Abstract
Background
Long chain fatty acid oxidation disorders (LC-FAOD) are caused by defects in the metabolic pathway that converts stored long-chain fatty acids into energy, leading to a deficiency in mitochondrial energy production during times of physiologic stress and fasting. Severe and potentially life threatening clinical manifestations include rhabdomyolysis, hypoglycemia, hypotonia/weakness, cardiomyopathy and sudden death. We present the largest cohort of patients to date treated with triheptanoin, a specialized medium odd chain (C7) triglyceride, as a novel energy source for the treatment of LC-FAOD.
Methods
This was a retrospective, comprehensive medical record review study of data from 20 of a total 24 patients with LC-FAOD who were treated for up to 12.5 years with triheptanoin, as part of a compassionate use protocol. Clinical outcomes including hospitalization event rates, number of hospitalization days/year, and abnormal laboratory values were determined for the total period of the study before and after triheptanoin treatment, as well as for specified periods before and after initiation of triheptanoin treatment. Other events of interest were documented including rhabdomyolysis, hypoglycemia, and cardiomyopathy.
Results
LC-FAOD in these 20 subjects was associated with 320 hospitalizations from birth to the end date of study. The mean hospitalization days/year decreased significantly by 67% during the period after triheptanoin initiation (n=15; 5.76 vs 17.55 vs; P=0.0242) and a trend toward a 35% lower hospitalization event rate was observed in the period after triheptanoin initiation compared with the before-treatment period (n=16 subjects >6 months of age; 1.26 vs 1.94; P=0.1126). The hypoglycemia event rate per year in 9 subjects with hypoglycemia problems declined significantly by 96% (0.04 vs 0.92; P=0.0091) and related hospitalization days/year were also significantly reduced (n=9; 0.18 vs 8.42; P=0.0257). The rhabdomyolysis hospital event rate in 11 affected subjects was similar before and after treatment but the number of hospitalization days/year trended lower in the period after triheptanoin initiation (n=9; 2.36 vs 5.94; P=0.1224) and peak CK levels trended toward a 68% decrease from 85,855 to 27,597 units in 7 subjects with reported peak CK values before and after treatment (P=0.1279). Triheptanoin was generally well tolerated. Gastrointestinal symptoms were the most commonly reported side effects.
Conclusions
This retrospective study represents the largest analysis reported to date of treatment of LC-FAOD with triheptanoin. The data suggest that triheptanoin improves the course of disease by decreasing the incidence and duration of major clinical manifestations and should be the focus of prospective investigations. Significant heterogeneity in the routine clinical care provided to subjects during the periods studied and the natural variation of clinical course of LC-FAODs with time emphasize the need additioanl study of the use of triheptanoin.
Keywords: Long Chain Fatty Acid Oxidation Disorder, triheptanoin, retrospective medical chart review, safety and effectiveness
Introduction
Inherited autosomal recessive defects in the mitochondrial long-chain fatty acid oxidation pathways are collectively referred to as long-chain fatty acid oxidation disorders (LC-FAOD). LC-FAODs are caused by enzyme deficiencies of the carnitine cycle or the mitochondrial β-oxidation pathway that converts fatty acids into energy, leading to deficiencies in mitochondrial energy metabolism [1]. Partial or incomplete oxidation of fatty acids leads to accumulation of high concentrations of metabolic intermediates in blood and organs and potentially devastating systemic effects such as hypoglycemia and acidosis [2].
LC-FAODs are characterized clinically by episodic crises of energy metabolism, particularly during periods of physiologic stress, infection, or fasting. Symptoms vary by age of onset and the clinical phenotype evolves over time. Neonates and young children may exhibit hypoglycemia, hepatic dysfunction, rhabdomyolysis, and cardiomyopathy while adolescents and adults primarily experience exercise or stress-induced rhabdomyolysis, which may be severe [3].
The current standard of care for LC-FAOD is dietary management. Treatment may vary according to the specific disorder and the severity of the underlying enzyme deficiency, but may include a low fat, high carbohydrate diet, the avoidance of fasting, and supplementation with carnitine and/or medium even chain triglyceride (MCT) oil, along with aggressive treatment of co-morbid illness [4, 5]. The effectiveness of therapies has largely been reported on a case-by-case basis with little information available from well-controlled clinical studies. A retrospective analysis of 187 clinically diagnosed patients with LC-FAOD concluded that mortality rates have not changed overall for patients during the past 30 years, even with an evolving standard of care, and overall there is greater than 50% mortality in each decade, with some indications having mortality in the 60–95% range during their period of review [2]. However, newborn screening and early treatment may reduce mortality and improve outcomes [6], although long-term experience has not yet been published. Despite diagnosis and treatment from the newborn period, one study demonstrated that FAOD subjects still had a major decompensation rate of ∼25% in the two years after birth [7]. Thus, patients require better treatment options, particularly to prevent the major decompensation events that lead to hospitalization and major morbidity and mortality.
The primary objective of this study was to evaluate the impact of triheptanoin treatment on the major clinical manifestations of LC-FAOD by performing a comprehensive and systematic medical chart review of patients treated for up to 12.5 years with triheptanoin through a compassionate use program.
Patients and methods
Study population
Twenty patients with LC-FAOD (11 males, 9 females) of 24 total patients that were treated with triheptanoin long-term as part of a compassionate use program were included in a retrospective medical chart review (Table 1). Patients were treated at Children’s Hospital of Pittsburgh of UPMC (University of Pittsburgh Medical Center, Pittsburgh, PA) with a majority of patients having transferred from Baylor Research Institute (Dallas, TX), but the subjects were originally identified by referrals for compassionate use from throughout the US. Patients with acute metabolic symptoms were treated emergently by the local metabolic specialist. All patients had a confirmed diagnosis of one of the LC-FAOD disorders: mitochondrial trifunctional protein (TFP) deficiency, carnitine palmitoyltransferase deficiencies (CPT II), very long chain acyl-CoA dehydrogenase (VLCAD) deficiency, isolated long-chain 3-hydroxy-acyl-CoA dehydrogenase (LCHAD) deficiency, or carnitine acylcarnitine translocase (CACT) deficiency. Written informed consent was obtained from participants or from a legally authorized representative for participants who were under the age of 18. The study was reviewed and approved by the Institutional Review Board at the University of Pittsburgh.
Table 1.
