Abstract
There is currently significant demand for minimally invasive thyroid surgery; however the majority of proposed surgical approaches necessitate a compromise between minimal tissue dissection with a visible cervical scar or extensive tissue dissection with a remote, hidden scar. The development of transoral endoscopic thyroid surgery however provides an approach which is truly minimally invasive, as it conceals the incision within the oral cavity without significantly increasing the amount of required dissection. The transoral endoscopic approach however presents multiple technical challenges, which could be overcome with the incorporation of a robotic operating system. This manuscript summarizes the literature on the feasibility and current clinical experience with transoral robotic thyroid surgery.
Keywords: Transoral, robotic, minimally invasive, endoscopic, thyroidectomy, thyroid surgery
Introduction
Surgical management of thyroid disease has undergone radical changes over the last century. Once feared, with a high mortality rate, thyroidectomies are now a routine procedure with more than 80,000 undertaken in the United States annually (1). As described by Kocher in the late 1880s, most cases are performed through a transcervical approach, which results in scarring that some patients find unsightly and difficult to conceal (2). Given the current epidemic of thyroid disease diagnosis and the young age of these patients, there is significant demand for minimally invasive surgery (3). Robotic transoral thyroid surgery provides an exciting minimally invasive alternative to the current transcervical approach to the thyroid gland.
History
To be considered minimally invasive, a procedure should be safe, respect surgical planes, minimize surgical trauma, tissue dissection, and avoid scarring (4,5). Since 1997, approximately 20 different thyroidectomy techniques have been proposed as minimally invasive alternatives to conventional transcervical thyroidectomies (4,6). These approaches, however, necessitate a compromise between minimal tissue dissection with a visible cervical scar and extensive tissue dissection with a remote, hidden scar. In 2007, Witzel et al. published a report where the feasibility of performing transoral endoscopic thyroid surgery was demonstrated in human cadavers and a porcine model was developed. In this natural orifice transluminal endoscopic surgical (NOTES) approach, the thyroid is accessed in the subplatysmal plane in the submental region through an incision made in the floor of the mouth (7). The advantages of this approach is the concealment of the incision within the floor of the mouth without significantly increasing the amount of required dissection and access to both sides of the neck. Since the publication of this proof of concept, there have been multiple reports in the literature on the use of transoral endoscopic thyroid surgery and different approaches to the thyroid gland in cadaver and porcine models. The first clinical use of transoral endoscopic thyroid surgery was reported by Wilhelm et al. (8). In this series of eight patients, there was a conversion rate of 38% and permanent recurrent laryngeal nerve injury in 13%. Subsequently, two small case series have been published on the clinical use of transoral endoscopic thyroid surgery. Nakajo et al. reports a case series of eight patients who underwent transoral endoscopic thyroid surgery; although, in this method, two Kirschner wires were inserted through the cervical skin for retraction (9). The average time for a simple hemithyroidectomy (five patients) was 208 minutes and for subtotal thyroidectomy with central node dissection (three patients), 361 minutes. All patients had “sensory disorder” around the chin which persisted for more than six months. One patient developed a laryngeal palsy but no patient had mental nerve palsy. There were no reported postoperative infections. Woo reports on the use of transoral endoscopic thyroid surgery in a patient with papillary thyroid micro-carcinoma (10). This procedure did not require any incision in the neck and was performed in 120 minutes. There were no reported complications and the patient remained disease free with 12 months of follow-up. Although transoral endoscopic thyroid surgery provides a remarkably direct and minimally invasive approach to the thyroid gland, the high rate of reported complications is concerning for the safety of the approach. Furthermore, current technology limits surgeons to non-wristed instruments and requires manual control of the endoscope whilst performing dissection. This results in greater tissue strain and manipulation due to the long fulcrum of the rigid endoscopic instruments and the need to simultaneously manage multiple tasks whilst performing dissection, increasing the complexity and required learning curve for the procedure (4).
