Supplemental Digital Content is available in the text.
Key Words: programmed cell death 1 ligand 1 (PD-L1), programmed cell death 1 (PD-1), immunohistochemistry (IHC)
Abstract
Nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune checkpoint inhibitor antibody, developed by Bristol-Myers Squibb Inc., has activity across non–small cell lung cancer (NSCLC) histologies and is Food and Drug Administration approved for treatment of metastatic squamous NSCLC with progression on or after platinum-based chemotherapy. PD-L1 has been investigated as a potential biomarker to predict clinical response to nivolumab in clinical settings. We report an automated PD-L1 immunohistochemistry (IHC) assay, which was developed to detect cell surface PD-L1 in formalin-fixed paraffin-embedded human tumor tissue specimens using Dako’s Autostainer Link 48. The primary antibody for this assay is a rabbit monoclonal anti-human PD-L1 antibody, clone 28-8. The specificity of 28-8 for PD-L1 was demonstrated by antigen competition and genetic deletion of PD-L1 in tumor cell lines. The specificity of the PD-L1 IHC assay was further evaluated in a collection of 30 normal human tissues. The PD-L1 IHC assay was optimized for high sensitivity and precision in routine application. A pathology scoring and interpretation method specific to nivolumab clinical studies was adopted for the assay. The analytical performance of the assay was validated for application in the determination of PD-L1 status in human NSCLC specimens. The clinical application of the assay and scoring method was further validated in 3 Clinical Laboratory Improvement Amendments certified labs. The assay is currently being investigated in a variety of clinical studies for use as an in vitro diagnostic to select and stratify patients for treatment with the anti-PD-1 therapeutic antibody, nivolumab.
Programmed cell death 1 (PD-1) and its ligands are checkpoint regulators in immune cells.1,2 PD-L1 (CD274), the PD-1 ligand, can also be expressed on the surface of tumor cells and engages PD-1 on the surface of the effector immune cells to evade an antitumor immune response.3-9 The expression of PD-L1 has been reported in a number of human malignancies including non–small cell lung cancer (NSCLC). Immunoassays using different primary antibodies, assay formats, and scoring approaches have been reported to assess the prevalence of PD-L1 positivity in NSCLC. An immunohistochemistry (IHC) assay was used to detect PD-L1 protein expression in the cytoplasm and on the cell surface of tumor specimens in 2 small NSCLC cohorts, one with 52 frozen sections10 and the other with 109 formalin-fixed paraffin-embedded (FFPE) sections.11 Specimens from both cohorts were collected pretreatment and staged I to IV based on The American Joint Committee on Cancer Staging classification. The estimated PD-L1 positivity rate was similar approximately 50% from both studies using the median value as an arbitrary cut-point. In 1 study, an analysis of squamous NSCLC FFPE specimens found 19.6% PD-L1 positivity (42/214, membrane and circumferential staining).12 Another study assessed PD-L1 expression in 120 NSCLC FFPE specimens and observed a positivity rate of 57.5% (staining in tumor cytoplasm and/or cell membrane).13 In this study, PD-L1 expression was significantly correlated with degree of tumor cell differentiation and stage of tumor node metastasis. A more recent study using a quantitative fluorescence assay analyzed 2 cohorts (n=340 and 204) of stage I to IV NSCLC in FFPE tissue microarrays.14 The PD-L1 positivity was measured by the overall fluorescent signals in tumor tissue compartment above the expression threshold based on normal tissue compartment and the average was approximately 30%. Multiple factors may contribute to the varied PD-L1 prevalence reported across these studies including differences in antibodies, assay methods, stages of the tumors, and treatments before sample collection [Bristol-Myers Squibb Inc. (BMS)/Dako presentation, 2013 Society for Immunotherapy of Cancer annual meeting].15
A PD-L1 IHC assay has been used to determine PD-L1 expression in a clinical phase 1 multiple dose escalation study of nivolumab in patients with previously treated advanced melanoma, NSCLC, RCC, CRPC, or CRC [Bristol-Myers Squibb Inc. (BMS)/Dako presentation, 2013 World Conference on Lung Cancer, online publication].16 There were 63 subjects with NSCLC (29 squamous, 34 nonsquamous), who provided archival tumor samples evaluable for analysis of PD-L1 expression. Using a 5% threshold to define PD-L1-positive status, 31 of 63 evaluable NSCLC subjects (49%) were found to be PD-L1 positive, including 15 squamous subjects and 16 nonsquamous subjects. Using a 1% expression threshold to define PD-L1-positive status, 35 of 63 evaluable NSCLC subjects (56%) were found to be PD-L1 positive, including 18 squamous subjects and 17 nonsquamous subjects.
