Abstract
Mass stranding of cetaceans (whales and dolphins), in close association with the activity of naval sonar systems, has been reported on numerous occasions. Necropsy showed bubble-associated lesions similar to those described in human decompression sickness (DCS). We examined the hypothesis that exposure to underwater sound may potentiate DCS. Rats were subjected to immersion and simulated dives with and without simultaneous acoustic transmissions at pressure levels and frequencies of 204 dB/8 kHz and 183.3 dB/15 kHz. DCS severity was assessed using the rotating wheel method. Recording of somatosensory evoked potentials (SSEPs) was employed under general anesthesia as an electrophysiological measure of neurologic insult. A significantly higher rate of decompression sickness was found among animals exposed to the 204-dB/8-kHz sound field. Significantly higher pathological SSEPs scores were noted for both underwater sound protocols. Pathological SSEPs scores in animals immersed during the acoustic transmissions, but without changes in ambient pressure, were comparable to those observed in animals exposed to the dive profile. The results demonstrate induction of neurological damage by intense underwater sound during immersion, with a further deleterious effect when this was combined with decompression stress. The study outcome has potential implications for human diving safety and may provide an explanation for the mass stranding of cetaceans purportedly associated with sonar activity.
Keywords: bioeffect, decompression sickness, rectified diffusion, somatosensory evoked potentials, underwater acoustics
mass strandings of cetaceans (whales and dolphins) have been recorded over many decades on the vulnerable north- and southeastern shores of the United States and in New Zealand and Australia, with most of the stranded animals showing no significant pathology (Bogomolni et al. 2010; Bradshaw et al. 2006). Recently, alarming numbers of mass stranding events have been reported in close association with the activity of mid-frequency (1–10 kHz) naval sonar systems (Balcomb and Claridge 2001; Parsons et al. 2008). Necropsy of these marine mammals revealed gas bubble-associated lesions and fat embolism in the blood vessels and parenchyma of vital organs, not unlike those described in human divers suffering from decompression sickness (DCS) (Fernández et al. 2004, 2005; Jepson et al. 2003). B-mode ultrasound scanning of live-stranded dolphins demonstrated the presence of bubbles, which was later corroborated by computerized tomography and necropsy of the dead animals (Dennison et al. 2012).
The accepted pathogenesis of DCS involves tissue injury resulting either directly or indirectly from the formation of inert gas bubbles during decompression (Francis and Gorman 1993). Bubble nuclei will grow to macroscopic size only under conditions of supersaturation, which develop when the partial pressure of the inert gas in the tissues is higher than that in the ambient atmosphere (Crum and Mao 1996). Symptoms and signs in the human include joint pain, skin rash, neurological dysfunction varying in severity from limb numbness to paralysis and hemiplegia, and, in some cases, cardiopulmonary collapse (Melamed et al. 1992).
Previous studies have shown nitrogen supersaturation even during routine foraging dives of marine mammals, which probably avoid DCS by physiological adaptation and diving behavior dictated by evolutionary processes (Hooker et al. 2012; Houser et al. 2001; Moore and Early 2004; Ridgway and Howard 1979; Rommel et al. 2006). In the human diver, nitrogen supersaturation may develop during the ascent from a dive using compressed air SCUBA (self-contained underwater breathing apparatus). The presence of inert gas bubbles in the circulation has even been demonstrated for the decompression phase of dives carried out with meticulous adherence to the decompression stops stipulated by widely employed decompression tables (Melamed et al. 1992).
The necropsy findings in stranded cetaceans may be explained by changes in diving behavior that could lead to critical levels of nitrogen supersaturation, thereby increasing the risk of DCS (Fernández et al. 2004; Rommel et al. 2006). An alternative explanation for the massive presence of nitrogen bubbles may be the expansion of existing inert gas microbubbles by means of rectified diffusion, secondary to the activity of underwater sonar. This may produce DCS-like infarcts (Crum et al. 2005; Houser et al. 2001). Rectified diffusion is the mechanism whereby growth of gas bubbles takes place in a liquid on exposure to a sufficiently intense sound field (Leighton 1994). When a bubble is forced to oscillate about an equilibrium value, it first contracts, the partial pressure of the gas in the interior of the bubble increases, and gas diffuses from the bubble to the tissue. The bubble then expands, the partial pressure of the gas in the interior decreases, and gas diffuses into the bubble. Because the total number of molecules transported through the interface in a given time is proportional to the surface area of the bubble, more gas will enter during the expansion phase than leaves during contraction so that over a complete cycle, there will be a net increase in the number of gas molecules within the bubble. The main effect contributing to bubble expansion, once it has been initiated, is diffusion of the gas in the liquid, which is proportional to its gradient. When the bubble expands, the gas concentration in its immediate vicinity, and thus diffusion toward the bubble, will increase and contribute to bubble enlargement.
Bubble nuclei already present in the marine mammal or human diver's tissues might destabilize and grow under the influence of ultrasound. Significant bubble production under these circumstances has been demonstrated in studies of an in vitro agar-based gel (Crum et al. 1987; Daniels et al. 1987), ex vivo tissues and the whole organ (Crum et al. 2005), and in vivo prawn, guinea pig, and rat models (Arieli et al. 2000; Shupak et al. 2002; ter Haar et al. 1982).
The suggested mechanism of inert gas bubble expansion might explain the lethal effects of exposure to sonar activity on marine mammals. No less important, it has potential implications for the safety of humans diving in the vicinity of sonar transmissions or exposed to the action of acoustic beacons, which are employed under water for diving surveillance, signaling, and navigation (Rooney 1979; Smith and Hunter 1980).
We examined the hypothesis that exposure to mid- and high-frequency sound during a controlled, simulated dive in the adult male rat model may potentiate clinical DCS and interfere with neuronal activity, as reflected by changes in somatosensory evoked potentials (SSEPs).
MATERIALS AND METHODS
Animals
A total of 189 male albino rats (Rattus norvegicus, Sprague-Dawley strain) weighing 350–400 g were used in the study, 53 animals in the preliminary experiment and 136 in the main investigation. Before the experimental sessions, rats were housed in an accredited animal care facility and had access to food and water ad libitum. The experimental procedures were approved by the Israel Ministry of Defense Animal Care Committee and were conducted in accordance with the principles of laboratory animal care (National Research Council 2011).
Exposure Pool and Animal Cage
A custom-designed trapezoid-shaped pool, anechoic and temperature-controlled, was filled with water and placed inside a 150-l hyperbaric chamber (Roberto Galeazzi, La Spezia, Italy). The pool measured 150 × 130 cm and was 90 cm deep. A round wire mesh cage 20 cm in diameter, suspended above the pool, was lowered by means of a pulley construction and immersed in the water as required. One animal at a time was put into the cage and immersed head-out in the pool. The animal was unrestrained and could move about freely inside the cage. Water heated to 37°C circulated continuously to keep the animal normothermic during the experiment (flow-type heater, model MTI-H20-R1; MTI, Kfar Hanagid, Israel).