Patient demographics and disease history
| Characteristic | N= 20 |
|---|---|
| Age, years, median (range)a | 12.7 (1.6 to 60.9) |
| Sex, female, n (%) | 9 (45%) |
| Ethnicity/Race | |
| White/Non-Hispanic | 19 (95%) |
| Other/Hispanic | 1 (5%) |
| LC-FAOD diagnosis type, n (%) | |
| MTFP | 2 (10%) |
| CPT1 | 0 |
| CPT2 | 3 (15%) |
| VLCAD | 9 (45%) |
| LCHAD | 5 (25%) |
| CACT | 1 (5%) |
| Family history of LC-FAOD, n (%) | 9 (45%) |
| Age at symptom onset, median (range) | 0 (0 to 9.75) |
| < 1 year | 16 (80%) |
| 10 years | 1 (5%) |
| unknown | 3 (15%) |
| Age at diagnosis | |
| ≤ 1 year | 14 (70%) |
| >1 year to ≤ 5 years | 3 (15%) |
| > 5 years to ≤ 10 years | 1 (5%) |
| > 10 years | 2 (10%) |
| Method of diagnosisb | |
| Prenatal screening | 2 (10%) |
| Newborn screening | 9 (45%) |
| Acylcarnitine profile | 15 (75%) |
| Skin biopsy | 12 (60%) |
| Other | 4 (20%) |
| MCT use | 17 (85%) |
Age at time of chart review
Some patients were diagnosed by more than one method
Data extraction and analysis
A systematic, protocol-driven, comprehensive medical chart review of patients’ records was used to establish a clinical baseline on standard of care treatment and then document the clinical outcomes after initiating triheptanoin treatment. Data from 120 individual patient medical charts were extracted to approximately 1600 case report forms covering 330 patient-years of time. The median pretreatment period was 3.7 years (range 0.05–51.39) and the median triheptanoin treatment time was 8.7 years (range 0.5–12.5). Seventeen of the 20 subjects had 5 years or longer duration of treatment using triheptanoin.
Data collection and analysis focused on major clinical events including hospitalizations and emergency room admissions related to FAOD disease and related complications, including: 1) rhabdomyolysis, 2) hypoglycemia and 3) cardiomyopathy. Events that included documented verbatim terms from medical staff such as “rhabdomyolysis” or “hypoglycemia” were included even when a correlated lab result was not available. Number of hospitalizations (including emergency department visits), days of hospitalization, and laboratory values were abstracted. The number and severity of events [defined as creatine kinase (CK) levels > 5,000U/L] associated with rhabdomyolysis events occurring pre- and post- triheptanoin treatment initiation] were compared. Hypoglycemia was not plausibly analyzed by glucose levels due to emergency interventions that affect glycemia during these medical events, however, when available, blood glucose levels < 60 mg/dL were recorded. For infants who received triheptanoin in the first six months of life (n=4), the time period prior to initiation of triheptanoin treatment was not sufficient to enable an accurate comparison of events pre and post treatment, so analyses of infants were conducted separately for events of interest. Hospitalization event rates were calculated as the number of events occurring during the risk duration/length of risk duration in years. Pre-triheptanoin risk duration was calculated as follows: (First dose date – date of birth)/365. Similarly, post-triheptanoin risk duration was calculated as (last medical record date – first dose date +1)/365. If a patient discontinued triheptanoin treatment, the last dose date was substituted for the last medical record date to calculate the post-triheptanoin risk duration; Only 2 patients discontinued triheptanoin during the treatment period, neither for reasons related to side effects of the medication. Event rate comparisons before and after starting triheptanoin treatment were made for those patients who experienced those particular events in the pre-treatment period. As a sensitivity analysis, event rates were also calculated using a narrower defined time window of 2 years before and 2 years after initiating triheptanoin treatment, to minimize changes that might be due to phenotype evolution as the subjects age. Windows of time more narrow than 2 years did not include a sufficient number of events to be reliable.
Volume and dose of triheptanoin over time were collected for each subject. Side effects associated with triheptanoin were abstracted.
Pairwise t-tests were used to compare rates of hospitalizations per year and the number of hospitalization days per year pre- and post-triheptanoin treatment for the following events: total events, hypoglycemia, rhabdomyolysis, and cardiomyopathy using a statistical analysis plan, but no adjustments were made for multiplicity of these analyses as this is a hypothesis-generating retrospective study.
Results
Baseline demographics and disease characterization
The clinical phenotype of LC-FAODs can change with age. To minimize this effect we only analyzed the clinical course for the two years before and after the start of triheptanoin. Medical history of patients before triheptanoin treatment initiation was characteristic of patients with LC-FAOD (Table 2). Events of rhabdomyolysis, muscle pain, and hypoglycemia were reported for at least half of patients. Four patients had a history of cardiomyopathy. Four patients were less than 6 months of age at initiation of triheptanoin and had more limited pretreatment history, and their outcomes were analyzed separately.
Table 2.
FAOD Symptom History Before Triheptanoin Treatment
| Sign/Symptom | N=20 n (%) |
|---|---|
| Rhabdomyolysis1 | 11 (55%) |
| Muscle pain | 11 (55%) |
| Hypoglycemia2 | 10 (50%) |
| Poor feeding/poor weight gain | 9 (45%) |
| Hepatomegaly | 8 (40%) |
| Hypotonia | 8 (40%) |
| Respiratory distress | 7 (35%) |
| Altered mental status/coma | 7 (35%) |
| Muscle weakness | 7 (35%) |
| Developmental delay | 6 (30%) |
| Exercise intolerance | 5 (25%) |
| Cardiomyopathy3 | 4 (20%) |
| Abnormal gait | 3 (15%) |
| Hemolysis, elevated liver enzymes, Low platelets | 3 (15%) |
| Seizures | 3 (15%) |
| Peripheral neuropathy | 2 (10%) |
| Retinopathy | 1 (5%) |
| Other | 13 (65%) |
A CK > 5,000 U/L or notation of “rhabdomyolysis” in the medical record were counted as events, even if a coinciding CK level was not documented.
Hypoglycemia was defined as glucose <60 mg/dL or “hypoglycemia” noted in the clinical note.
Limited 2D echo data were available. An ejection fraction < 40 was considered severe cardiomyopathy.
Twenty of 24 patients consented for the chart review, and from these patients 1600 case report forms were generated covering 330 years of patient records, containing data on 320 hospitalizations in 120 individual medical charts. An assessment of completeness based on the contiguity of time intervals found 78% of charts were complete or nearly complete. The relatively limited number of missing data appeared to be random and thus was not expected to impact the interpretation of the results. There was no particular pattern to the 4 patients that did not consent in terms of their apparent outcomes.
Demographics and clinical characteristics of patients included in the chart review cohort are shown in Tables 1 and 2. The median age of patients at the time of data abstraction, as calculated from date of birth, was 12.7 years (range=1.6 to 60.9 yrs). Patients included 11 males and 9 females, and all but one patient were Caucasian. LC-FAOD diagnoses were varied. Nearly half of patients (n=9, 45%) had a family history of LC-FAOD. Of note, over half of the patients were identified symptomatically rather than through newborn screening. This was primarily because expanded newborn screening by tandem mass spectrometry to enable detection of LC-FAODs was introduced at various times across the US during the period of study. For patients diagnosed through clinical symptoms, the episode leading to the diagnosis was not included in Table 2.
Review of clinical data showed inconsistent documentation of dietary history; however, 85% of patients received MCT (Table 2). Almost all patients were noted to be on a low fat high carbohydrate diet. Nine patients were noted to have gastrosotomy tubes at various times during the study. No other routine dietary changes as a result of study participation were made over the 4 year time span of the record review as best as can be discerned from the medical records. Moreover, there was significant dietary heterogeneity across the multiple caregivers and many years represented in the study.
