | 15.2 | 9.2 | |
| Ethnicity | |||
| Non-Turkmen | 24.4 | 26.4 | <0.001 |
| Turkmen | 75.6 | 73.6 | |
| Place of Residence | |||
| Rural | 81.5 | 78.8 | <0.001 |
| Urban | 18.5 | 21.2 | |
| Wealth | |||
| Low | 28.5 | 29.7 | <0.001 |
| Low-Medium | 19.6 | 20.6 | |
| Medium-High | 24.8 | 24.6 | |
| High | 27.1 | 25.2 | |
| Education | |||
| No School | 49.1 | 85.7 | <0.001 |
| Primary/middle school | 34.6 | 11.7 | |
| High school | 12.0 | 2.1 | |
| University | 4.4 | 0.5 | |
| Cigarette Smoking | |||
| Never | 61.7 | 98.5 | <0.001 |
| ≤5 pack-years | 11.6 | 1.0 | |
| 5.1 to 10 pack-years | 5.7 | 0.2 | |
| 10.1 to 20 pack-years | 8.3 | 0.2 | |
| >20.1 pack-years | 12.7 | 0.1 | |
| Physical Activity | |||
| Irregular non-intense | 35.6 | 80.6 | <0.001 |
| Regular non-intense | 42.8 | 15.6 | |
| Irregular or regular intense | 21.6 | 3.9 | |
| Body Mass Index | |||
| <18.5 (underweight) | 5.9 | 4.0 | <0.001 |
| 18.5 to 24.9 (normal) | 45.3 | 28.9 | |
| 25 to 29.9 (overweight) | 34.2 | 33.7 | |
| ≥30 (obese) | 14.6 | 33.4 | |
| Waist Circumference (WHO Criteria) | |||
| Normal | 66.2 | 8.9 | <0.001 |
| At Risk | 33.8 | 91.1 | |
| Waist to Hip Ratio (WHO Criteria) | |||
| Normal | 25.7 | 10.7 | <0.001 |
| At Risk | 74.3 | 89.3 | |
| Pictogram at Age 15 | |||
| 1 (slimmest) | 10.2 | 28.7 | <0.001 |
| 2 | 25.8 | 20.1 | |
| 3 | 30.4 | 13.3 | |
| 4 | 19.3 | 9.3 | |
| 5 | 8.9 | 8.8 | |
| 6 | 3.7 | 7.6 | |
| 7 | 1.8 | 4.2 | |
| 8 | 0.0 | 3.1 | |
| 9 | 0.0 | 4.8 | <0.001 |
| Pictogram at Age 30 | |||
| 1 (slimmest) | 1.4 | 5.8 | |
| 2 | 12.5 | 20.1 | |
| 3 | 31.6 | 22.4 | |
| 4 | 32.3 | 17.6 | |
| 5 | 16.4 | 15.3 | |
| 6 | 4.8 | 10.4 | |
| 7 | 1.1 | 4.8 | |
| 8 | 0.0 | 2.4 | |
| 9 | 0.0 | 1.1 | |
| Pictogram at the Time of Interview | |||
| 1 (slimmest) | 4.6 | 7.9 | <0.001 |
| 2 | 15.0 | 11.4 | |
| 3 | 22.4 | 16.5 | |
| 4 | 27.0 | 19.6 | |
| 5 | 20.6 | 20.3 | |
| 6 | 8.7 | 13.5 | |
| 7 | 1.6 | 6.7 | |
| 8 | 0.0 | 2.5 | |
| 9 | 0.0 | 1.6 | |
| Change in Pictogram from Age 15 to 30 | |||
| Major decrease (>2) | 0.7 | 5.4 | <0.001 |
| Slight decrease (≤2) | 13.7 | 19.3 | |
| No change | 29.9 | 19.9 | |
| Slight increase (≤2) | 51.7 | 45.2 | |
| Major increase (>2) | 4.1 | 10.2 | |
| Change in Pictogram from Age 30 to the Time of Interview | |||
| Major decrease (>2) | 4.4 | 9.9 | <0.001 |
| Slight decrease (≤2) | 23.6 | 19.8 | |
| No change | 33.3 | 19.5 | |
| Slight increase (≤2) | 35.8 | 36.4 | |
| Major increase (>2) | 2.9 | 14.4 | |
| Hypertension | |||
| Normotensive | 62.5 | 53.8 | <0.001 |
| Hypertensive | 37.5 | 46.2 | |
| Self-Reported Diabetes Mellitus | |||
| Non-diabetic | 94.8 | 91.8 | <0.001 |
| Diabetic | 5.2 | 8.2 | |
| Diabetes Duration (years) | |||
| 1–5 | 3.1 | 5.4 | <0.001 |
| 6–10 | 1.2 | 1.7 | |
| 11–20 | 0.7 | 0.9 | |
| ≥21 | 0.2 | 0.1 | |
Data in this table are baseline data, i.e. information at the time of enrollment in the study, except for tobacco use data, which are based on tobacco use before HD being diagnosed.
Comparison between men and women.
The total number of participants who reported a history of HD was 3050 (6.1%). Associations between anthropometric indices and physical activity with prevalent HD are shown in Table 2. In both men and women, BMI, WC, and WHR were associated with HD in both the unadjusted and adjusted analyses. Higher BMI was significantly associated with increased prevalence of HD. Women who were underweight also had an increased prevalence of HD, although this relationship was not apparent for men. Both men and women demonstrated a linear increase in HD prevalence with increasing WC and WHR. There was an inverse relationship between physical activity and HD for both men and women. Traditional CVD risk factors such as hypertension, diabetes, and cigarette smoking were associated with HD among men and women (results not shown). There was no evidence of interaction when physical activity was included in the statistical model (results not shown).