Historically, the 5% expression threshold was utilized by several investigators,17,18 and while the rationale was not described it has been presumed that this value was above the “noise,” or intrinsic variability of the entire assay process, and would yield a reproducible result. The 1% and 5% expression thresholds were selected by BMS based on preliminary data from early-phase clinical studies indicating both a reasonable frequency of PD-L1 positivity that effectively divided the patient population, and a numerically higher objective response rate among PD-L1-positive subjects relative to PD-L1-negative subjects. In this report, we present the specificity, sensitivity, analytical performance, and validation of this assay as an aid to determine PD-L1 status in patients with NSCLC using the 1% and 5% expression thresholds.
MATERIALS AND METHODS
Principles of the PD-L1 IHC Assay
This PD-L1 IHC assay utilizes a monoclonal rabbit antibody directed against the extracellular domain of human PD-L1. The negative control reagent is a monoclonal rabbit IgG isotype control (DA1E; Cell Signaling Technology, Danvers, MA). Deparaffinization, rehydration, and target retrieval was performed in the PT Link (Dako PT100). Slides were then processed on the Autostainer Link 48 (Dako AS480) using an automated staining protocol validated for the PD-L1 IHC assay. The IHC staining protocol includes sequential application of a peroxidase-blocking reagent, PD-L1 primary antibody or negative control reagent, mouse anti-rabbit IgG linker, visualization reagent consisting of secondary antibody molecules and horseradish peroxidase coupled to a polymer backbone, 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogen reagent with hydrogen peroxide substrate, and a DAB enhancer which modifies the color of the precipitated chromogen. Automated staining runs included a control slide containing a PD-L1-positive cell line (NCI-H226) and a PD-L1-negative cell line (MCF-7) to serve as run controls. Reagents utilized in addition to the PD-L1 assay components included a wash buffer specially formulated for automated IHC staining, and a hematoxylin counterstain. IHC-stained slides were mounted in nonaqueous, permanent mounting media.
Generation of PD-L1 Primary Antibody 28-8
Hybridoma clones were generated after immunization of rabbits with purified recombinant human PD-L1 containing the extracellular domain of huPD-L1 (Phe19-Thr239). Candidate clones were screened by IHC using parental and engineered cell lines with or without expression of huPD-L1 or huPD-L2, and positive and negative human normal and tumor specimens. Anti-PD-L1 antibody, clone 28-8, was identified as the best candidate in multiple screenings and showed no reactivity to huPD-L2 (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/AIMM/A92). A stable transfectant monoclonal anti-PD-L1 clone 28-8 was produced for the PD-L1 IHC assay (Abcam, Cambridge, MA).
Specimen Preparation for Routine Application
All specimens used in these studies were FFPE. Sections were cut at 4 μm thickness, placed on charged slides, and dried in an oven for 1 hour. Human tissues sections were dried at 56 to 60°C and experimental cell lines were dried at 40 to 44°C. The cut sections were stored in dark at 2 to 8°C and used for the PD-L1 IHC assay within 4 months.
Generation of Tumor Cell Line FFPE Blocks With Engineered PD-L1 and PD-L1 Knock-out
Full-length human PD-L1 cDNA were transfected into parental HT-29 cell line. Transfected cells were enriched by Protein A Dynabeads (Life Technologies, Carlsbad, CA), and single cell cloned using limiting dilutions.
PD-L1 knock-out clones were generated from ES-2 and L2987 parental lines using a transcription activator-like effector nucleases (TALENs) genomic targeting approach.19 TALE from the plant pathogenic bacterium Xanthomonas, fused to the catalytic domain of FokI endonuclease, was constructed to recognize PD-L1 gene (Cd274) sequences. FokI cleaves only when present as a dimer, allowing precise recognition of sequences on opposing DNA strands. The resulting chromosomal double-stranded breaks elicit an error-prone cellular DNA repair mechanism known as nonhomologous end-joining. This mechanism was utilized to generate cell lines with frame-shift mutations in the PD-L1 gene locus Cd274. TALEN constructs targeting exon4 of human Cd274, transcript variant 1 (NM_014143.3), were designed and constructed by Cellectis (Paris, France) in the vector pTAL.CMV.T7.v2. TALENs were designed to recognize the following sequences:
TALEN1-Left: 5′-TGGATCCAGTCACCTCT-3′
TALEN1-Right: 5′-TAGCCCTCAGCCTGACA-3′
TALEN2-Left: 5′-TGAGGGCTACCCCAAGG-3′
TALEN2-Right: 5′-CAAGCAGTGACCATCAA-3′
ES-2 and L2987 cells were transfected with the TALEN pairs. Transfected cells were single cell cloned using limiting dilutions. PCR was performed across the target site using Accuprime pfx polymerase (Life Technologies) and primers: Cd274 2s: 5′-GGCAGAGCTAGCAGGTGTTC-3′; Cd274 2a: 5′-GGATGAATGGAGGTGAGGAA-3′. PCR amplicons were sequenced to confirm the mutations.
