Abstract
Facial flushing in rosacea is often induced by trigger events. However, trigger causation mechanisms are currently unclear. This study tested the central hypothesis that rosacea causes sympathetic and axon reflex-mediated alterations resulting in trigger-induced symptomatology. Twenty rosacea patients and age/sex-matched controls participated in one or a combination of symptom triggering stressors. In protocol 1, forehead skin sympathetic nerve activity (SSNA; supraorbital microneurography) was measured during sympathoexcitatory mental (2-min serial subtraction of novel numbers) and physical (2-min isometric handgrip) stress. In protocol 2, forehead skin blood flow (laser-Doppler flowmetry) and transepithelial water loss/sweat rate (capacitance hygrometry) were measured during sympathoexcitatory heat stress (whole body heating by perfusing 50°C water through a tube-lined suit). In protocol 3, cheek, forehead, forearm, and palm skin blood flow were measured during nonpainful local heating to induce axon reflex vasodilation. Heart rate (HR) and mean arterial pressure (MAP) were recorded via finger photoplethysmography to calculate cutaneous vascular conductance (CVC; flux·100/MAP). Higher patient transepithelial water loss was observed (rosacea 0.20 ± 0.02 vs. control 0.10 ± 0.01 mg·cm−2·min−1, P < 0.05). HR and MAP changes were not different between groups during sympathoexcitatory stressors or local heating. SSNA during early mental (32 ± 9 and 9 ± 4% increase) and physical (25 ± 4 and 5 ± 1% increase, rosacea and controls, respectively) stress was augmented in rosacea (both P < 0.05). Heat stress induced more rapid sweating and cutaneous vasodilation onset in rosacea compared with controls. No axon reflex vasodilation differences were observed between groups. These data indicate that rosacea affects SSNA and that hyperresponsiveness to trigger events appears to have a sympathetic component.
Keywords: supraorbital nerve, rosacea triggers, mental stress, physical stress, heat stress, autonomic dysfunction, sympathetic nervous system
rosacea is a chronic inflammatory skin disease affecting 2.3 to 10% of workers in Germany, Ireland, and Sweden in well-controlled large cohort studies (Tan and Berg 2013) and a projected 16 million Americans (National Rosacea Society 2013a). Rosacea causes both increased financial burden and reduced quality of life (Aksoy et al. 2010; Beikert et al. 2013). Rosacea's etiology and mechanisms are unclear; however, trigger events such as physical, mental, and thermal stress cause symptomatology including facial flushing (Del Rosso 2012; Del Rosso et al. 2013). Neural reflexes (sympathetic and local axon) may contribute to rosacea symptomatology, since these reflexes are actively engaged in symptom-mediating stress responses induced by triggers.
Physical, mental, and thermal stress increase skin sympathetic outflow (Cui et al. 2006; Muller et al. 2013; Vissing and Hjortso 1996; Wilson et al. 2006), which can be quantified directly via postganglionic skin sympathetic nerve activity (SSNA) and indirectly via sympathetic end-organ responses (skin blood flow and sweat gland secretions). It is currently unknown whether rosacea patients have altered skin sympathetic outflow during triggering stresses. One difficulty in addressing this question is that SSNA contains multiple signals and is not uniform across the body (Charkoudian and Wallin 2014; Iwase 2009), whereas rosacea symptoms are normally confined to the head and neck (Pelle 2012). Fortunately, Nordin and colleagues adapted microneurography to impale cutaneous nerves from the head (Nordin 1994; Nordin et al. 1986; Nordin and Thomander 1989); only the supraorbital nerve contained suitable and measurable efferent sympathetic outflow traffic (Nordin 1990). Thus quantifying supraorbital skin sympathetic outflow during baseline and trigger stimuli may give insight into potential rosacea mechanisms.
Face-related SSNA has only rarely been described, and never in a dermatology population with facial involvement. In contrast, skin blood flow has been partially described in the literature (Drott et al. 2002; Guzman-Sanchez et al. 2007; Mark et al. 2003; Shanler and Ondo 2007; Sibenge and Gawkrodger 1992). Many of these studies, however, are difficult to interpret because of lack of controls and inappropriate use of laser-Doppler methodology. For example, blood pressure and laser-Doppler flowmetry must be assessed simultaneously to interpret sympathetic-induced flux changes (e.g., increased arterial blood pressure can increase flux independent of cutaneous vasodilation), and blood flow standardization must be employed, since absolute values are meaningless unless blood vessel density and other parameters are known (but are unknown in all noninvasive studies). Although also under sympathetic control (Shibasaki et al. 2006), sweat gland function, to our knowledge, has not been evaluated in rosacea.
Axon reflexes involve local peripheral neural circuits where a cutaneous afferent stimulus causes a local sympathetic-like end-organ response, yet these sensory-motor responses are not mediated by brain stem autonomic control centers (Holzer 1997; Rubino and Burnstock 1996). Noxious axon reflexes associated with stinging induced by cholinergic iontophoresis are augmented in severe vs. milder rosacea (Drummond and Su 2012). It is possible that in some patients with rosacea, triggers are mediated via sympathetic-like axon reflexes involving warmth, which activate transient receptor potential vanilloid subtype (TRPV) ion channels in cutaneous afferents, inducing vasodilation (Wong and Fieger 2010). We recently quantified cheek and forehead nonnoxious axon reflexes via laser-Doppler flowmetry, local heating, and topical afferent nerve block (Metzler-Wilson et al. 2012). It is possible that this nonnoxious test can identify the sympathetic-like effects of axon reflexes in trigger events.
