Abstract
Objectives
Precise characterization of cognitive outcomes and factors that contribute to cognitive variability will enable better understanding of disease progression and treatment effects in mucopolysaccharidosis type I (MPS I). We examined the effects on cognition of phenotype, genotype, age at evaluation and first treatment, and somatic disease burden.
Methods
Sixty patients with severe MPS IH (Hurler syndrome treated with hematopoietic cell transplant and 29 with attenuated MPS I treated with enzyme replacement therapy), were studied with IQ measures, medical history, genotypes. Sixty-seven patients had volumetric MRI. Subjects were grouped by age and phenotype and MRI and compared to 96 normal controls.
Results
Prior to hematopoietic cell transplant, MPS IH patients were all cognitively average, but post-transplant, 59% were below average, but stable. Genotype and age at HCT were associated with cognitive ability. In attenuated MPS I, 40% were below average with genotype and somatic disease burden predicting their cognitive ability. White matter volumes were associated with IQ for controls, but not for MPS I. Gray matter volumes were positively associated with IQ in controls and attenuated MPS I patients, but negatively associated in MPS IH.
Conclusions
Cognitive impairment, a major difficulty for many MPS I patients, is associated with genotype, age at treatment and somatic disease burden. IQ association with white matter differed from controls. Many attenuated MPS patients have significant physical and/or cognitive problems and receive insufficient support services. Results provide direction for future clinical trials and better disease management.
1. Introduction
We present results from a comprehensive multicenter study of cognitive function covering the spectrum of severity in mucopolysaccharidosis type I (MPS I) using a uniform testing protocol with associated neuroimaging volumes. As the severity of MPS I is usually defined on the basis of cognitive deficits, effective treatment of patients with severe disease must access the brain. To date, only hematopoietic cell transplantation (HCT) has demonstrated benefit to the brain, however, emerging new treatments may be effective such as intrathecal enzyme administration, substrate reduction drugs, chaperone molecules, and gene therapies. The goal of this study is to provide knowledge to guide future clinical trials and disease management. Defining age-related changes in cognition and neuroimaging will benefit clinical trials by providing comparisons based on current standard of care. Furthermore, such knowledge will help physicians and health care providers to better counsel parents on long-term outcomes of treatments and those interventions that may provide benefit.
MPS I is an autosomal recessive error of lysosomal glycosaminoglycan catabolism resulting from deficient α-L-iduronidase enzyme activity, and the consequent accumulation of heparan and dermatan sulfate [1]. Damage to multiple organs results from primary storage and secondary pathogenic cascades, occurring across the full spectrum of disease severity. For severe MPS I (Hurler syndrome, MPS IH), HCT prevents progressive physical and cognitive decline and death [1]. Data are sparse regarding rate of decline in untreated patients as HCT has become the standard of care [2].
As the attenuated forms, Hurler-Scheie and Scheie syndrome, are a spectrum [3] without clearly distinguishing diagnostic definitions [1], we combined them as MPS Iatt. MPS Iatt has a later onset than MPS IH, a longer life span, and variable effect on brain [1,3]. Recently, an MPS Iatt genotype was described in a group of patients with distinctive severe cognitive and behavioral impairment [4]. The treatment standard for MPS Iatt is enzyme replacement therapy (ERT) [1], although it does not cross the blood-brain barrier to adequately treat cognitive dysfunction [5].
Cognitive ability is a functional marker used to identify brain disease severity in MPS I [6,7], which impacts long-term outcomes including educational achievement, life skills, and quality-of-life. However, for cognitive data to be a precise measurement of disease progression and treatment outcome, rigorous assessment guidelines must be followed [8]. With careful control over assessment tools and examiners, our goals were to characterize the degree of impairment in treated MPS I and identify factors associated with outcome, provide comparative historical cognitive data regarding untransplanted MPS IH children, and explore the association of cognitive ability with brain volumes. We hypothesized that cognitive outcomes will vary by disease phenotype and genotype, age at evaluation, cumulative somatic disease burden, the age at first treatment, and correlate with white and gray matter volumes.
2. Materials and methods
2.1. Subjects
Ninety-two subjects with MPS I from 5 centers were enrolled in “Longitudinal Studies of Brain Structure and Function in MPS Disorders” NCT01870375 of the Lysosomal Disease Network (Rare Disease Clinical Research Network-RDCRN). Inclusion criterion was documented MPS I by enzyme assay or genotyping. Exclusion criterion was noncompliance with neuropsychological testing. Two patients who had invalid testing due to behavioral noncompliance and one with MPS IH, treated with ERT only, were excluded.
Cross-sectional data were analyzed from initial visits or if imaging was unavailable for that visit, a subsequent visit. Eighty-nine had cognitive and medical data; 67 had analyzable neuroimaging data; all were collected within a three month window. All centers had IRB approval. Consents were signed at each institution including permission to share de-identified data with the RDCRN Data Monitoring and Coordination Center and the University of Minnesota for analysis.
Comparative historical cognitive data were collected from medical records of 22 MPS IH patients, all deceased with known dates of death, without any treatment, evaluated between 1985 and 1995 in an NINDS-supported study of HCT outcomes. Genotyping and MRI data were unavailable.
Patients were categorized by age at visit and disease phenotype. For the MPS IH patients <2 years of age, data prior to HCT after 12 weeks of ERT were used. Because of rapid brain maturation during the period of ages 2 to 5 [8,9], and the use of different tests after age 6, all patients were categorized into two age groups: ages 2-<6 and ≥6-25. Four MPS Iatt patients (all diagnosed with Scheie syndrome) ≥ age 25 were included for descriptive purposes only as no comparable MPS IH or control groups were available.
Three separate IRB-approved studies provided normal control subjects with cognitive and volumetric MRI data acquired with comparable sequences. The control sample included 34 4-8 year olds and 62 10-24 year olds.
2.2 Procedures
2.2.1 Cognitive testing
Table 1 describes the measures. Three patients had incomplete IQ testing; an IQ estimate was made by pro-rating completed subtests. Note that the term IQ is used to denote cognitive ability scores from all tests used.
Table 1.