ES-2 clone T1-1 was determined to have 73% Cd274 knock-out with 2 different Cd274 edited sequences leading to a 5 bp deletion (73% of the TOPO clones sequenced), and a 6 bp deletion (27%) which maintains the open-reading frame for Cd274. ES-2 clone T1-22 was determined to have 100% Cd274 knock-out with 8 different Cd274 edited sequences leading to 298 bp deletion (29%), 202 bp deletion (23%), 55 bp deletion (23%), 25 bp deletion (18%), 5 bp deletion (4%), 5 bp deletion/1 bp insertion (1%), 4 bp deletion (1%), and 375 bp deletion (1%). L2987 clone L2-10 was determined to have 100% Cd274 knock-out with 3 different Cd274 edited sequences leading to 5 bp deletion (53%), 7 bp deletion (40%), and 268 bp insertion (7%). L2987 clone L2-14 was determined to have 100% Cd274 knock-out with 2 different Cd274 edited sequences leading to 11 bp deletion (54%), and a 124 bp insertion (46%). No wild-type Cd274 exon4 sequences were observed in any TOPO clones originating from the PCR amplicon obtained from these clones.
PD-L1 expression of all the parental and genetically engineered clones was verified using the Fluorescence-Activated Cell Sorter (FACS) staining with a PE-labeled antibody to PD-L1 (clone 29E.1A3.; BioLegend, San Diego, CA).
Antigen Competition of PD-L1 IHC Staining
Recombinant human PD-L1 protein (hPDL1-TVMV-His) was used as the antigen for PD-L1 antibody competition in IHC staining. The recombinant human PD-L1 is comprised of the PD-L1 extracellular domain linked to a His-tag through a 4 amino acid linker. The anti-PD-L1 primary antibody solution with antigen competition was prepared with 10× (4 µg/mL) and 50× (20 μg/mL) molar excess of the antigen containing additional nonspecific blocking reagents 2% BSA, 3% PEG, 0.1% Tween, 0.2% casein, and 0.015 mol/L sodium azide. The 28-8 primary antibody solution with addition of antigen was preincubated at room temperature for 1 hour before IHC staining procedures.
Statistical Methods for Agreement Analysis of Repeatability Tests
Positive/negative results of PD-L1 tumor scores were determined based on the expression level thresholds. For each repeatability test, the number of total nonredundant pair-wise comparisons (T), concordant negative pair-wise comparisons (CN), and concordant positive pair-wise comparisons (CP), and discordant pair-wise comparisons (Disc) for a given specimen were calculated. No reference result was assumed for each test. Therefore, average percent agreement was calculated for Negative Percent Agreement (ANA), Positive Percent Agreement (APA), and Overall Percent Agreement (OA) as the following20:
The 95% confidence intervals for ANA, APA, and OA were calculated based on the percentile bootstrap method. Each dataset was randomly sampled from, with replacement, to produce 10,000 bootstrap datasets. The frequency of CNs, CPs, and Discs for each bootstrap dataset was calculated. Using the frequencies, ANA, APA, and OA were calculated for each bootstrap dataset. Percent agreements from bootstrap datasets were rank ordered, and the 2.5 and 97.5 percentiles were used for the lower and upper bounds of the confidence intervals.
RESULTS
Primary antibody concentration and incubation times for assay components were optimized for maximum sensitivity with minimum background staining on human tumor specimens covering a wide dynamic range of PD-L1 expression. A software program for the PD-L1 IHC assay was developed and validated for use on the Autostainer Link 48. The components and assay conditions for the PD-L1 IHC assay are presented in Table 1.
TABLE 1.