Table 2.
Association of anthropometric indices and physical activity with prevalent heart disease in Golestan Cohort Study.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HD Cases^ No. (%) N=1264 |
Controls^ No. (%) N=19964 |
Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
HD Cases^ No. (%) N=1786 |
Controls^ No. (%) N=27021 |
Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
|
| BMI | ||||||||
| <18.5 (underweight) | 62 (4.9) | 1195 (95.1) | 0.98 (0.75 – 1.29) | 0.91 (0.68 – 1.20) | 70 (6.1) | 1083 (93.9) | 1.37 (1.05 – 1.78) | 1.36 (1.03 – 1.78) |
| 18.5 to 24.9 (normal) | 480 (5.0) | 9132 (95.0) | Reference | Reference | 375 (4.5) | 7941 (95.5) | Reference | Reference |
| 25 to 29.9 (overweight) | 493 (6.8) | 6778 (93.2) | 1.38 (1.21 – 1.58) | 1.30 (1.13 – 1.49) | 606 (6.2) | 9095 (93.8) | 1.41 (1.24 – 1.61) | 1.32 (1.15 – 1.52) |
| ≥30 (obese) | 229 (7.4) | 2859 (92.6) | 1.52 (1.29 –
1.79) P trend: <0.001 |
1.37 (1.15 –
1.64) P trend: <0.001 |
736 (7.6) | 8901 (92.4) | 1.75 (1.54 –
1.99) P trend: <0.001 |
1.60 (1.40 –
1.84) P trend: <0.001 |
| Waist Circumference Quintiles (men; women) | ||||||||
| Q1 (<92.0cm; <92.0cm) | 191 (4.6) | 3928 (95.4) | Reference | Reference | 304 (5.5) | 5257 (94.5) | Reference | Reference |
| Q2 (92.0 – 95.9; 92.0 – 96.9cm) | 178 (4.8) | 3523 (95.2) | 1.04 (0.84 – 1.28) | 1.03 (0.83 – 1.27) | 291 (5.5) | 4977 (94.5) | 1.01 (0.86 – 1.19) | 1.06 (0.89 – 1.26) |
| Q3 (96.0 – 99.9; 97.0 – 101.9cm) | 245 (6.1) | 3793 (93.9) | 1.33 (1.09 – 1.61) | 1.34 (1.09 – 1.66) | 360 (6.0) | 5619 (94.0) | 1.11 (0.95 – 1.30) | 1.22 (1.04 – 1.44) |
| Q4 (100.0 – 104.9; 102.0 – 107.9cm) | 323 (6.7) | 4527 (93.3) | 1.47 (1.22 – 1.76) | 1.50 (1.23 – 1.83) | 375 (6.5) | 5365 (93.5) | 1.21 (1.03 – 1.41) | 1.37 (1.16 – 1.62) |
| Q5 (≥105.0cm; ≥108.0cm) | 325 (7.2) | 4185 (92.8) | 1.60 (1.32 –
1.92) P trend: <0.001 |
1.59 (1.30 –
1.95) P trend: <0.001 |
456 (7.3) | 5797 (92.7) | 1.36 (1.17 –
1.58) P trend: <0.001 |
1.48 (1.25 –
1.74) P trend: <0.001 |
| Waist to Hip Ratio Quintiles (men and women) | ||||||||
| Q1 (<0.88) | 156 (3.7) | 4051 (96.3) | Reference | Reference | 203 (3.7) | 5350 (96.3) | Reference | Reference |
| Q2 (0.88 – 0.93) | 190 (4.7) | 3859 (95.3) | 1.28 (1.03 – 1.59) | 1.20 (0.96 – 1.49) | 264 (4.6) | 5511 (95.4) | 1.26 (1.05 – 1.52) | 1.04 (0.86 – 1.27) |
| Q3 (0.94 – 0.97) | 242 (5.6) | 4118 (94.4) | 1.53 (1.24 – 1.87) | 1.31 (1.06 – 1.62) | 337 (6.0) | 5268 (94.0) | 1.68 (1.41 – 2.01) | 1.22 (1.02 – 1.47) |
| Q4 (0.98 – 1.02) | 309 (7.3) | 3910 (92.7) | 2.05 (1.68 – 2.50) | 1.61 (1.31 – 1.99) | 428 (7.3) | 5426 (92.7) | 2.08 (1.75 – 2.47) | 1.33 (1.11 – 1.59) |
| Q5 (>1.02) | 364 (8.3) | 4017 (91.7) | 2.35 (1.94 –
2.85) P trend: <0.001 |
1.55 (1.26 –
1.90) P trend: <0.001 |
554 (9.2) | 5456 (90.8) | 2.68 (2.27 –
3.16) P trend: <0.001 |
1.41 (1.18 –
1.68) P trend: <0.001 |
| Physical Activity a | ||||||||
| Irregular non-intense | 695 (9.2) | 6840 (90.8) | Reference | Reference | 1503 (6.5) | 21609 (93.5) | Reference | Reference |
| Regular non-intense | 413 (4.6) | 8664 (95.4) | 0.47 (0.41 – 0.53) | 0.59 (0.52 – 0.68) | 241 (5.4) | 4128 (94.6) | 0.82 (0.71 – 0.94) | 0.82 (0.70 – 0.96) |
| Irregular or regular intense | 153 (3.3) | 4429 (96.7) | 0.34 (0.28 –
0.41) P trend: <0.001 |
0.49 (0.41 –
0.60) P trend: <0.001 |
39 (3.5) | 1074 (96.5) | 0.52 (0.38 –
0.72) P trend: <0.001 |
0.72 (0.52 –
1.01) P trend: 0.002 |
Numbers may not add up to the total numbers due to missing data in some variables.
