Abstract
The purpose of this study was to examine how sex and apolipoprotein E (APOE) genotype contribute to individual differences in spatial learning and memory. The associations of APOE genotype with neurocognitive function have been well studied among the elderly but less is known at earlier ages. Young adults (n = 169, 88 females) completed three neurocognitive tasks: Mental Rotation, Spatial Span, and Memory Island, a spatial navigation test. Males outperformed females on all three tasks: finding the hidden targets more quickly on Memory Island (Cohen's d = 0.62) and obtaining higher scores on Mental Rotation (d = 0.54) and Spatial Span (d = 0.37). In contrast, no significant effects of APOE were observed. The identified sex differences elaborate upon past literature documenting sexually dimorphic performance on specific neurobehavioral tasks.
Keywords: apolipoprotein E, sex differences, young adults, learning, memory
INTRODUCTION
Sex differences in spatial memory are among the most widely reported and studied of cognitive sex differences (Andreano & Cahill, 2009). Included among the most sexually dimorphic types of memory are spatial rotation and object location (Linn & Petersen, 1985; Voyer, Postma, Brake, & Imperato-McGinley, 2007). Spatial rotation tasks require participants to “mentally rotate” geometric figures to determine whether a figure is different from a target figure or simply rotated three dimensionally in space. Robust and replicable sex differences favoring males have been found for this task (Linn & Petersen, 1985), but research suggests that performance on spatial rotation tests may be more sexually dimorphic (i.e., larger effect sizes) than performance on visuospatial working memory (e.g., spatial span) tasks (Andreano & Cahill, 2009).
It must also be noted that, even with mental rotation type tests, the magnitude of the sex difference is dependent on the stimuli employed (i.e., largest with polygons and more modest with cube drawings or animal stimuli) (Jansen-Osmann & Heil, 2007), individual differences in confidence (Estes & Felker, 2012), and can be ameliorated with targeted computerized (Cherney, 2008; Feng, Spence, & Pratt, 2007) or non-computerized experiences (Tzuriel & Egozi, 2010). Performance on navigation tasks, another sub-category of spatial memory, also shows a clear male advantage. Male rats consistently outperform females in navigating through the Morris Water Maze (Perrot-Sinal, Kostenuik, Ossenkopp, & Kavaliers, 1996). Human studies of navigation through virtual environments also display similar sex differences favoring males (Acevedo, Piper, Craytor, Benice, & Raber, 2010; Astur, Ortiz, & Sutherland, 1998; Berteau-Pavy, Park, & Raber, 2007; Piper et al., 2011a). While males typically outperform females in tests of spatial navigation and mental rotation, significant female advantages have been documented in object location memory (Andreano & Cahill, 2009; Berteau-Pavy et al., 2007; Piper et al., 2011a; Piper, Yasen, & Miller, 2011b; Silverman, Choi, & Peters, 2007).
An apolipoprotein is a specialized protein that binds lipids, specifically fat and cholesterol, to transport lipids through the lymphatic and circulatory systems. The apolipoprotein E (APOE) gene has three common alleles: ε2, ε3, and ε4. Compared to the ε3 allele, the ε4 allele is associated with poor performance in neurocognitive domains, particularly in delayed episodic memory (De Blasi et al., 2009), and increased risk of developing late-onset Alzheimer's disease (Laws, Hone, Gandy, & Martins, 2003). The effects of ε4 on spatial and non-spatial memory have been extensively studied among the elderly (Berteau-Pavy et al., 2007; Reiman et al., 1996) and, to a lesser extent, child populations (Ruiz et al., 2010; Taylor et al., 2011). Berteau-Pavy et al., assessed cognitive function in non-demented elderly individuals and found that non-ε4 carriers consistently outperformed ε4 carriers on a spatial memory task (Memory Island) but not on other neurocognitive tests, including facial recognition or spatial span. Similarly, ε4+ children did not show a target quadrant preference during Memory Island assessments unlike their peers without an ε4 allele (Acevedo et al., 2010). These findings suggest that the ε4 allele may be associated with meaningful changes in cognitive performance, even in those without Alzheimer's disease. Importantly, Swan, Lessov-Schlagger, Carmelli, Schellenberg, and La Rue (2005) conducted a longitudinal analysis comparing change in cognitive performance of elderly ε4 carriers and non-carriers over the course of four years and identified a pattern of results that was task and sex dependent. Male ε4 carriers showed a greater decline in performance on executive function and verbal memory than non-carriers, specifically on the delayed symbol substitution and Color-Word Interference tasks. Female carriers experienced greater decline on part B of the Trail Making Test than non-carriers. These results, as well those by others (Beydoun et al., 2012), suggest that ε4 may affect elderly women and men differently as they age.