Summary of cognitive ability tests used by age groupings
| Tests used in current study | Age Range | Number | Scores reported |
|---|---|---|---|
| Mullen Scales of Early Learning (MSEL) [11] | <4 years | 31 | Early Learning Composite (ELC) a Expressive Languageb Visual Receptionb |
| Wechsler Preschool and Primary Scale of Intelligence - III (WPPSI-III) [12] | 4-<6 years | 8c | Full Scale IQ Verbal IQ Performance IQ |
| Wechsler Abbreviated Scale of Intelligence (WASI) [13] | ≥6 years | 50d | Full Scale IQ Verbal IQ Performance IQ |
| Tests for historical controls | |||
| Bayley Scales of Infant Development, first edition [14] | 7-41 months plus 3 older impaired patients (50, 60, 91 months) | 21 | Mental Development Index or Developmental Quotient for older impaired patients (ratio of age equivalent to chronological age)a |
| Stanford Binet Intelligence Scale Fourth Edition. [15] | 46 months | 1 | Composite Scorea |
Proxy for IQ
Proxy for Verbal and Performance IQs
5 year olds had the WPPSI
Out of level testing: One child had the WPPSI and another the MSEL
2.2.2 Medical/treatment history
Caretaker interview and medical records provided medical and treatment history. Data were collated into the MPS Physical Symptom Scale (PSS), designed to reflect somatic disease burden, and is in the process of validation [16]. The PSS summary score is based on 4 domains (skeletal/orthopedic, vision, hearing, cardiorespiratory), enumeration of surgeries, and the presence of hydrocephalus and/or cord compression.
Age at evaluation, age at first treatment and genotype were collected. Genotypes (Supplementary Table 1) for the MPS IH patients were categorized as severe (homozygous for severe mutations, W402X or Q70X or deletions) or mild (one or more mutations previously associated with the attenuated phenotype). Three MPS IH patients for whom a second mutation could not be found also were categorized as mild. In the MPS Iatt group, patients were classified based on the presence of the L238Q mutation, a severe Hurler-Scheie form [4]. All MPS Iatt patients had one or two missense mutations. Additionally, we collected frequency and percents of school age children (ages 6-25) who received special services, including special education, speech therapy, physical therapy, occupational therapy, and behavioral therapies.
2.2.3 MRIs
All MRIs were acquired on 3 Tesla scanners with prescribed sequences established by our center and adjusted for either Siemens (Trio or Skyra) or Phillips scanners. For young patients requiring anesthesia, clinical scans using the same sequences were utilized with IRB permission. Each center provided quality-control scans to determine whether the scan sequences were acceptable for analysis or needed adjustment. Volumes were determined by FreeSurfer Image Analysis Suite version 5.3 from MPRAGE (magnetization-prepared rapid acquisition with gradient echo) sequences [17]. Due to young age of some subjects and structural abnormality causing the automated parcellation to fail, many scans were manually adjusted and re-run. Total gray and white matter volumes were calculated. In 13 patients < 2, and 9 patients > 2 years of age, volumetric analysis failed because of insufficient gray-white differentiation or no scan was available.
2.3 Statistical analysis
Descriptive statistics were tabulated separately for different age groups among MPS IH and MPS Iatt as well as pre-transplant MPS IH patients. These included the mean and standard deviation for continuous variables and frequency for categorical variables. P-values for differences in means across multiple groups in IQ and volumes were based on an F test while pair-wise comparisons were evaluated using a t-test with unequal variance and Welch degrees of freedom. First order linear trends were based on least squares simple regression estimates. Adjusted analyses for IQ were evaluated separately for MPS IH and MPS Iatt groups based on linear regression and the t-distribution with corresponding model degrees of freedom for confidence intervals and P-values. All analyses were conducted using R v2.15.2 [18].
3. Results
3.1 Descriptive information regarding the subsamples can be found in Table 2
Table 2.
Descriptive data: values are mean (SD) or N (%) where indicated.
| Group | Historical controls | Pre-HCT≤2 | MPSIH 2-<6 | MPSIH ≥6-25 | MPSIatt <6 | MPSIatt ≥6-25 | MPSIatt >25 |
|---|---|---|---|---|---|---|---|
| Treatment | None | ERT | HCT | HCT | ERT | ERT | ERT |
| N | (N=22) | (N=14) | (N=19) | (N=27) | (N=4) | (N=21) | (N=4) |
| Female | 11 (50.0%) | 10 (71.4%) | 9 (47.4%) | 14 (51.9%) | 2 (50.0%) | 10 (47.6%) | 3 (75.0%) |
| Male | 11 (50%) | 4 (28.6%) | 10 (52.6%) | 13 (48.1%) | 2 (50.0%) | 11 (52.4%) | 1 (25.0%) |