Triheptanoin therapy
A summary of triheptanoin treatment history is shown in Table 3. A total of 13 patients (65%) initiated treatment before age 6, and three patients initiated treatment after 16 years of age, including two patients in their 60s. Most patients (85%) had received triheptanoin treatment for at least 5 years. The triheptanoin dose target was 30–35% of calories, although some patients did not achieve this level. The median age at triheptanoin treatment initiation was 3.7 years (range=0 to 51.4), and median time on treatment was 8.7 years (range=0.5 to 12.5). The dose applied ranged from 0.17 mL/kg to 5.62 mL/kg (5.4 to 228.0 g/day) and changed over time for a given patient with no apparent pattern with regard to age at start or duration of treatment. The mean (SD) dose was 69.9 (41.1) g/day. Triheptanoin was usually gradually introduced in patients not on MCT oil. In patients on MCT, a direct exchange was made with triheptanoin, and the dose was titrated to the target dose. If symptoms occurred at a higher dose, it was titrated down to a tolerable dose, and then titrated up slowly. In some patients, soluble fiber was added to slow gut transit time. Oil administration varied widely among patients, with most taking it in several divided doses daily mixed with food or a liquid. Compliance with the prescribed dose between yearly study site visits, was not able to be ascertained.
Table 3.
Triheptanoin Treatment History
| Age at start of treatment |
Duration of Triheptanoin Treatment | ||||
|---|---|---|---|---|---|
| < 1 year (n) |
1–2 years (n) |
2–5 years (n) |
> 5 years (n) |
Total (n) |
|
| 0–1 month | - | - | - | 2 | 2 |
| 1 month–<2 years | 1 | - | 1 | 3 | 5 |
| 2 to <6 yrs | 6 | 6 | |||
| 6 to <12 yrs | - | - | - | 4 | 4 |
| 12–16 yrs | - | - | - | - | - |
| >16 yrs | - | 1 | 2 | 3 | |
| Total (n) | 1 | - | 2 | 17 | 20 |
Events
Overall hospitalizations
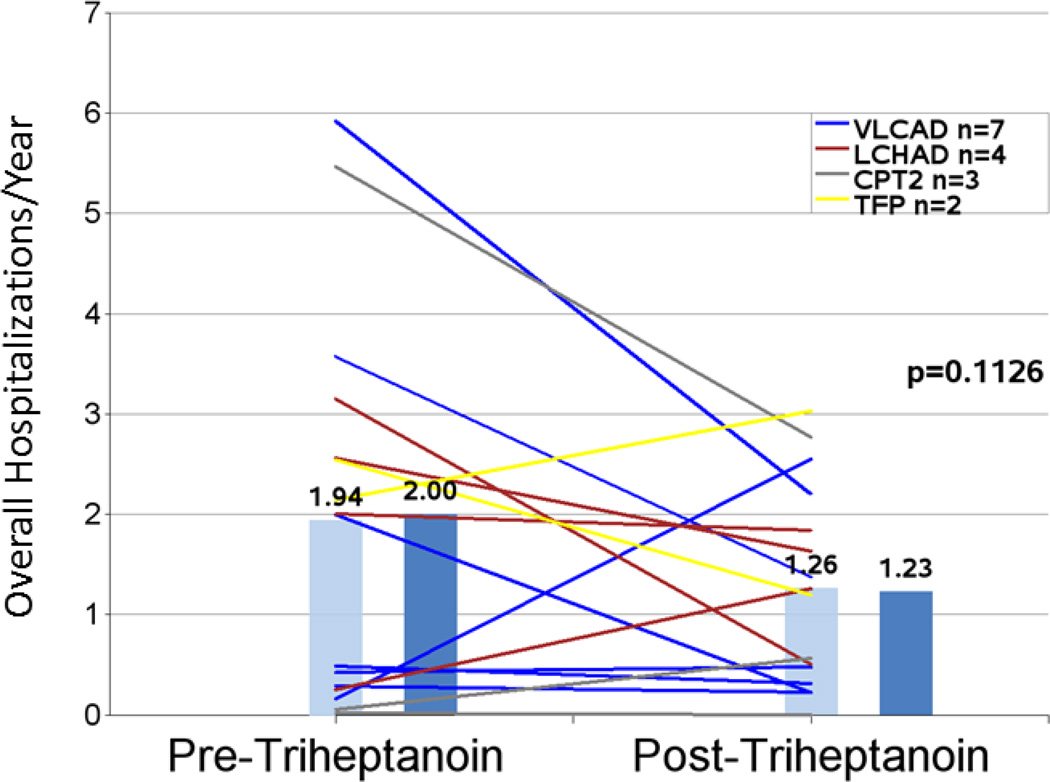
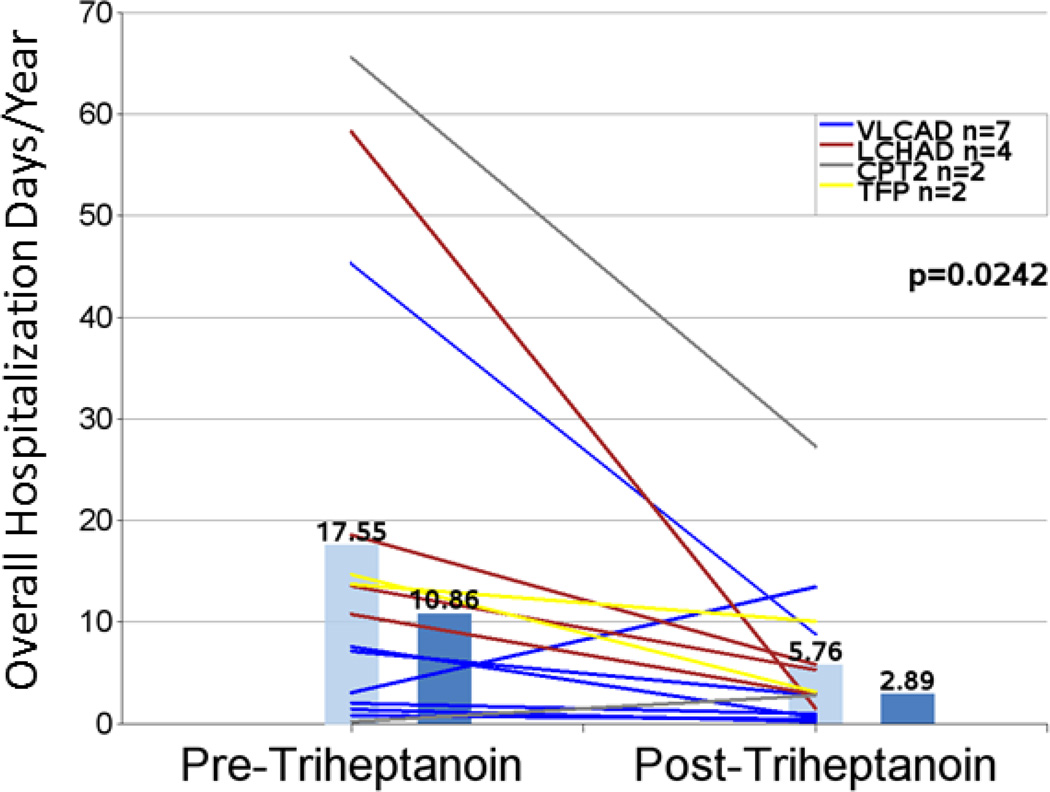
A trend toward a 35% reduction in mean hospitalizations per year from 1.94 to 1.26 (n=16; P=0.1126) was observed following initiation of treatment with triheptanoin compared with the pretreatment period (Figure 1 and Table 4). The decrease in hospitalizations was accompanied by a significant 67% reduction in mean total hospital days per year after triheptanoin treatment initiation (n=15; 17.55 vs 5.76; P=0.0242) (Figure 2 and Table 4). Similar results were found when the analysis was limited to hospitalizations occurring in the 2-year periods before and after triheptanoin initiation with reductions in both total hospitalizations/year (n= 9; 2.22 vs 1.28; P=0.1050) and total hospitalization days/year (n=8; 16.75 vs 10.13; P=0.0541).