While the results from these studies demonstrated that ε4 may affect neurobehavioral performance in older populations, less is known about the cognitive effects of ε4 within the young adult population. Recent investigations have suggested that ε4 carriers may actually have better episodic memory (Mondadori et al., 2007), elevated Performance IQ (Yu, Lin, Chen, Hong, & Tsai, 2000), and a tendency towards attaining more education than non-carriers during young adulthood (Hubacek et al., 2001). Mondadori et al. noted that while ε4 is related to several harmful biological effects, the question still remained as to why this uniquely human isoform of APOE has persisted through the generations. This group found that ε4 carriers exhibited better performance on delayed (episodic), but not on immediate, (working) memory tasks. In contrast, no significant differences were identified in cognitive performance in an index of educational ability in Spanish adolescents (Ruiz et al., 2010). Volume decrements in the hippocampus, a structure important for spatial memory, were documented in ε4, relative to ε2, young adults although no significant differences in intellectual function were observed (Alexopoulos et al., 2011). Further, strong relationships between APOE genotype and levels of HDL and LDL cholesterol were identified but no evidence was found to suggest that APOE was associated with IQ scores in children (Tzuriel & Egozi, 2010). Similarly, a meta-analysis found no consistent neurocognitive effects of APOE among children and young-adults (Ihle et al., 2012).
The objective of this study was to address a gap in the APOE memory literature and determine whether sex and APOE polymorphisms influenced performance on spatial memory tasks in young adults. Based on past studies documenting performance differences between ε4 carriers and non-carriers (Acevedo et al., 2010; Berteau-Pavy et al., 2007) as well as neuroimaging data (Alexopoulos et al., 2011) and a large neurobehavioral literature (Andreano & Cahill, 2009; Linn & Petersen, 1985), it was hypothesized that there would be a male advantage and that non-carriers would outperform ε4 carriers.
METHOD
Participants
A total of 169 college students (88 females), age 18 to 22 years, received course credit as incentive for completing the cognitive testing, which took approximately 1.5 h. Further information about the participants is shown in Table 1.
Table 1.
Participant characteristics. eε2/ε4 (N = 3) individuals were excluded
| Females (N = 88) | Males (N = 81) | |
|---|---|---|
| Mean (SEM) | Mean (SEM) | |
| Age (years) | 18.8 (0.1) | 19.0 (0.1) |
| Laterality Index (SEM) | 0.74 (0.03) | 0.55 (0.06)* |
| ε: 2+ N (%e) | 9 (5.4%) | 6 (3.6%) |
| 3/3 N (%) | 54 (32.5%) | 57 (34.3%) |
| 4+ N (%)e | 23 (13.9%) | 17 (10.2%) |
| Video Game: Age 1st played (years) | 8.8 (0.4) | 6.5 (0.3)** |
| Hours/day (maximum) | 3.9 (0.3) | 8.3 (0.4)** |
| Hours last week | 0.2 (0.1) | 4.3 (0.6)** |
p < .05
p < .005.
Procedure
Saliva samples were collected at the beginning of the session. APOE genotyping was performed using polymerase chain reaction. Handedness was determined based on a standardized inventory (Oldfield, 1971). Information on handedness was obtained because an earlier study identified an advantage on spatial memory tests among left-handers (Piper et al., 2011a). Video game usage was evaluated to assess if this variable influenced Memory Island performance by asking participants: (1) the first video game ever played; (2) the age of first exposure to video games; (3) the most number of hours played in one day; (4) the most number of hours played in one week; (5) to list their favorite video games; (6) the number of hours spent playing last week; and (7) to list the games played in the preceding week. Data about video game experience were collected as this is important to fully interpret spatial navigation endpoints (Astur et al., 1998). All procedures were completed in accordance with the Code of Ethics of the World Medical Association Declaration of Helsinki and were approved by the IRB of Willamette University.
Measures
Participants were seated at one of eight computer stations and tested concurrently on three spatial function tests: Mental Rotation, Spatial Span, and Memory Island. These tasks differ in their spatial demands with Mental Rotation requiring three-dimensional visualization while the other tasks are two-dimensional. The results of another spatial test (Novel Image Novel Location) completed by these participants are available elsewhere (Piper et al., 2011b).
Mental Rotation
The pen and paper version of the Mental Rotation Task contains 20 objects in five sets of four. Each item contains a 3-dimensional target figure, two rotated versions of the target figure, and two incorrect “distracter” figures. Participants were to choose which two objects matched the target object. Participants were given three minutes to complete 20 of these problems. One point was given for each correct response, and participants were instructed to complete the problems as quickly as possible without compromising accuracy (Vandenberg & Kuse, 1978). This version of the task was chosen because these stimuli show the most robust sex differences (Jansen-Osmann & Heil, 2007).