Our aim was to test whether rosacea triggers induce hyperresponsive sympathetic and local axon reflex responses via fundamental alterations in neural reflexes. These exaggerated sympathetic and sympathetic-like responses may trigger symptomatology and local inflammation and, over time, cause disease progression. Thus, to identify whether changes in sympathetic and axon reflex responses occur in rosacea, we tested the following hypotheses: 1) We hypothesized that augmented supraorbital SSNA and cutaneous end-organ responses to mental and physical stress would occur in rosacea vs. controls. Mental and physical stressors were used as sympathoexcitatory triggers during direct supraorbital sympathetic outflow quantification. 2) We hypothesized that augmented cutaneous end-organ responses to whole body thermal stress would occur in rosacea vs. controls. Thermal stress was also used as a sympathoexcitatory trigger, but because supraorbital SSNA recordings cannot be maintained for the time necessary for a whole body heating protocol in our hands, only indirect supraorbital sympathetic outflow was quantified. 3) We hypothesized that greater skin blood flow reflex changes would result from local heating in affected compared with unaffected skin and controls. Nonnoxious local heating can engage axon reflexes, producing sympathetic-like responses.
METHODS
Participants
Eleven otherwise healthy erythematotelangiectatic rosacea patients (3 women, 8 men) and 11 age/sex-matched healthy controls participated in protocol 1. Two to 4 wk later, 16 of these participants (8 rosacea, 8 control) participated in protocol 2. Nine otherwise healthy erythematotelangiectatic rosacea patients (5 women, 4 men) and 9 age/sex-matched healthy controls participated in protocol 3. One protocol 3 control participant's data were not analyzed due to a technical issue. By design, there were no significant age differences (P = 0.808), but patients were significantly heavier than controls (P = 0.015); collapsed means ± SD for all protocols were age = 35 ± 15 and 25 ± 5 yr and body mass index = 24 ± 4 and 25 ± 4 kg/m2 for women and men, respectively.
Participant health was assessed by medical history and physical exam including 12-lead electrocardiogram, and rosacea classification was determined on the basis of established guidelines (Wilkin et al. 2002). Exclusion criteria were smoking; pregnancy; hypertension; obesity; neural, cardiovascular, gastrointestinal, hematological, musculoskeletal, or other dermatological diseases; or medications or supplements that could not be temporarily withheld. All experiments conformed to the Declaration of Helsinki and were approved by the local Institutional Review Board. Each participant gave both written and verbal informed consent to participate in this study.
Participants refrained from caffeine, alcohol, and exercise for 12–24 h and prescription drugs for at least 4 half-lives prior to testing conducted in the late morning or early afternoon in a temperature- and humidity-controlled laboratory (23–25°C, relative humidity <50%). All participants rested awake and supine for at least 30 min before experimentation.
Measurements
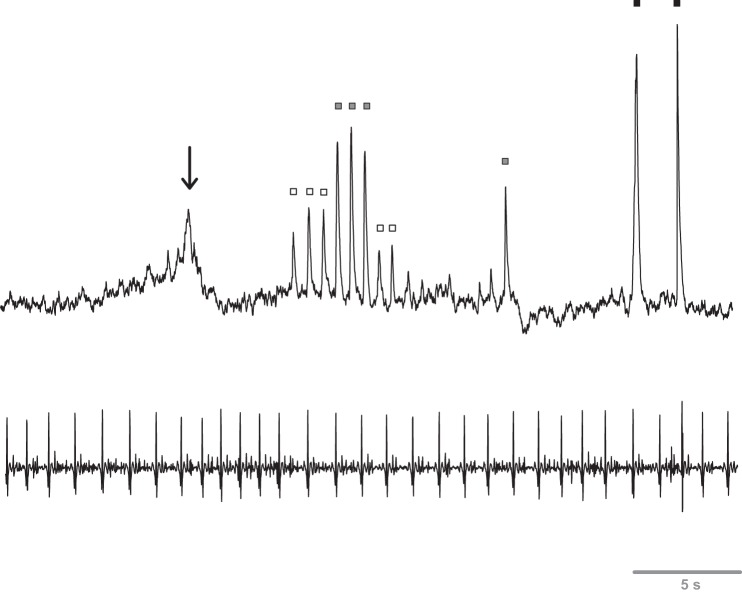
The supraorbital nerve tract was first identified and mapped via external stimulation using a square-wave electrical current (Grass Telefactor, West Warwick, RI) delivered via wand electrode. Nerve stimulation of this type produces afferent sensations and small muscle contractions. Multiunit supraorbital SSNA was measured by inserting a custom sterile tungsten microneurography electrode (impedance 2 MΩ, shaft diameter 0.2 mm; FHC, Bowdoin, ME) through the skin without local anesthesia. A ground electrode was then inserted close to the recording electrode but outside the sensory innervation area. The recording electrode was then positioned within the nerve fascicle and adjusted until SSNA bursts were clearly identified using previously established criteria (Delius et al. 1972; Hagbarth et al. 1972). In brief, these included 3:1 signal-to-noise ratio; skin afferent recordings; non-pulse-synchronous SSNA induction by startle, inspiratory gasp, and handgrip; and no relation to chemoreflex or baroreflex perturbations. Figure 1 depicts a representative tracing of baseline and during skin afferent stimulation. The nerve signal was amplified, passed through a bandpass filter (500–5,000 Hz), and integrated with a 0.1-s time constant (Iowa Bioengineering, Iowa City, IA). Mean voltage neurograms were visually displayed and recorded on a data acquisition system (Biopac Systems, Goleta, CA). The nerve signal was also routed to a loudspeaker for monitoring throughout the study.
Fig. 1.
Representative neurogram (first row) and ECG tracing (second row) during baseline and skin afferent stimulation. Arrow denotes a spontaneous baseline burst, and squares represent light (open squares), moderate (shaded squares), and firm (solid squares) rhythmical brush strokes with a calligraphy brush within the area of innervation of the supraorbital nerve. Note that bursts are not related to ECG r waves, and cutaneous mechanoreceptor activation is related to amplitude.
Skin blood flow was indexed via integrated laser-Doppler flow probes (Moor Instruments, Wilmington, DE) placed on skin sites devoid of visible superficial and telangiectatic veins. In protocol 3, optical sending and receiving fibers were housed in an 8-mm disk containing a heating element (Moor Instruments). Transepidermal water loss (TEWL) and sweat rate were measured via capacitance hygrometry using a custom ventilated chamber (protocols 1 and 2: 0.5- and 2.83-cm2 chamber windows, protocol 3: 0.6-cm2 chamber window) perfused with N2 (150 ml/min). Consistent with the literature, we define TEWL as water loss during nonstimulated baseline conditions and sweat rate as water loss during sweat gland engagement during sympathoexcitatory conditions. Chamber humidity was measured using a high-sensitivity humidity-temperature probe (Sable Systems International, Las Vegas, NV) connected in series with the chamber.