| Age at diagnosis (years) (4 missing) | n.a. | 0.63 (0.38) | 1.05 (0.57) | 0.95 (0.70) | 2.44 (0.42) | 5.28 (3.29) | 21.75 (7.81) |
| Age at first treatment (years) | n.a. | 1.13 (0.54) | 1.62 (0.67) | 1.38 (0.79) | 2.52 (0.94) | 9.06 (4.32) | 33.58 (10.22) |
| Age at evaluation (years) | 2.28 (1.44) | 1.02 (0.54) | 3.83 (1.13) | 11.45 (4.04) | 4.29 (0.78) | 15.51 (5.30) | 41.07 (10.49) |
| Years since first treatment | n.a. | −0.11 (0.09) | 2.21 (1.14) | 10.07 (4.14) | 1.77 (1.59) | 6.45 (3.47) | 7.48 (2.10) |
| Physical Symptom Score (PSS) | n.a. | 6.64 (1.22) | 7.74 (1.82) | 9.52 (2.46) | 5.75 (2.63) | 8.90 (2.90) | 10.75 (2.87) |
| Hydrocephalus | 0 (0%) | 1 (5%)1 | 4 (15%)1 | 0 (0%) | 5 (24%)2 | 2 (50%)2 | |
| Cervical cord compression | 0 (0%) | 1 (5%) | 3 (11%) | 1 (25%) | 6 (25%) | 3 (75%) | |
| Mild or Moderate Hearing loss3 | 7 (50%) | 16 (84%)4 | 14 (52%)4 | 1 (25%) | 15 (71%)5 | 2 (50%) | |
| IQ | 67 (17.37) | 90.50 (15.62) | 76.32 (19.86) | 76.22 (20.08) | 109 (14.45) | 91.10 (17.19) | 96.50 (16.52) |
| Verbal IQ | n.a. | 91.64 (15.66) | 81.72 (18.73) | 82.76 (16.12) | 110 (15.29) | 91.57 (16.68) | 98.25 (10.78) |
| missing verbal IQ | n.a. | 0 (0.00%) | 1 (5.26%) | 2 (7.41%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Non-verbal IQ | n.a. | 96.04 (15.26) | 83.26 (21.08) | 80.76 (14.18) | 105 (13.20) | 91.95 (17.32) | 95.75 (20.04) |
| missing non-verbal IQ | n.a. | 0 (0.00%) | 0 (0.00%) | 2 (7.41%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| IQ Normal (85-115) | 4 (14%)6 | 10 (71.4%) | 6 (31.6%) | 11 (40.7%) | 4 (100.0%) | 12 (57.1%) | 3 (75.0%) |
| IQ Borderline (70-85) | 7 (25%)6 | 3 (21.4%) | 5 (26.3%) | 6 (22.2%) | 0 (0.0%) | 5 (23.8%) | 1 (25.0%) |
| IQ Impaired (<70) | 17 (61%)6 | 1 (7.1%) | 8 (42.1%) | 10 (37.0%) | 0 (0.0%) | 4 (19.0%) | 0 (0.0%) |
| MPS-IH | |||||||
| No ERT pre-HCT | n.a. | 0 (0.0%) | 0 (0.0%) | 19 (70.4%) | |||
| ERT pre-HCT | n.a. | 14 (100.0%) | 14 (73.7%) | 5 (18.5%) | |||
| Missing data ERT pre-HCT | n.a. | 0 (0.0%) | 5 (26.3%) | 3 (11.1%) | |||
| Engraftment <90% | 3 (15.8%) | 6 (22.2%) | |||||
| ≥90% | 13 (68.4%) | 17 (63.0%) | |||||
| Missing data for engraftment | 3 (15.8%) | 4 (14.8%) | |||||
| Severe genotype (derived)7 | n.a. | 10 (71.4%) | 13 (68.4%) | 12 (44.4%) | |||
| Other genotype (derived)8 | n.a. | 1 (7.1%) | 3 (15.8%) | 4 (14.8%) | |||
| Missing genotype | n.a. | 3 (21.4%) | 3 (15.8%) | 11 (40.7%) | |||
| MPS-Iatt | |||||||
| No L238Q | 4 (100.0%) | 12 (57.1%) | 4 (100.0%) | ||||
| L238Q | 0 (0.0%) | 6 (28.6%) | 0 (0.0%) | ||||
| Missing genotype | 0 (0.0%) | 3 (14.3%) | 0 (0.0%) | ||||
| MRI Volumes | N=14 | N=26 | N=4 | N=18 | N=4 | ||
| Cortex (mL) | n.a. | n.a. | 565 (61.12) | 556 (71.29) | 581 (94.70) | 516 (63.64) | 454 (16.43) |
| White matter (mL) | n.a. | n.a. | 311 (43.95) | 364 (54.43) | 354 (51.58) | 401 (65.44) | 419 (52.03) |
All hydrocephalus was diagnosed pre-transplant; 2 (50%) of these patients had a shunt
4 of 7 (35%) MPS Iatt patients had shunts
No patient had severe hearing loss.
MPS IH 10/30 (33%) wore hearing aids
MPS Iatt 2/15 (13%) wore hearing aids
N=22; 28 observations
either both nonsense, deletions, or splice site mutations
either one missense or known mild mutation or missing data
3.2 Cognitive outcomes
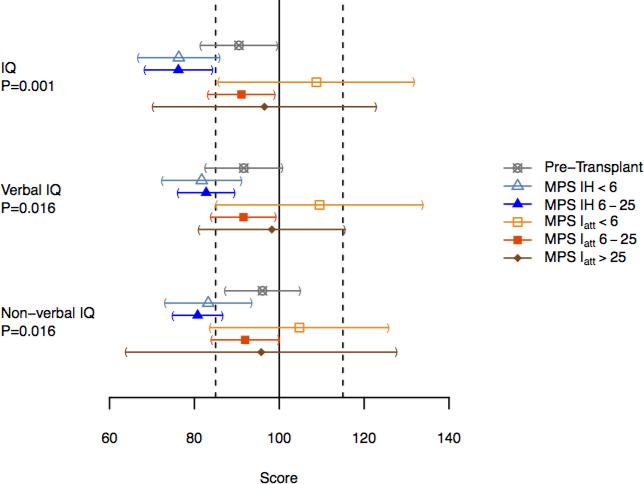
Pre-HCT, MPS IH are within the average range, although 2/3 of a standard deviation below the population mean. Post-HCT, their mean IQ was below the average range compared to normative data (Figure 1). For all groups, no differences in verbal and nonverbal (performance) IQ patterns were found.
Figure 1.
Means and confidence limits for the five groups of patients with P-values for differences in means across groups.
3.3 IQ by mutation
Post-HCT MPS IH patients with severe genotypes had a mean (SD) full scale IQ of 77.9 (14.4), those with a missense and nonsense mutation combined with those who had one mutation whose severity was unknown, had an IQ of 95.2 (11.9), and those with no genotype data, 70.1 (21.8).
We previously reported that in MPS Iatt, the L238Q mutation paired with a nonsense mutation presents with a distinctive phenotype with low IQ and psychiatric problems [4]. Compared to the rest of the ≥6-25 MPS Iatt group who have other mutations, the L238Q mean IQ is 72 and non-L238Q is 100 (difference of 28; 95%CI: 18, 38; P<0.001).
3.4 Medical and treatment data for MPS IH
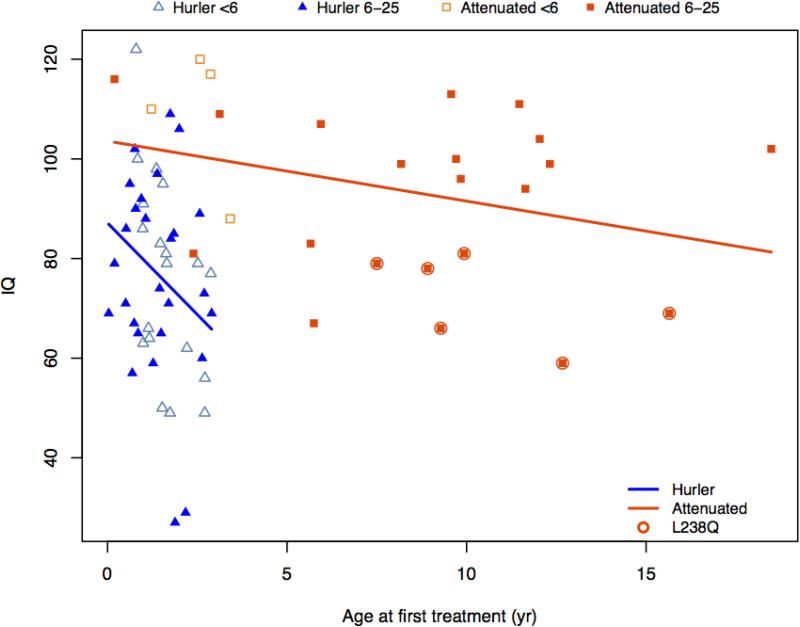
IQ decreased with increasing age at HCT (Figure 2). Outcomes were not significantly associated with HCT regimen (data in Supplementary Table 2) including irradiation (Mean IQ for nine children who had radiation was 69.9 versus 79.4 without, P=0.161). Note that 2 subjects who had radiation had 2 transplants and one had HCT at 32 months. However, removing these subjects did not substantially change these results.