Spatial Span
This test provides an evaluation of visual-spatial memory and is a computerized version of the Corsi Block Tapping task (Shiels et al., 2008). An array of 10 gray squares was presented to participants on the computer screen. For each trial, a yellow smiley face illuminated the squares one at a time for 1-sec. For the forward span task, participants were instructed to remember the exact order in which the squares were illuminated and use the computer mouse to click on the gray squares in the same order in which the smiley face appeared. In the backward condition, participants were asked to click on the squares in the reverse order in which the smiley face illuminated the blocks. Visual feedback was presented after each trial indicating a correct or incorrect response as well as the amount of points earned. Participants received one point for each correct trial. The level of difficulty increased as participants advanced in the task, requiring participants to remember longer sequences of illuminated blocks (maximum = 8 locations) with two trials completed at each level of difficulty. The task terminated when both trials within a difficulty level were incorrect. A screenshot with task stimuli may be found elsewhere (Shiels et al., 2008).
Memory Island
Participants were trained to navigate using a joystick to a target location marked with a flag (visible trial). Four different target objects were used during the visible training trials. After completing the four visible trials, participants navigated to a hidden target without a flag in four trials (hidden trials). The participants had to remember the location of the hidden target and how to navigate there. The location of the hidden target was kept constant. If the participant was unable to locate the target object in less than 2 min, an arrow appeared at the top of the computer screen to guide them to the location of the object. For each trial, the latency to reach the target was recorded. Upon completion of the last hidden trial, participants received a final 30-second probe trial with the target object removed to measure spatial memory. The duration spent in each quadrant of Memory Island (the target quadrant which previously contained the item of interest and the non-target quadrants) was quantified. Importantly, Memory Island provides both distant and local cues and therefore provides an index of egocentric and allocentric memory. Further information describing this test is available elsewhere (Rizk-Jackson et al., 2006). A video with the participant's perspective is contained in Piper et al. (2011a). A male advantage has been identified on Memory Island among 7-9 year old children (Acevedo et al., 2009), young-adult community college students (Rizk-Jackson et al., 2006), and in nondemented elderly (age 62-92) participants (Berteau-Pavy et al., 2007).
Statistical Analyses
All analyses were conducted with Systat version 13.0 (Chicago, IL) with the exception of a mediation analysis which was completed with SPSS version 20.0 with the Preacher and Hayes (2004) macro. Three genotypes were defined: (1) ε3/ε3 carriers (N = 111, 54 females); (2) ε3/ε4 (N = 38) and ε4/ε4 (N = 2) carriers were combined as ε4+ carrier (23 females); (3) ε2/ε3 (N = 13) and ε2/ε2 (N = 2) carriers were combined as ε2+ carriers (9 females). Participants that were ε2/ε4 (N = 3) were excluded from APOE analyses as has also been done by others (Rebeck, Kindy, & LaDu, 2002; Swan et al., 2005). Although the retention interval between the hidden and probe Memory Island trials was manipulated (immediate versus 30-60 minutes), this delay had no significant effect on the percent time in the target and non-target [(left + right + opposite)/3] quadrants and was not incorporated into the analyses. As Memory Island has not been extensively used with this age group, correlations were determined between dependent measures and also with demographic characteristics with p < .05 considered statistically significant. The sample in the present endeavor is over three-fold larger than was employed in a prior report that identified both sex and APOE effects with Memory Island (Acevedo et al., 2010). Quantification of video game experience and usage is contained in Table 1. Variability was expressed as the SEM. Group differences were expressed in terms of Cohen's d effect size measure with 0.20 considered small, 0.50 as medium, and 0.80 as large. A male advantage was anticipated for all tasks with a large effect size for mental rotation, moderate to large for spatial navigation, and modest for spatial span (Andreano & Cahill, 2009). The post-hoc power of key findings was determined with G*Power 3.1 with an alpha of .05 (Faul, Erdfelder, Lang, & Buchner, 2007). Two complementary analyses, analysis of covariance and a mediation analysis (Preacher & Hayes, 2004), were conducted to examine the relationship between video game experience and sex differences on the dependent measures.
RESULTS
There was no significant sex difference in age or APOE genotype, χ2(1) = 1.37, but males had a significantly smaller (i.e., more left-handed) Laterality Index. Females played their first video-game when they were over two years older than males. The most amount of time males spent playing a video-game in a single week was twice as large as that of females. Similarly, the duration of video-game experience in the preceding week was over 20-fold greater in males (Table 1).