Preliminary Study
Preliminary experiments were conducted on 53 rats to find a compression-decompression profile that would result in signs of DCS in about half of the animals with a minimal mortality rate. Such a protocol was required for the main study, whose purpose was to examine the possible influence of underwater sound on the occurrence of DCS. This had to be accomplished by using a compression-decompression profile that was not extreme, producing a high rate of DCS and death. A previously published rat DCS model was adopted as the initial format (Lillo and Parker 2000). Animals were randomly allocated to each of the experimental simulated dive profiles using a computerized “randomizer” (http://www.random.org/sequences). After the completion of each preliminary experiment, which included exposure to the simulated dive profile and assessment of DCS by the rotating wheel method as detailed below, the animal was euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/1.5 kg body wt).
Assessment of DCS.
DCS was assessed using the rotating wheel method, based on previously reported studies (Kayar et al. 1998; Lillo and Parker 2000). A pneumatic rotating cylindrical cage was constructed from two commercial running wheels (diameter 21 cm, width 21 cm). A door was cut in each running wheel to enable the rat to be placed inside. The engine of a pneumatic drill was adjusted with transmission wheels and a rubber band to run the cylindrical cage, while the rotation rate was controlled using a pressure gauge. After decompression to atmospheric pressure, the rat was freed from the exposure cage and placed inside the cylindrical cage rotating at a perimeter speed of ∼3 m/min for 0.5 h. This was established to be a sufficient length of time for almost all cases of DCS to become evident (Lillo et al. 1985). The active role of the rat, stepping as it advances inside the rotating wheel, should reveal DCS-induced cardiopulmonary and motor derangements that might remain undiagnosed with the animal at rest.
Signs of DCS, as adopted from Lillo and Parker (2000), consisted of walking difficulties, abnormal breathing pattern, forelimb and/or hind limb paralysis, rolling about in the rotating wheel, convulsions, and death. Outcome was divided into three categories: “no DCS,” “DCS excluding death,” and “death” when signs of DCS culminated in death. Before the experiment commenced, the animal was placed in the rotating cage to ensure a normal motion pattern.
Main Studies
The rat was immersed head-out in the wire mesh cage inside the exposure pool and was subjected to the simulated dive profile designed to induce decompression sickness while exposed to transmission from an acoustic beacon. Six groups of 10 animals were assessed for DCS after immersion or a simulated dive with or without exposure to sound, and 6 additional groups of 10 animals underwent examination of SSEPs. Animals in the Control group were immersed only, without exposure to sound. Those in the 8 kHz group were immersed while exposed to sound at 8 kHz. Rats in the 15 kHz group were immersed while exposed to sound at 15 kHz. Animals in the diving group made a simulated dive, without exposure to sound. Those in the Diving + 8 kHz group made a simulated dive while exposed to sound at 8 kHz. Rats in the Diving + 15 kHz group made a simulated dive while exposed to sound at 15 kHz. Animals were randomly assigned to each of the study groups using a computerized “randomizer” (http://www.random.org/sequences).
Sound exposure.
An acoustic beacon, 116 mm in diameter, was submerged in the exposure pool at a depth of 20 cm and at a distance of 100 cm from the animal's cage. Intermittent sounds at a pressure level of 204 dB re 1 μPa (16 kPa) and a frequency of 8 kHz or 188.3 dB re 1 μPa (2.6 kPa) and a frequency of 15 kHz, measured at the animal's abdomen with a duration of 100 ms, were transmitted omnidirectionally in a duty cycle of 10% from commencement to termination of the immersion or simulated dive for a total of 34 min and 6 s. Unless otherwise indicated, all sound pressure level (SPL) values in this text refer to 1 μPa at the exposure site. The sound source was a commercially available acoustic beacon used for underwater communication (model TSE-4; L-3 Communications ELAC Nautik, Kiel, Germany). SPLs were measured at the level of the animal's abdomen by a hydrophone (model 8105; Brüel & Kjær, Nærum, Denmark) and were repeatedly verified before each individual exposure. Sound at 8 kHz/204 dB represents the typical transmission of naval sonar, which has been associated with numerous incidents of cetacean stranding. Sound at 15 kHz/188.3 dB is in the transmission range of commonly used underwater navigation devices employed by human divers (Salami et al. 2010; Smith and Hunter 1980; Watts 1999).
Assessment of DCS.
The rat was removed from the immersion cage and placed inside the rotating cage for 30 min. DCS severity was scored as described above.
Examination of SSEPs.
SSEPs were examined under intraperitoneal anesthesia 30 min after completion of the immersion or the simulated dive.
Recording and scoring of SSEPs.
Recording of SSEPs was employed as an electrophysiological measure of neurological insult. Clinical studies have consistently found that abnormalities in the SSEPs are associated with spinal cord posterior column injuries, particularly in severe cases of DCS. This method has been successfully employed in DCS research in the rat and the dog (Francis et al. 1990; Hyldegaard et al. 1994; Katsenelson et al. 2009; Shupak et al. 2003; Sykes et al. 1986) and in the clinical evaluation of acute DCS in humans (Murrison et al. 1995).
For recording of the SSEPs, rats were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). Ketamine-xylazine anesthesia has no significant effect on peripheral neural conduction (Hayton et al. 1999) and has been successfully used in the past for recording SSEPs in the same animal species (Shupak et al. 2003). We waited 15 min after the immersion was completed before commencing anesthesia because preliminary trials showed that a shorter interval resulted in a high mortality rate, probably due to the combination of decompression stress and anesthesia. The SSEPs recording electrodes were then placed in position, and wave registration commenced 30-min postimmersion. To avoid any effect of alterations in body temperature on wave latency and amplitude of the SSEPs, temperature was maintained at 37°C by means of a thermostatically heated pad (Oro and Haghighi 1992). During recording, the heating pad was disconnected to eliminate electrical interference.
Stimuli were electrical square wave pulses of 100-μs duration and at a repetition rate of 4 pulses/s, administered using a disposable monopolar needle with cable electrodes. The electrodes were positioned subcutaneously between the medial malleolus and the Achilles tendon for hind paw peroneal nerve stimulation and in the palmar aspect of the wrist for forepaw median nerve stimulation, as described previously (Shupak et al. 2003).
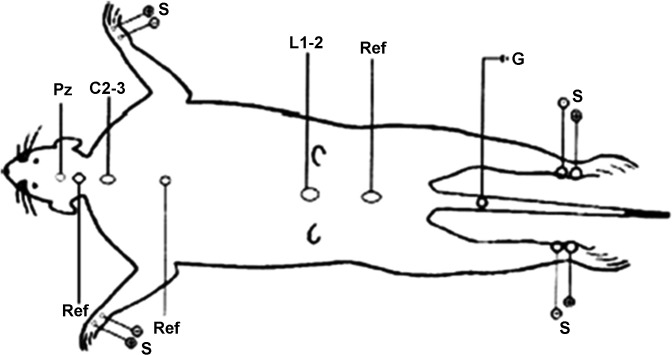
Alternate bilateral stimulation of the upper and lower limbs was delivered at a suprathreshold intensity sufficient to cause moderate contraction of the digits and the whole limb. Recording electrodes were placed transcutaneously over the C2-3 and L1-2 intervertebral spaces and on the skull vertex over the motor cortex (Pz). References for the Pz, C2-3, and L1-2 recordings were placed subcutaneously 1.5 cm below the Pz recording position, between the scapulas, and between the iliac crests, respectively. A common ground electrode was placed below the tail root (Fig. 1). Impedance of the recording and reference electrodes was maintained below 5 kΩ. Analysis time was 21 ms, and 500 repetitions per trial were averaged. To avoid possible side or limb bias, stimulation and recording order were random among the animals. Each averaged recording was replicated and superimposed to verify reproducibility of waveform, amplitude, and latency.