Adjusted for age, ethnicity, place of residence, education level, economic status, cigarette smoking, physical activity, hypertension, and self-reported diabetes.
Adjusted results were additionally adjusted for current BMI.
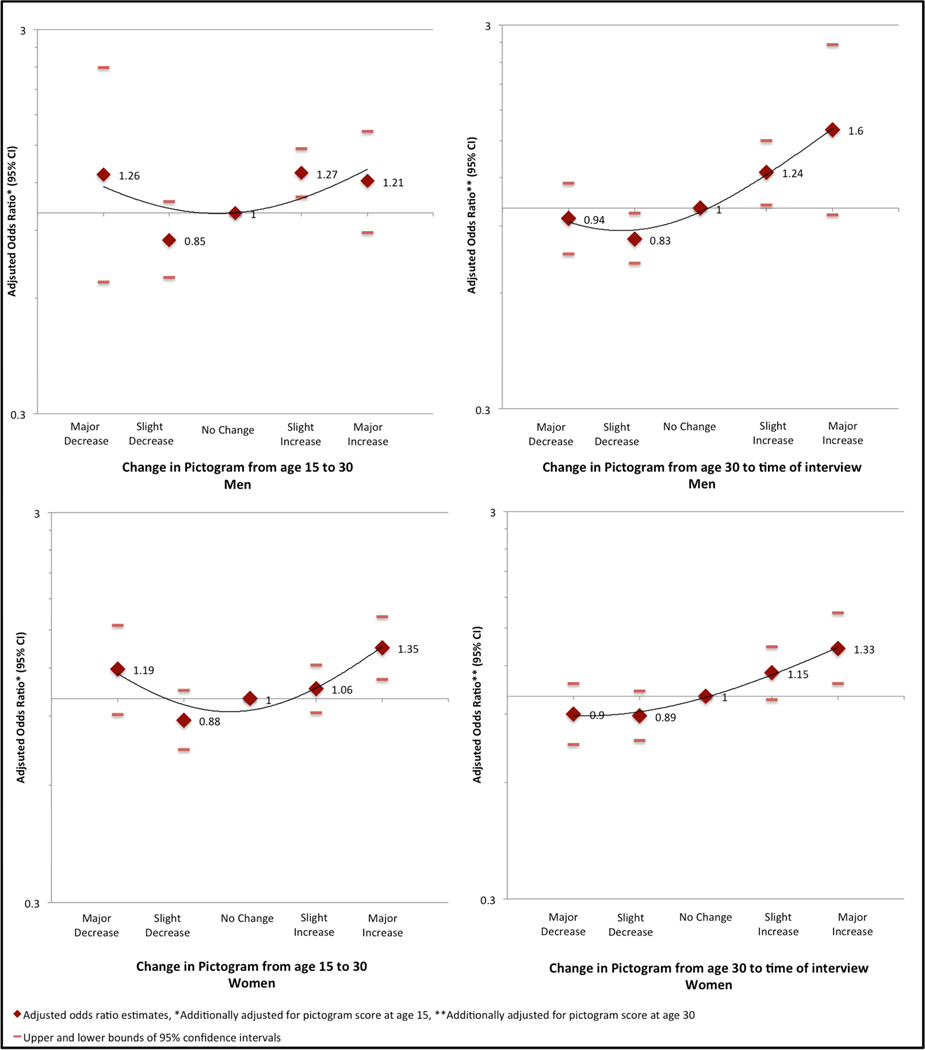
Associations of prevalent HD with body size perception and temporal change in body size perception are shown in Table 3. For men, body size perception at ages 15, 30, and at the time of interview had a U-shaped association with HD in the unadjusted analyses. In the adjusted analyses, the odds ratios were attenuated but the U-shaped relationships remained. For women, there was no association between body size perception at age 15 and prevalent HD. At age 30, associations between women’s body size perception and HD were noted with the slimmest size as well as heavier body size (images 6 and 7), but not the heaviest (images 8 and 9). However, the number of participants in the latter group was small. Body size perception for women at the time of interview demonstrated a positive association with HD prevalence (P for trend < 0.001). For men and women, change in body size perception from age 15 to 30 (controlling for body size perception at age 15) appeared to have a U-shaped relationship, with both major increase and major decrease associated with increased prevalence of HD (Figure 3, Table 3). The odds ratios, however, were not statistically significant except for women reporting a major increase. Change in body size perception from age 30 to the time of interview for both men and women (controlling for body size perception at age 30) had a J-shaped relationship with HD (Figure 3, Table 3). There was a significant positive association between HD prevalence and increase in body size perception from age 30 to the time of interview, but no significant association with decrease in body size perception during this same time period.
Table 3.