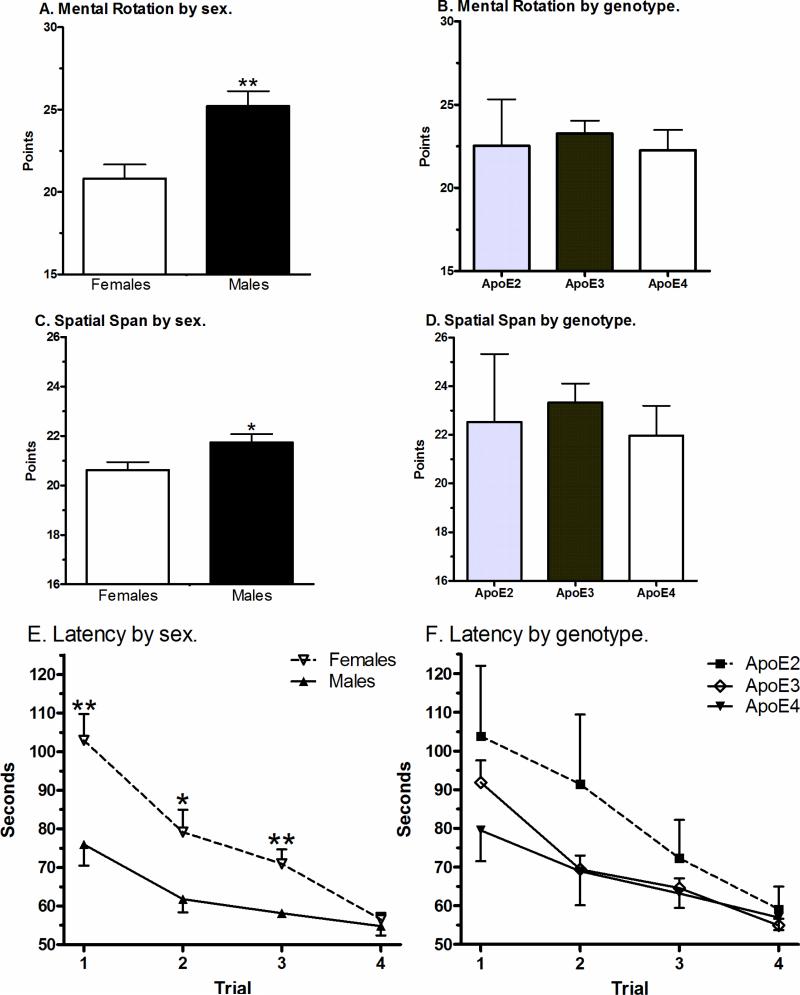
Clear sex, but not APOE genotype, differences were evident (Fig. 1). Males showed the anticipated elevation on the Mental Rotation Test, t(166) = 3.53, p < .001, Cohen's d = 0.54, Power = 0.94, Fig. 1A. A smaller, but still significant, male advantage was also observed on Spatial Span, t(164) = 2.35, p < .05, d = 0.37, Power = .66, Fig. 1C. Males reached the hidden target sooner on three of the four hidden (i.e., spatial learning) Memory Island trials (Fig. 1E) and the total hidden trial latency was approximately one minute lower (59.8 sec), t(164) = 3.77, p < .0005, d = 0.62, Power = .98. Further analyses were completed with video-game experience included as a covariate. The male advantage was retained with the variance attributable to age at first video-game (p < .0005), maximum hours lifetime (p < .05), or the hours last week (p < .0005) removed.
Figure 1.
Neurocognitive performance on the Mental Rotation Test (A & B), Spatial Span (C & D), and on the hidden trials of Memory-Island (E,F) show sex but not APOE effects. See Table 1 for the N/cell (*p < .05, **p < .005).
On the probe (spatial memory retention) trial, males traveled faster than females (8.1 ± 0.2 versus 7.5 ± 0.2 virtual units/sec), t(162) = 2.32, p < .05, d = 0.36, Power = .63. The percent time in the target quadrant (82.5 ±1.8) was greater than the average percent time in the non-target quadrant (5.8 ±1.6), t(165) = 31.19, p < .0005. There was no significant difference in the percent time in the target quadrant based on sex and the ε3+ group did not differ from the ε2+ or ε4 groups (Table 2).
Table 2.
Percent time in the target and non-target quadrants of Memory Island during the probe trial.
| Target | Left | Right | Opposite | |
|---|---|---|---|---|
| Genotype | Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) |
| ε2 | 74.1 (5.8) | 2.6* (1.5) | 10.5* (4.4) | 12.8* (3.2) |
| ε3 | 81.8 (2.4) | 5.3* (1.5) | 3.3* (0.6) | 9.7* (1.5) |
| ε4 | 87.0# (3.5) | 1.8* (0.6) | 4.5* (2.4) | 6.8* (2.3) |
| Sex | ||||
| Female | 80.2 (2.7) | 3.8* (1.4) | 4.6* (1.3) | 11.4* (1.6) |
| Male | 85.1 (2.5) | 4.4* (3.7) | 3.7* (1.0) | 6.8* (1.8) |
p < .0005 versus target
p = .058 versus ε2.