Fig. 1.
Somatosensory evoked potential (SSEP) recording montage showing placement of the recording electrodes. Pz, electrode placed over the motor cortex; C2-3, electrode placed over the cervical intervertebral spaces; L1-2, electrode placed over the lumbar intervertebral spaces; Ref, reference electrodes placed between the scapulas and iliac crests; G, common ground electrode; S, stimulation electrodes for median nerve (fore paw) and peroneal nerve (hind paw).
For analysis of the SSEPs, wave peaks were selected when the statistical correlation between the same peaks in two subsequent recordings was 0.75 or greater. A peak latency more than two standard deviations from the mean latency of the Control group (immersion only) was defined as abnormal. Lumbar spine SSEPs are characterized by a three-wave response, whereas cervical spine and cortical stimulation are followed by a biphasic wave response (Fig. 2). Normal SSEPs, when all peaks could be identified and their latency was within two standard deviations, were summed up to a total of seven evoked waves from each side. Each abnormal or nondetectable wave was assigned a score of 1; thus the severity of abnormal SSEPs was scored from 0 to 14 for each animal.
Fig. 2.
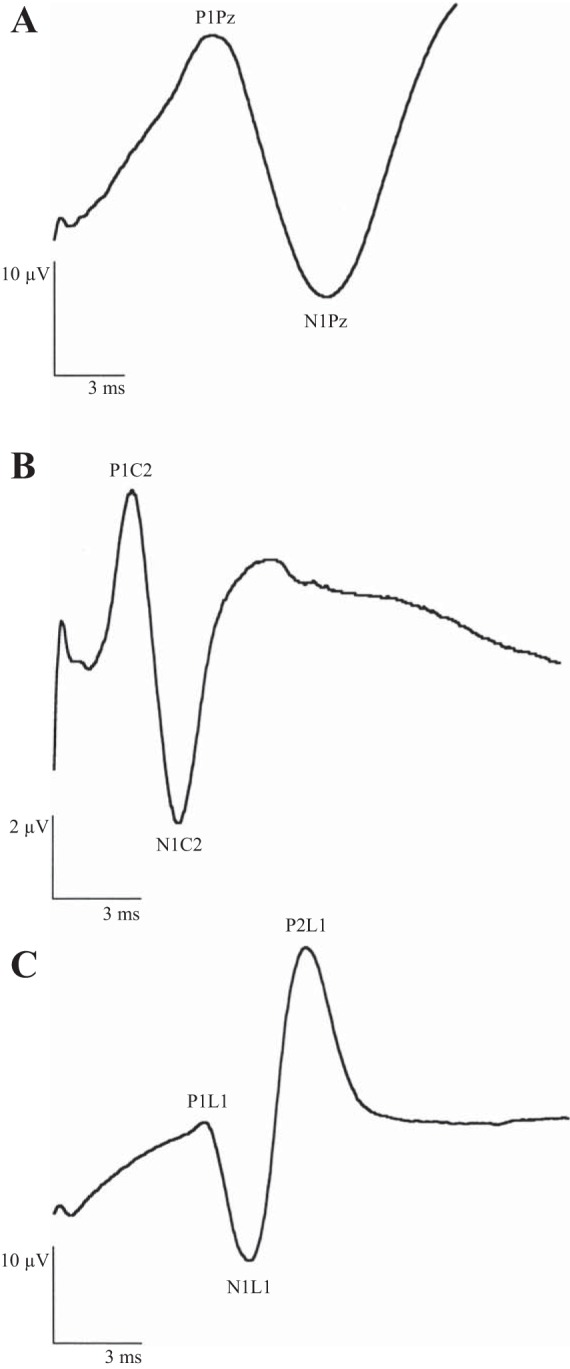
Normal SSEP wave forms. A: cortical (Pz) recording in response to stimulation of the median nerve. B: cervical spinal cord (C2-3) recording in response to stimulation of the median nerve. C: lumber spinal cord (L1-2) recording in response to stimulation of the peroneal nerve.
At the end of the study, animals were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/1.5 kg body wt).
Statistical Analysis
The χ2 test was employed to compare the occurrence of DCS between the groups in the DCS assessment study. For SSEPs, nonparametric ANOVA (Kruskal-Wallis test) and Dunn's multiple comparisons test were used to analyze the variance in pathological scores between the groups. A P value <0.05 was considered statistically significant.
RESULTS
Preliminary Study
The compression-decompression profile suggested by Lillo and Parker (2000) resulted in the immediate death of 53% of the animals. This profile did not therefore comply with the goal of a DCS rate of ∼ 50% with minimal mortality, which was our requirement for assessment of DCS and SSEPs and one of the main conditions of the study.
Additional simulated dive profiles were tested (profiles B–E, detailed in Table 1). The profile found to be suitable was compression at a rate of 10 atmospheres absolute (ATA) per minute to 6.5 ATA, remaining at this bottom pressure for 33 min, and decompression to an ambient pressure of 1 ATA at a rate of 10 ATA/min (profile E, Table 1).
Table 1.
Preliminary study: the compression-decompression profiles examined
| Profile | No. of Animals | Compression Rate, ATA/min | Bottom Pressure, ATA | Bottom Time, min | Decompression Rate, ATA/min | No DCS | DCS | Death |
|---|---|---|---|---|---|---|---|---|
| A* | 15 | 10 | 8 | 33 | 20 | 2 | 5 | 8 |
| B | 10 | 10 | 7 | 33 | 20 | 5 | 4 | 1 |
| C† | 6 | 10 | 6.5 | 33 | 6.6 | 2 | 0 | 4 |
| D | 10 | 10 | 6.5 | 33 | 20 | 5 | 0 | 5 |
| E | 12 | 10 | 6.5 | 33 | 10 | 7 | 5 | 0 |
Compression-decompression profile adopted from Lillo and Parker (2000).
This experiment included a smaller number of animals because a high mortality rate was already observed among the first 6 animals.
ATA, atmospheres absolute; DCS, decompression sickness.
Main Studies
Assessment of DCS.
Rats in the Control, 8 kHz, and 15 kHz groups showed no overt signs of DCS. Thirty percent of the animals in the Diving group were diagnosed with DCS. A similar incidence of DCS was observed in the Diving + 15 kHz group. The Diving + 8 kHz group had a DCS rate of 30% and mortality of 20% (Fig. 3). All cases of DCS and death occurred within 30 min of decompression. The higher incidence of DCS and death among rats in the Diving + 8 kHz group reached statistical significance compared with the 8 kHz and Diving groups (P < 0.02, χ2 test). No significant difference in the incidence of DCS was found between the Control, 15 kHz, Diving, and Diving + 15 kHz groups.