Figure 1.
Overall hospitalization event rates in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life.
Table 4.
Summary of Major Medical Events in the Periods Before and After Initiation of Triheptanoin
| Description of Event | n | Pre-treatment | Post-treatment | % Decrease |
P value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Mean total hospitalizations/year1 | 16 | 1.94 (1.890) | 1.26 (0.992) | 35% | 0.1126 |
| Mean total hospital days/year 1,2 | 15 | 17.55 (21.259) | 5.76 (7.114) | 67% | 0.0242 |
| Mean infant total hospitalizations/year 3 | 4 | 13.01 (8.079) | 1.36 (1.480) | 90% | 0.0892 |
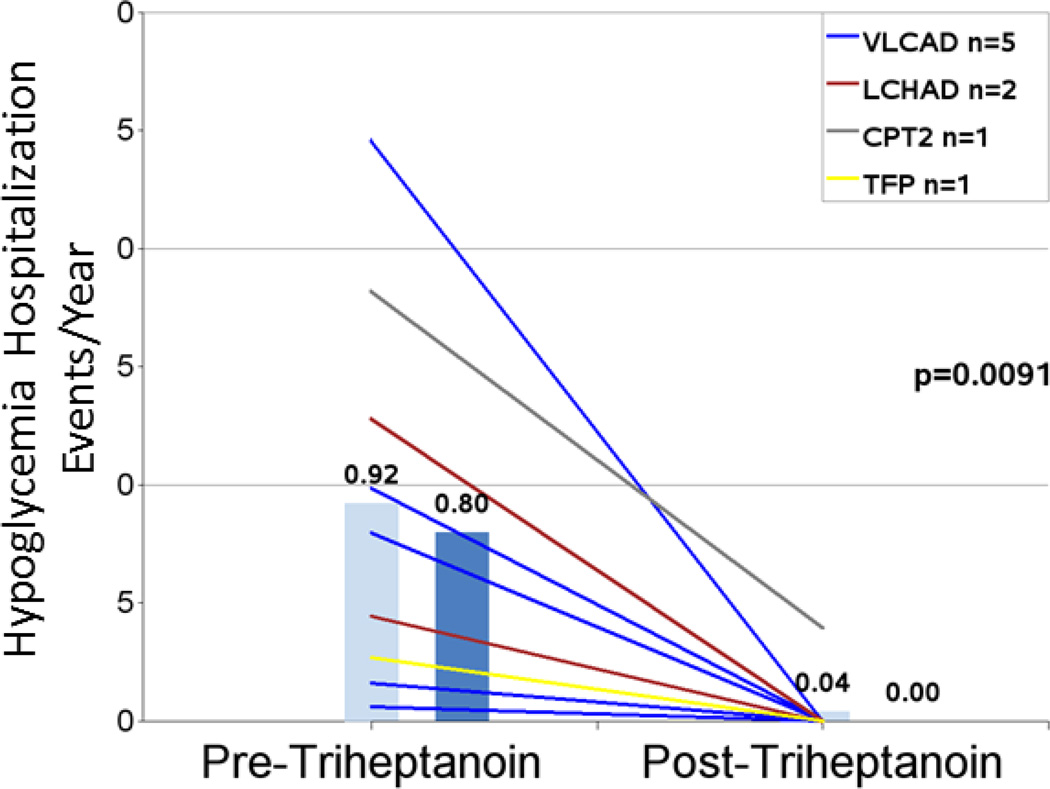
| Mean hypoglycemia events/year 1,2,4 | 9 | 0.92 (0.815) | 0.04 (0.132) | 96% | 0.0091 |
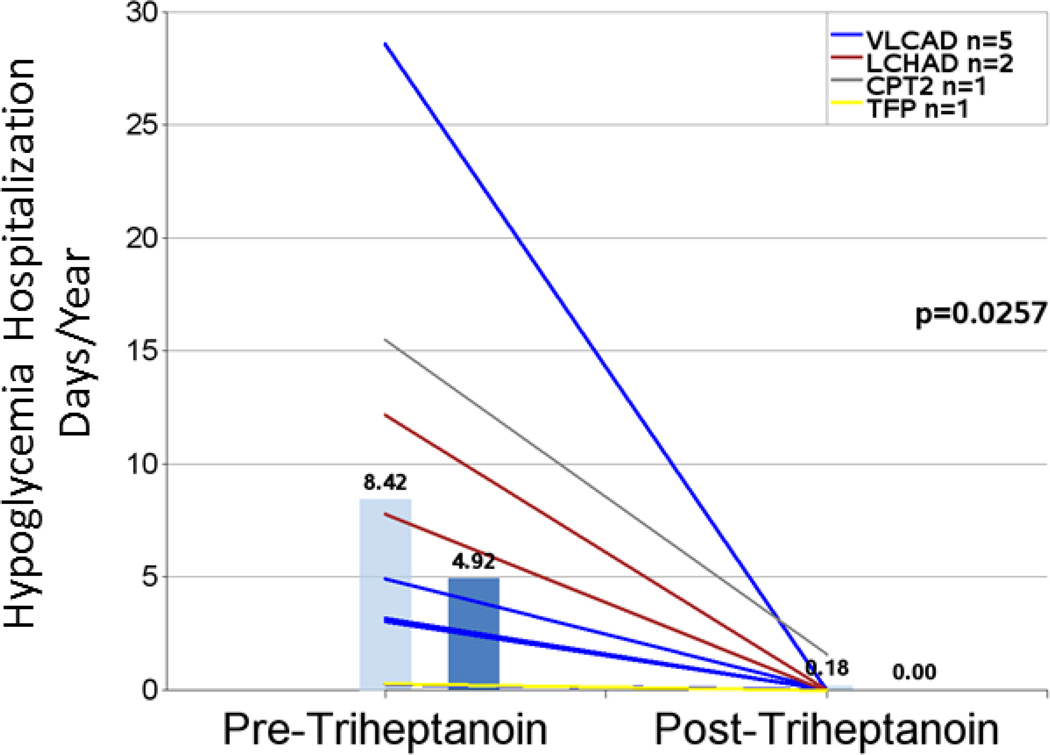
| Mean hypoglycemia total hospital days/year1,4 | 9 | 8.42 (9.178) | 0.18 (0.527) | 98% | 0.0257 |
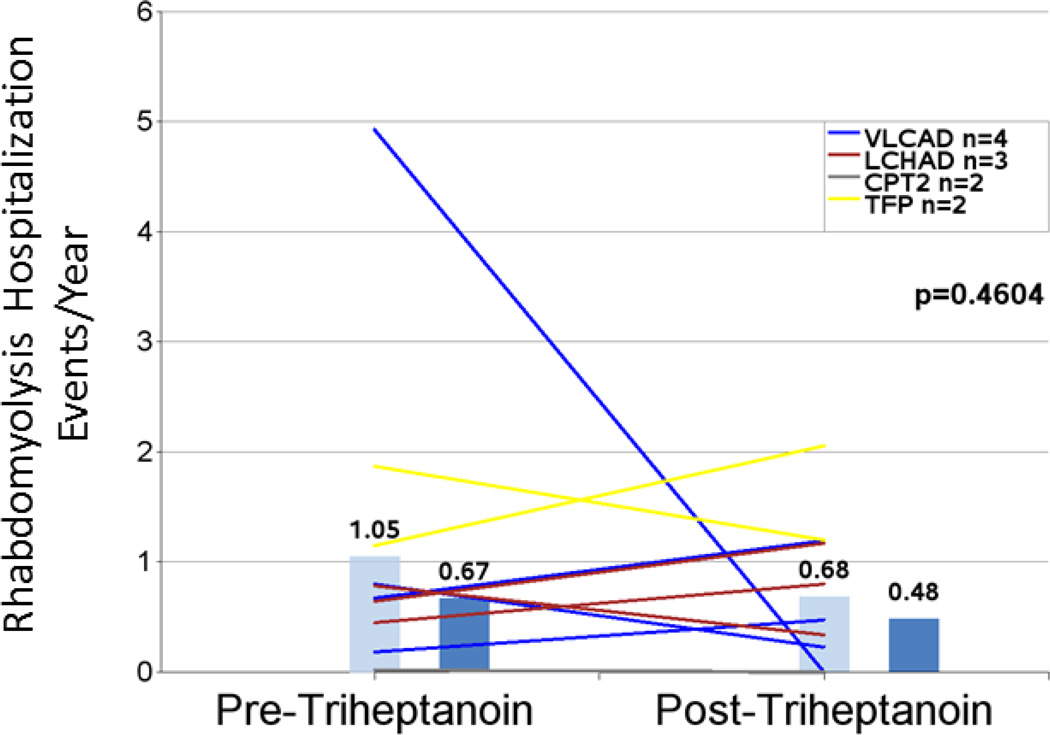
| Mean rhabdomyolysis events/year1,5 | 11 | 1.05 (1.394) | 0.68 (0.665) | 35% | 0.4604 |
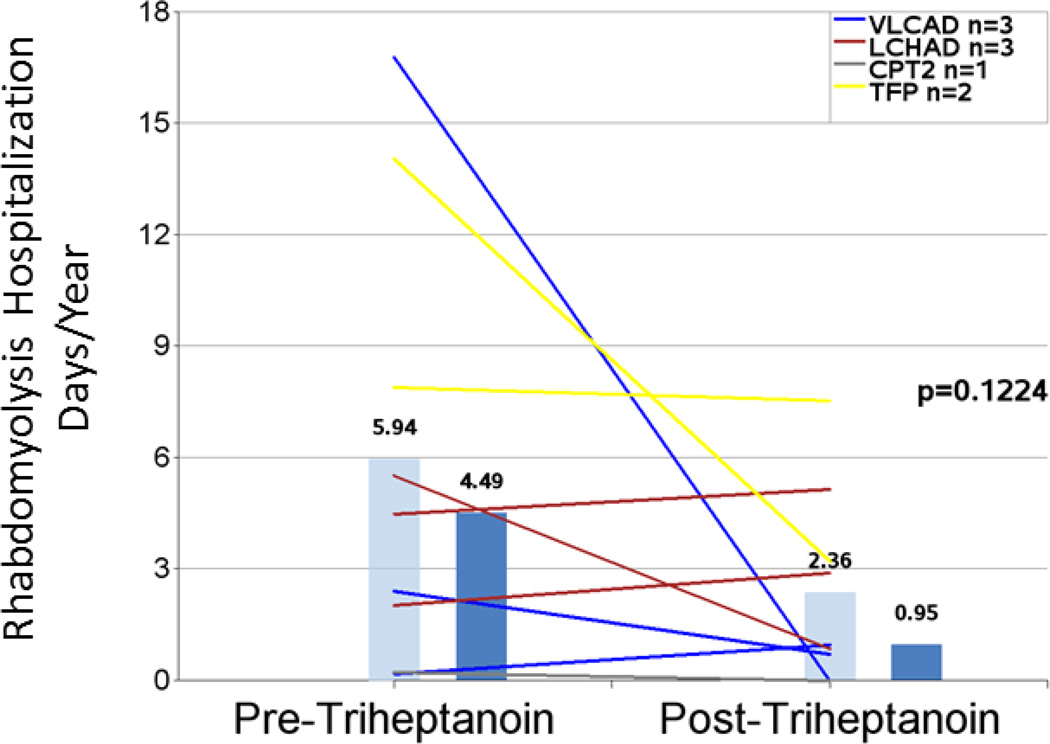
| Mean rhabdomyolysis total hospital days/year1,5 | 9 | 5.94 (5.949) | 2.36 (2.588) | 60% | 0.1224 |
| Mean peak creatine kinase (units) for rhabdomyolysis events1,5 | 7 | 85,855 (95,700) | 27,597 (16,022) | 68% | 0.1279 |
Excludes data for 4 infants dosed within first 6 months of life.
Excludes hospitalizations with unknown discharge dates.
Four infants were dosed within the first 6 months of life.
Includes only those patients with hypoglycemia events prior to treatment.
Includes only those patients with rhabdomyolysis events prior to treatment.
Figure 2.
Total hospitalization days per year in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life.