A correlational analysis between the behavioral and demographic variables was also completed (Table 3). Individuals who had spent more time playing video-games had a lower latency to reach the hidden targets on Memory Island. Similarly, the amount of recent video-game experience was positively correlated with Spatial Span and Mental Rotation tests. Performance on the Mental Rotation test showed modest, but significant, associations with that on the Spatial Span but not the Memory Island test.
Table 3.
Correlation matrix examining the associations among performance on Memory Island (MI), the Mental Rotation Test, computerized Spatial Span, and participant characteristics.
| A. | B. | C. | D. | E. | F. | G. | H. | |
|---|---|---|---|---|---|---|---|---|
| A. MI hidden latency | +1.00 | |||||||
| B. MI probe (% time in target) | −0.24** | +1.00 | ||||||
| C. Mental Rotation | −0.09 | +0.00 | +1.00 | |||||
| D. Forward Spatial Span | −0.22** | +0.17* | +0.18* | +1.00 | ||||
| E. Backward Spatial Span | −0.14 | +0.17* | +0.26** | +0.46** | +1.00 | |||
| F. Laterality Index | +0.07 | +0.04 | −0.08 | −0.09 | −0.03 | +1.00 | ||
| G. Video-Game last week (hrs) | −0.07 | −0.13 | +0.20* | +0.16* | +0.20* | −0.10 | +1.00 | |
| H. Video-Game maximum (hrs) | −0.39** | +0.05 | +0.14 | +0.17* | +0.18* | −0.17* | +0.38** | +1.00 |
p < .05
p < .005.
Finally, a mediation analysis (Preacher & Hayes, 2004) was completed as a secondary analysis. The sex difference on total latency to reach the hidden target was still significant with recent video game experience included as a putative mediator (β = -69.1, S.E. = 18.5, p < .0005) as was mental rotation (β = 3.5, S.E. = 1.4, p < .05) but spatial span was not (β = 0.6, S.E. = 0.5). The significance of the video game experience mediational effect on spatial span was again confirmed with a bootstrapping method with 1,000 replication samples of N = 165 with a 95% confidence interval of the indirect effect (0.04 to 1.10).
DISCUSSION
The two central questions evaluated in this study were: (1) Are there sex differences in performance on spatial learning and memory tasks in young adults? (2) Does APOE affect neurobehavioral spatial function within this population? The three spatial tasks chosen differed in that spatial span and Memory Island are two-dimensional while mental rotation is three-dimensional. Further, mental rotation has a very limited memory component whereas spatial span and Memory Island provided an index of working memory. The presence of clear sex differences without significant effects of APOE genotype on performance supported our first, but not second, hypothesis. This outcome is congruent with an earlier report showing sexually dimorphic visual recognition memory on the Novel Image Novel Location test, but with no effects of APOE (Piper et al., 2011b).
This investigation showed significant and robust sex differences. A large male advantage for the Mental Rotation task was identified, which was consistent with expectations (Andreano & Cahill, 2009). In addition, participants in the Astur, Tropp, Sava, Constable, and Markus (2004) report who performed well on the mental rotation task also found the hidden platform sooner during the virtual maze task, a result that was quite different than the present findings where no significant correlation was obtained. It should, therefore, be emphasized that the virtual maze of Astur only has distal visual cues and therefore requires the formation of a mental map. In contrast, Memory Island has many local cues and we strongly suspect that subjects were using a strategy (e.g., “head towards the glass sculpture and then turn left”). Further research where participants complete different virtual mazes is warranted but, until then, we suspect that the Astur maze, like the Morris water maze, is a clearer index of allocentric memory. Alternatively, Memory Island, like real-world navigation, can involve egocentric or allocentric memory. Significant sex differences obtained in the Memory Island task were consistent with earlier findings (Acevedo et al., 2010). These outcomes were also congruent with faster and more accurate performance by males than females across the lifespan in virtual mazes (Berteau-Pavy et al., 2007; Driscoll, Hamilton, Yeo, Brooks, & Sutherland, 2005; Piper et al., 2011a; Rizk-Jackson et al., 2006).