Figure 2.
IQ and age at first treatment
Engraftment was unrelated to IQ; 77% of patients had >90% donor engraftment. Four children had 2 transplants; all were cognitively impaired with a mean IQ of 62.6 (range 40-64, age at 2nd HCT 17-34 months). Compared to those with a single transplant (mean IQ of 77.6) the difference of 15.4 showed a trend to significance (p=0.060).
The somatic disease burden as measured by the PSS increased with age (Table 2). Despite reports of hearing loss, neither parents nor examiners found any patients unable to hear adequately. All patients had some degree of corneal clouding whose severity was difficult to quantify, but did not interfere with testing. Two had corneal transplants; two had additional visual handicaps.
3.5 Medical and treatment data for MPS Iatt
All MPS Iatt patients were on ERT; age at first treatment shows a small association in Figure 2 between younger age and higher IQ. The PSS score increased with age (Table 2). All patients had some degree of corneal clouding; one had an additional visual handicap. Many patients had hearing loss of mild to moderate degree, but only 2 wore hearing aids (Table 2).
3.6 Special education and therapy services
90% of MPS IH patients in the ≥6-25 age group received special education, speech, occupational, and physical therapy. 70% of MPS Iatt patients in the same age group received such services. Results can be found in Supplementary Table 3.
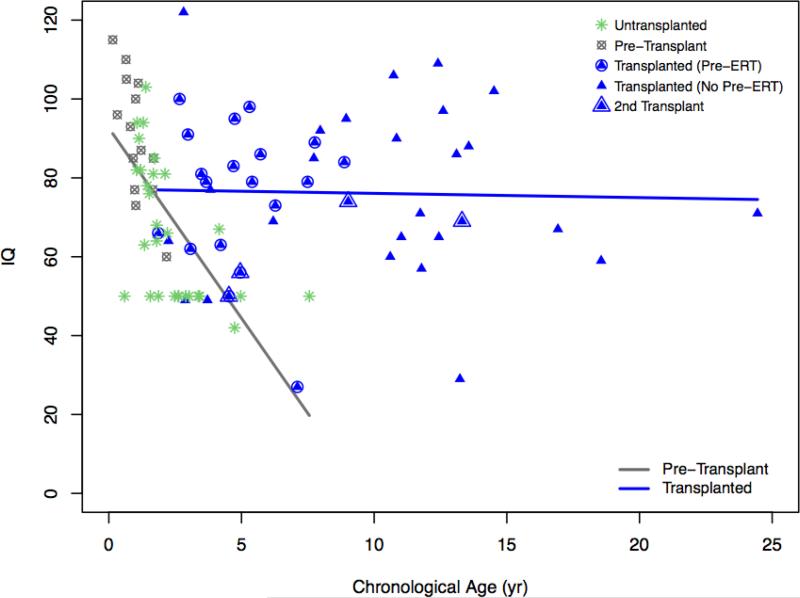
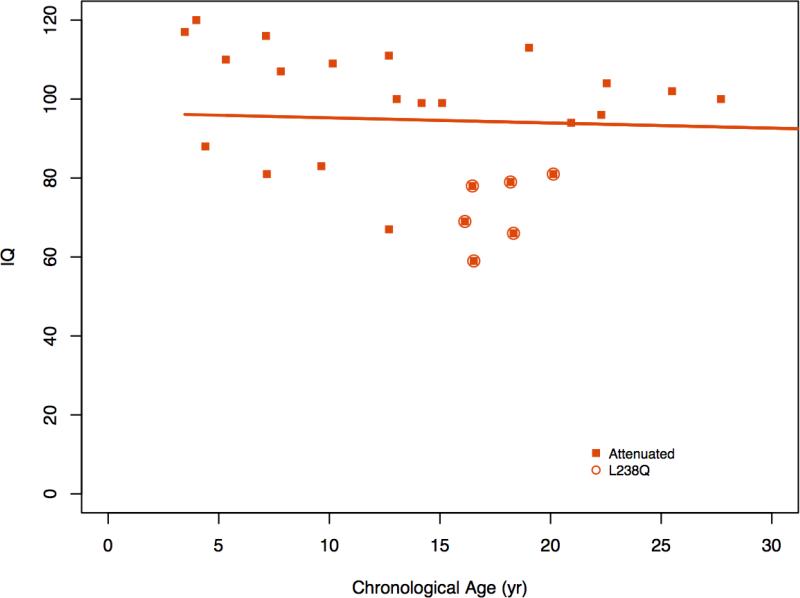
3.7 Age and cognitive ability
In Figure 3a, MDI/IQ is plotted for 22 untransplanted MPS IH patients which showed a steep decline with age. In contrast the post-HCT MPS IH patients showed no association between age and cognitive ability reflecting stable IQ. Similarly, no discernible association of age with IQ is seen in MPS Iatt patients (Figure 3b).
Figure 3a.
Age and IQ in MPS IH
Figure 3b.
Age and IQ in MPS Iatt
3.8 Predictors of IQ
The contribution to IQ of genotype, disease burden, age at first treatment and age at evaluation is shown in Table 3. For both MPS IH and MPS Iatt, genotype has the largest association with IQ. In addition, for MPS IH, each year delay in treatment is associated with an IQ lower by 9 points on average. When radiation was included in the adjusted analysis of differences in IQ, estimates did not change meaningfully and the estimated association of radiation versus not, was −8.3 (P=0.167).
Table 3.