Hypoglycemia
Nine patients had hypoglycemia events in the pretreatment period. Age at time of events ranged from birth to 6 years, consistent with the phenotype of LC-FAOD. The hypoglycemia event rate (hospitalization events/year) decreased by 96% from 0.92 pretreatment to 0.04 post-triheptanoin treatment (n=9; P=0.0091; Figure 3 and Table 4). Mean hospitalization days/year for hypoglycemia were significantly reduced by 98% in the post-treatment period (n= 9; 8.42 vs 0.18, P=0.0257; Figure 4 and Table 4). Eight of the nine patients had no hypoglycemia events in the post-treatment observation period, including one patient who had 11 events in the pretreatment period and 0 events after initiating triheptanoin. Hypoglycemic events in this patient were reported from ages 11 months to 3 years, and the patient initiated triheptanoin treatment at 4.5 years old.
Figure 3.
Hospitalizations/year due to hypoglycemia in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life and include only those patients with events in the pretreatment period.
Figure 4.
Hypoglycemia-related hospitalization days per year in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life and include only those patients with events in the pretreatment period.
A similar pattern of response was observed when the analyses were limited to the time period of 2 years before and 2 years after triheptanoin treatment initiation: hospitalization rate (n=3; 1.00 vs 0.00; P=0.1835) and hospitalization days/year (n=3; 7.17 vs 0.00; P=0.2824); however, this group included only 3 patients. Data on serum glucose levels corresponding to hypoglycemia events were limited.