Fig. 3.
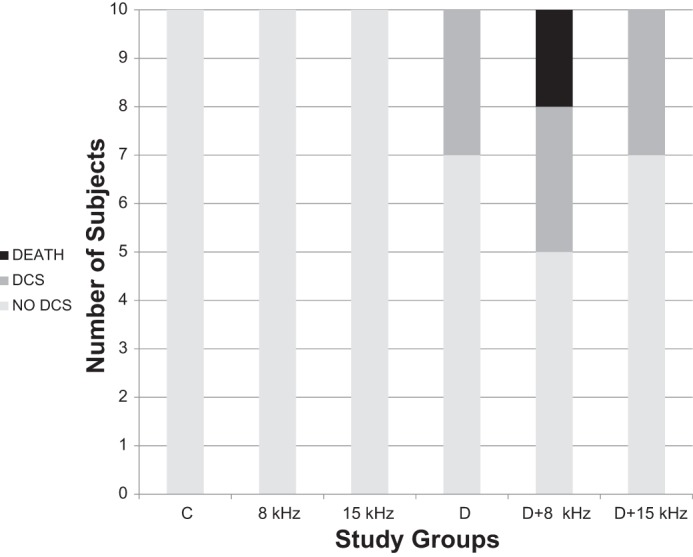
Incidence of decompression sickness (DCS) in the different groups during 30-min walking in the rotating cage. Groups: C, Control (immersion only); 8 kHz, immersion while exposed to transmission at 8 kHz; 15 kHz, immersion while exposed to transmission at 15 kHz; D, Diving (simulated dive); D+8 kHz, simulated dive while exposed to transmission at 8 kHz; D+15 kHz, simulated dive while exposed to transmission at 15 kHz. Outcomes: no DCS, no sign of DCS; DCS, symptoms of DCS (excluding death); death, symptoms of DCS culminating in death.
Assessment of SSEPs.
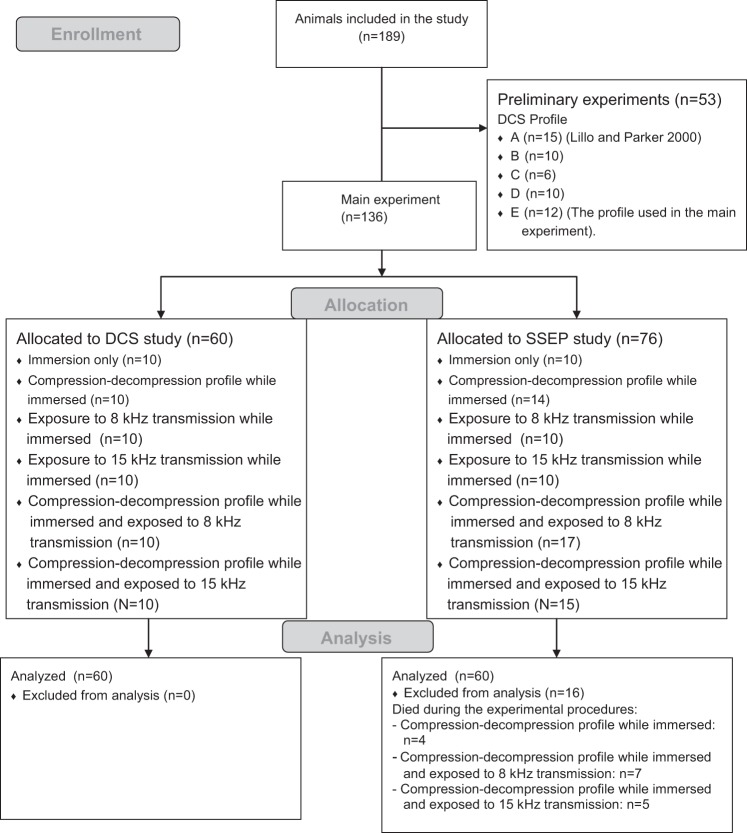
Recording of the evoked potentials had to be from live animals. For that reason, rats which succumbed to DCS were replaced by others that were exposed to the same experimental conditions. Figure 4 is a flow diagram depicting the animals' progress through the preliminary and main studies.
Fig. 4.
Flow diagram depicting the animals' progress through the preliminary and main studies (n = no. of animals).
Nonparametric ANOVA (Kruskal-Wallis test) showed significant variance in pathological scores for SSEPs between the Control, 8 kHz, Diving, and Diving + 8 kHz groups (P < 0.0001). This variance was explained by the differences between the Control group and the 8 kHz, Diving, and Diving + 8 kHz groups (P < 0.05, P < 0.01, and P < 0.001, respectively, Dunn's multiple comparisons test).
Comparison of the SSEPs scores in the 8 kHz, Diving, and Diving + 8 kHz groups showed a significant difference (P < 0.01); the multiple comparisons test indicated a significantly higher score in the Diving + 8 kHz group compared with the 8 kHz and Diving groups (P < 0.05).
Significant variance was also found in SSEPs scores between the Control, 15 kHz, Diving, and Diving + 15 kHz groups (P < 0.0001). This stemmed from the differences between the Control group and the 15 kHz, Diving, and Diving + 15 kHz groups (P < 0.05, P < 0.05, and P < 0.001, respectively, Dunn's multiple comparisons test).
Comparison of the SSEPs scores in the 15 kHz, Diving, and Diving + 15 kHz groups showed a significant difference (P < 0.01); the multiple comparisons test indicated a significantly higher score in the Diving + 15 kHz group compared with the 15 kHz and Diving groups (P < 0.01 and P < 0.05, respectively).
Pathological scores for SSEPs in the 8 kHz and 15 kHz groups were comparable with those found in the Diving group. These scores were significantly higher than those found for the Control group (P < 0.0005, Kruskal-Wallis test; P < 0.01 for multiple comparisons of the Control group with the 8 kHz, 15 kHz, and Diving groups, Dunn's multiple comparisons test). SSEPs scores are presented in Table 2 and Fig. 5.
Table 2.
SSEP scores
| Study Group | SSEP Score |
|---|---|
| Control | 0.8 ± 1.13*†‡ |
| 8 kHz | 4.8 ± 2.04*‡ |
| 15 kHz | 4.7 ± 2.4†‡ |
| Diving | 5.2 ± 2.35*†‡ |
| Diving +8 kHZ | 8.4 ± 3.2* |
| Diving +15 kHz | 8.5 ± 1.78† |
Data are means ± SD of somatosensory evoked potential (SSEP) scores.
Kruskal-Wallis test, P < 0.0001; Dunn's multiple comparisons test: Control vs. 8 kHz, P < 0.05; Control vs. Diving, P < 0.01; Control vs. Diving +8 kHz, P < 0.001;
Kruskal-Wallis test, P < 0.0001; Dunn's multiple comparisons test: Control vs. 15 kHz, P < 0.05; Control vs. Diving, P < 0.05; Control vs. Diving +15 kHz, P < 0.001;
Kruskal-Wallis test, P < 0.0005; Dunn's multiple comparisons test: Control vs. 8 kHz, P < 0.01; Control vs. 15 kHz, P < 0.01; Control vs. Diving, P < 0.01.