Beat-by-beat finger arterial blood pressure was measured via volume-clamp photoplethysmography that accounted for the vertical distance between the finger and heart (Finapres Medical Systems, Amsterdam, The Netherlands). Heart rate (HR) was derived in real time from the arterial pressure waveform (Finapres Medical Systems), and cardiac output was derived by ModelFlow methodology (Bogert and van Lieshout 2005). Oral temperature was measured via a thermocouple (Omega, Stamford, CN; protocol 2) located in the sublingual sulcus, and local skin temperature was measured via a thermocouple (Omega) or thermistor (YSI, Yellow Springs, OH) secured with surgical tape. All temperature data were routed to a bioamplifier (Sable Systems International, protocols 1 and 2; Biopac Systems, protocol 3).
Experimental Protocols
Protocol 1.
SSNA was measured in the supraorbital nerve (Fig. 1). A laser-Doppler flow probe for skin blood flow, ventilated capsule for TEWL/sweat rate, and thermistor for skin temperature were placed within the typical area of supraorbital innervation on the contralateral forehead because ipsilateral sensory-related mechanoreceptive afferents could not be avoided. After SSNA verification, baseline measurements were obtained, followed by mental arithmetic, re-baseline, and then handgrip protocols.
MENTAL ARITHMETIC.
To induce mental stress, participants continuously performed serial subtractions of the number 7 or 11 from a multiple-digit number for 2 min, with a novel starting number provided every ∼30 s. Participants indicated answers via finger gesture of the last numerical digit to avoid jaw or arm movement, which could cause microneurography electrode artifact. Because of occasional baseline neurogram shift and/or if the participant did not give mental task maximal effort, in some subjects the task was repeated after ∼15 min re-baseline. This type of mental arithmetic increases fibular, tibial, and supraorbital SSNA (Iwase et al. 1997; Muller et al. 2013; Nordin 1990).
HANDGRIP.
To induce physical stress, participants performed 2-min isometric handgrip. Participants performed maximal voluntary contractions while force was measured via compression dynamometry (Biopac Systems) to determine a target submaximal force of 30%. Handgrip force feedback was provided via verbal cues. This task increases fibular SSNA in an effort-dependent manner that is not related to the amount of muscle mass involved (Silber et al. 2000; Vissing and Hjortso 1996; Vissing et al. 1991; Wilson et al. 2006).
Protocol 2.
Thermoregulatory effectors (skin blood flow and sweating) were engaged by whole body heating. Each participant wore a high-density tube-lined suit (Med-Eng Systems, Ottawa, Canada) that covered the entire body except the hands, feet, and head and allowed for skin temperature control via changing the temperature of water perfusing the suit. Following a thermoneutral baseline (34°C water perfusion), whole body heating was performed by 50°C water perfusion. This method generally increases skin temperature to ∼38°C and core temperature by ∼1.0°C above baseline after 30–60 min of heating. Because of pronounced symptom triggering and heat intolerance in participants with rosacea, whole body heating was often terminated before the ∼1.0°C temperature increase (average heating ∼30 min). At the completion of whole body heating, 24°C water was perfused to cool the participant. Whole body heating increases SSNA, cutaneous vascular conductance (CVC), and sweat rate (Cui et al. 2006; Normell and Wallin 1974; Wilson et al. 2001) and appears to follow a pattern of initial suppression of SSNA and then dramatically increased internal temperature (Iwase et al. 2002).
Protocol 3.
Local cutaneous vasodilation was engaged via direct skin heating within focal forehead, cheek, forearm, and palm areas while skin blood flows were indexed via laser-Doppler flowmetry; these sites included two facial sites that can flush with rosacea and both glabrous and hairy control sites. Local heating protocols can be completed and interpreted in these skin sites (Metzler-Wilson et al. 2012). Following 6 min at baseline, local skin temperature was standardized to 32°C for 6 min. Skin temperature was then increased by 0.1°C/2-3 s and maintained at 42°C for 30 min. Increasing skin temperature at this rate ensured that pain fibers were not activated, because pain induction can alter the skin blood flow (Magerl and Treede 1996). After 30 min or when red blood cell flux plateaued, local skin temperature was increased to 43°C and maintained for 6 min to verify achievement of maximal skin blood flow. Local heating induces axon reflex vasodilation followed by sustained nitric oxide-mediated vasodilation, previously identified via sensory afferent and nitric oxide synthase blockade (Johnson et al. 2014).
Data Analysis
Data were acquired at 1,000 (protocols 1 and 2) or 62.5 Hz (protocol 3) with the use of a data acquisition system and analyzed with commercially available software (Biopac Systems). Rosacea and control subject characteristics were evaluated by two-tailed independent t-test. Critical P value was 0.05. Data are means ± SE unless otherwise noted.
Protocol 1.
Supraorbital SSNA was calculated as the integral of the integrated SSNA neurogram (Young et al. 2009), averaged in 15-s bins, normalized to baseline, and expressed as percent change from baseline. Normalization accounts for individual SSNA variation and electrode locations within the nerve. CVC was calculated as red blood cell flux multiplied by 100, and then that quantity was divided by mean arterial pressure (MAP). TEWL and sweat rate were determined by the product of measured N2 flow rate and humidity change across the skin surface, divided by the skin area exposed to N2. HR, cardiac output, MAP, CVC, and sweat rate were averaged in 1-min bins and expressed as absolute change from baseline. Group differences were tested via Wilcoxon signed-rank test (SSNA) and independent t-test (HR, cardiac output, MAP, CVC, and TEWL/sweat rate).
Protocol 2.