Association of body size perception and temporal change in body size perception with prevalent heart disease in Golestan Cohort Study.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HD Cases^ No. (%) N=1264 |
Controls^ No. (%) N=19964 |
Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
HD Cases^ No. (%) N=1786 |
Controls^ No. (%) N=27021 |
Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
|
| Pictogram at Age 15 | ||||||||
| 1 (slimmest) | 157 (7.3) | 1999 (92.7) | 1.41 (1.16 – 1.72) | 1.25 (1.02 – 1.53) | 563 (6.8) | 7708 (93.2) | 1.24 (1.06 – 1.47) | 1.08 (0.91 – 1.28) |
| 2 | 312 (5.7) | 5169 (94.3) | 1.09 (0.93 – 1.27) | 1.06 (0.90 – 1.24) | 299 (5.2) | 5478 (94.8) | 0.93 (0.78 – 1.17) | 0.91 (0.76 – 1.09) |
| 3 | 339 (5.3) | 6096 (94.7) | Reference | Reference | 212 (5.5) | 3626 (94.5) | Reference | Reference |
| 4 | 245 (6.0) | 3841 (94.0) | 1.15 (0.97 – 1.36) | 1.09 (0.92 – 1.30) | 147 (5.5) | 2534 (94.5) | 0.99 (0.80 – 1.23) | 0.97 (0.78 – 1.21) |
| 5 | 122 (6.4) | 1773 (93.6) | 1.24 (1.00 – 1.53) | 1.13 (0.91 – 1.41) | 184 (7.2) | 2357 (92.8) | 1.34 (1.09 – 1.64) | 1.20 (0.98 – 1.48) |
| 6 | 52 (6.7) | 726 (93.3) | 1.29 (0.95 – 1.74) | 1.00 (0.73 – 1.37) | 138 (6.3) | 2052 (93.7) | 1.15 (0.92 – 1.43) | 1.00 (0.80 – 1.26) |
| 7 | 33 (8.9) | 338 (91.1) | 1.76 (1.21 – 2.55) | 1.21 (0.82 – 1.79) | 79 (6.5) | 1134 (93.5) | 1.19 (0.91 – 1.55) | 0.96 (0.72 – 1.26) |
| 8 | - | - | - | - | 61 (6.9) | 829 (93.2) | 1.26 (0.94 – 1.69) | 0.88 (0.65 – 1.20) |
| 9 | - | - | - | - | 103 (7.4) | 1291 (92.6) | 1.36 (1.07 – 1.74) | 0.94 (0.73 – 1.21) |
| Pictogram at Age 30 | ||||||||
| 1 (slimmest) | 23 (7.7) | 275 (92.3) | 1.54 (1.00 – 2.40) | 1.50 (0.95 – 2.35) | 121 (7.2) | 1056 (92.8) | 1.41 (1.14 – 1.75) | 1.39 (1.12 – 1.72) |
| 2 | 143 (5.4) | 2507 (94.6) | 1.06 (0.86 – 1.29) | 1.03 (0.84 – 1.27) | 318 (5.5) | 5474 (94.5) | 1.06 (0.90 – 1.24) | 1.05 (0.89 – 1.23) |
| 3 | 344 (5.1) | 6367 (94.9) | Reference | Reference | 337 (5.2) | 6123 (94.8) | Reference | Reference |
| 4 | 375 (5.5) | 6465 (94.5) | 1.07 (0.92 – 1.25) | 1.02 (0.87 – 1.19) | 285 (5.6) | 4794 (94.4) | 1.08 (0.92 – 1.27) | 1.07 (0.91 – 1.27) |
| 5 | 267 (7.7) | 3205 (92.3) | 1.54 (1.31 – 1.82) | 1.26 (1.07 – 1.50) | 279 (6.4) | 4116 (93.6) | 1.23 (1.05 – 1.45) | 1.15 (0.97 – 1.36) |
| 6 | 83 (8.2) | 929 (91.8) | 1.65 (1.29 – 2.12) | 1.14 (0.90 – 1.49) | 237 (7.9) | 2760 (92.1) | 1.56 (1.31 – 1.85) | 1.28 (1.07 – 1.53) |
| 7 | 25 (11.3) | 197 (88.7) | 2.35 (1.53 – 3.61) | 1.41 (0.90 – 2.20) | 121 (8.7) | 1270 (91.3) | 1.73 (1.39 – 2.15) | 1.23 (0.99 – 1.54) |
| 8 | - | - | - | - | 61 (8.9) | 622 (91.1) | 1.78 (1.34 – 2.37) | 1.17 (0.87 – 1.57) |
| 9 | - | - | - | - | 27 (8.4) | 295 (91.6) | 1.66 (1.10 – 2.50) | 0.99 (0.65 – 1.51) |
| Pictogram at the Time of Interview | ||||||||
| 1 (slimmest) | 64 (6.5) | 920 (93.5) | 1.34 (1.01 – 1.78) | 1.08 (0.81 – 1.45) | 153 (6.7) | 2119 (93.3) | 1.23 (1.00 – 1.51) | 1.02 (0.83 – 1.27) |
| 2 | 159 (5.0) | 3028 (95.0) | 1.01 (0.82 – 1.24) | 0.94 (0.76 – 1.17) | 162 (4.9) | 3119 (95.1) | 0.88 (0.72 – 1.08) | 0.81 (0.66 – 0.99) |
| 3 | 235 (4.9) | 4524 (95.1) | Reference | Reference | 263 (5.5) | 4486 (94.5) | Reference | Reference |
| 4 | 341 (6.0) | 5388 (94.1) | 1.22 (1.03 – 1.45) | 1.18 (0.99 – 1.41) | 299 (5.3) | 5343 (94.7) | 0.95 (0.80 – 1.13) | 0.94 (0.79 – 1.12) |
| 5 | 278 (6.3) | 4087 (93.6) | 1.31 (1.09 – 1.57) | 1.14 (0.95 – 1.38) | 359 (6.2) | 5465 (93.8) | 1.12 (0.95 – 1.32) | 1.06 (0.89 – 1.25) |