Adjusted linear regression results for IQ separately for MPS-IH and MPS-IA (excluding the four over 25 years of age).
| Covariate | Mean Difference in IQ (95% CI) | P-value |
|---|---|---|
| MPS-IH (2-<25 years of age) | ||
| Severe mutation (vs. not severe) | −16.0 (−29.0, −3.0) | 0.016 |
| Missing mutations (vs. not severe) | −23.1 (−38.7, −7.5) | 0.004 |
| PSS | 0.3 (−2.9, 3.5) | 0.846 |
| Age at first treatment (per year) | −8.1 (−14.7, −1.6) | 0.014 |
| Age (per year) | −0.1 (−1.2, 0.9) | 0.804 |
| MPS-IA (2-<25 years of age) | ||
| Severe mutation (vs. not) | −20.2 (−32.2, −8.2) | 0.001 |
| PSS | −3.5 (−6.3, −0.7) | 0.014 |
| Age at first treatment (per year) | −1.6 (−3.3, 0.2) | 0.079 |
| Age (per year) | 1.7 (0.1, 3.2) | 0.036 |
In MPS Iatt, in addition to the L238Q genotype, somatic burden of disease contributes significantly, with IQ lower on average by 3 points for each PSS point. Additionally, a trend was seen for age at first treatment, with IQ lower on average by 1.6 points for each year of delay of treatment. For age at evaluation, when adjusted for genotype, PSS, and age of first treatment, a significant but small effect was found with IQ higher on average by 1.7 points per year of age.
3.9 IQ and MRI volumes
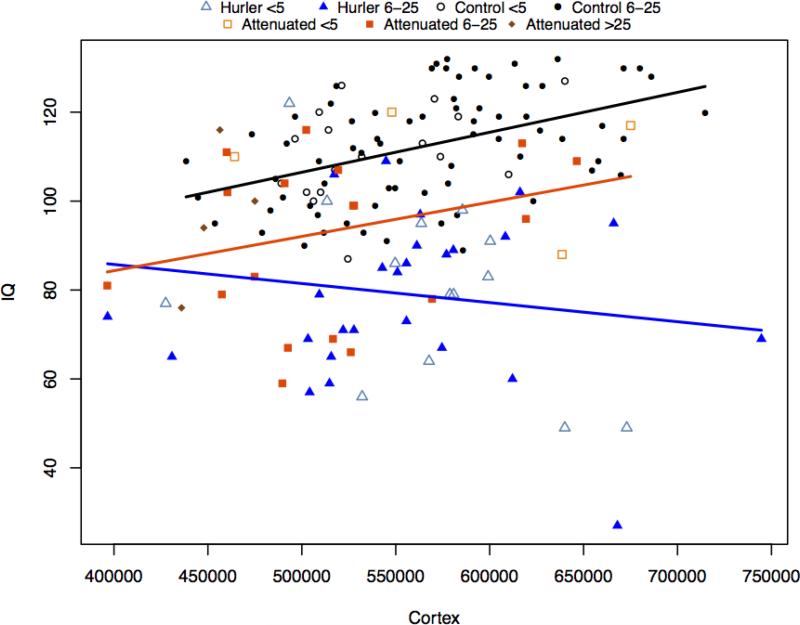
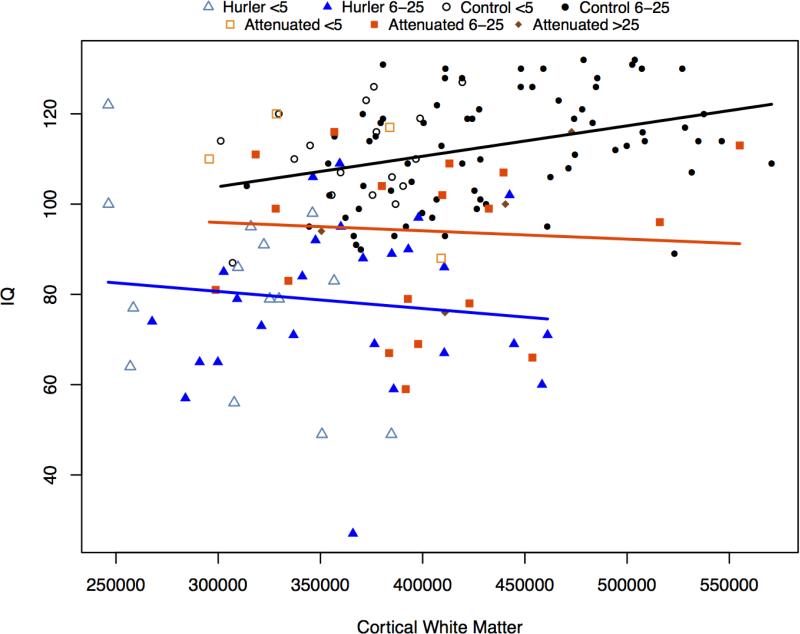
White matter (WM) volumes were associated with IQ for controls, but not for MPS I. Gray matter (GM) volumes were related to IQ in controls and MPS Iatt, but not in MPS IH. (Figures 4a; 4b; Table 4. )
Figure 4a.
IQ and cortical gray matter volumes
Figure 4b.
IQ and cortical white matter volumes
Table 4.
P-values of difference in slopes for IQ versus volumes.
| Comparison of slopes | P value | ||
|---|---|---|---|
| IQ | Cortex- Gray Matter | Control vs. MPS Iatt | 0.512 |
| Control vs. MPS IH | 0.009 | ||
| MPS Iatt vs. MPS IH | 0.069 | ||
| IQ | Cortical White Matter | Control vs. MPS Iatt | 0.218 |
| Control vs. MPS IH | 0.057 | ||
| MPS Iatt vs. MPS IH | 0.598 |
4. Discussion
We have systematically examined the cognitive ability together with medical history and brain imaging data, of a large multicenter cohort of MPS I patients across the broad spectrum of disease severity. Although HCT halts cognitive decline in MPS IH, significant residual cognitive and physical abnormalities indicate again the need for earlier and better treatment [19-22]. The prospective assessment of cognitive function of MPS Iatt patients indicates many unmet needs in this population especially the somatic disease burden and its impact on cognition.
Cognitive ability is a standard and sensitive variable that can be used as an indicator of disease progression and responsiveness to treatment [8,23]. Functionally, quality-of-life, self-sufficiency and educational attainment are dependent on cognitive ability. We now have a cohort of adolescent MPS I patients with multiple medical problems and invasive treatments who are transitioning to adulthood; this knowledge about their cognitive status may provide additional impetus to enhance their chances of success.