CVC and sweat rate were determined as in protocol 1. HR, cardiac output, MAP, facial skin temperature, oral temperature, CVC, and sweat rate were averaged in 1-min bins throughout the thermal stress. Data from the final minute of heat stress were compared via independent t-test. The onset thresholds of thermoregulatory effectors were determined as the time when the variable exponentially increased from baseline. These thresholds can provide an indirect indication of central sympathetic outflow to sweat glands and cutaneous blood vessels (Shibasaki et al. 2006).
Protocol 3.
CVC for each site was determined as in protocol 1 and expressed as a maximal percentage (derived from the highest plateau value). Averages of 60-s bins were obtained during initial baseline, 32°C standardization, plateau at 42°C, and plateau at 43°C; additionally, 5-s bins were obtained during the site's initial peak. HR and MAP were also averaged in 60-s bins. Two-way (skin site × time) repeated-measures ANOVA was utilized, with Student-Newman-Keuls post hoc analysis when significant main effects were observed.
RESULTS
Protocol 1
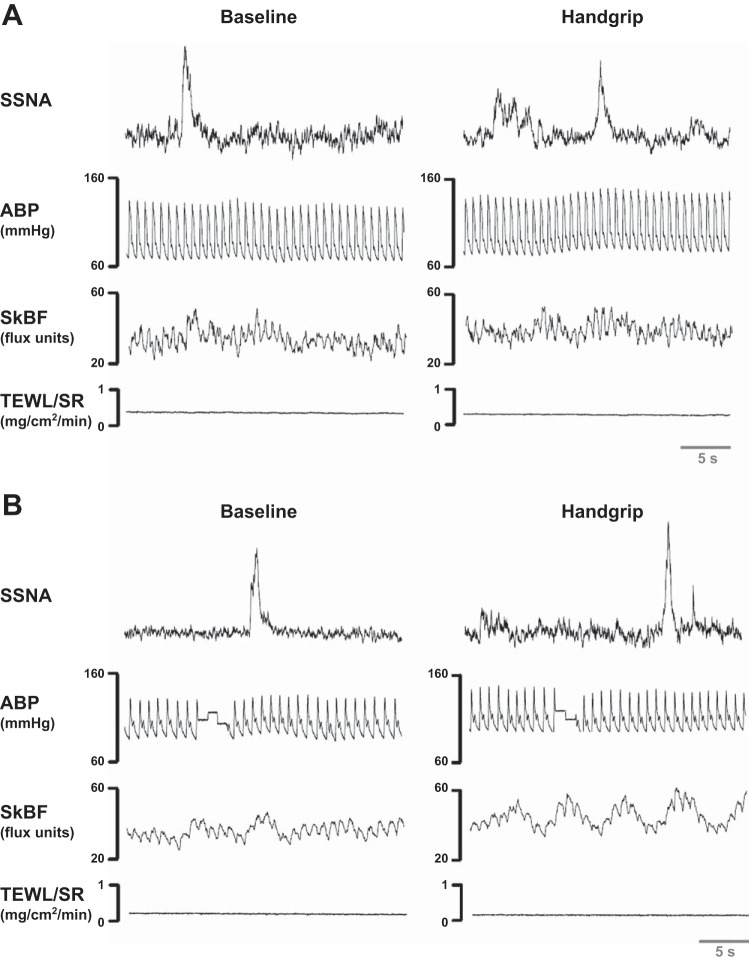
Augmented supraorbital SSNA responses occurred in rosacea compared with controls during both physical (Figs. 2 and 3A) and mental (Fig. 3B) stress. Because mental stress responses can vary and some nerve recordings were difficult to maintain, correlation coefficients were obtained on the percent increase in supraorbital SSNA within the initial portion of mental arithmetic between multiple bouts in some subjects. These correlations were 0.96 in rosacea (n = 7) and 0.87 in controls (n = 4), indicating high between-bout reproducibility. During the baseline period preceding mental stress, rosacea patients showed higher forehead TEWL than controls (0.20 ± 0.02 vs. 0.10 ± 0.01 mg·cm−2·min−1; P = 0.03), and baseline CVC was similar (36 ± 5 vs. 28 ± 3 flux/mmHg, for rosacea and controls, respectively; P = 0.18). Thermoregulatory effectors did not significantly change during physical or mental stress in the normothermic laboratory environment (ΔCVC <1% of baseline and Δsweat rate <0.01 mg·cm−2·min−1 for all subjects and conditions). During the physical stress protocol, the groups displayed similar forehead temperatures during baseline (33.18 ± 0.35 and 33.29 ± 0.27°C) and during the handgrip stressor (33.16 ± 0.36 and 33.39 ± 0.28°C, for rosacea and controls, respectively; P = 0.60). Similarly, during the mental stress protocol, the groups displayed similar forehead temperatures during baseline (33.23 ± 0.29 and 33.38 ± 0.28°C) and during the stressor (33.20 ± 0.29 and 33. ± 0.48°C, for rosacea and controls, respectively; P = 0.55). HR and MAP changes from baseline were not different between rosacea and controls during either stressor. Change in HR (handgrip: 4 ± 2 and 7 ± 1 beats/min, P = 0.28; mental arithmetic: 11 ± 2 beats/min for both groups, P = 0.92) and change in MAP (handgrip: 6 ± 1 and 9 ± 2 mmHg, P = 0.21; mental arithmetic: 9 ± 2 and 11 ± 2 mmHg, P = 0.37; for rosacea and controls, respectively) were not different between groups during either stress, whereas change in cardiac output was higher during mental arithmetic (1.2 ± 0.3 vs. 0.4 ± 0.2 l/min, P = 0.04) but not handgrip (0.3 ± 0.1 vs. 0.2 ± 0.1 l/min, P = 0.59) in rosacea.
Fig. 2.
Representative neurograms during baseline and physical stress for rosacea (A) and age/sex-matched controls (B). The first row depicts the representative skin sympathetic nerve activity (SSNA) tracing during baseline (left) and during a sympathoexcitatory response to physical stress involving 2 min of 30% maximal isometric handgrip (right) in one rosacea and one control subject. The second row depicts the arterial blood pressure (ABP) waveform. The third and fourth rows depict the corresponding skin blood flow (SkBF) and transepidermal water loss/sweat rate (TEWL/SR) from cutaneous end-organs on the contralateral side of the forehead.