| 6 | 146 (7.9) | 1694 (92.1) | 1.66 (1.34 – 2.05) | 1.32 (1.06 – 1.65) | 285 (7.3) | 3616 (92.7) | 1.34 (1.13 – 1.60) | 1.20 (1.01 – 1.44) |
| 7 | 36 (10.6) | 302(89.4) | 2.29 (1.58 – 3.32) | 1.51 (1.03 – 2.22) | 148 (7.7) | 1785 (92.3) | 1.32 (1.15 – 1.74) | 1.18 (0.95 – 1.46) |
| 8 | - | - | - | - | 67 (9.2) | 660 (90.8)) | 1.73 (1.31 – 2.29) | 1.31 (0.98 – 1.75) |
| 9 | - | - | - | - | 50 (10.7) | 417 (89.3) | 2.05 (1.49 – 2.81) | 1.36 (0.98 – 1.90) |
| Change in Pictogram from Age 15 to 30 c | ||||||||
| Major decrease (>2) | 13 (9.1) | 130 (90.9) | 1.86 (1.03 – 3.32) | 1.26 (0.66 – 2.39) | 112 (7.2) | 1438 (92.8) | 1.24 (0.99 – 1.55) | 1.19 (0.91 – 1.54) |
| Slight decrease (≤2) | 138 (4.8) | 2768 (95.2) | 0.92 (0.75 – 1.14) | 0.85 (0.68 – 1.07) | 295 (5.3) | 5269 (94.7) | 0.89 (0.76 – 1.05) | 0.88 (0.74 – 1.05) |
| No change | 324 (5.1) | 6009 (94.9) | Reference | Reference | 339 (5.9) | 5400 (94.1) | Reference | Reference |
| Slight increase (≤2) | 715 (6.5) | 10239 (93.5) | 1.29 (1.13 – 1.48) | 1.27 (1.10 – 1.47) | 791 (6.1) | 12209 (93.9) | 1.03 (0.91 – 1.18) | 1.06 (0.92 – 1.22) |
| Major increase (>2) | 68 (7.9) | 795 (92.1) | 1.60 (1.21 – 2.08) | 1.21 (0.89 – 1.63) | 249 (8.5) | 2693 (91.5) | 1.47 (1.24 – 1.74) | 1.35 (1.11 – 1.62) |
| Change in Pictogram from Age 30 to Time of Interview d | ||||||||
| Major decrease (>2) | 85 (9.0) | 856 (91.0) | 2.24 (1.74 – 2.88) | 0.94 (0.76 – 1.16) | 232 (8.1) | 2627 (91.9) | 1.76 (1.47 – 2.12) | 0.90 (0.75 – 1.08) |
| Slight decrease (≤2) | 321 (6.4) | 4676 (93.6) | 1.55 (1.32 – 1.82) | 0.83 (0.72 – 0.97) | 361 (6.4) | 5325 (93.6) | 1.36 (1.15 – 1.59) | 0.89 (0.77 – 1.03) |
| No change | 299 (4.2) | 6760 (95.8) | Reference | Reference | 268 (4.8) | 5352 (95.2) | Reference | Reference |
| Slight increase (≤2) | 494 (6.5) | 7090 (93.5) | 1.58 (1.36 – 1.82) | 1.24 (1.02 – 1.50) | 583 (5.6) | 9886 (94.4) | 1.18 (1.02 – 1.37) | 1.15 (0.98 – 1.34) |
| Major increase (>2) | 58 (9.4) | 557 (90.6) | 2.35 (1.75 – 3.16) | 1.60 (0.96 – 2.67) | 342 (8.2) | 3818 (91.8) | 1.79 (1.52 – 2.11) | 1.33 (1.08 – 1.64) |
Numbers may not add up to the total numbers due to missing data in some variables.
Adjusted for age, ethnicity, place of residence, education level, economic status, cigarette smoking, physical activity, hypertension, and self-reported diabetes.
Adjusted results were additionally adjusted for the pictogram score at age 15.
Adjusted results were additionally adjusted for the pictogram score at age 30.
Figure 3.
Association between prevalent heart disease and change in body size perception over time for men and women.
Controlling for current BMI (at the time of interview) attenuated the association for body size perception and temporal change in perception with HD but did not change the above patterns (Supplementary Table).
Discussion
We report a positive association between anthropometric indices—BMI, WC, and WHR—and prevalent HD for both men and women from Golestan, Iran. In addition, underweight women were more likely to have HD. Notably, we further report a U-shaped relationship between HD prevalence and change in body size perception between adolescence and early adulthood for both men and women. The association between change in body size perception between early and late adulthood and HD was J-shaped for men and women. Traditional CVD risk factors such as hypertension, diabetes mellitus, cigarette smoking, and physical inactivity were associated with HD prevalence among men and women.