Components for the PD-L1 IHC Assay

Results of PD-L1 IHC assay stained slides were interpreted using light microscopy, inspecting the entire section using ×4 objectives and turned to ×10, ×20, and ×40 gradually to examine the PD-L1 staining. Positive PD-L1 staining is defined as complete and/or partial circumferential linear plasma membrane staining at any intensity that can be differentiated from background and diffuse cytoplasmic staining. Granular staining in the cytoplasm was not considered as positive staining although it can be attributed to cytoplasmic PD-L1 protein. Negative staining is defined as no staining of the plasma membrane. Positive PD-L1 was computed for tumor cells in tissues with a minimum of 100 evaluable tumor cells. When applicable, the staining intensity of PD-L1 was graded in a 0 to 3 grading scale; the staining intensity values are 0 (no staining), 1+ (weak), 2+ (moderate), and 3+ (strong) (Fig. 1, top). The percentage of viable tumor cells of the entire specimen that exhibit PD-L1 plasma membrane staining at any intensity was recorded as the PD-L1 tumor score (Fig. 1, bottom). The percentage of positive tumor PD-L1 cells was determined using 1% increment for scores ranging from 0 to 10%. A 10% increment was applied for percentage scores >10% (rounding to the nearest 10%); however, 1% increments was used when applicable. The specimen is considered to be PD-L1 positive if determined to be greater than or equal to the threshold tumor score, or PD-L1 negative if determined to be less than the threshold tumor score. The estimated number of viable tumor cells in the tumor space was used as the denominator for computation of the score.
FIGURE 1.
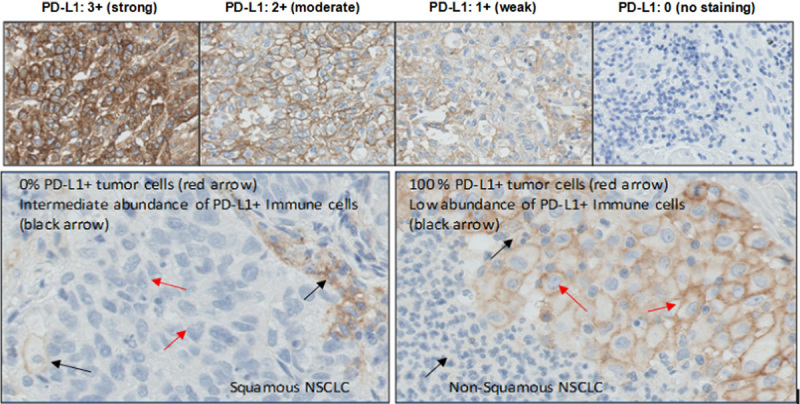
Positive PD-L1 membrane staining in NSCLC tumor tissues illustrating intensity grades (top, ×20) and PD-L1 tumor scores (bottom, ×40). NSCLC indicates non–small cell lung cancer; PD-L1, programmed cell death 1 ligand 1.
Variable degrees of PD-L1 surface membrane expression were also detected on immune cells, specifically histiocytes (macrophages) and lymphocytes, and when present were recorded in accord with the principles set forth by the “Immunoscore Consortium.”21 These data, however, have not at this point been incorporated into the formal scoring algorithm that forms the basis of the present report.
The PD-L1 IHC assay specifically detects PD-L1 membrane protein expressed in tumor cells in FFPE blocks. HT-29 is a human colorectal tumor cell line that showed nondetectable PD-L1. Three stable HT-29 cell lines overexpressing full-length human PD-L1 were generated and showed differential expression of PD-L1 by FACS analysis (see the Materials and methods section). FFPE cell pellets of the parental and PD-L1 overexpressing cell lines were stained by the PD-L1 IHC assay. The level of PD-L1 detected by IHC was consistent with the PD-L1 level detected by FACS, and was abolished by the addition of PD-L1 antigen to the protein-rich antibody diluent (see the Materials and methods section, Fig. 2).
FIGURE 2.
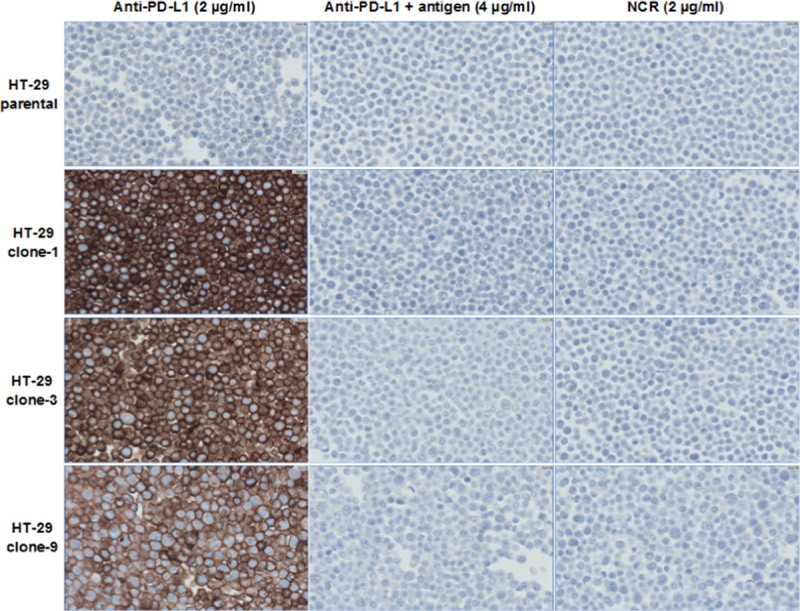
Photomicrographs (×40) of HT-29 parental and PD-L1 overexpressing FFPE cell pellets stained by the PD-L1 IHC assay with and without antigen competition. FFPE indicates formalin-fixed paraffin-embedded; IHC, immunohistochemistry; NCR, negative control reagent; PD-L1, programmed cell death 1 ligand 1.