In 2010, Richmon et al. proposed modifying the transoral endoscopic thyroid surgery approach by incorporating a robotic operating system (4). The use of robots in remote thyroid surgery is not novel and has since been demonstrated to be safe and to provide oncologic outcomes that are equivalent to open thyroidectomy with improved cosmesis, patient satisfaction and quality of life (2). Currently the da Vinci system is the only Food and Drug Administration (FDA)-approved robotic system for surgery in humans (3). The system provides a high-resolution, 3-dimentional image, which provides the surgeon with both depth perception and high magnification which aids with tissue handling, and identification of structures such as the recurrent laryngeal nerve and parathyroid glands. The system has wristed instruments, tremor filtration and precise robotic movement which allows for a degree of control and accuracy currently not available with endoscopic equipment. These characteristics lend themselves to a transoral approach to the thyroid gland and overcome limitations seen with the video-assisted approach.
Surgical technique
Richmon et al. conducted a feasibility study for robotic transoral thyroid surgery in two cadavers (4). In this model, a 1.5-cm incision was made along the lingual frenulum just posterior to the mandible. The midline raphe between the genioglossus muscle was bluntly divided under direct visualization and the dissection was continued between the geniohyoid and mylohyoid muscle until the subplatysmal plane was identified and a subplatysmal pocket was created. Two 1.5-cm incisions were placed in the gingival-buccal sulcus at the level of the first molar to avoid the mental nerve, and blunt dissection was undertaken. These two pockets were connected with the submental pocket already created. The da Vinci system was then brought in and a 0 degree endoscope was advanced through the midline incision and into the submental, subplatysmal pocket. A Maryland dissector was placed through the left gingival-buccal incision and a bipolar forceps through the right gingival-buccal incision. Under endoscopic visualization, these instruments were used to dissect down to the level of the thyroid cartilage notch, where the strap muscles were identified and the midline raphe divided to expose the thyroid gland. Part of the superior and medial insertion of the sternothyroid muscle on the thyroid cartilage was divided to facilitate exposure. The overlying skin had to be manually elevated by an assistant to maintain the working space, as a seal could not be created to maintain CO2 pressure. The superior lobe of the right thyroid gland was grasped and retracted inferomedially, this was continued until the superior vascular pedicle was identified and cauterized. The recurrent laryngeal nerve was then identified at its entry point and traced inferiorly using gentle blunt dissection. The gland was then retracted further medially, allowing for a capsular dissection. The isthmus was divided and the lobe was freed entirely. The left thyroid lobe was removed in a similar fashion and both lobes were delivered through the midline incision of the floor of the mouth. Richmon et al. however noted from this approach that placement of the camera through the floor of the mouth led to restricted movement due to collisions with the nose and maxilla (11). As a result, they subsequently modified their approach and placed the three ports through gingival-buccal incisions, all anterior to the mandible (Figures 1,2). The lateral ports were positioned posterior to the mental nerve and the endoscope port was placed in the midline between the fasciculus of the mentalis muscles. The dissection was continued around the inferior aspect of the mandible and a submental pocket created. Using this approach also allowed creation of a seal and CO2 insufflation at 8 L/min was sufficient to maintain a working cavity which removed the need for external retraction. Excellent mobility and view of the central cavity was provided by this approach, and a right thyroid lobectomy and central neck dissection was successfully performed (Figures 3,4).
Figure 1.