Age at evaluation was not associated with cognitive impairment after treatment in MPS IH. Although cross-sectional, this data provides further evidence of stability of cognitive functions after HCT [19,24,25]. This contrasts with the untransplanted MPS IH group whose cognitive ability declines with age. All untransplanted children were in the impaired range (> two SDs below the mean) after 27 months of age. In our pre-HCT group, younger than the historical controls, all children <12 months and 4/7 of those 12-24 months of age are in the average range. The oldest child in the pre-HCT group was the most impaired. The pre-HCT group is more intact than the historical controls, possibly because of younger age, more aggressive medical care and the beneficial effects of peri-HCT ERT [26-28].
Having two transplants was associated with lower IQ; older age at second transplant likely contributed. Radiation was associated with an 8 point lower IQ but was not statistically significant in an adjusted analysis. The overlap of radiation use, two HCTs, and older age at HCT in our patients makes it difficult to separate their effects on IQ in such a small group. Those who had two transplants were necessarily older and more likely to have radiation in their pre-transplant regimen.
Most of the children post-HCT functioned below the average range, more than 90% requiring special education or therapy services. The most salient predictor of cognitive outcome was genotype, followed by age at HCT. When patients are homozygous for nonsense mutations or other mutations, resulting in the most profound absence of a-L-iduronidase enzyme activity, severe disease is consistently present [29]. We found cognitive impairments are greater in those homozygous for nonsense, deletion, or splice site mutations than a combination of a nonsense and a missense or with one unknown mutation. Our findings are consistent with those of Terlato and Cox [29] who state that unless the patient has 2 nonsense mutations which results in a severe phenotype, genotype/phenotype correlations are variable; also they state that splice site and insertion/deletion mutations will result in a severe phenotype unless paired with a missense mutation. As the IQ of those missing genotype data is similar to the severe group, we assume that they had a preponderance of severe mutations. The relationship between age at HCT and IQ has been reported previously [19,21,22,30], and speaks to the necessity of earlier diagnosis and newborn screening, especially with known mutations causing severe disease. While HCT prevents disease-associated mortality and arrests cognitive decline, better and earlier treatments are needed to ameliorate functional impairments.
Although unrelated to IQ, PSS scores increase with age. Symptoms were primarily orthopedic with multiple surgeries resulting in significant physical handicap. Many MPS IH children in our sample had mild or moderate hearing loss. Previous reports indicate that after HCT, hearing loss stabilizes or improves (19,31). Our patients’ parents felt that hearing loss was stable and was not reported as a major problem. Most of the MPS IH patients in our sample had corneal clouding and 2 patients had received corneal grafts. Parents did not report worsening of visual difficulties, consistent with reports in the literature [19]. HCT prevented hydrocephalus, but not cord compression. As in other studies [19,20], hydrocephalus was seen pre-HCT, but new instances of hydrocephalus were not diagnosed among patients who had received HCT. Consistent with the literature [19,32] two patients had surgery for cervical cord compression so HCT may not prevent it entirely [33].
MPS Iatt patients are heterogeneous in all aspects; cognitive ability, somatic disease burden, and genotypes. The 2 <6 year old patients all have normal range IQs. In the ≥6-25 group, 43% have either borderline or impaired cognitive ability although as a group their mean IQ is in the average range. The 4 patients over 25 years are normal or borderline in IQ though this may reflect ascertainment bias due to being conditional on survival to an older age. While Vijay and Wraith indicate in their series that a few patients had cognitive impairment [3]; our rate of 43% is higher than expected. Although most MPS Iatt patients were found to have significant physical problems and some had cognitive problems, they did not receive as much educational or therapeutic support as the MPS IH patients. Very little attention has been paid to the immense educational needs of these attenuated patients. More school interventions and accommodations are warranted.
All MPS Iatt patients were on ERT with small association between age of treatment initiation and IQ when adjusted. PSS is associated with age at evaluation and indicates a significant somatic disease burden [3,34]. PSS was found to be a significant predictor of IQ. There are a number of possible reasons for this. Hearing loss and hydrocephalus may have effects on cognitive performance. Seventy percent of MPS Iatt patients in the ≥6-25 year group have mild or moderate hearing loss which is higher than previously reported [3] and while previous reports make no mention of hydrocephalus [3,34], we found 24% diagnosed with it. This may explain in part the significant association of the PSS score with cognitive ability. Another possible reason for the association between PSS and IQ is that physical disability may cause children and adolescents to suffer psychologically and have decreased motivation to perform. We did not present behavioral data in this paper; we will do that in a subsequent paper and further investigate that hypothesis.
The L238Q mutation has a significant impact on cognitive ability [17], suggesting that for this subgroup of MPS Iatt who have a phenotype closer to MPS IH, brain treatment is necessary. Vijay and Wraith report that one patient with MPS Iatt has been transplanted with good results, and Aldhoven suggests that those with a phenotype closer to that of MPS IH could be considered for HCT on an individual basis [3,19].
A growing body of evidence supports white matter abnormalities in MPS I [35-38]. While we found controls showed an association between the larger volumes of white matter and higher IQs, in both MPS groups the relationship is inverted, although slopes were significantly different only between the MPS IH and controls. One can surmise that development of white matter may be abnormal in MPS I, but further work is needed to identify the mechanism. The positive association of gray matter volumes with IQ is similar for controls and MPS Iatt, but MPS IH shows a nonsignificant but slightly negative association. Smaller volumes and better functional outcomes might be associated with clearance of storage material. Clearance has been documented histologically in high dose and intrathecal enzyme-treated MPS I animal models [39,40].
Although secondary effects of chemotherapy associated with HCT in MPS IH may contribute to cognitive problems associated with white matter abnormalities [41-43], the cognitive impairment associated with MPS I is disease-related [35]. Numerous pathological processes have been proposed as mechanisms such as storage and deficiency in the degradation of heparan sulfate and dermatan sulfate, especially meningeal storage [44] which may cause hydrocephalus; abnormalities in pathways leading to metabolism of gangliosides associated with abnormal early neural development [45]; inflammatory processes and oxidative stress [46,47]; alteration in membrane/cell wall permeability [46]; changes in homeostasis of ions leading to apoptosis [48]; and abnormal signaling [48]. Understanding how these neuropathological mechanisms affect functional outcomes may eventually yield more focused treatments of the brain.