Fig. 5.
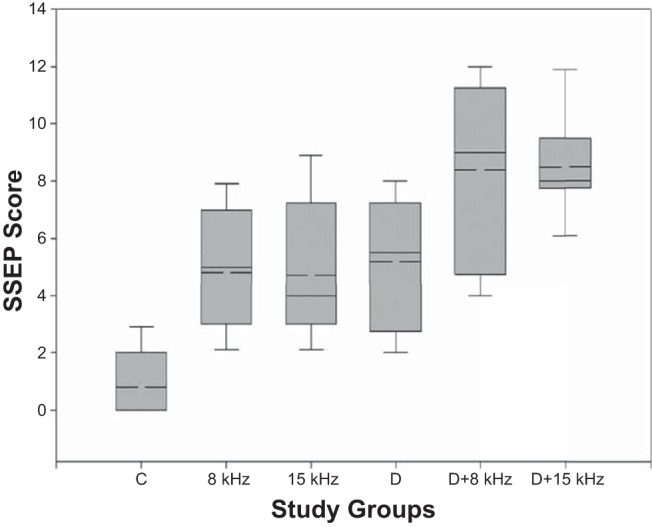
SSEP scores for the different groups. The boundary of the box closest to zero indicates the 25th percentile, the solid line within the box marks the median, the dashed line marks the mean, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles, respectively.
DISCUSSION
A theoretical physical model (Crum and Mao 1996) demonstrated that at normal fluid saturations, bubble growth could be initiated by SPLs as low as 180 dB re 1 μPa at 1 m (1 kPa), whereas significant and rapid growth would take place at exposure levels >200 dB (10 kPa). Supersaturation of the inert gas under the same conditions resulted in considerable bubble growth at lower SPLs and with shorter exposure times. For example, a 1-μm bubble (nucleus) in a fluid with a dissolved gas concentration of 125% was predicted to increase its diameter 20-fold in about 20 s at exposure levels ranging from 150 to 190 dB (0.03 to 3 kPa).
There are a number of studies which corroborate these theoretical considerations. Extensive bubble production was demonstrated ex vivo in blood and liver tissue and in the whole kidney compressed to 5–8 ATA for 1–3 h, with subsequent exposure to sound transmission at 37 kHz, 214 dB (50 kPa) (Crum et al. 2005). In prawns compressed to 2 ATA for 10 min (Arieli et al. 2000), a significant increase was reported in the mean volume of bubbles under acoustic transmission at 37 kHz, 243 to 249 dB (1,412 to 2,818 kPa). Significant bubble production was found in the guinea pig when 750-kHz sound was employed at 200 dB (10 kPa) (ter Haar et al. 1982). In rats compressed to 4 and 5 ATA for 60 min while exposed to sound at 37 kHz/208.9 dB (28.2 kPa), vital microscopy of the intestinal mesentery showed a significant increase in both bubble density and diameter (Shupak et al. 2002).
Inert gas supersaturation, which may play a crucial role in bubble growth secondary to acoustically induced agitation, is commonly encountered in both marine mammals and human divers. During regular diving, the calculated intramuscular nitrogen tensions for the dolphin, beaked whale, and blue whale reach ∼300% of their level on the surface (Dennison et al. 2012; Hooker et al. 2009; Houser et al. 2001; Kvadsheim et al. 2012). In human divers, widely employed decompression schedules allow for ∼160% nitrogen supersaturation during ascent from a dive (Tikuisis and Gerth 2003).
Hence, both theoretical models and previous ex and in vivo studies imply that under conditions of inert gas supersaturation, which are often present during a dive, existing bubbles in marine mammals and the human diver may be activated and grow to a significant degree, secondary to underwater sound transmissions. However, the production and growth of intra- and extravascular inert gas bubbles does not always result in overt DCS. Several precordial Doppler and ultrasonic imaging studies demonstrated the presence of bubbles in asymptomatic human divers, whereas clinical signs of DCS were correlated only with the highest bubble scores (Bayne et al. 1985; Daniels 1984; Eckenhoff et al. 1990; Kumar et al. 1997; Nishi et al. 2003).
The present study demonstrates clinically significant consequences of bubble enlargement, as put forward in the hypothesis. Namely, the occurrence of overt illness or death during the simulated dive profile designed to induce DCS was higher in animals subjected to sound transmission at 204 dB/8 kHz. It may be interesting to note that this higher incidence of DCS was observed only for the mid-frequency transmission (8 kHz), which matches the frequency range of naval active sonar that has been associated with the mass stranding of cetaceans (DeRuiter et al. 2013; Fahlman et al. 2014; Houser et al. 2001; Moretti et al. 2014; Salami et al. 2010; Sivle et al. 2012).
Because no measurement such as actual bubble imaging or quantification was employed in the present study, it cannot be proved that sound-activated bubble growth was the causative mechanism of the insult in the rat. Nevertheless, in the context of previous studies showing inert gas bubble growth secondary to intense underwater sound transmissions in various animal models (Arieli et al. 2000; Shupak et al. 2002; ter Haar et al. 1982), this should at least be considered a possible mechanism contributing to the evolution of the insult.
We found significantly higher pathological scores for SSEPs in the rats diving while exposed to the underwater sound fields of 204 dB/8 kHz and 188.3 dB/15 kHz compared with the “immersion only” condition. These scores were also higher than those found in the “diving only” group. This indicates a significant contribution of sound transmission to the development of the neurologic insult, in addition to the dysfunction caused by decompression stress per se.
Significantly higher pathological scores were found for SSEPs in the immersed rats exposed to 8-kHz and 15-kHz transmissions compared with the Control group. Although the growth of micronuclei to form bubbles has previously been demonstrated at normal inert gas saturation, in such a case significant bubble expansion would be anticipated only for sound pressures >210 dB (Crum and Mao 1996). Thus these SSEPs scores, which were similar to those found in animals exposed to the simulated dive profile resulting in DCS, require further explanation.
It has previously been hypothesized that nonauditory effects of sound exposure may be produced by activation of cortico-hippocampal areas (Talpalar and Grossman 2005) or by the influence of vibration on central nervous system (CNS) tissues and straining of nerve fibers by the mechanical forces induced (Gennarelli 1993; McIntosh et al. 1996; Steevens et al. 1999). The pathological changes we observed in SSEPs also may be related to significant effects of ultrasound on cell metabolism as reported in a number of studies, among others an increase in transepithelial calcium ion transport, resulting in decreased cellular gap-junction permeability (Dinno et al. 1989), generation of free radicals with irreversible inactivation of luciferase and ATPase (Matthews et al. 1993), and lipid and protein peroxidation (Al-Karmi et al. 1994). Finally, a recent model of cell vibration, under sound transmission of tens to hundreds of kilohertz at 1 W/cm2 (186 dB re 1 μPa), suggests that the mechanism may be vibration-induced alterations in neuronal cell function, including inhibition of action potentials (Or and Kimmel 2009). The authors propose the theory that due to the inherently inhomogeneous nature of the cell cytoplasm, acoustically driven vibrations may induce relative motion between various intracellular elements that would affect their function.