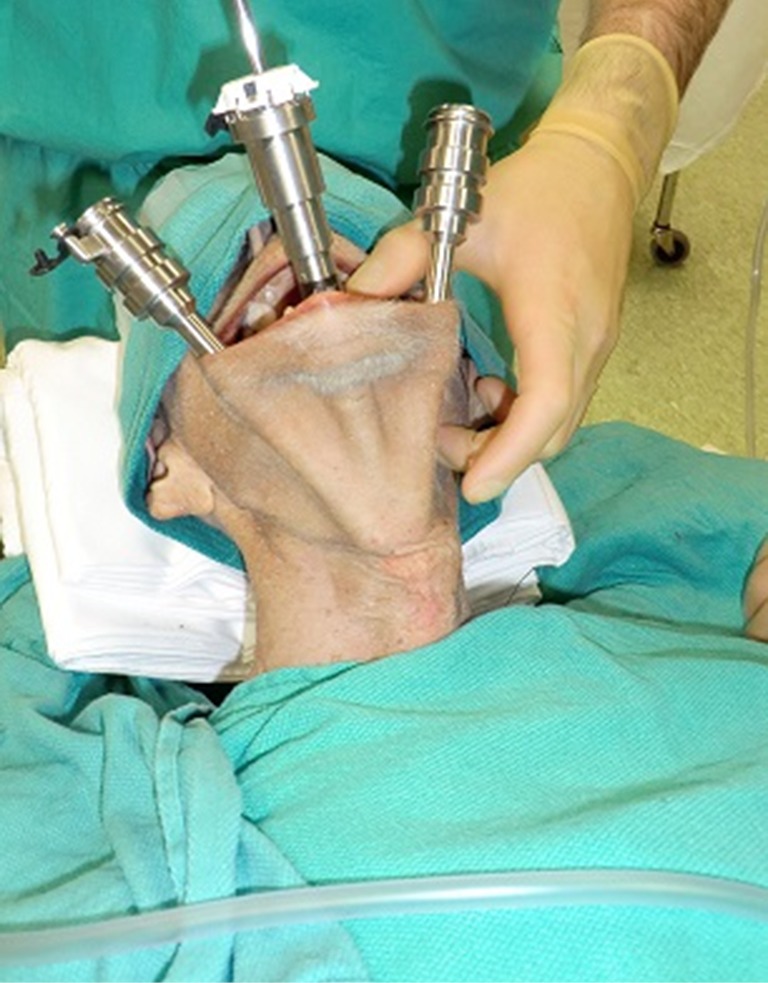
Cadaver with three robotic ports placed anterior to the mandible to access the central neck.
Figure 2.
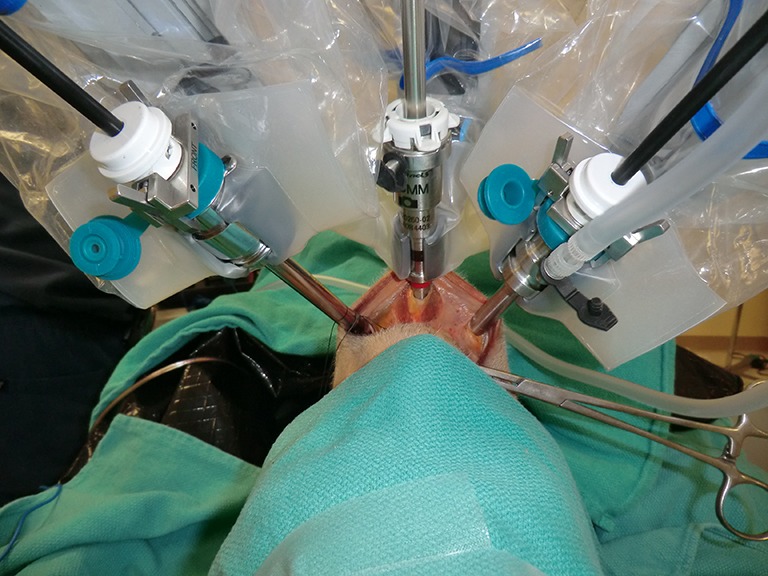
The robot docked with the three ports placed through the vestibule anterior to the mandible. The central camera port enters between the fasciculus of the mentalis muscles.
Figure 3.
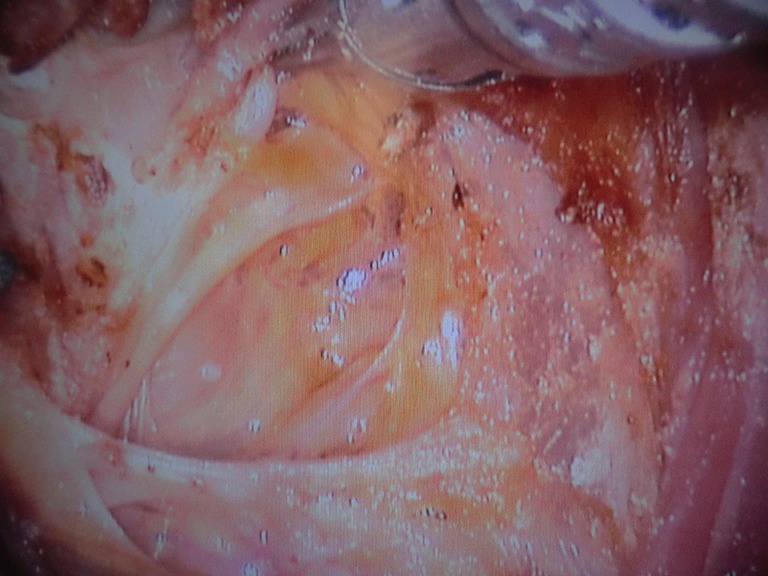
View through the console of the right recurrent laryngeal nerve after a central neck dissection has been performed.