The neural substrates responsible for sexually dimorphic behaviors have yet to be conclusively characterized. Total brain size is about ten percent larger in males (Giedd, Raznahan, Mills, & Lenroot, 2012) but volumetric analysis of specific structures (e.g., the hippocampus) reveals limited evidence for sex differences (Giedd et al., 1996; Uematsu et al., 2012). Clear differences were apparent using functional magnetic resonance imaging, which revealed that males showed greater parietal activation than females whereas females, relative to males, demonstrated heightened right frontal activity during mental rotation (Weiss et al., 2003). Virtual maze learning and memory present nontrivial technical challenges to translation into a neuroimaging environment but a preliminary report showed sexually dimorphic activation in the hippocampus, parahippocampus, and cingulate cortex (Sneider, Sava, Rogowska, & Yurgelun-Todd, 2011). A pronounced behavioral advantage for males is apparent with Memory Island at prepubescent ages (Acevedo et al., 2010; Piper et al., 2011a). Similarly, a sex difference in mental rotation is evident among first-graders (Tzuriel & Egozi, 2010) and using the preferential gaze methodology in infants (Moore & Johnson, 2008; Quinn & Liben, 2008).
Importantly, the present findings contradicted some past literature documenting the effects of APOE on memory tasks (Herz & Beffert, 2000), including spatial performance using Memory Island (Acevedo et al., 2010; Berteau-Pavy et al., 2007). Importantly, these results were congruent with others documenting an absence of APOE associated neurobehavioral effects, particularly at pre-senescent ages (Alexopoulos et al., 2011; Deary et al., 2002; Ihle et al., 2012; Matura et al., 2014; Piper et al., 2011; Westlye, Reinvang, Rootwelt, & Espesth, 2012). Evoked response potential research suggests that cognitive impairment associated with ε4 is age-dependent and thus not detected during young adulthood (Yu et al., 2000). Ruiz et al. (2010) proposed that the ε4 allele alone is not responsible for the decreased cognitive performance observed in many APOE studies. Instead, the findings from this team suggest that the combined presence of ε4 and the methylenetetrahydrofolate reductase 677 TT genotype may be responsible for decreased cognitive performance. Another possible explanation for the lack of genotype effects on performance is that ε4 carriers may find ways to compensate for the disparity seen during childhood, essentially eliminating measureable memory disadvantages by the time they reach young adulthood. The lack of neurobehavioral differences in this study may suggest that, in a young adult sample, there might be transient behavioral differences as a function of APOE or only behavioral differences on select cognitive tests. Taylor et al. (2011) determined that APOE genotype was strongly associated with levels of HDL and LDL cholesterol, but not with IQ, memory tasks, or performance on standardized school assessments among approximately 4,000 children. Our null findings suggest that the detrimental effects of ε4 on cognitive performance may be transient or not appear until later in life. Of course, this interpretation might contradict earlier findings with primary school children (Acevedo, 2010; Oria et al., 2005). Importantly, as these earlier reports contained both a large number of measures and very small sample sizes, they may reflect Type I errors. A recently completed meta-analysis concluded that APOE is not associated with individual differences in executive function using a wide variety of spatial and non-spatial measures with children, adolescents, or young adults (Ihle, Bunce, & Kliegel, 2012). The present neurobehavioral findings, as well as those of others (Alexopoulos et al., 2011; Westlye et al., 2012), were consistent with that outcome.
The characteristics of the sample employed are also worthy of consideration. The participants were obtained from a selective private school where many are from middle or upper socioeconomic households. As computerized training can reduce sex differences in spatial cognition (Feng et al., 2007; Green & Bevelier, 2003), another possibility is that high technological familiarity may have obscured detection of genotype differences. Extensive computerized experience, particularly with the first person perspective style games that benefit visuospatial capabilities (Cherney, 2008; Spence, Yu, Feng, & Marshman, 2009), would also be much less likely among pre-adolescent or the elderly samples where an effect of ε4 was observed (Acevedo et al., 2010; Berteau-Pavy et al., 2007).
This report indicated that increased video game experience was associated with finding the hidden targets more efficiently. Although there were clear sex differences in both video game experience and Memory Island performance, ANCOVA and mediation analyses indicated that these factors were independent in this sample (see Astur et al., 2004 for analogous findings). However, we strongly recommend that other investigators employing Memory Island, particularly with non-senescent aged samples, carefully take this variable into consideration. Also noteworthy, researchers have not been able to uniformly identify a male advantage in the Spatial Span task (Acevedo et al., 2010; Berteau-Pavy et al., 2007; Farrell, Busch, Medina, Bartok, & Krikorian, 2006; Piper et al., 2011a) or have documented relatively modest sex differences (Andreano & Cahill, 2009). As this effect was both relatively small (d = 0.34) and mediated by prior video game experience, the larger sample size in this report relative to prior investigations (Acevedo et al., 2010; Berteau-Pavy et al., 2007; Farrell et al., 2006; Piper et al., 2011a) or participant age could be important.