Rhabdomyolysis
Hospitalization event rates for rhabdomyolysis did not appear to change substantially and were 1.05 events/year pretreatment and 0.68 events/year post-triheptanoin (−35%; n=11; P=0.4604) for eleven patients with events in the pretreatment period (Figure 5 and Table 4). The mean hospital days/year trended toward a decrease of 60% after initiation of triheptanoin from 5.94 to 2.36 (n=9; P=0.1224) (Figure 6 and Table 4). A similar pattern was observed when the narrower 2-year intervals before and after triheptanoin initiation were analyzed (2.00 vs 1.20; −40%; n= 5; P=0.1951 for hospitalization events/year and 13.00 vs 4.25 hospital days/year [−67%; n=4; P=0.1635]), though the smaller number of events reduces power for detecting the difference.
Figure 5.
Hospitalizations/year due to rhabdomyolysis events in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life and include only those patients with rhabdomyolysis events in the pretreatment period.
Figure 6.
Rhabdomyolysis-related hospitalization days per year in the periods before and after triheptanoin initiation. Light blue bars are means. Dark blue bars are medians. Lines represent individual patients. Data excludes patients dosed with triheptanoin within the first 6 months of life and include only those patients with rhabdomyolysis events in the pretreatment period.
CK is a biomarker of muscle damage that is elevated with rhabdomyolysis events and was recorded when available during the hospitalization events. Among 7 patients for whom peak CK levels during rhabdomyolysis events were recorded, mean peak CK levels decreased from 85855 U/L pretreatment to 27597 U/L post treatment (P=0.1279).
Cardiomyopathy
Cardiomyopathy is frequent in LC-FAOD but most of the patients in this study had inadequate formal documentation of cardiac function over time to meet the criteria of the retrospective follow up study. Therefore, we (JV, EM, SD, AB) abstracted all records for all patients followed in our clinic and treated with triheptanoin for a history of cardiomyopathy. In total, we had records on 14 patients with a history of cardiomyopathy treated with triheptanoin (Table 5). Six of these patients participated in the retrospective study, while the others came to our care after the retrospective study was started and were therefore not included. One patient (11; from the retrospective study) is notable because in spite of dramatic improvement in muscle tone and glucose control, her heart function did not recover from very poor pretreatment function. She ultimately required transplant. All other patients either improved (8) or remained stable (3). Two patients had resolved their cardiomyopathy before starting triheptanoin and their function has remained stable on treatment.
Table 5.
Cardiomyopathy and triheptanoin treatment
| Patient ID (Current Age) |
Diagnosis | Participated in Retrospective Study |
Ejection Fraction Pretreatment (%) |
Ejection Fraction Post treatment (%) |
|---|---|---|---|---|
| 3 (6 yrs) | CACT | Y | 35 | 67 |
| 7 (8 yrs) | LCHAD | Y | 37 | “Normal” |
| 11 (9 yrs) | CPT2 | Y | 31 | Transplanted |
| 13 (12 yrs) | VLCAD | Y | 40 | 61 |
| 15 (19 yrs)* | VLCAD | N | Resolved before C7 | |
| 19 (23 yrs)* | VLCAD | Y | Resolved before C7 | |
| 21 (18 yrs)* | VLCAD | N | Persistent cardiomyopathy | Resolved |
| 24 (3 yrs) | VLCAD | Y | 65 | 63 |
| 25 (11 yrs) | VLCAD | N | 59 | 52 |
| 26 (3 yrs) | VLCAD | N | “Very low” | 71 |
| 28 (34 yrs) | VLCAD | N | 35 | 35 |
| 29 (2 yrs) | VLCAD | N | 44 | 46–53 |
| 32 (35 yrs) | TFP | N | 45 | 50–55 |
| 33 (18 mths) | LCHAD | N | 22 | 56 |
C7=triheptanoin
Results for these patients are also included in [9]. Patient 21 withdrew from triheptanoin therapy at age 18 yrs.
Patients initiating triheptanoin as infants
Four patients in this retrospective study began treatment with triheptanoin before 6 months of age with a median 9.5 years of post-treatment follow up and were analyzed separately due to the shorter pretreatment period. Three of the four patients had an older affected sibling, thus enabling early diagnosis and treatment. Among these patients the overall hospitalization event rate was 13.01 events/year pretreatment vs 1.36 events/year post treatment (P=0.0892). The mean number of hospital days/year was reduced by 91% (108.21 vs 9.93 days/year; P=0.1684) after treatment initiation, but variability in the pretreatment period was high and the number of patients was relatively small.
Safety and tolerability of triheptanoin
Side effects associated with triheptanoin treatment are shown in Table 6. The most common side effects were diarrhea (60%) and gastrointestinal upset (55%), consistent with oral or G tube administration of an oil. None of the GI intolerance was reported to result in discontinuation of therapy. The most frequent GI symptoms were pain or cramping; vomiting was rare, and usually due to an intercurrent illness or reflux. Diarrhea and loose stools were frequent, but could not readily be distinguished from symptoms due to other illness, e.g, viral gastroenteritis, which is a relatively common cause of acute metabolic decompensation. Gastric pain or cramping, thought to be due the gastrin response to oil and consequent increased gut transit time, has also been observed in patients on MCT.
Table 6.
Side Effects Related to Triheptanoin Treatment
| Recorded side effect | Patients with event N=20 n (%) |
|---|---|
| Diarrhea | 12 (60%) |
| GI upset | 11 (55%) |
| None | 7 (35%) |
| Frequent loose stools | 3 (15%) |
| Lethargy | 2 (10%) |
Discussion
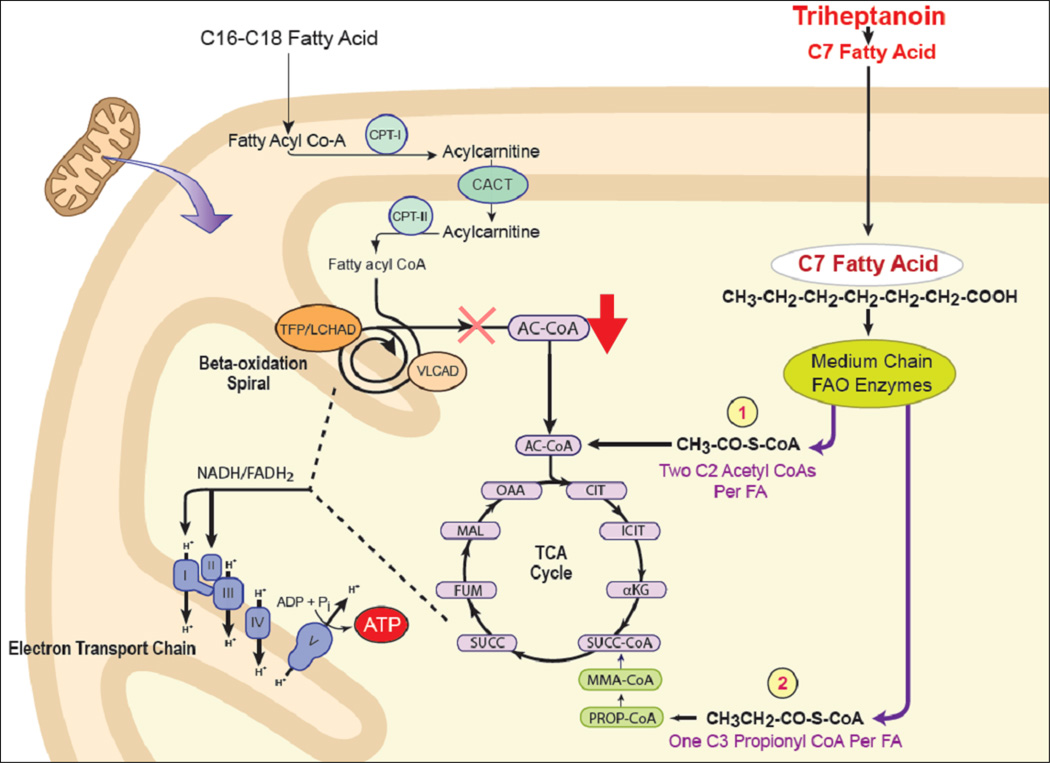
The proposed mechanism of action of triheptanoin is illustrated in Figure 7. FAO generates energy during fasting and stress by directly supplying reducing equivalents for mitochondrial oxidative phosphorylation (through the acyl-CoA dehydrogenase and 3-hydroxyacyl-CoA dehydrogenase reactions) as well as providing acetyl-CoA through the tricarboxylic acid (TCA) cycle, which in turn generates addtitional reducing equivalents for oxidative phosphorylation. Loss of the former, along with impaired ketone body production, leads to increased demand on the TCA cycle for energy production. Supplementation with MCT oil provides a two-carbon substrate (acetyl-CoA) for the TCA cycle, but not a three carbon substrate, eventually leading to the depletion of the latter. In contrast, triheptaonin generates two molecules of acetyl-CoA and one of propionyl-CoA (an odd chain substrate eventually converted to succinate). This effect, known as anaplerosis, maintains the appropriate substrate balance for the TCA cycle, improving energy generation for gluconeogenesis.