Figure 4.
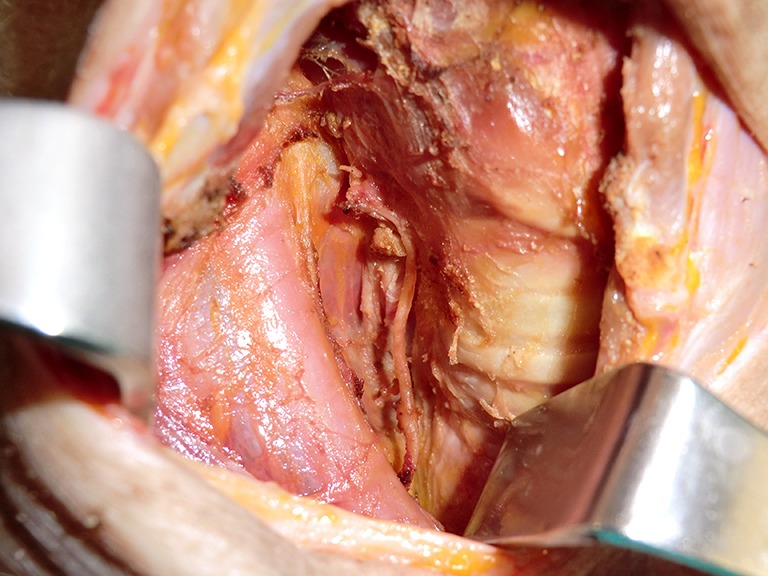
An external incision was made after the transoral robotic thyroidectomy to demonstrate the completeness of the thyroidectomy, nerve dissection, and central neck dissection.
The feasibility of transoral robotic thyroid surgery was further supported by Hoon Yub Kim and his group at Korea University who created a porcine model for the procedure (12). Transoral robotic total thyroidectomies were successfully performed in seven pigs. Follow-up examinations were performed for seven days and followed by autopsy. The first three cases were noted to develop seromas post operatively (one of which was infected), as a result, in case 4 and onwards a drain was placed at time of surgery and no further seroma occurred. The recurrent nerves were noted to be intact in all cases. The two cadavers and one porcine study provide proof of concept for robotic transoral thyroid surgery and serve to identify advantages and potential pitfalls for the clinical application of this approach.
The clinical impact of robotic transoral thyroid surgery remains hard to predict. Since being described in the literature in 2010, actual clinical introduction has been slow. However, Dr. Kim’s group recently reported their initial series of robotic transoral thyroidectomy in living patients. They used transgingival-buccal approach for robotic assisted surgery in four patients, although an accessory port was placed in the axilla, which was used to retract the strap muscles laterally (13). Of the four cases, two were right lobectomies, one was a left lobectomy and one was a left lobectomy with a central neck dissection. All four cases were completed robotically, operative time ranged from 190-390 minutes, with console time ranging from 74-230 minutes. In two cases, the ipsilateral mental nerve was torn (repaired intraoperatively) and stretched in one case. In these cases, patients had paresthesia in a portion of their lower lip and chin but all improved by post-operative week four. There were no cases of temporary or permanent vocal cord palsy and no reported post-operative infections.