Fig. 3.
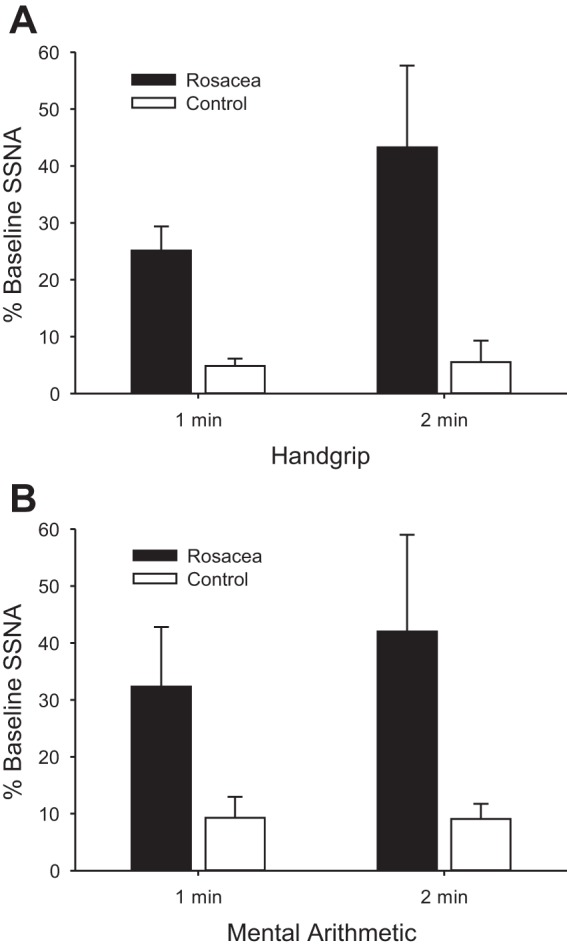
Changes in skin sympathetic nerve activity (SSNA) during physical stress involving 2 min of 30% maximal isometric handgrip (A) and mental stress involving 2 min of mental arithmetic (B) for rosacea and age/sex-matched controls. Values are means ± SE. In A, significant differences occur between group (rosacea vs. control, P < 0.01), across time (P = 0.03), and in the interactive term (P = 0.03). In B, significant differences occur between group (rosacea vs. control, P = 0.04), but there is no significant difference across time (P = 0.37) or in the interactive term (P = 0.35).
Protocol 2
Whole body heating occurred until internal temperature increased 1°C from baseline or subjects self-terminated the protocol (duration: 16–47 min for rosacea and 27–53 min for controls). During the thermoneutral baseline period, oral (36.1 ± 0.5 and 36.0 ± 0.2°C, P = 0.84) and forehead skin (33.7 ± 0.3 and 33.8 ± 0.4°C, for rosacea and controls, respectively; P = 0.39) temperatures were not different between groups. Whole body heating increased oral (0.94 ± 0.37 and 0.88 ± 0.12°C; P = 0.88) and forehead skin (1.51 ± 0.26 and 1.17 ± 0.48°C, for rosacea and controls, respectively; P = 0.21) temperature equally in both groups. Whole body heating increased HR (27 ± 1 and 28 ± 4 beats/min; P = 0.90) and cardiac output (1.92 ± 0.31 and 1.64 ± 0.34 l/min; P = 0.55) similarly in both groups but had a variable effect on MAP (−6 ± 3 and 4 ± 6 mmHg, for rosacea and controls, respectively; P = 0.19). During whole body heating, the sweating onset threshold in rosacea occurred at 10:53 ± 1:44 compared with 18:04 ± 2:34 (min:s) in controls (P = 0.02). However, sweating only trended higher during thermal stress in rosacea compared with controls (0.72 ± 0.19 and 0.41 ± 0.14 mg·cm−2·min−1; P = 0.10). The cutaneous vasodilation onset threshold during whole body heating occurred at 12:19 ± 1:57 in rosacea compared with 19:59 ± 1:52 (min:s) in controls (P < 0.01). Whole body heating increased CVC to a greater extent in rosacea compared with controls (451 ± 40 and 352 ± 33% increase from baseline; P = 0.04).
Protocol 3
CVC was not different between rosacea and controls within a given skin site during initial baseline or 32°C standardization. Similarly, initial peak response to local heating was not different between rosacea and controls within a given skin site (Table 1). Plateau values for each skin site, expressed as percent increases from baseline, were not different between groups (forehead, P = 0.66; cheek, P = 0.23; forearm, P = 0.77; and palm, P = 0.67). HR did not change across the local heating protocol (rosacea: 62 ± 4 to 62 ± 5 beats/min, P = 0.84; control: 65 ± 4 to 65 ± 4 beats/min, P = 0.90; for baseline and end-heating, respectively), but MAP did increase slightly in rosacea (rosacea: 89 ± 4 to 93 ± 5 mmHg, P = 0.02; control: 95 ± 3 to 97 ± 3 mmHg, P = 0.32; for baseline and end-heating, respectively). No pain was reported during increasing skin temperature or with prolonged 42–43°C local heating.
Table 1.