One previous study investigated the neurological impact of underwater sound in submerged rats under ambient conditions. The animals were subjected to underwater sound of 180 (1 kPa) and 194 dB (5 kPa) at frequencies of 150 and 250 Hz, respectively. The exposure at 180 dB/150 Hz resulted in transient, minor motor deficits that resolved by 14 days following sound exposure (Laurer et al. 2002). The authors pointed out the similarity between their findings of transient neurological deficits and those described in animal models and human patients suffering from mild traumatic brain injury.
A single clinical case series reported on two divers suffering from transient, acute neurological symptoms following short no-decompression dives to 10 and 20 m while exposed to underwater transmissions at 160 dB (0.1 kPa)/240 Hz and 181 dB (1.1 kPa)/1,000 Hz (Steevens et al. 1999). The overt symptoms resolved within 30 min in both cases. However, both divers reported subtle neurological abnormalities, including headache and cognitive impairment weeks to months after the exposures and a single episode of a complex partial seizure 16 mo later in one diver.
Over the past two decades, there have been numerous reports of the mass stranding of cetaceans in association with naval sonar activity, becoming the subject of intense public opinion and scientific discussion (Balcomb and Claridge 2001; Dalton 2003; Di Guardo and Marruchella 2005; Frantzis 1998; Malakoff 2001, 2002; Parsons et al. 2008; Piantadosi and Thalmann 2004; Simmonds and Lopez-Jurado 1991). Several explanations have been suggested for this disturbing phenomenon. Significant changes have been observed in the diving behavior of marine mammals exposed to mid-frequency sonar transmissions (DeRuiter et al. 2013; Goldbogen et al. 2013; Sivle et al. 2012; Tyack et al. 2011). The described deviation from normal diving behavior may have led to extreme levels of nitrogen supersaturation, resulting in bubble formation by off-gassing and DCS-like insults (Dennison et al. 2012; Fahlman et al. 2014; Fernández et al. 2004, 2005; Hooker at al. 2012; Jepson et al. 2003; Parsons et al. 2008; Talpalar and Grossman 2005).
On the other hand, theoretical models, in vitro studies, and ex and in vivo studies have shown the feasibility of bubble agitation and growth by rectified diffusion secondary to intense underwater sound (Arieli et al. 2000; Crum and Mao 1996; Crum et al. 1987, 2005; Daniels et al. 1987; ter Haar et al. 1982) with resultant DCS and subsequent stranding (Fernández et al. 2005; Houser et al. 2001). The present study demonstrates in vivo the facilitation of DCS by underwater sound transmissions in the frequency and intensity range of sonar activity purportedly associated with cetacean stranding. Because abnormalities in the SSEPs were also noted in animals exposed to underwater sound despite the absence of nitrogen supersaturation, other underlying mechanisms such as an increase in calcium ion transport or the generation of free radicals (Al-Karmi et al. 1994; Dinno et al. 1989; Matthews et al. 1993), vibration effects (Gennarelli 1993; McIntosh et al. 1996; Or and Kimmel 2009; Steevens et al. 1999) or cortico-hippocampal stimulation (Talpalar and Grossman 2005) may have produced the CNS insult, in addition to the proposed mechanism of rectified diffusion.
Conclusions
Underwater sound transmissions with frequency and intensity characteristics similar to those of active naval sonars and diving navigation systems may induce neurological damage during immersion and facilitate overt DCS. Our results indicate a deleterious interaction between intense underwater sound fields and the vital body systems of a small terrestrial mammal, either directly or via inert gas bubble growth. This may contribute to our understanding of the mass stranding of cetaceans, purportedly associated with the deployment of naval sonar, and may also have potential implications for human diving safety.
GRANTS
This study was supported by a research grant from the Israel Defense Forces Medical Corps and from the Israel Ministry of Defense Directorate of Defense Research and Development Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T., H.S.-B., and A.S. conception and design of research; D.T., D.H., Y.A., and A.S. analyzed data; D.T., D.H., Y.A., and A.S. interpreted results of experiments; D.T. and A.S. prepared figures; D.T., H.S.-B., D.H., Y.A., and A.S. edited and revised manuscript; D.T., H.S.-B., D.H., Y.A., and A.S. approved final version of manuscript; H.S.-B. performed experiments; A.S. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Richard Lincoln of the Israel Naval Medical Institute for help in editing the manuscript.
REFERENCES
- Al-Karmi AM, Dinno MA, Stoltz DA, Crum LA, Matthews JC. Calcium and the effects of ultrasound on frog skin. Ultrasound Med Biol 20: 73–81, 1994. [DOI] [PubMed] [Google Scholar]
- Arieli Y, Arieli R, Shupak A. Can high-frequency sound affect gas-bubble dynamics? A study in the intact prawn Palaemon elegans. Ultrasound Med Biol 26: 1511–1515, 2000. [DOI] [PubMed] [Google Scholar]
- Balcomb KC 3rd, Claridge DE. A mass stranding of cetaceans caused by naval sonar in the Bahamas. Bahamas J Sci 8: 2–12, 2001. [Google Scholar]
- Bayne CG, Hunt WS, Johanson DC, Flynn ET, Weathersby PK. Doppler bubble detection and decompression sickness: a prospective clinical trial. Undersea Biomed Res 12: 327–332, 1985. [PubMed] [Google Scholar]
- Bogomolni AL, Pugliares KR, Sharp SM, Patchett K, Harry CT, LaRocque JM, Touhey KM, Moore M. Mortality trends of stranded marine mammals on Cape Cod and southeastern Massachusetts, USA, 2000 to 2006. Dis Aquat Organ 88: 143–155, 2010. [DOI] [PubMed] [Google Scholar]
- Bradshaw CJ, Evans K, Hindell MA. Mass cetacean strandings–a plea for empiricism. Conserv Biol 20: 584–586, 2006. [DOI] [PubMed] [Google Scholar]
- Crum LA, Bailey MR, Guan J, Hilmo PR, Kargl SG, Matula TJ, Sapozhnikov OA. Monitoring bubble growth in supersaturated blood and tissue ex vivo and the relevance to marine mammal bioeffects. Acoust Res Lett Online 6: 214–220, 2005. [Google Scholar]
- Crum LA, Daniels S, ter Haar GR, Dyson M. Ultrasonically induced gas bubble production in agar based gels: Part II. Theoretical analysis. Ultrasound Med Biol 13: 541–554, 1987. [DOI] [PubMed] [Google Scholar]