Although the sample was quite sufficient to detect sex differences, the possibility certainly exists that genotype effects are much more subtle and would have been observed with a larger sample. However, increasing the sample size with adolescent/young-adult populations has tended to decrease the likelihood that APOE effects are observed (Ihle et al., 2012). Importantly, a very well powered investigation (N ≈ 4,000 children) documented no significant effects of APOE on IQ, short-term memory, working memory, or school achievement (Taylor et al., 2011), indicating that the present findings may also reflect a true null result and not a Type II error.
Conclusion
Sex differences were identified in mental rotation, spatial span, and Memory Island performance in young adults. In contrast, we found no evidence of significant APOE effects. Future studies may be directed at characterizing the neural substrates and also how computerized experiences contribute to these sexually dimorphic behaviors.
Acknowledgements
Oregon Health and Science University receives a modest, one-time licensing fee from some laboratories that use Memory Island.
Footnotes
Authors ALY, JKM, and BJP declare that they have no competing interests.
REFERENCES
- Acevedo SF, Piper BJ, Craytor M, Benice T, Raber J. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatric Research. 2010;67:293–299. doi: 10.1203/PDR.0b013e3181cb8e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos P, Richter-Schmidinger T, Horn M, Maus S, Reichel M, Sidiropoulos C, et al. Hippocampal volume differences between healthy young apolipoprotein E ε2 and ε4 carriers. Journal of Alzheimer’s Disease. 2011;26:207–210. doi: 10.3233/JAD-2011-110356. [DOI] [PubMed] [Google Scholar]
- Andreano J, Cahill L. Sex influences on the neurobiology of learning and memory. Learning & Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Astur R, Ortiz M, Sutherland R. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behavioural Brain Research. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Astur R, Tropp J, Sava S, Constable R, Markus E. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behavioural Brain Research. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE ε4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Boueiz A, Abougergi MA, Kitner-Triolo MH, Beydoun HA, Resnick SM, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment and decline. Neurobiology & Aging. 2012;33:720–731. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney ID. Mom, let me play more computer games: They improve my mental rotation skills. Sex Roles. 2008;59:776–786. [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, et al. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- De Blasi S, Montesanto A, Martino C, Dato S, De Rango F, Bruni AC, et al. APOE polymorphism affects episodic memory among non-demented elderly subjects. Experimental Gerontology. 2009;44:224–227. doi: 10.1016/j.exger.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton D, Yeo R, Brooks W, Sutherland R. Virtual navigation in humans: The impact of age, sex, and hormones on place learning. Hormones and Behavior. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Estes Z, Felker S. Confidence mediates the sex difference in mental rotation performance. Archives of Sexual Behavior. 2012;41:557–570. doi: 10.1007/s10508-011-9875-5. [DOI] [PubMed] [Google Scholar]
- Farrell KP, Busch RM, Medina KL, Bartok JA, Krikorian R. Developmental normative data for the Corsi Block-tapping task. Journal of Clinical and Experimental Neuropsychology. 2006;28:1043–1052. doi: 10.1080/13803390500350977. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feng J, Spence I, Pratt J. Playing an action video game reduces gender differences in spatial cognition. Psychological Science. 2007;18:50–855. doi: 10.1111/j.1467-9280.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of Sex Differences. 2012;3:19. doi: 10.1186/2042-6410-3-19. doi:10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4-18 years. Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Green CS, Bevelier D. Action video game modifies visual spatial attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Herz J, Beffert U. Apolipoprotein E receptors: Linking brain development and Alzheimer's disease. Nature Reviews Neuroscience. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- Hubacek J, Pitha J, Škodová Z, Adámková V, Lánská V, Poledne R. A possible role of apolipoprotein E polymorphism in predisposition to higher education. Neuropsychobiology. 2001;43:200–203. doi: 10.1159/000054890. [DOI] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M. APOE ε4 in early life: A meta-analysis. Neuropsychology. 2012;26:267–277. doi: 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- Jansen-Osmann P, Heil M. Suitable stimuli to obtain (no) gender differences in the speed of cognitive processes involved in mental rotation. Brain and Cognition. 2007;64:217–227. doi: 10.1016/j.bandc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Laws S, Hone E, Gandy S, Martins R. Expanding the association between the APOE gene and the risk of Alzheimer's disease: Possible roles for APOE promoter polymorphisms and alterations in APOE transcription. Journal of Neurochemistry. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- Linn M, Petersen A. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Development. 1985;56:1479–1498. [PubMed] [Google Scholar]