The link between obesity and CVD has been previously affirmed [1,12–14]. Our study further supports this association, demonstrating increasing prevalence of HD with higher BMI, WC, and WHR. While several investigators have reported a monotonic positive relationship between obesity and CVD, there have been other more recent reports that indicate a U-shaped relationship between BMI and CVD mortality [1,15–19,22,31–34]. A large pooled analysis was conducted by the Prospective Studies Collaboration, with cohorts mostly from western Europe and North America, demonstrating a U-shaped relationship between BMI and all-cause mortality, but more of a linear relationship with cardiovascular mortality [33]. Another study assessed different communities from Asia—Chinese, Japanese, Korean, Indian, and Bangladeshi—and concluded a greater risk of all-cause mortality for both extremes of low and high BMI for East Asians [15]. Regional differences were evident in this study as they found that South Asians (Indian and Bangladeshi) had a higher mortality risk associated only with lower BMI. Additional investigations have shown a J-shaped relationship between BMI and mortality, either from ischemic stroke or all-cause mortality [17,22]. In contrast to other reported findings, an inverse U-shaped relationship between BMI and CAD was noted in one study evaluating African Americans undergoing cardiac catheterization, suggesting that being overweight may increase the risk of CAD compared to lean or obese [34].
The majority of investigations evaluating BMI with mortality or CAD have focused on a single BMI measurement. Relatively few studies have investigated the impact of changes in BMI over time—in particular, the lifelong burden of obesity, being underweight, or drastic changes toward either end of the BMI spectrum and later development of CVD. A pooled analysis of four prospective cohort studies showed that a longer duration of obesity starting from childhood portends increased CVD risk as an adult [35]. Yet the risk of CVD was diminished, equaling that of adults who were never obese, for individuals who were obese at childhood but had a normal BMI in adulthood. Conversely, obese adults were at significantly higher risk of hypertension, diabetes, and dyslipidemia independent of childhood weight. A recent German study demonstrated a U-shaped relationship between BMI and mortality and a J-shaped relationship between BMI and occupational disability, with the associations being stronger when accounting for changes in BMI over time [24]. These cohorts were from high-income countries and individuals from each cohort were mostly white, possibly limiting generalizability to LMICs or different ethnic backgrounds. Others have reported an incremental, putatively linear, increase in CVD prevalence and mortality with increasing BMI over time [19–21]. A few studies have shown inconclusive or contradictory results [23, 31].
In our study, we utilized a proxy measure for BMI—body size perception—at younger ages in order to evaluate the association between body habitus at different periods of life, as well temporal change of body habitus, and prevalence of HD. The Nurses’ Health Study employed similar methods, investigating the risk of developing type 2 diabetes in adulthood using pictogram identification for body fatness at younger ages as a proxy for childhood obesity [36]. This study revealed that increasing body size in childhood is associated with greater risk of diabetes in adulthood. Similar to previous studies on changes in BMI over time and HD, the study population was based in a high-income country.
To our knowledge, our results demonstrate the first description of a U-shaped or J-shaped relationship between temporal change in body size perception and prevalent HD in a LMIC population. In particular, there appears to be a different relationship between HD and body size perception earlier in life versus later in adulthood for men and women. The J-shaped relationship between HD and change in body size perception from early and late adulthood supports the concept of cumulative overweight and prolonged exposure to metabolic abnormalities, especially during the adult years. It is possible that the age range during which adiposity increases may be an important factor in determining subsequent risk of CVD [37,38]. These results, if confirmed in prospective studies, would have substantial implications for developing life course- and gender-specific strategies to promote cardiovascular health in LMICs both at the individual and population level.
Strengths of this study include a large sample size of the adult population, including both rural and urban inhabitants. Second, current anthropometric indices were measured by trained staff and not obtained through self-report. In addition, baseline face-to-face interviews were conducted allowing for greater participation from individuals with lower education levels.
There are several limitations to consider. First, we used self-reported history of HD rather than an objective assessment. Since previous Iranian studies have reported HD prevalence rates of greater than 13%, our use of HD by self-report might have underestimated HD prevalence [39]. Unless there was differential under-reporting according to anthropometric measure categories, this should not lead to systematic bias in our reported estimates of association. In addition, our study is limited by a lack of specificity in our assessment of HD. By design in the GCS, data on IHD and heart failure (HF) were collected as a combined variable since it was expected that a substantial proportion of study participants, particularly from rural areas, would not be able to distinguish between IHD and HF when asked about cardiac history. We therefore used HD as a combined variable of IHD and HF. In the few available reports from Iran, HF has been associated with IHD in 60–65% of admitted patients, with similar proportions observed in studies from adjacent countries (40–60%) [40–43]. In addition, the most recent available national mortality data (2004) indicate that IHD was the cause of 21.8% of all mortality cases in Iran, approximately 7-times more than hypertensive heart disease (which included heart failure) [44]. Therefore, we believe that IHD is likely far more common than HF in this study, and a considerable proportion of HF patients also had IHD.
Another limitation is the problem of reverse causation; it is possible that individuals with HD may subsequently change anthropometric indices. The impact of this limitation is less likely for the temporal change in body size perception at ages 15 and 30, as HD in young people is uncommon, and none of the HD cases in our study were first diagnosed at the age of 30 or earlier (data not shown) [45]. In addition, we did not further adjust for other medical comorbidities, which may influence the relationship between anthropometric indices, especially at lower BMI, and prevalence of HD. The analysis may have also been limited by the lack of availability of lipid levels for each individual. Another limitation involves the use of pictograms as surrogate markers of BMI at younger ages instead of an objective measurement of BMI. While the use of pictograms is a validated study tool for estimates of current BMI [28, 29], it has not been validated for objective measurements of BMI earlier in life, and therefore may be less accurate when representing anthropometric indices earlier in life due to recall error or bias. Finally, body size perception could reflect either accurate assessment of BMI or a psychological judgment, and there may be greater variability between different ethnic backgrounds pertaining to body shape perception and social definitions of beauty. These may affect the accuracy of self-assessments via pictograms at any age, which could potentially lead to differential misclassification and bias [46]. However, we adjusted our results for ethnicity, so any major effect of such variations on the observed associations is unlikely.