- Crum LA, Mao Y. Acoustically enhanced bubble growth at low frequencies and its implications for human diver and marine mammal safety. J Acoust Soc Am 99: 2898–2907, 1996. [DOI] [PubMed] [Google Scholar]
- Dalton R. Scientists split over regulations on sonar use. Nature 425: 549, 2003. [DOI] [PubMed] [Google Scholar]
- Daniels S. Ultrasonic monitoring of decompression procedures. Philos Trans R Soc Lond B Biol Sci 304: 153–175, 1984. [DOI] [PubMed] [Google Scholar]
- Daniels S, Blondel D, Crum LA, ter Haar GR, Dyson M. Ultrasonically induced gas bubble production in agar based gels: Part I. Experimental investigation. Ultrasound Med Biol 13: 527–539, 1987. [DOI] [PubMed] [Google Scholar]
- Dennison S, Moore MJ, Fahlman A, Moore K, Sharp S, Harry CT, Hoppe J, Niemeyer M, Lentell B, Wells RS. Bubbles in live-stranded dolphins. Proc Biol Sci 279: 1396–1404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuiter SL, Southall BL, Calambokidis J, Zimmer WM, Sadykova D, Falcone EA, Friedlander AS, Joseph JE, Moretti D, Shirr GS, Thomas L, Tyack PL. First direct measurements of behavioural responses by Cuvier's beaked whales to mid-frequency active sonar. Biol Lett 9: 2130223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guardo G, Marruchella G. Sonars, gas bubbles, and cetacean deaths (Letter). Vet Pathol 42: 517, 2005. [DOI] [PubMed] [Google Scholar]
- Dinno MA, Dyson M, Young SR, Mortimer AJ, Hart J, Crum LA. The significance of membrane changes in the safe and effective use of therapeutic and diagnostic ultrasound. Phys Med Biol 34: 1543–1552, 1989. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Olstad CS, Carrod G. Human dose-response relationship for decompression and endogenous bubble formation. J Appl Physiol 69: 914–918, 1990. [DOI] [PubMed] [Google Scholar]
- Fahlman A, Tyack PL, Miller PJO, Kvadsheim PH. How man-made interference might cause gas bubble emboli in deep diving whales. Front Physiol 5: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A, Arbelo M, Deaville R, Patterson IAP, Castro P, Baker JR, Degollada E, Ross HM, Herráez P, Pocknell AM, Rodríguez E, Howie FE, Espinosa A, Reid RJ, Jaber JR, Martin V, Cunningham AA, Jepson PD. Pathology: whales, sonar and decompression sickness. Nature 428: 1–2, 2004. [DOI] [PubMed] [Google Scholar]
- Fernández A, Edwards JF, Rodríguez F, Espinosa de los Monteros A, Herráez P, Castro P, Jaber JR, Martín V, Arbelo M. “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (family Ziphiidae) exposed to anthropogenic sonar signals. Vet Pathol 42: 446–457, 2005. [DOI] [PubMed] [Google Scholar]
- Francis TJ, Gorman DF. Pathogenesis of the decompression disorders. In: The Physiology and Medicine of Diving (4th ed), edited by Bennett PB and Elliott DH. London: Saunders, 1993, p. 454–480. [Google Scholar]
- Francis TJ, Griffin JL, Homer LD, Pezeshkpour GH, Dutka AJ, Flynn ET. Bubble-induced dysfunction in acute spinal cord decompression sickness. J Appl Physiol 68: 1368–1375, 1990. [DOI] [PubMed] [Google Scholar]
- Frantzis A. Does acoustic testing strand whales? Nature 392: 29, 1998. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA. Mechanisms of brain injury. J Emerg Med 11, Suppl 1: 5–11, 1993. [PubMed] [Google Scholar]
- Goldbogen JA, Southall BL, DeRuiter SL, Calambokidis J, Friedlaender AS, Hazen EL, Falcone EA, Schorr GS, Douglas A, Moretti DJ, Kyburg C, McKenna MF, Tyack PL. Blue whales respond to simulated mid-frequency military sonar. Proc Biol Sci 280: 20130657, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton SM, Kriss A, Muller DP. Comparison of the effects of four anaesthetic agents on somatosensory evoked potentials in the rat. Lab Anim 33: 243–251, 1999. [DOI] [PubMed] [Google Scholar]
- Hooker SK, Baird RW, Fahlman A. Could beaked whales get the bends? Effect of diving behaviour and physiology on modelled gas exchange for three species: Ziphius cavirostris, Mesoplodon densirostris and Hyperoodon ampullatus. Respir Physiol Neurobiol 167: 235–246, 2009. [DOI] [PubMed] [Google Scholar]
- Hooker SK, Fahlman A, Moore MJ, Aguilar de Soto N, Bernaldo de Quirós Y, Brubakk AO, Costa DP, Costidis AM, Dennison S, Falke KJ, Fernandez A, Ferrigno M, Fitz-Clarke JR, Garner MM, Houser DS, Jepson PD, Ketten DR, Kvadsheim PH, Madsen PT, Pollock NW, Rotstein DS, Rowles TK, Simmons SE, Van Bonn W, Weathersby PK, Weise MJ, Williams TM, Tyack PL. Deadly diving? Physiological and behavioural management of decompression stress in diving mammals. Proc Biol Sci 279: 1041–1050, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser DS, Howard R, Ridgway S. Can diving-induced tissue nitrogen supersaturation increase the chance of acoustically driven bubble growth in marine mammals? J Theor Biol 213: 183–195, 2001. [DOI] [PubMed] [Google Scholar]
- Hyldegaard O, Møller M, Madsen J. Protective effect of oxygen and heliox breathing during development of spinal decompression sickness. Undersea Hyperb Med 21: 115–128, 1994. [PubMed] [Google Scholar]
- Jepson PD, Arbelo M, Deaville R, Patterson IAP, Castro P, Baker JR, Degollada E, Ross HM, Herráez P, Pocknell AM, Rodríguez F, Howie FE, Espinosa A, Reid RJ, Jaber JR, Martin V, Cunningham AA, Fernández A. Gas-bubble lesions in stranded cetaceans. Nature 425: 575–576, 2003. [DOI] [PubMed] [Google Scholar]
- Katsenelson K, Arieli R, Arieli Y, Abramovich A, Feinsod M, Tal D. Hyperbaric oxygen pretreatment according to the gas micronuclei denucleation hypothesis reduces neurologic deficit in decompression sickness in rats. J Appl Physiol 107: 558–563, 2009. [DOI] [PubMed] [Google Scholar]
- Kayar SR, Miller TL, Wolin MJ, Aukhert EO, Axley MJ, Kiesow LA. Decompression sickness risk in rats by microbial removal of dissolved gas. Am J Physiol Regul Integr Comp Physiol 275: R677–R682, 1998. [DOI] [PubMed] [Google Scholar]
- Kumar VK, Billica RD, Waligora JM. Utility of Doppler-detectable microbubbles in the diagnosis and treatment of decompression sickness. Aviat Space Environ Med 68: 151–158, 1997. [PubMed] [Google Scholar]
- Kvadsheim PH, Miller PJO, Tyack PL, Sivle LD, Lam FPA, Fahlman A. Estimated tissue and blood N2 levels and risk of decompression sickness in deep-, intermediate-, and shallow-diving toothed whales during exposure to naval sonar. Front Physiol 3: 125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurer HL, Ritting AN, Russ AB, Bareyre FM, Raghupathi R, Saatman KE. Effects of underwater sound exposure on neurological function and brain histology. Ultrasound Med Biol 28: 965–973, 2002. [DOI] [PubMed] [Google Scholar]