Figure 7.
Anaplerotic effect of triheptanoin metabolism. Fatty acid oxidation provides reducing equivalents directly to the electron transport chain and supplies substrates to the tricarboxylic acid (TCA) cycle. Oxidation of even chain fats produces only acetyl-CoA and the TCA cycle can ultimately become depleted of odd chain carbon species with excessive catabolism in patients with LC-FAODs. In contrast, triheptanoin provides both acetyl-CoA and propionyl-CoA, preserving TCA cycle function.
Our retrospective chart review presents data from 20 patients with LC-FAOD in the US before and after receiving triheptanoin treatment for up to 13 years under a compassionate use protocol. LC-FAODs are associated with frequent complications and hospitalizations with 320 hospitalizations charted for these 20 subjects. This retrospective analysis included a review of pretreatment medical records allowing us to establish a clinical baseline for the population while on standard of care, before initiation of triheptanoin. Most patients were diagnosed before one year of age through a combination of methods; nearly half were diagnosed through newborn screening and/or had a family history of the disease. As expected, rhabdomyolysis, muscle pain, and hypoglycemia were common, occurring in at least half of patients in the time period examined. Hepatomegaly was reported in 40% of the population, less than half the incidence reported in a French study of pediatric patients who were diagnosed based on clinical symptoms [2]. This difference may be due to the earlier diagnosis of patients in our cohort. Most patients in our study initiated triheptanoin therapy before age 6. Treatment prior to triheptanoin included MCT and a low fat/high carbohydrate diet in the majority of patients.
Overall, we found a significant 67% reduction in hospitalization days/year in the period after triheptanoin was initiated and a trend toward a 35% reduction in the hospitalization rate. A sensitivity analysis for a narrower time period of 2 years before and after triheptanoin did find consistent results, although the power of these analyses is reduced due to the low number of total events. These hospitalization results are consistent with prior reports for some of these patients [8–10], but had not been systematically quantified in the past publications. Although a change of this magnitude would be clinically important, the verification of these results requires further evaluation.
Triheptanoin use was associated with near elimination of hospitalizations for hypoglycemic events. Given the known gluconeogenic capacity of triheptanoin, as well its effect on increasing liver glycogen in treated rats [11] such an impact on hypoglycemia is not surprising. In some patients, this effect appeared profound. However, this finding must also be viewed in the context of the changing phenotype of FAOD over time. Serious hypoglycemic events occur most commonly in young patients and often decrease or disappear in older patients [2, 6, 12]. Thus, some portion of the observed effect could be due to the increasing age of the patients over time, although the profound degree of the effect seems unlikely to be based on aging alone, particularly in one case reviewed. The hypoglycemic event rates remained lower post-treatment even when the analysis was conducted looking only at the 2 years before and after initiation of triheptanoin treatment, an analysis that minimizes the effects of age on phenotype, but patient and event numbers were small.
Rhabdomyolysis was the most common clinical manifestation of FAOD reported in the pretreatment period. Although no reduction in rhabdomyolysis hospitalization rates was demonstrated in the period after initiating triheptanoin treatment, the number of hospitalization days/year due to rhabdomyolysis trended toward a reduction of 60%. The unchanged frequency of hospital admissions may reflect the practice of admitting patients for early intervention, with shorter hospital stays because the events were less severe. This finding on the number of rhabdomyolysis hospitalization days was supported by a reduction in maximum CK levels associated with rhabdomyolysis events after treatment, suggesting triheptanoin reduced the severity of rhabdomyolysis in some patients.
There were insufficient data in this study to draw conclusions about the potential impact of triheptanoin treatment for cardiomyopathy. However, of the LC-FAOD patients followed at our center and treated with triheptanoin, four presented with severe symptoms of cardiac failure have had a rapid improvement in cardiac function in response to triheptanoin treatment (Table 5, patients 3, 21, 26, and 33). In total, we have followed 14 LC-FAOD patients with cardiomyopathy, and all but one improved or had stable echo parameters. One patient ultimately required heart transplant; her ejection fraction prior to starting triheptanoin suggested irreversible tissue damage. However, this patient had improved in other aspects of LC-FAOD disease. The impact of triheptanoin on cardiomyopathy resolution deserves further study based on these cases. Of note, the onset of cardiomyopathy in a VLCAD−/− mouse model has been demonstrated to be associated with depletion of Krebs cycle intermediates [13], for which the anaplerotic effects of triheptanoin could be beneficial.
Four patients in the study began receiving triheptanoin when they were less than 6 months old. Two of these infants were detected by newborn screening, and had a history of previously affected older siblings, one was diagnosed prenatally, and had a deceased older sibling; and one patient presented clinically at 9 days of age. Because of the very short medical history available before treatment, and the fact that pretreatment events occurred at such a young age, it is difficult to draw meaningful conclusions about the effects of triheptanoin in this population versus prior treatment. However, the trends towards low hospitalization event rates and hospitalization days/year in these young patients are encouraging. Longer term treatment and follow-up of LC-FAOD sibling subjects with early lethal forms of disease could be important in assessing the impact of triheptanoin in the critical neonatal and infantile period.
Triheptanoin appeared to be well tolerated. The most commonly recorded side effects were diarrhea and gastrointestinal upset, which occurred in about half of patients. In a third of patients, it was noted that triheptanoin had no side effects. Gastrointestinal events would not be unexpected following administration of an oil such as triheptanoin. Side effects with MCT oil, are limited by mixing with an appropriate food or liquid, and a similar effect has been reported with triheptanoin use [10]. However, we were unable to capture additional information on administration practices in this study from the original source medical charts.