To demonstrate the specificity of the PD-L1 IHC assay in detecting endogenous PD-L1 protein expressed in tumor cells, FFPE cell pellets of the parental and knock-out cell lines were stained by the PD-L1 IHC assay. Human ovarian clear cell carcinoma tumor cell line ES-2 and human lung adenocarcinoma tumor cell line L2987 showed high level of PD-L1 expression by FACS analysis. PD-L1 gene expression was genetically disrupted and knocked out by a genomic editing approach as described in the Materials and methods section. The knock-out of the PD-L1 expression in the 2 cell lines was verified by direct DNA deep sequencing and FACS analysis of the cells (see the Materials and methods section). Membrane staining of PD-L1 was not detectable in the 100% knock-out cell pellets. Membrane staining of PD-L1 in the parental cell lines was abolished by the addition of PD-L1 antigen to the protein-rich antibody diluent (see the Materials and methods section, Fig. 3). Because the knock-out cells were selected by cell cloning, it is likely that a low percentage of incomplete knock-out cells were present that were below the detection limit of FACS and batch sequencing. Very faint blush cytoplasmic staining was observed in some knock-out cells, and is thus nonspecific for PD-L1. To demonstrate that PD-L1 antigen specifically blocked binding of PD-L1 antibody clone 28-8 to PD-L1, IHC testing with a pair of broadly expressed control epithelial biomarkers CK8/CK18 was performed with and without competition by PD-L1 antigen. The addition of PD-L1 antigen to the CK8/CK18 cocktail did not block the IHC staining of CK8 and CK18, further supporting the specificity of PD-L1 antibody 28-8 in detecting PD-L1 by the PD-L1 IHC assay (Supplemental Figs. 2A, B, Supplemental Digital Content 2, http://links.lww.com/AIMM/A93).
FIGURE 3.
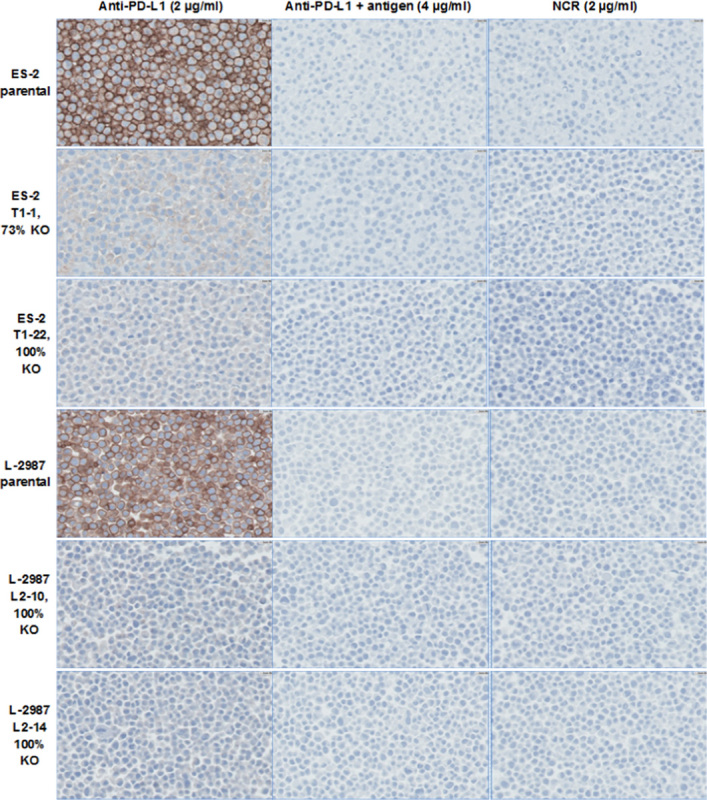
Photomicrographs (×40) of parental and PD-L1 knock-out (KO)-stable FFPE cell pellets stained by the PD-L1 IHC assay. FFPE indicates formalin-fixed paraffin-embedded; IHC, immunohistochemistry; NCR, negative control reagent; PD-L1, programmed cell death 1 ligand 1.