- Leighton TG. The Acoustic Bubble. San Diego, CA: Academic, 1994, p. 379–407. [Google Scholar]
- Lillo RS, Flynn ET, Homer LD. Decompression outcome following saturation dives with multiple inert gases in rats. J Appl Physiol 59: 1503–1514, 1985. [DOI] [PubMed] [Google Scholar]
- Lillo RS, Parker EC. Mixed-gas model for predicting decompression sickness in rats. J Appl Physiol 89: 2107–2116, 2000. [DOI] [PubMed] [Google Scholar]
- Malakoff D. A roaring debate over ocean noise. Science 291: 576–578, 2001. [DOI] [PubMed] [Google Scholar]
- Malakoff D. Suit ties whale deaths to research cruise. Science 298: 722–723, 2002. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Harder WL, Richardson WK, Fisher RJ, Al-Karmi AM, Crum LA, Dinno MA. Inactivation of firefly luciferase and rat erythrocyte ATPase by ultrasound. Membr Biochem 10: 213–220, 1993. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest 74: 315–342, 1996. [PubMed] [Google Scholar]
- Melamed Y, Shupak A, Bitterman H. Medical problems associated with underwater diving. N Engl J Med 326: 30–35, 1992. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Early GA. Cumulative sperm whale bone damage and the bends. Science 306: 2215, 2004. [DOI] [PubMed] [Google Scholar]
- Moretti D, Thomas L, Marques T, Harwood J, Dilley A, Neales B, Shaffer J, McCarthy E, New L, Jarvis S, Morrissey R. A risk function for behavioral disruption of Blainville's beaked whales (Mesoplodon densirostris) from mid-frequency active sonar. PLoS One 9: e85064, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrison A, Glasspool E, Francis J, Sedgwick M. Somatosensory evoked potentials in acute neurological decompression illness. J Neurol 242: 669–676, 1995. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals (8th ed). Washington, DC: National Academies, 2011. [Google Scholar]
- Nishi RY, Brubakk AO, Eftedal OS. Bubble detection. In: Bennett and Elliott's Physiology and Medicine of Diving (5th ed), edited by Brubakk AO and Neuman TS. Edinburgh, UK: Saunders, 2003, p. 501–529. [Google Scholar]
- Or M, Kimmel E. Modeling linear vibration of cell nucleus in low intensity ultrasound field. Ultrasound Med Biol 35: 1015–1025, 2009. [DOI] [PubMed] [Google Scholar]
- Oro J, Haghighi SS. Effects of altering core body temperature on somatosensory and motor evoked potentials in rats. Spine (Phila Pa 1976) 17: 498–503, 1992. [DOI] [PubMed] [Google Scholar]
- Parsons EC, Dolman SJ, Wright AJ, Rose NA, Burns WC. Navy sonar and cetaceans: just how much does the gun need to smoke before we act? Mar Pollut Bull 56: 1248–1257, 2008. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Thalmann ED. Pathology: whales, sonar and decompression sickness. Nature 428: 1, 2004. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Howard R. Dolphin lung collapse and intramuscular circulation during free diving: evidence from nitrogen washout. Science 206: 1182–1183, 1979. [DOI] [PubMed] [Google Scholar]
- Rommel SA, Costidis AM, Fernández A, Jepson PD, Pabst DA, McLellan WA, Houser DS, Cranford TW, van Helden AL, Allen DM, Barros NB. Elements of beaked whale anatomy and diving physiology and some hypothetical causes of sonar-related stranding. J Cetacean Res Manag 7: 189–209, 2006. [Google Scholar]
- Rooney JA. Biological Effects of Ultrasound and their Relevance to the Establishment of Safety Standards for the Exposure of Divers to Intense Ultrasound. NSMRL Report no. 893 Groton, CT: Naval Submarine Medical Research Laboratory, 1979. [Google Scholar]
- Salami A, Dellepiane M, Barbierato M, Freda P, Crippa B, Guastini L, Mora R. The effect of active sonar for the protection of moored and anchored warships on the human hearing. Eur Arch Otorhinolaryngol 267: 207–211, 2010. [DOI] [PubMed] [Google Scholar]
- Shupak A, Arieli Y, Bitterman H, Brod V, Arieli R, Rosenhause G. High-frequency sound field and bubble formation in a rat decompression model. Ultrasound Med Biol 28: 655–660, 2002. [DOI] [PubMed] [Google Scholar]
- Shupak A, Pratt H, Arieli Y, Tal D. High-frequency sound transmissions under water and risk of decompression sickness. Ultrasound Med Biol 29: 119–125, 2003. [DOI] [PubMed] [Google Scholar]
- Simmonds MP, Lopez-Jurado LF. Whales and the military (Letter). Nature 351: 448, 1991. [Google Scholar]
- Sivle LD, Kvadsheim PH, Fahlman A, Lam FP, Tyack PL, Miller PJ. Changes in dive behavior during naval sonar exposure in killer whales, long-finned pilot whales, and sperm whales. Front Physiol 3: 400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, Hunter WL Jr. On the Effects of Exposure to Intense Underwater Sound on Navy Divers. A Report of a Conference on the Bio-Effects of Sound. NSMRL Memorandum Report 80-1 Groton, CT: Naval Submarine Medical Research Laboratory, 1980. [Google Scholar]
- Steevens CC, Russell KL, Knafelc ME, Smith PF, Hopkins EW, Clark JB. Noise-induced neurologic disturbances in divers exposed to intense water-borne sound: two case reports. Undersea Hyperb Med 26: 261–265, 1999. [PubMed] [Google Scholar]
- Sykes JJW, Hallenbeck JM, Leitch DR. Spinal cord decompression sickness: a comparison of recompression therapies in an animal model. Aviat Space Environ Med 57: 561–568, 1986. [PubMed] [Google Scholar]
- Talpalar AE, Grossman Y. Sonar versus whales: noise may disrupt neural activity in deep-diving cetaceans. Undersea Hyperb Med 32: 135–139, 2005. [PubMed] [Google Scholar]
- ter Haar G, Daniels S, Eastaugh KC, Hill CR. Ultrasonically induced cavitation in vivo. Br J Cancer 45, Suppl 5: 151–155, 1982. [PMC free article] [PubMed] [Google Scholar]
- Tikuisis P, Gerth WA. Decompression theory. In: Bennett and Elliott's Physiology and Medicine of Diving (5th ed), edited by Brubakk AO and Neuman TS. Edinburgh, UK: Saunders, 2003, p. 419–454. [Google Scholar]
- Tyack PL, Zimmer WMX, Moretti D, Southall BL, Claridge DE, Durban JW, Clark CW, D'Amico A, DiMarzio N, Jarvis S, McCarthy E, Morrissey R, Ward J, Boyd IL. Beaked whales respond to simulated and actual navy sonar. PLoS One 6: e17009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AJ. Jane's Underwater Warfare Systems (11th ed). Coulsdon, Surrey, UK: Jane's Information Group, 1999, p. 100–111. [Google Scholar]