CVC responses to nonnoxious local heating in rosacea and age/sex-matched control subjects
| CVC, %max |
||||||||
|---|---|---|---|---|---|---|---|---|
| Forehead |
Cheek |
Forearm |
Palm |
|||||
| Rosacea | Control | Rosacea | Control | Rosacea | Control | Rosacea | Control | |
| Baseline | 25 ± 10 | 17 ± 5 | 11 ± 4 | 19 ± 4 | 5 ± 1 | 12 ± 6 | 23 ± 7 | 17 ± 5 |
| 32°C standardization | 23 ± 8 | 17 ± 4 | 16 ± 4 | 25 ± 4 | 7 ± 2 | 7 ± 2 | 19 ± 5 | 13 ± 4 |
| Initial peak | 68 ± 9 | 75 ± 5 | 77 ± 8 | 78 ± 5 | 54 ± 9 | 65 ± 5 | 74 ± 6 | 77 ± 6 |
Values are means ± SE of cutaneous vascular conductance (CVC) to maximal blood flow for the particular site. Baseline indicates CVC responses at individual nonstandardized skin temperature (rosacea = 30.7 ± 0.4, 29.1 ± 0.5, 29.1 ± 0.5, and 29.4 ± 0.9°C, and control = 30.2 ± 0.3, 29.5 ± 0.3, 28.8 ± 0.2, and 28.8 ± 0.6°C for forehead, cheek, forearm, and palm, respectively). 32°C standardization is when local heaters were engaged to standardize blood flow between subjects and sites. Initial peak refers to the first peak in the local heating response.
DISCUSSION
This study aimed to determine if rosacea causes changes in sympathetic outflow and axon reflex-mediated vasodilation. We believe this is the first direct quantification of a facial nerve's sympathetic outflow in rosacea. Our primary new findings are 1) trigger-induced supraorbital sympathetic outflow hyperresponsiveness in rosacea during physical and mental stress, 2) increased sympathetic drive in rosacea demonstrated by earlier onset of sweating and cutaneous vasodilation during whole body heat stress, and 3) similar axon reflex-mediated vasodilation in rosacea during nonpainful local heating in both affected and nonaffected skin. Thus it appears that sympathetic outflow is affected by rosacea, whereas local axon reflexes remain intact.
Rosacea presentation often suggests a neurological component in addition to the traditionally defined inflammatory disease process, because sympathoexcitatory triggers induce flushing (National Rosacea Society 2013b) and associated sympathectomies decrease symptoms (Drott et al. 2002; Schram and James 2012). Providing further indication of a neural link, Schwab et al. (2011) identified increased myelinated neuron number in erythematotelangiectatic rosacea compared with healthy skin. However, providing direct evidence of sympathetic outflow differences in the face is difficult. To our knowledge, only Nordin (1990) has identified supraorbital SSNA. SSNA contains sympathetic signals to sweat glands, cutaneous blood vessels, and piloerector muscles and is often used as a central sympathetic outflow index because it is not governed by reflexes (e.g., baroreflexes and chemoreflexes) that influence sympathetic outflow via muscle or renal sympathetic nerve activity (Menon et al. 2007; Vissing 2000; Vongpatanasin et al. 1999). Less is known about supraorbital SSNA, because facial skin may or may not resemble peripheral hairy or glabrous skin as indexed via fibular and tibial nerves. Because this study provides the first sympathetic outflow quantification in rosacea, it has the potential to directly evaluate rosacea's neurological component.
Augmented supraorbital SSNA increases were observed during isometric handgrip exercise in rosacea compared with controls, which widened further as handgrip duration increased. To our knowledge, supraorbital SSNA has never been assessed during physical stress. Handgrip has been used extensively to induce fibular SSNA increases (Vissing 2000; Wilson et al. 2006); it may be more reproducible and tolerable for patients than mental or thermal stress. Arousal level likely dictates the increased supraorbital SSNA, but whether supraorbital SSNA tracks previous fibular SSNA observations (Seals 1993; Vissing and Hjortso 1996) is unclear. Physical exercise including “carrying and lifting” is a trigger event for many rosacea patients (National Rosacea Society 2013b), and this higher outflow may be one reason.
We also observed augmented supraorbital SSNA increases during mental stress in rosacea compared with controls. Previously, increased supraorbital (Nordin 1990) and fibular (Muller et al. 2013) SSNA have been identified in healthy subjects during mental stress. Iwase et al. (1997) also identified increased tibial SSNA in plantar hyperhidrosis. Because of likely proximity differences in needle placement within a nerve and the current recommended SSNA analysis procedures that evaluate the integral rather than burst frequency, it is difficult to determine if rosacea subjects had increased baseline supraorbital SSNA. As a general qualitative observation, supraorbital baseline activity was fairly low in both groups (Fig. 2B). However, in response to mental arithmetic, SSNA increased in both groups, with dramatically increased responses in rosacea that persisted throughout the task. This higher outflow may be a reason that mental stress is often a rosacea symptom trigger.
We employed a number of improvements in skin blood flow assessment compared with previous studies in this area: 1) CVC calculation rather than obtaining only flux measures, which are significantly affected by MAP, and 2) skin blood flow standardization to either baseline or maximal CVC to more accurately compare both persons and skin sites with varying blood vessel densities. Cutaneous vasodilation occurred sooner and CVC increased to a greater extent in rosacea compared with controls during whole body heating. Subjects with rosacea increased oral temperature to the same degree as age/sex-matched controls despite being heated for less time. It is possible that this higher skin blood flow observed in rosacea aided in the observed heat gain during the thermal stress. However, we cannot confirm this because we do not have an index of skin blood flow in non-facial skin. Baseline CVC was not significantly different in rosacea compared with controls in either protocol. This is not unusual, because visible surface veins and telangiectases were avoided and no patients actively flushed during normothermic baseline.
Whole body heating induced symptoms such as flushing in most rosacea participants, and many self-terminated the heating protocol. In contrast to thermal stress, neither of the protocol 1 stressors appeared to cause flushing or change CVC in patients or controls despite increased SSNA. Nordin (1990) observed weak vasoconstriction and vasodilation during stressful perturbations when linking supraorbital SSNA to end-organ responses. Nordin's skin blood flow data, however, are more difficult to interpret because of lack of accounting for blood pressure changes and because of use of an atypical skin blood flow standardization procedure. In normothermic conditions, it is not unusual to observe a lack of change in CVC within the area of innervation of the common fibular nerve during isometric exercise (Wilson et al. 2006). Although an increase in SSNA with no end-organ change seems paradoxical, local factors can have dramatic effects on skin perfusion. Local skin temperature dramatically affects peripheral skin blood flow and alters the sympathetic drive-to-CVC relationship (Wenger et al. 1985). Room temperature was ∼23°C in all conditions, which is in the range to suppress CVC, but during whole body heating, forehead skin temperature increased indirectly because of warm blood perfusing the skin. The precise role of local temperature in modulating facial skin blood flow responses remains unknown.