The PD-L1 IHC assay was used to detect PD-L1 in 30 different “normal” human FFPE specimens. The PD-L1 IHC assay detected PD-L1 protein localized in the plasma membrane of cell types known to express the PD-L1 antigen such as immune cells and cells of epithelial origin (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/AIMM/A94). Cytoplasmic staining was noted in some cell types but was not evaluated as positive staining. Background staining was <1 grade in all specimens tested.
The PD-L1 IHC assay detected PD-L1 plasma membrane protein in FFPE NSCLC specimens with desired dynamic range and showed 73% PD-L1 positivity rate for 1% threshold and 45% positivity rate for 5% threshold in a collection of 186 commercial archival NSCLC specimens representing a variety of the histologic subtypes and tumor stages (Supplemental Fig. 3, Supplemental Digital Content 4, http://links.lww.com/AIMM/A95). Validation of assay precision was demonstrated by repeatability tests (Table 2). The robustness of the assay conditions was tested in a range of assay parameters near the optimal conditions. The range of assay conditions meeting acceptable performance specifications was identified.
TABLE 2.
Analytical Precision of the PD-L1 IHC Assay in Validation Study
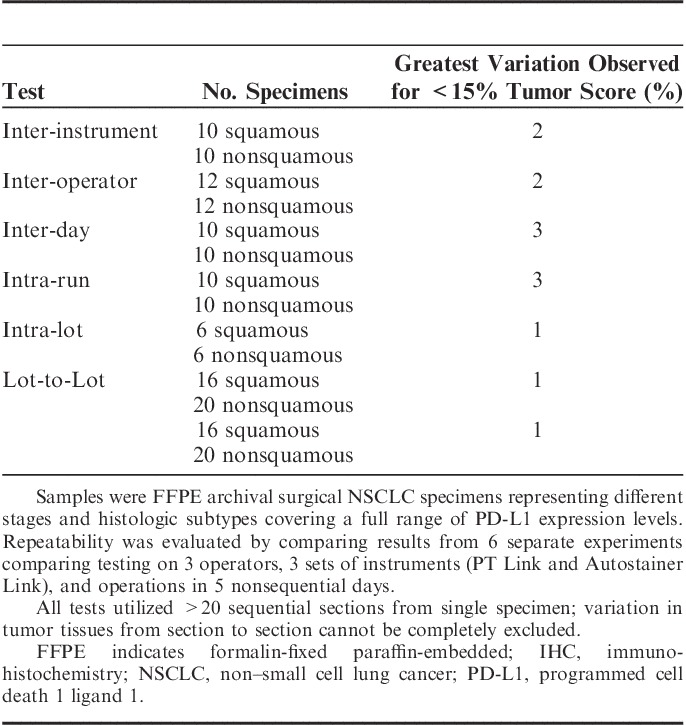
The use of PD-L1 IHC assay was validated for the detection of PD-L1 expression at 1% and 5% thresholds to determine PD-L1 status. The precision of the assay on the PD-L1-positive/PD-L1-negative determination was demonstrated by repeatability tests within the same IHC run and runs across 3 instruments, 3 analysts, and 5 nonconsecutive testing days. Each test included 20 to 24 unique NSCLC specimens representing different stages and histologic subtypes covering a full range of PD-L1 expression levels with a minimum of 25% of the PD-L1 scores within 10% PD-L1 score range around the cut-off threshold. Each of the repeatability tests showed 100% agreement in pair-wise comparison of all nonredundant pairs of the PD-L1 staining scored by 1 observer using a comparative microscope, demonstrating high analytical precision of the assay excluding observer variability.