Although pending publication, Dr. Kim presented his team’s experience of performing nine transoral robotic hemi-thyroidectomies without the need for an accessory port at the Johns Hopkins Hospital Transoral Robotic Surgery Symposium in Baltimore MD, July 24-25, 2014 (Figures 5,6,7). All cases were completed without the need to open, and three cases also included central neck dissection. The average time for the whole procedure was 234 minutes with on average 126 minutes at the consol. There were no recurrent nerve injuries although 4 mental nerves were torn and 2 stretched. These case series, although promising, highlight the significant amount of time required for a robotic transoral approach, which is comparable to transoral endoscopic thyroid surgery and significantly slower than an open procedure. Furthermore, the high rate of mental nerve injury precludes this approach with current instrumentation. The known literature regarding transoral robotic thyroidectomy is highlighted in Table 1.
Figure 5.
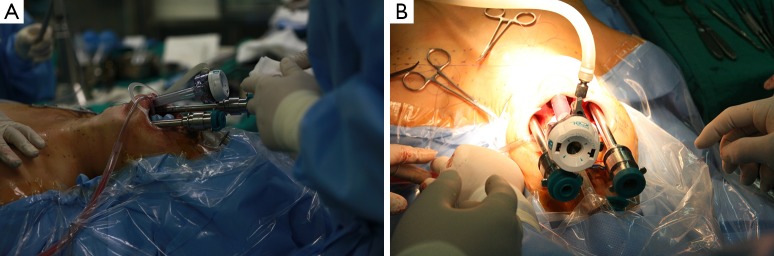
Port placement of robotic transoral periosteal thyroidectomy using three ports; (A) lateral and (B) anterior views. Three robotic arms were placed through the transoral ports.
Figure 6.
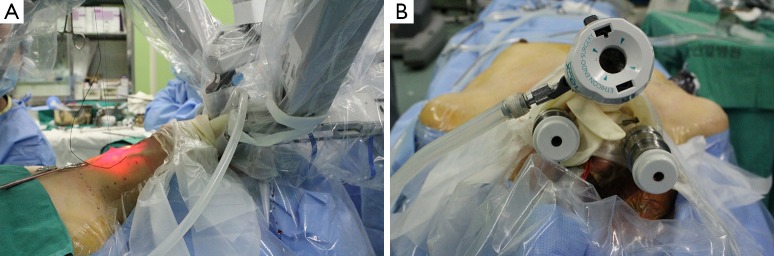
View during the robotic transoral periosteal thyroidectomy using three ports; (A) lateral and (B) anterior views. Three robotic arms were placed through the transoral ports. The da Vinci robotic system was docked at the patient’s left side. The robotic camera was inserted through the central port.
Figure 7.
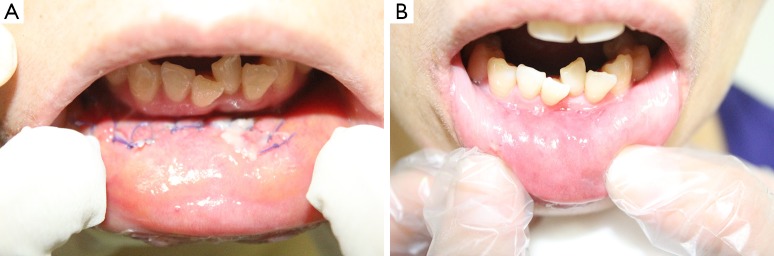
(A) The incision scar of the patient undergone robotic transoral periosteal thyroidectomy (TOPOT) (postoperative 1st day); (B) the incision scar of the patient is almost imperceptible (postoperative 2nd week).
Table 1. Summary of robotic transoral thyroid surgery literature.
| Author | Year | Country | Model [n] | Indication [n] | Operation [n] | Complication [n] |
|---|---|---|---|---|---|---|
| Richmon (4) | 2011 | USA | Cadaver [2] | Feasibility study [2] | Total thyroidectomy [2] | NA |
| Richmon (11) | 2011 | USA | Cadaver [2] | Feasibility study [2] | Total thyroidectomy [2] | NA |
| Central neck dissection [2] | ||||||
| Lee (12) | 2014 | Korea | Porcine [7] | Saftey [7] | Total Thyroidectomy [7] | Seroma [3] |
| Lee (13) | 2015 | Korea | Cadaver [8] | Feasibility study [8] | Total Thyroidectomy [8] | NA |
| Porcine [7] | Feasibility study [7] | Total Thyroidectomy [9] | None | |||
| Human [4] | Follicular adenoma [1] | Lobectomy [4] | Mental nerve stretching [3] | |||
| Papillary carcinoma [1] | Central neck [1] | Mental nerve tear [1] | ||||
| Nodular hyperplasia [2] |
NA, not applicable.