TEWL was increased in rosacea compared with controls. TEWL changes are often interpreted to indicate an impaired skin barrier. Jappe et al. (2005) suggested that rosacea results in a disrupted and inflamed facial skin barrier which could increase susceptibility to contact dermatitis and result in a more vigorous response to cutaneous irritation. Skin barrier issues in rosacea could prove clinically useful and warrant additional testing. To our knowledge, alterations in sweat rate responses in rosacea have not previously been identified during triggers. This may be because the more sophisticated methodology (i.e., capacitance hygrometry) used in this study can detect subtle water vapor content changes, and the dry N2 provides a high gradient for evaporation without saturation. Other methodologies such as skin conductance (or its reciprocal: skin resistance) can be significantly affected by ionic fluid changes such as blood beneath the skin surface. During protocol 1, sweat rates were not significantly different between groups. Sweating increased dramatically during whole body heating and trended higher in rosacea. Additionally, the sweating threshold decreased (i.e., sweating was initiated earlier) in rosacea during whole body heating, providing evidence of increased central sympathetic outflow to the region during heat stress. We are unaware of reports of sudomotor dysfunction in rosacea; however, since we identified changes in SSNA in rosacea, and SSNA includes sudomotor activity, future investigations into possible alterations of sudomotor activity in rosacea may be warranted.
Contrary to our hypothesis, nonnoxious local heating did not induce greater axon reflex changes in skin blood flow in affected compared with unaffected skin and compared with controls. The local heating response has been well-documented in dorsal and ventral hairy forearm skin (Johnson et al. 2014) and in the cheek and forehead (Metzler-Wilson et al. 2012). The vasodilatory response to local warming is biphasic; topical afferent anesthetics can block the initial peak, thereby implicating a local axon reflex mechanism. Local heat stimulates TRPV receptors on warm sensory afferents. TRPV subtypes 1–4 are upregulated in affected skin in erythematotelangiectatic rosacea (Sulk et al. 2012). Despite this, we did not observe a difference in initial peaks, indicating intact axon reflexes in rosacea. The second peak during local heating is primarily nitric oxide-mediated. Currently, it is unknown whether nitric oxide-mediated vasodilation is altered in rosacea. Local heating responses are central nervous system-independent as demonstrated by preservation despite nerve blocks ((Johnson et al. 2014). Previous studies indicate that noxious heat increases skin blood flow in affected vs. unaffected skin in patients with papulopustular, but not erythematotelangiectatic, rosacea (Guzman-Sanchez et al. 2007). Additionally, noxious stinging induced by cholinergic iontophoresis augments axon reflexes in severe vs. milder rosacea (Drummond and Su 2012). Thus axon reflex responses may be altered differently depending on rosacea classification and the presence of pain.
This was the first study to directly quantify sympathetic outflow in vivo in rosacea. During both handgrip and mental arithmetic, individuals with rosacea had augmented supraorbital SSNA compared with controls. This suggests hyperresponsive sympathetic outflow in rosacea. Indirect evidence of hyperresponsiveness was also collected during whole body heating, where sweating and cutaneous vasodilation occurred earlier in rosacea compared with controls. Nonnoxious local heating-induced axon reflexes were not different. Taken together, these results imply that sympathetic nerve activity is affected by rosacea, whereas local heating-induced skin axon reflexes remain intact. Given that sympathectomy decreases flushing, sympathoexcitatory trigger events induce flushing, and individuals with rosacea have hyperresponsive sympathetic outflow to triggers, the neural influence in rosacea may need to be revisited in addition to the previously demonstrated changes in inflammatory mediators.
GRANTS
This project was supported by a grant from the National Rosacea Society (to T. E. Wilson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.-W., K.T., D.L.S., and T.E.W. conception and design of research; K.M.-W., K.T., S.M., A.J.J., O.D., and T.E.W. performed experiments; K.M.-W., K.T., S.M., A.J.J., O.D., and T.E.W. analyzed data; K.M.-W., K.T., O.D., and T.E.W. interpreted results of experiments; K.M.-W. and T.E.W. prepared figures; K.M.-W., K.T., and T.E.W. drafted manuscript; K.M.-W., K.T., D.L.S., S.M., A.J.J., O.D., and T.E.W. edited and revised manuscript; K.M.-W., K.T., D.L.S., S.M., A.J.J., and T.E.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We express appreciation to Michael Tomc, DO, Andrew LePorte, and Aiwane Iboaya for technical assistance and to the subjects for their willing participation.