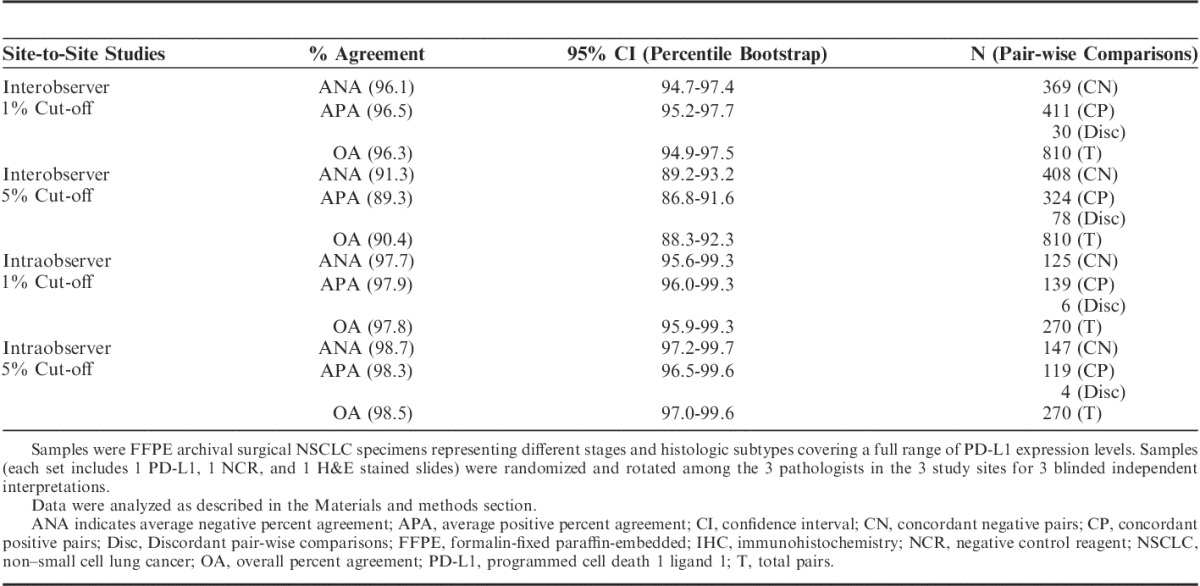
The performance of the assay for PD-L1 status determination based on 1% and 5% thresholds was established for day-to-day, site-to-site, observer-to-observer reproducibility on the Dako Autostainer Link 48 by conducting a blinded study in 3 external Clinical Laboratory Improvement Amendments certified clinical labs where the laboratory technicians and pathologists were trained and certified for use of this assay. The lower bound of the 95% percentile bootstrap confidence interval computed on pair-wise percent agreement was >85% for all comparisons, as presented in Table 3 for day-to-day/site-to-site comparison and in Table 4 for observer-to-observer comparison.
TABLE 3.
Site-to-Site Reproducibility of the PD-L1 IHC Assay on NSCLC
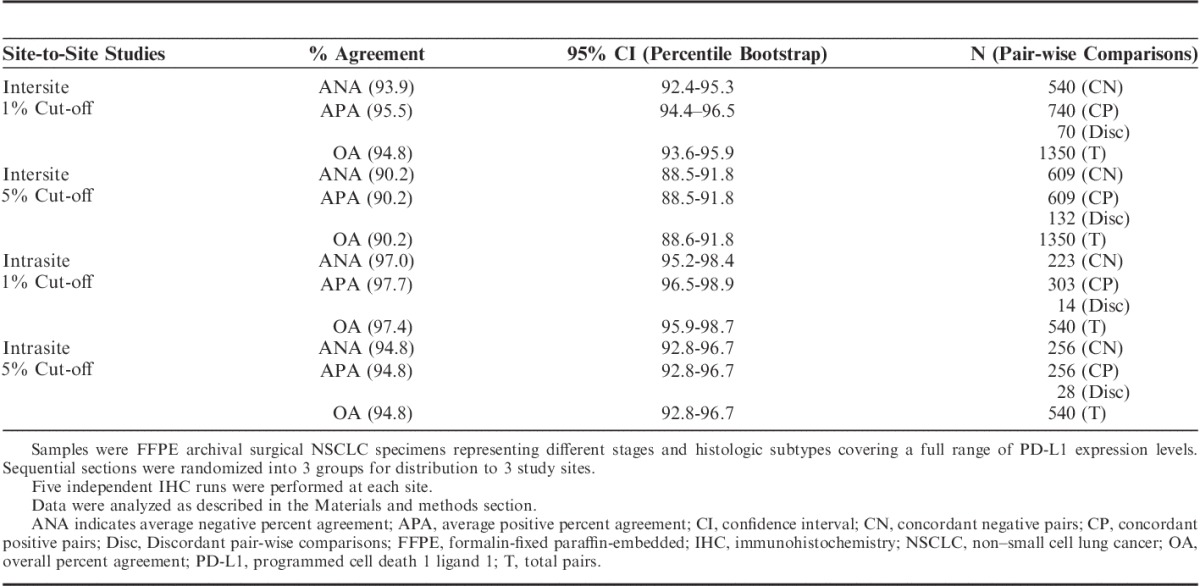
TABLE 4.