Some limitations of this study are that it is cross-sectional; we have not included data regarding home environment, school environment, other concomitant illnesses; and or other cognitive domains such as attention and memory which deserve consideration. Finally, bias in enrollment may also influence these results as the majority of data were collected at one institution. The most impaired patients were not likely to have enrolled in this study, especially if travel was required.
In conclusion, we found that cognitive performance is below average but is relatively stable after treatment in MPS I. One year after HCT, IQ is stabilized in MPS IH although below average. Associations of IQ with MRI white matter brain volumes in both MPS I groups differ from controls and suggest a lack of expected white matter development. In MPS IH but not in MPS Iatt there is evidence for lack of development of cortical gray matter. Age at transplant for MPS IH and somatic disease burden as reflected in PSS score for MPS Iatt are important predictors of cognitive performance. We need to increase awareness of the high rates of cognitive impairment, hydrocephalus, hearing loss, and somatic disease burden in MPS Iatt.
The benefits of newborn screening for MPS I, given knowledge of the correlation of specific genotypes with severity, will allow for earlier treatment. New treatments that benefit brain function are needed to improve outcomes. Cognitive performance will have utility as an important endpoint in such clinical trials. Increasing awareness of cognitive limitations in MPS I patients by physicians and health care providers will enable them to provide focused interventions that may benefit long-term outcomes in MPS I.
Supplementary Material
Highlights.
Genotype and age at transplant age are associated with IQ outcome in MPS IH
Genotype, disease burden, and ERT start age predict IQ outcome in attenuated MPS I
Brain white matter development differs from age-matched controls in both MPS I groups
Acknowledgments
We thank Brenda Diethelm-Okita, David Erickson, and Evelyn Redtree in the Lysosomal Disease Network office at the University of Minnesota for administrative assistance. We thank James Provenzale, M.D. (Duke University) and Bryon Mueller, Ph.D. (University of Minnesota) neuroimaging consultation, Kelly King, Ph.D. (University of Minnesota) for neuropsychological assistance, and Renee Cooksley for assistance with genotyping. We are also grateful to the Center for Neurobehavioral Development, the Center for Magnetic Resonance Research, and the Minnesota Supercomputer Center for the provision of infrastructure for this research.
Funding sources:
• The Lysosomal Disease Network supported this study through “Longitudinal Studies of Brain Structure and Function in the Mucopolysaccharidoses” (E. Shapiro, P.I.). The Lysosomal Disease Network (U54NS065768) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH (C. Whitley P.I.).
• Genzyme/Sanofi supported this study as an investigator initiated research grant– (Dr. Shapiro)
• This project was supported in part (Dr. Rudser) by the National Center for Advancing Translational Sciences, National Institutes of Health, through University of Minnesota -CTSI Grant Number NCATS UL1TR000114. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
• This project was supported in part (Dr. Harmatz) by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
• The National MPS Society (imaging, genotyping, and 4-7 control study) (Dr. Shapiro)
• The Ryan Foundation for Orphan Diseases (genotyping, and 4-7 control study) (Dr. Shapiro)
• NIH-NS29099:Value of Transplant for Storage Diseases 1991-1996
Imaging Controls:
• NIH 5R01MH060662 (Dr. Wozniak)
• NIH (5P41RR008079, 5K12RR023247, P30-NS057091, & MO1-RR00400) (Dr. Lim)
• Shire (4-7 control study) (Dr. Nestrasil)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Number: NCT01870375
Contributor Information
Elsa G. Shapiro, University of Minnesota
Igor Nestrasil, University of Minnesota
Kyle Rudser, University of Minnesota
Kathleen Delaney, University of Minnesota
Victor Kovac, University of Minnesota
Alia Ahmed, University of Minnesota
Brianna Yund, University of Minnesota
Paul J. Orchard, University of Minnesota
Julie Eisengart, University of Minnesota
Gregory R. Niklason, University of Minnesota
Julian Raiman, Hospital for Sick Children, University of Toronto, Toronto, CA
Eva Mamak, Hospital for Sick Children, Toronto, CA
Morton J. Cowan, UCSF Benioff Children's Hospital, University of California San Francisco
Mara Bailey-Olson, UCSF Benioff Children's Hospital, University of California San Francisco.
Paul Harmatz, UCSF Benioff Children's Hospital Oakland
Suma P. Shankar, Emory University.
Stephanie Cagle, Emory University
Nadia Ali, Emory University
Robert D. Steiner, Oregon Health & Science University.
Jeffrey Wozniak, University of Minnesota
Kelvin O. Lim, University of Minnesota
Chester B. Whitley, University of Minnesota
References
- 1.Muenzer J, Wraith JE, Clarke LA. The International Consensus Panel on the Management and Treatment of Mucopolysaccharidosis I. Mucopolysaccharidosis I: management and treatment. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 2.Boelens J, Prasad V, Tolar J, Wynn R, Peters C. Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin North Am. 2010;57:123–45. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94:872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Whitley CB, Cooksley R, et al. Neurocognitive and neuropsychiatric phenotypes associated with the mutation L238Q of the alpha-L-iduronidase gene in Hurler-Scheie syndrome. Mol Genet Metab. 2014;111:123–127. doi: 10.1016/j.ymgme.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Sawaf S, Mayatepek E, Hoffmann B. Neurological findings in Hunter disease: pathology and possible therapeutic effects reviewed. J Inherit Metab Dis. 2008;31(4):473–480. doi: 10.1007/s10545-008-0878-x. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro E, Balthazor M. Metabolic and neurodegenerative disorders of childhood. In: Taylor G, Ris D, Yeates K, editors. Pediatric neuropsychology: research, theory and practice. Guilford Press; New York, NY: 1999. pp. 171–205. [Google Scholar]
- 7.Shapiro EG, Lockman LA, Balthazor M, Krivit W. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis. 1995;18:413–429. doi: 10.1007/BF00710053. [DOI] [PubMed] [Google Scholar]
- 8.Delaney K, Rudser K, Yund B, Whitley C, Haslett P, Shapiro E. Methods in neuropsychological assessment in children with neurodegenerative disease: Sanfilippo syndrome. J Inherit Metab Dis Reports. 2013 doi: 10.1007/8904_2013_269. DOI/10.1007/8904_2013_269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol psychol. 2000;54(1):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 11.Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines MN: 1995. [Google Scholar]