REFERENCES
- Aksoy B, Altaykan-Hapa A, Egemen D, Karagoz F, Atakan N. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol 163: 719–725, 2010. [DOI] [PubMed] [Google Scholar]
- Beikert FC, Langenbruch AK, Radtke MA, Augustin M. Willingness to pay and quality of life in patients with rosacea. J Eur Acad Dermatol Venereol 27: 734–738, 2013. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the finger. Exp Physiol 90: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 290: H1601–H1609, 2006. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol 5: 16–25, 2012. [PMC free article] [PubMed] [Google Scholar]
- Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield L, Stein-Gold L, Berson D, Zaenglein A. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis 92: 234–240, 2013. [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972. [DOI] [PubMed] [Google Scholar]
- Drott C, Claes G, Rex L. Facial blushing treated by sympathetic denervation–long-lasting benefits in 831 patients. J Cosmet Dermatol 1: 115–119, 2002. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Su D. Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res 304: 133–137, 2012. [DOI] [PubMed] [Google Scholar]
- Guzman-Sanchez DA, Ishiuji Y, Patel T, Fountain J, Chan YH, Yosipovitch G. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol 57: 800–805, 2007. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972. [DOI] [PubMed] [Google Scholar]
- Holzer P. Control of the cutaneous vascular system by afferent neurons. In: Autonomic Innervation of the Skin, edited by Morris JL and Gibbons IL. Amsterdam, The Netherlands: Harwood Academic, 1997. [Google Scholar]
- Iwase S. [Clinical application of skin sympathetic nerve activity]. Brain Nerve 61: 243–253, 2009. [PubMed] [Google Scholar]
- Iwase S, Cui J, Wallin BG, Kamiya A, Mano T. Effects of increased ambient temperature on skin sympathetic nerve activity and core temperature in humans. Neurosci Lett 327: 37–40, 2002. [DOI] [PubMed] [Google Scholar]
- Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J Auton Nerv Syst 64: 65–73, 1997. [DOI] [PubMed] [Google Scholar]
- Jappe U, Schnuch A, Uter W. Rosacea and contact allergy to cosmetics and topical medicaments–retrospective analysis of multicentre surveillance data 1995–2002. Contact Dermatitis 52: 96–101, 2005. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Minson CT, Kellogg DL Jr.. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. [DOI] [PubMed] [Google Scholar]
- Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 497: 837–848, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Sparacio RM, Voigt A, Marenus K, Sarnoff DS. Objective and quantitative improvement of rosacea-associated erythema after intense pulsed light treatment. Dermatol Surg 29: 600–604, 2003. [DOI] [PubMed] [Google Scholar]
- Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li JL, Victor RG, Vongpatanasin W. Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am Coll Cardiol 50: 626–633, 2007. [DOI] [PubMed] [Google Scholar]
- Metzler-Wilson K, Kellie LA, Tomc C, Simpson C, Sammons D, Wilson TE. Differential vasodilatory responses to local heating in facial, glabrous and hairy skin. Clin Physiol Funct Imaging 32: 361–366, 2012. [DOI] [PubMed] [Google Scholar]
- Muller MD, Sauder CL, Ray CA. Mental stress elicits sustained and reproducible increases in skin sympathetic nerve activity. Physiol Rep 1: e00002, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Rosacea Society. For Patients (Online). http://www.rosacea.org/patients/index.php [20 Dec 2013a].
- National Rosacea Society. Rosacea Triggers Survey (Online). http://www.rosacea.org/patients/materials/triggersgraph.php [20 Dec 2013b].
- Nordin M. Intrafascicular recordings of afferent multi-unit activity from the human supraorbital nerve. Acta Physiol Scand 151: 507–514, 1994. [DOI] [PubMed] [Google Scholar]
- Nordin M. Sympathetic discharges in the human supraorbital nerve and their relation to sudo- and vasomotor responses. J Physiol 423: 241–255, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Hagbarth KE, Thomander L, Wallin U. Microelectrode recordings from the facial nerve in man. Acta Physiol Scand 128: 379–387, 1986. [DOI] [PubMed] [Google Scholar]
- Nordin M, Thomander L. Intrafascicular multi-unit recordings from the human infra-orbital nerve. Acta Physiol Scand 135: 139–148, 1989. [DOI] [PubMed] [Google Scholar]
- Normell LA, Wallin BG. Sympathetic skin nerve activity and skin temperature changes in man. Acta Physiol Scand 91: 417–426, 1974. [DOI] [PubMed] [Google Scholar]
- Pelle MT. Rosacea. In: Fitzpatrick's Dermatology in General Medicine, edited by Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, and Wolff K. New York, NY: McGraw-Hill, 2012. [Google Scholar]
- Rubino A, Burnstock G. Capsaicin-sensitive sensory-motor neurotransmission in the peripheral control of cardiovascular function. Cardiovasc Res 31: 467–479, 1996. [PubMed] [Google Scholar]
- Schram AM, James WD. Neurogenic rosacea treated with endoscopic thoracic sympathectomy. Arch Dermatol 148: 270–271, 2012. [DOI] [PubMed] [Google Scholar]
- Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, Rivier M, Carlavan I, Rossio P, Metze D, Buddenkotte J, Cevikbas F, Voegel JJ, Steinhoff M. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 15: 53–62, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. Influence of force on muscle and skin sympathetic nerve activity during sustained isometric contractions in humans. J Physiol 462: 147–159, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol 143: 1369–1371, 2007. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol 100: 1692–1701, 2006. [DOI] [PubMed] [Google Scholar]
- Sibenge S, Gawkrodger DJ. Rosacea: a study of clinical patterns, blood flow, and the role of Demodex folliculorum. J Am Acad Dermatol 26: 590–593, 1992. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sinoway LI, Leuenberger UA, Amassian VE. Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol 88: 126–134, 2000. [DOI] [PubMed] [Google Scholar]
- Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, Nowak P, Voegel JJ, Buddenkotte J, Steinhoff M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Investig Dermatol 132: 1253–1262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol 69: S27–35, 2013. [DOI] [PubMed] [Google Scholar]
- Vissing SF. Neural control of skin circulation. In: Exercise and Circulation in Health and Disease, edited by Saltin B, Boushel R, Secher N, and Mitchell JH. Champaign, IL: Human Kinetics, 2000, p. 103–111. [Google Scholar]
- Vissing SF, Hjortso EM. Central motor command activates sympathetic outflow to the cutaneous circulation in humans. J Physiol 492: 931–939, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res 69: 228–238, 1991. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation 100: 497–502, 1999. [DOI] [PubMed] [Google Scholar]
- Wenger CB, Bailey RB, Roberts MF, Nadel ER. Interaction of local and reflex thermal effects in control of forearm blood flow. J Appl Physiol 58: 251–257, 1985. [DOI] [PubMed] [Google Scholar]
- Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, Powell F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol 46: 584–587, 2002. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol 536: 615–623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Dyckman DJ, Ray CA. Determinants of skin sympathetic nerve responses to isometric exercise. J Appl Physiol 100: 1043–1048, 2006. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CN, Keller DM, Crandall CG, Fadel PJ. Comparing resting skin sympathetic nerve activity between groups: caution needed. J Appl Physiol 106: 1751–1752; author reply 1753, 2009. [DOI] [PubMed] [Google Scholar]