Observer-to-Observer Reproducibility of the PD-L1 IHC Assay on NSCLC
DISCUSSION
We have developed an automated PD-L1 IHC assay, using rabbit monoclonal clone 28-8 to detect cell surface PD-L1 in FFPE human tumor tissue specimens using the Dako Autostainer Link 48. We demonstrated that the assay specifically detects PD-L1 plasma membrane protein expressed in tumor cells. The assay showed high analytical precision and day-to-day, site-to-site, and observer-to-observer reproducibility for determination of PD-L1 status based on 1% and 5% cut-off thresholds.
A number of preanalytical variable factors outside our design control process can potentially compromise the reproducibility of the PD-L1 IHC assay results. Our standard procedure for preanalytical processing of tumor biopsies is to immerse biopsy samples into 10% neutral buffered formalin for fixation within 30 minutes of collection and to keep the samples immersed for 24 to 48 hours before paraffin embedding. We attempted to assess the impact of sample ischemia time and fixation method on the PD-L1 staining using freshly excised human tonsil and frozen NSCLC tumor tissues processed with variable conditions. In this work, sample ischemia time is defined as the period of the time when tissues are removed from in vivo conditions and kept under in vitro conditions to the time when tissues are placed in fixative reagents. Compromised staining results were not observed in the testing of a range of ischemia time for up to 12 hours and fixation time in 10% neutral buffered formalin for up to 72 hours.
Another preanalytical variable factor is the presence of tumor heterogeneity in PD-L1 expression. We attempted to assess the variability of multiple samples from the same case and the variability between primary versus metastatic sample types. With a collection of commercial archival FFPE samples including 30 cases of multisamples per case and 20 pairs of matched primary versus metastatic tissues, we observed discordant PD-L1 results in 6% of the multisampled cases and 30% of the matched primary versus metastatic cases. Continued study of tumor heterogeneity and its impact on PD-L1 expression will aid in development of appropriate sample qualification criteria for PD-L1 IHC assays.
The PD-L1 IHC assay is currently being used in late-stage nivolumab clinical studies of multiple indications including NSCLC. The clinical validation of the assay and its utility in identifying patients for treatment with nivolumab have been demonstrated in 2 phase III trials in previously treated NSCLC with distinct histologies.6,7 The clinical utility of this assay is being further evaluated in NSCLC clinical trials in the first-line setting.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Henrik Winther, Rosanne Welcher, and Dave Stanforth for critical review of the manuscript and their generous support to the work presented here; John Feder and Gabe Mintier (Bristol-Myers Squibb) for generating knock-out cell lines, Malini Venkata (Biocon-Bristol-Myers Squibb Research Center) for preparing tonsils at different ischemia and fixation times, Dako histology team for procurement of tissues and preparation of cut sections for this study, especially Lenka Van Alphen, Rosie Sheridan, and Dominique Burns; Dako operations team for the production of the reagents for this assay; Asterand Bioscience (Detroit, MI) and Cooperative Human Tissue Network which is funded by the National Cancer Institute for providing tissue samples for this study; Malinka Jansson for suggestions to the statistical methods used for the data analysis; and project management support from Sam An.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.appliedimmunohist.com.
C.T. is a consultant to Dako North America contracted through Keck School of Medicine of the University of Southern California. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 6.Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs. docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl): abstract 8009. [Google Scholar]
- 7.Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl): abstract LBA109. [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 9.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. [DOI] [PubMed] [Google Scholar]
- 10.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. [DOI] [PubMed] [Google Scholar]
- 11.Mu C-Y, Huang J-A, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. [DOI] [PubMed] [Google Scholar]
- 12.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14:157–163. [DOI] [PubMed] [Google Scholar]
- 13.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. [DOI] [PubMed] [Google Scholar]
- 14.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosso J, Inzunza DH, Wu Q, et al. Programmed death-ligand 1 (PD-L1) expression in various tumor types. J Immunother Cancer. 2013;1(suppl 1):P53. [Google Scholar]
- 16.Antonia SJ, Grosso JF, Horak CE, et al. Association of tumor pd-l1 expression and immune biomarkers with clinical activity in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. J Thoracic Oncology. 2013;8(suppl 2):P907. [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JC, Tan S, Qiao G, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. [DOI] [PubMed] [Google Scholar]
- 20.CLSI. Quality assurance for design control and implementation of immunohistochemistry assays; approved guideline—second edition. I/LA28-A2, Vol. 19 No. 26:112–113. [Google Scholar]
- 21.Immunoscore. Immunoscore as a new possible approach for cancer classification. Available at: http://www.immunoscore.org. Accessed May 28, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