Conclusions
We found a significant association between BMI, WC, and WHR with prevalent HD. Among men, there was a U-shaped relationship between HD and body size perception at younger ages. For change in body size perception over time for men and women, there was a U-shaped relationship with HD from adolescence to early adulthood and a J-shaped pattern from early to late adulthood. Overweight and obesity appears to be a major contributor to the increase in prevalence of HD in LMICs. In addition, attention must be directed at men and women who are underweight to examine the factors that may contribute to poor nutritional status and increased CVD risk. Public health interventions focused on decreasing the cumulative burden of risk factors over a lifetime on cardiovascular morbidity and mortality may be an important component of cardiovascular risk reduction, particularly in LMICs.
Supplementary Material
We present a cross-sectional analysis of baseline data from a prospective study of adults in Golestan Province, Iran to investigate the relationship between prevalent heart disease (HD) and current anthropometric indices and body size perception over time from adolescence to adulthood in Iran.
Body size perception for age 15, age 30, and at the time of interview was assessed via pictograms.
For men and women, higher BMI, WC, and WHR were associated with HD.
Men had a U-shaped relationship between HD and body size perception at younger ages.
For change in body size perception, men and women demonstrated a U-shaped relationship with prevalent HD from adolescence to early adulthood, but a J-shaped pattern from early to late adulthood.
Acknowledgements
We would like to thank Goharshad Goglani, Behrooz Abaie, Dariush Nasrollahzadeh, Haji-amin Marjani, Ali Yoonessi, Mohsen Sadatsafavi, Ramin Shakeri, Alireza Sadjadi, Amir Sharifi, and the Golestan Cohort Study Center staff from the Digestive Disease Research Center of Tehran University of Medical Sciences for their dedicated work. Also, we thank the Golestan University of Medical Sciences, Gorgan, Iran and the local health networks and health workers (Behvarzes) in the study area for their assistance in recruitment of participants.
Grant Support: The Golestan Cohort Study was supported by Tehran University of Medical Sciences (grant No: 81/15), Cancer Research UK (grant No: C20/A5860), the Intramural Research Program of the US National Cancer Institute, and International Agency for Research on Cancer. RV is supported by the Fogarty International Center of the National Institutes of Health under Award Number K01 TW 009218 - 03. The content is solely the responsibility of the authors and the funding sources had no role in the design, conduct, statistical analysis and interpretation of results, or writing of the manuscript.
Abbreviations
- GCS
Golestan Cohort Study
- LMICs
Low- and middle-income countries
- HD
heart disease
- IHD
ischemic heart disease
- BMI
body mass index
- WC
waist circumference
- WHR
waist to hip ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors do not have any competing financial interests or other conflicts to disclose.
Contributor Information
Farhad Islami, Email: islamif@gmail.com.
Akram Pourshams, Email: akrampourshams@gmail.com.
Hossein Poutschi, Email: h.poustchi@gmail.com.
Hooman Khademi, Email: khademih@fellows.iarc.fr.
Mohammad Naeimi, Email: mohammadnaeimi@yahoo.com.
Akbar Fazel-Tabar Malekshah, Email: afmalekshah@yahoo.com.
Elham Jafari, Email: jafari_85120@yahoo.com.
Rasool Salahi, Email: salahi_md@yahoo.com.
Farin Kamangar, Email: kamangaf@mail.nih.gov.
Arash Etemadi, Email: arash.etemadi@nih.gov.
Paul D. Pharoah, Email: paul1@srl.cam.ac.uk.
Christian C. Abnet, Email: abnetc@mail.nih.gov.
Paul Brennan, Email: brennan@iarc.fr.
Sanford M. Dawsey, Email: dawseys@dcpcepn.nci.nih.gov.
Valentin Fuster, Email: valentin.fuster@mountsinai.org.
Paolo Boffetta, Email: paolo.boffetta@mssm.edu.
Reza Malekzadeh, Email: dr.reza.malekzadeh@gmail.com.