- Matura S, Prvulovic D, Hartmann D, Miller J, Scheibe M, O'Dwyer L, et al. Differential effects of the ApoE4 genotype on brain structure and function. Neuroimage. 2014;89:81–91. doi: 10.1016/j.neuroimage.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Mondadori C, de Quervain D, Buchmann A, Mustovic H, Willmer MA, Schmidt C, et al. Better memory and neural efficiency in young apolipoprotein E ε4 carriers. Cerebral Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Moore DS, Johnson SP. Mental rotation in human infants. Psychological Science. 2008;19:1063–1066. doi: 10.1111/j.1467-9280.2008.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oria R, Patrick P, Zhang H, Lomtz B, DeCastro C, Carlos MB, et al. ApoE4 protects the cognitive development in children with heavy diarrhea burdens in northeast Brazil. Pediatric Research. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal T, Kostenuik M, Ossenkopp K, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behavioral Neuroscience. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Craytor M, Murray P, Raber J. The use and validation of the spatial navigation Memory Island test in primary school children. Behavioural Brain Research. 2010;210:257–262. doi: 10.1016/j.bbr.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Edwards K, Curtiss A, McGinnis G, Raber J. Age, sex, and handedness differentially contribute to neurospatial function on the Memory Island and Novel-Image Novel-Location tests. Physiology and Behavior. 2011a;103:513–522. doi: 10.1016/j.physbeh.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Yasen A, Miller JK. Examination of sexually dimorphic behavior on the Novel-Image Novel-Location recognition memory test. Journal of Behavioral and Brain Science. 2011b;1:134–139. [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavioral Research Methods. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Liben LS. A sex difference in mental rotation in young infants. Psychological Science. 2008;19:1067–1070. doi: 10.1111/j.1467-9280.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- Rebeck G, Kindy M, LaDu MJ. Apolipoprotein E and Alzheimer’s disease: The protective effects of ApoE2 and E3. Journal of Alzheimer’s Disease. 2002;4:145–154. doi: 10.3233/jad-2002-4304. [DOI] [PubMed] [Google Scholar]
- Reiman E, Caselli R, Yun L, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. New England Journal of Medicine. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Rizk-Jackson A, Acevedo S, Inman D, Howieson D, Benice T, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behavioural Brain Research. 2006;173:181–190. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Castillo R, Labayen I, Moreno L, Fuentes M, Lamuño D, et al. Individual and combined effects of ApoE and MTHFR 677C/T polymorphisms on cognitive performance in Spanish adolescents: The AVENA study. Journal of Pediatrics. 2010;156:978–984. doi: 10.1016/j.jpeds.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk L, Lysczek C, Tannock R, Pelham W, Spencer S, et al. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2008;36:903–913. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman I, Choi J, Peters M. The hunter-gatherer theory of sex differences in spatial abilities: Data from 40 countries. Archive of Sexual Behavior. 2007;36:261–268. doi: 10.1007/s10508-006-9168-6. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Sava S, Rogowska J, Yurgelun-Todd DA. A preliminary study of sex differences in brain activation during a navigation task in healthy adults. Perceptual & Motor Skills. 2011;113:461–480. doi: 10.2466/04.22.24.27.PMS.113.5.461-480. [DOI] [PubMed] [Google Scholar]
- Spence I, Yu JJ, Feng J, Marshman J. Women match men when learning a spatial skill. Journal of Experimental Psychology: Learning Memory, and Cognition. 2009;35:1097–1103. doi: 10.1037/a0015641. [DOI] [PubMed] [Google Scholar]
- Swan G, Lessov-Schlaggar C, Carmelli D, Schellenberg G, La Rue A. Apolipoprotein E ε4 and change in cognitive functioning in community-dwelling older adults. Journal of Geriatric Psychiatry and Neurology. 2005;18:196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Taylor A, Guthrie P, Smith G, Golding J, Sattar N, Hingorania A, et al. IQ, educational attainment, memory, and plasma lipids: Associations with apolipoprotein E genotype in children. Biological Psychiatry. 2011;70:152–158. doi: 10.1016/j.biopsych.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzuriel D, Egozi G. Gender differences in spatial ability of young children: The effects of training and processing strategies. Child Development. 2010;81:1417–1430. doi: 10.1111/j.1467-8624.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLOS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg S, Kuse A. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual and Motor Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D, Postma A, Brake B, Imperato-McGinley J. Gender differences in object location memory: A meta-analysis. Psychonomic Bulletin and Review. 2007;14:23–38. doi: 10.3758/bf03194024. [DOI] [PubMed] [Google Scholar]
- Weiss E, Siedentopf CM, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, et al. Sex differences in brain activation pattern during a visuospatial cognitive task: A functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters. 2003;344:169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Reinvang I, Rootwelt H, Espesth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79:1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human colunteer apolipoprotein E ε4 and non-ε4 carriers. Neuroscience Letters. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]