A number of methodological caveats are associated with retrospective chart reviews. The most critical limitation of a retrospective study is that it is not randomized and so no ongoing control exists for these subjects for comparison. Medical chart reviews are also dependent on in the content of the medical charts and the care with which it is recorded. Most notably, only major events, such as hospitalizations can be reliably captured, and non-major events, which may still cause significant clinical burden to the patient, are more difficult to ascertain. In addition, in this study many patients were treated at local hospitals rather than at the academic medical center study site. Regardless, we were able to reach nearly 90% ascertainment of event records, an outcome we believe is high enough to be a fair representation of case outcomes. Further, important factors such as dosing compliance or metabolic control are difficult to quantify using chart reviews. We attempted to quantify severity of events by capturing metabolic markers such as CK levels for rhabdomyolysis, but not all events had associated markers reported.
In conclusion, this retrospective study is the largest and longest report to date of patients treated with triheptanoin. Treatment of LC-FAOD with triheptanoin appeared to improve the course of disease by decreasing the incidence of clinical manifestations, essentially eliminating hypoglycemia. However, episodes of rhabdomyolysis continued in most, albeit at reduced frequency and intensity, suggesting the need for additional therapies in conjunction with triheptanoin. Significant heterogeneity in the routine clinical care provided to subjects during the periods studied and the natural variation of clinical course of LC-FAODs with time emphasize the need for further study of the use of triheptanoin. As a retrospective study, the major value of this study is to inform future prospective clinical trials.
Highlights.
Fatty acid oxidations are serious inborn errors of metabolism that are significant causes of morbidity and mortality in patients of all ages. Dietary therapy has been only partially successful in preventing symptoms. We present a retrospective analysis of the use of a novel therapy for fatty acid oxidation disorders, triheptanoin. This medication was effective in nearly eliminating episodes of low blood sugar and decreased muscle symptoms and hospital days for patients during acute episodes. Additional clinical trials are planned.
Acknowledgments
Ultragenyx Pharmaceutical Inc. sponsored this study. Medical writing assistance was provided by Holly Brenza Zoog and Anne Morris. The authors thank Brian King, Natasha Dutta, Andrea Schatz, Julie Scoggin, and Pam Walentynowicz for help with data abstraction and Jennifer Sniadecki for statistical support. The authors also thank Mary Wallace, RD for help in accessing medical records for some patients. JV was supported in part by PHS grant NIH-R01-DK78775.
Abbreviations
- CATR
Carnitine Acylcarnitine Translocase
- CM
Cardiomyopathy
- CRF
Case Report Forms
- ECHO
Echocardiography
- ER
Emergency room
- LC-FAOD
Long Chain Fatty Acid Oxidation Disorder
- MCT
Medium Chain Triglyceride
- TFP
Trifunctional Protein
- CK
Creatine kinase
- CPT
Carnitine Palmitoyltransferase Deficiencies
- ICU
Intensive care unit
- IQR
Interquartile range
- IV
Intravenous
- LCHAD
Long-Chain 3-Hydroxy-acyl-CoA Dehydrogenase
- VLCAD
Very Long Chain Acyl-CoA Dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
DM, KH, and EK are employees and shareholders of Ultragenyx Pharmaceutical Inc.
References
- 1.Spiekerkoetter U. Mitochondrial fatty acid oxidation disorders: Clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. Journal of inherited metabolic disease. 2010;33:527–532. doi: 10.1007/s10545-010-9090-x. [DOI] [PubMed] [Google Scholar]
- 2.Baruteau J, Sachs P, Broue P, Brivet M, Abdoul H, Vianey-Saban C, Ogier de Baulny H. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: A french pediatric study of 187 patients. Journal of inherited metabolic disease. 2013;36:795–803. doi: 10.1007/s10545-012-9542-6. [DOI] [PubMed] [Google Scholar]
- 3.Spiekerkoetter U, Bastin J, Gillingham M, Morris A, Wijburg F, Wilcken B. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. Journal of inherited metabolic disease. 2010;33:555–561. doi: 10.1007/s10545-010-9188-1. [DOI] [PubMed] [Google Scholar]
- 4.Roe C, Ding J. Mitochondrial fatty acid oxidations disorders. In: Scriver C, editor. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 2001. pp. 2297–2326. [Google Scholar]
- 5.Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, Das A, Haase C, Hennermann JB, Karall D, de Klerk H, Knerr I, Koch HG, Plecko B, Roschinger W, Schwab KO, Scheible D, Wijburg FA, Zschocke J, Mayatepek E, Wendel U. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. Journal of inherited metabolic disease. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- 6.Wilcken B. Fatty acid oxidation disorders: Outcome and long-term prognosis. Journal of inherited metabolic disease. 2010;33:501–506. doi: 10.1007/s10545-009-9001-1. [DOI] [PubMed] [Google Scholar]
- 7.Lindner M, Gramer G, Haege G, Fang-Hoffmann J, Schwab KO, Tacke U, Trefz FK, Mengel E, Wendel U, Leichsenring M, Burgard P, Hoffmann GF. Efficacy and outcome of expanded newborn screening for metabolic diseases--report of 10 years from south-west germany. Orphanet journal of rare diseases. 2011;6:44. doi: 10.1186/1750-1172-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roe CR, Mochel F. Anaplerotic diet therapy in inherited metabolic disease: Therapeutic potential. Journal of inherited metabolic disease. 2006;29:332–340. doi: 10.1007/s10545-006-0290-3. [DOI] [PubMed] [Google Scholar]
- 9.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. The Journal of clinical investigation. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roe CR, Yang BZ, Brunengraber H, Roe DS, Wallace M, Garritson BK. Carnitine palmitoyltransferase ii deficiency: Successful anaplerotic diet therapy. Neurology. 2008;71:260–264. doi: 10.1212/01.wnl.0000318283.42961.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu L, Zhang GF, Kombu RS, Allen F, Kutz G, Brewer WU, Roe CR, Brunengraber H. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal Effects on lipolysis rats. Ii, glucose production and liver acyl-coa profile. American journal of physiology. Endocrinology and metabolism. 2010;298:362–371. doi: 10.1152/ajpendo.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, Das A, Haase C, Hennermann JB, Karall D, de Klerk H, Knerr I, Koch HG, Plecko B, Roschinger W, Schwab KO, Scheible D, Wijburg FA, Zschocke J, Mayatepek E, Wendel U. Management and outcome in 75 individuals with long-chain fatty acid oxidation defects: Results from a workshop. Journal of inherited metabolic disease. 2009;32:488–497. doi: 10.1007/s10545-009-1125-9. [DOI] [PubMed] [Google Scholar]
- 13.Tucci S, Flogel U, Hermann S, Sturm M, Schafers M, Spiekerkoetter U. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-coa dehydrogenase deficient (vlcad(−/−)) mice. Biochimica et biophysica acta. 2014;1842:677–685. doi: 10.1016/j.bbadis.2014.02.001. [DOI] [PubMed] [Google Scholar]