- 12.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. Psychological Corporation; San Antonio TX: 2002. [Google Scholar]
- 13.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio TX: 1999. [Google Scholar]
- 14.Bayley N. Bayley Scales of Infant Development. Psychological Corporation; San Antonio TX: 1969. [Google Scholar]
- 15.Thorndike R L, Hagen E P, Sattler JM. Stanford-Binet Intelligence Scale: Fourth Edition. Riverside; Chicago: 1986. [Google Scholar]
- 16.Ahmed A, Kunin-Batson A, Redtree E, Whitley CB, Shapiro E. MPS (mucopolysaccharidosis) specific physical symptom score-development, reliability and validity. Mol Genet Metab. 2014;111(2):S17–S18. [Google Scholar]
- 17.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. URL http://www.R-project.org/ [Google Scholar]
- 19.Aldenhoven M, Boelens J, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol. Blood MarrowTransplant. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Souillet G, Guffon N, Maire I, et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 21.Peters C, Balthazor M, Shapiro E, et al. Outcome of unrelated donor bone marrow transplantation in forty children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 22.Peters C, Shapiro E, Anderson J, et al. Hurler Syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 23.Martin HR, Poe MD, Reinhartsen D, et al. Methods for assessing neurodevelopment in lysosomal storage diseases and related disorders: a multidisciplinary perspective. Acta Pediatr. 2008;97:69–75. doi: 10.1111/j.1651-2227.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 24.Lücke T, Das AM, Hartmann H, et al. Developmental outcome in five children with Hurler syndrome after stem cell transplantation: a pilot study. Dev Med Child Neurol. 2007;49(9):693–696. doi: 10.1111/j.1469-8749.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 25.Malm G, Gustafsson B, Berglund G, et al. Outcome in six children with mucopolysaccharidosis type IH, Hurler syndrome, after haematopoietic stem cell transplantation (HSCT). Acta Paediatr. 2008;97(8):1108–1112. doi: 10.1111/j.1651-2227.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 26.Eisengart JB, Rudser KD, Tolar J, et al. Enzyme replacement is associated with better cognitive outcomes after transplant in Hurler syndrome. J Pediatr. 2013;162(2):375–380. doi: 10.1016/j.jpeds.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynn R, Mercer J, Page J. Use of enzyme replacement therapy (Laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J Pediatr. 2009;154:135–9. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Tolar J, Grewal S, Bjoraker K, et al. Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome. Bone Marrow Transplant. 2007;41:531–5. doi: 10.1038/sj.bmt.1705934. [DOI] [PubMed] [Google Scholar]
- 29.Terlato NJ, Cox GF. Can mucopolysaccharidosis type I disease severity be predicted based on a patient's genotype? A comprehensive review of the literature. Gen Med. 2003;5(4):286–294. doi: 10.1097/01.GIM.0000078027.83236.49. [DOI] [PubMed] [Google Scholar]
- 30.Poe MD, Chagnon SL, Escolar ML. Early treatment is associated with improved cognition in Hurler syndrome. Ann. Neurol. 2014. 2014;76(5):747–753. doi: 10.1002/ana.24246. [DOI] [PubMed] [Google Scholar]
- 31.Krivit W, Lockman LA, Watkins PA, Hirsch J, Shapiro EG. The future for treatment by bone marrow transplantation for adrenoleukodystrophy, metachromatic leukodystrophy, globoid cell leukodystrophy and Hurler syndrome. J Inherit Metab Dis. 1995;18(4):398–412. doi: 10.1007/BF00710052. [DOI] [PubMed] [Google Scholar]
- 32.Weisstein JS, Delgado E, Steinbach LS, et al. Musculoskeletal manifestations of Hurler syndrome: long-term follow-up after bone marrow transplantation. J Pediatr Orthop. 2004;24:97–101. doi: 10.1097/00004694-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Hite SH, Peters C, Krivit W. Correction of odontoid dysplasia following bone-marrow transplantation and engraftment (in Hurler syndrome MPS 1H). Pediatr Radiol. 2000;30:464–470. doi: 10.1007/s002470000210. [DOI] [PubMed] [Google Scholar]
- 34.Thomas JA, Beck M, Clarke JT, Cox GF. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33(4):421–427. doi: 10.1007/s10545-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro E, Guler OE, Rudser K. An exploratory study of brain function and structure in mucopolysaccharidosis type I: Long term observations following hematopoietic cell transplantation (HCT). Mol Genet Metab. 2012;107(1):116–121. doi: 10.1016/j.ymgme.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vedolin LI, Schwartz VD, Komlos M. Brain MRI in mucopolysaccharidosis Effect of aging and correlation with biochemical findings. Neurol. 2007;69(9):917–924. doi: 10.1212/01.wnl.0000269782.80107.fe. [DOI] [PubMed] [Google Scholar]
- 37.Matheus GM, Castillo JK, Smith, et al. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiol. 2004;46:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 38.Vite C, Nestrasil I, Mlikotic A, et al. Features of Brain MRI in Dogs with Treated and Untreated Mucopolysaccharidosis Type I. Compar Med. 2013;63:163–173. [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson P, McEntee M, Vogler C, et al. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou L, Herzog T, Koniar BL, Gunther R, Whitley CB. High-dose enzyme replacement therapy in murine Hurler syndrome. Mol Genet Metab. 2014;111:116–122. doi: 10.1016/j.ymgme.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson FS, Kunin-Batson AS, Perkins JL, et al. White versus gray matter function as seen on neuropsychological testing following bone marrow transplant for acute leukemia in childhood. Neuropsychiatr. Dis. Treat. 2008;4(1):283–288. doi: 10.2147/ndt.s2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr.Blood Cancer. 2009;52:159–164. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 43.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–949. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zafeiriou DI, Batzios SP. Brain and spinal MR imaging findings in mucopolysaccharidoses: a review. Am J Neuroradiol. 2013;34(1):5–13. doi: 10.3174/ajnr.A2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walkley SU. Secondary accumulation of gangliosides in lysosomal storage disorders. Sem Cell Dev Biol. 2004;15(4):433–444. doi: 10.1016/j.semcdb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Ohmi K, Greenberg DS, Rajavel KS, et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira VG, Martins AM, Micheletti C, D'Almeida V. Mutational and oxidative stress analysis in patients with mucopolysaccharidosis type I undergoing enzyme replacement therapy. Clin Chim Acta. 2008;387:75–79. doi: 10.1016/j.cca.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Campos D, Monaga M. Mucopolysaccharidosis type I: current knowledge on its pathophysiological mechanisms. Met Brain Disease. 2012;27(2):121–129. doi: 10.1007/s11011-012-9302-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.