References
- 1.Gaziano TA, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low-and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vedanthan R, Fuster V. Disease Prevention: The moving target of global cardiovascular health. Nat Rev Cardiol. 2009;6(5):327–328. doi: 10.1038/nrcardio.2009.48. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization; 2008. The global burden of disease: 2004 update. [Google Scholar]
- 4.Fuster V, Kelly BB, Vedanthan R. Global cardiovascular health: Urgent need for an intersectoral approach. J Am Coll Cardiol. 2011;58(12):1208–1210. doi: 10.1016/j.jacc.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 8.Nabipour I, Amiri M, Imami SR, et al. The metabolic syndrome and nonfatal ischemic heart disease, a population-based study. Int J Cardiol. 2007;118(1):48–53. doi: 10.1016/j.ijcard.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nozha MM, Arafah MR, Al-Mazrou YY, et al. Coronary artery disease in Saudi Arabia. Saudi Med J. 2004;25(9):1165–1171. [PubMed] [Google Scholar]
- 10.Farzadfar F, Murray C, Gakidou E, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: An observational study. Lancet. 2011;6736(11):61349–61344. doi: 10.1016/S0140-6736(11)61349-4. [DOI] [PubMed] [Google Scholar]
- 11.Hatmi ZN, Tahvildari S, Gafarzadeh Motlag A, et al. Prevalence of coronary artery disease risk factors in Iran: a population based survey. BMC Cardiovasc Disord. 2007;7:32. doi: 10.1186/1471-2261-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrami H, Sadatsafavi M, Pourshams A, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6:158. doi: 10.1186/1471-2458-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benderly M, Boyko V, Goldbourt U. Relation of body mass index to mortality among men with coronary heart disease. Am J Cardiol. 2010;106(3):297–304. doi: 10.1016/j.amjcard.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Funada S, Shimazu T, Kakizaki M, et al. Body mass index and cardiovascular disease mortality in Japan: The Ohsaki Study. Prev Med. 2008;47(1):66–70. doi: 10.1016/j.ypmed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Copeland W, Vedanthan R, et al. Association between body-mass index and cardiovascular disease mortality in East Asians and South Asians: A pooled analysis from the Asia Cohort Consortium. BMJ. 2013 doi: 10.1136/bmj.f5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365(10):901–908. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunnell DJ, Frankel SJ, Nanchahal K, et al. Childhood obesity and adult cardiovascular mortality: A 57-y follow-up study based on the Boyd Orr Cohort. Am J Clin Nutr. 1998;67(6):1111–1118. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- 22.Kivimaki M, Ferrie JE, Batty GD, et al. Optimal form of operationalizing BMI in relation to all-cause and cause-specific mortality: The original Whitehall Study. Obesity (Silver Spring) 2008;16(8):1926–1932. doi: 10.1038/oby.2008.322. [DOI] [PubMed] [Google Scholar]
- 23.Corrada MM, Kawas CH, Mozaffar F, et al. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claessen H, Brenner H, Drath C, et al. Repeated measures of body mass index and risk of health related outcomes. Eur J Epidemiol. 2012;27(3):215–224. doi: 10.1007/s10654-012-9669-7. [DOI] [PubMed] [Google Scholar]
- 25.Pourshams A, Khademi H, Malekshah AF, et al. Cohort profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39(1):52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socioeconomic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Geneva: 2008. Dec 8–11, Waist circumference and waist–hip ratio: report of a WHO expert consultation. [Google Scholar]
- 28.Stunkard AJ, Sorensen T, Schulsinger F. Use of a Danish Adoption Register for the study of obesity and thinness. Gen of Neuro and Psych Disorders. 1983:115–120. [PubMed] [Google Scholar]
- 29.Keshtkar AA, Semnani S, Pourshams A, et al. Pictogram use was validated for estimating individual's body mass index. J Clin Epidemiol. 2010;63(6):655–659. doi: 10.1016/j.jclinepi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:120. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 31.Jee SH, Pastor-Barriuso R, Appel LJ, et al. Body mass index and incident ischemic heart disease in south korean men and women. Am J Epidemiol. 2005;162(1):42–48. doi: 10.1093/aje/kwi166. [DOI] [PubMed] [Google Scholar]
- 32.Flint AJ, Rexrode KM, Hu FB, et al. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes Res Clin Pract. 2010;4(3):e171–e181. doi: 10.1016/j.orcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitlock G, LEwington S, Sherlicker P, et al. Body mass index and cause specific mortality in 900,000 adults: collaborate analysis of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams-Campbell LL, Peniston RL, Kim KS, et al. Body mass index and coronary artery disease in African-Americans. Obes Res. 1995;3(3):215–219. doi: 10.1002/j.1550-8528.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 36.Yeung EH, Zhang C, Louis GM, et al. Childhood size and life course weight characteristics in association with the risk of incident type 2 diabetes. Diabetes Care. 2010;33(6):1364–1369. doi: 10.2337/dc10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charakida M, Khan T, Johnson W, et al. Lifelong patterns of BMI and cardiovascular phenotype in individuals aged 60–64 years in the 1946 British birth cohort study: an epidemiological study. Lancet Diabetes Endocrinol. 2014 May 20;(14):70103–70102. doi: 10.1016/S2213-8587(14)70103-2. [DOI] [PubMed] [Google Scholar]
- 38.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;(3):280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelishadi R, Alikhani S, Delavari A, et al. Obesity and associated lifestyle behaviours in Iran: Findings from the first national non-communicable disease risk factor surveillance survey. Public Health Nutr. 2008;11(3):246–251. doi: 10.1017/S1368980007000262. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal AK, Venugopalan P, de Bono D. Prevalence and aetiology of heart failure in an Arab population. Eur J Heart Fail. 2001;3:301–305. doi: 10.1016/s1388-9842(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 41.Ergin A, Eryol NK, Unal S, et al. Epidemiological and pharmacological profile of congestive heart failure at Turkish academic hospitals. Anadolu Kardiyol Derg. 2004;4:32–38. [PubMed] [Google Scholar]
- 42.Khan H, Jan H, Hafizullah M. A hospital-based study on causes peculiar to heart failure. J Tehran Univ Heart Centr. 2009;1:25–28. [Google Scholar]
- 43.Al Habib KF, Elasfar AA, Al Backr H, et al. Design and preliminary results of the heart function assessment registry trial in Saudi Arabia (HEARTS) in patients with acute and chronic heart failure. Eur J Heart Fail. 2011;13:1178–1184. doi: 10.1093/eurjhf/hfr111. [DOI] [PubMed] [Google Scholar]
- 44.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: A science advisory from the American heart Association. Circulation. 2009;119(25):3263–3271. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 46.Field AE, Franko DL, Striegel-Moore RH, et al. Race differences in accuracy of self-reported childhood body size among white and black women. Obes Res. 2004;12(7):1136–1144. doi: 10.1038/oby.2004.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.