The da Vinci system provides a clear magnified 3-dimensional view of the surgical field and has wristed instruments to aid mobility in limited space. This, however, comes at the lack of tactile feedback. The use of the da Vinci system in robotic transoral thyroid surgery is limited by high cost, non-ideal instruments and lack of single port technology. This approach is also limited by the current need to dissect around the inferior aspect of the mandible to create a submental subplatysmal pocket, which is time-consuming and complex. Although the gingival-buccal approach removes the need to violate the tongue and the floor of mouth musculature, which might impact speech and swallowing, this anterior midline approach can cause cosmetic deformity of the mentum and the placement of lateral ports risk mental nerve injury. In addition the transoral approach risks contamination of the neck with oral bacterial flora, although this has not been witnessed with transoral approaches to the sumandibular or sublingual gland. Surgeons should however be cognizant of this risk, irrigate copiously, and select prophylactic antibiotic coverage for oral flora. The transoral robotic thyroid approach is the only remote approach with a midline point-of-view and equal access to both sides of the neck, a considerable advantage over the transaxillary and facelift approaches which have limited capacity to address the contralateral neck.
The future of transoral robotic thyroid surgery is likely to be shaped by trends in the treatment of thyroid cancer and methods of reimbursement. Adam et al. analyzed the National Cancer Database [2010-2011] and found that less than 1% of all thyroid cancer surgeries were performed robotically. Furthermore, most of these cases were limited to a few institutes, with the majority of cases being performed at low-volume institutions (2). Currently there are many barriers to the adoption and evolution of robotic thyroid surgery in the US, particularly with the current uncertainty in reimbursement changes and the move towards preferential referral to high volume centers. At this time, robotic transoral thyroid surgery still needs further investigation and development to ensure its outcomes, cost effectiveness, and safety.
Conclusions
Robotic transoral thyroid surgery has been demonstrated to be feasible in cadaver, porcine models and in patients. The approach combines the advantages of a “scarless”, remote access incision with the goals of minimally invasive surgery. Unfortunately, current instrumentation and technology make this approach technically difficult and place unacceptable risk on the mental nerve. Further refinements to overcome these obstacles are necessary prior to a more generalized adoption of this approach, which at this point remains experimental.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Becker AM, Gourin CG. New technologies in thyroid surgery. Surg Oncol Clin N Am 2008;17:233-48, x. [DOI] [PubMed] [Google Scholar]
- 2.Adam MA, Speicher P, Pura J, et al. Robotic thyroidectomy for cancer in the US: patterns of use and short-term outcomes. Ann Surg Oncol 2014;21:3859-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [DOI] [PubMed] [Google Scholar]
- 4.Richmon JD, Pattani KM, Benhidjeb T, et al. Transoral robotic-assisted thyroidectomy: a preclinical feasibility study in 2 cadavers. Head Neck 2011;33:330-3. [DOI] [PubMed] [Google Scholar]
- 5.Benhidjeb T, Wilhelm T, Harlaar J, et al. Natural orifice surgery on thyroid gland: totally transoral video-assisted thyroidectomy (TOVAT): report of first experimental results of a new surgical method. Surg Endosc 2009;23:1119-20. [DOI] [PubMed] [Google Scholar]
- 6.Dionigi G, Rovera F, Boni L. Commentary on transoral access for endoscopic thyroid resection: Witzel K, von Rahden BH, Kaminski C, Stein HJ (2008) Transoral access for endoscopic thyroid resection. Surg Endosc 22(8):1871-1875. Surg Endosc 2009;23:454-5; discussion 456. [DOI] [PubMed] [Google Scholar]
- 7.Witzel K, von Rahden BH, Kaminski C, et al. Transoral access for endoscopic thyroid resection. Surg Endosc 2008;22:1871-5. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm T, Metzig A. Endoscopic minimally invasive thyroidectomy (eMIT): a prospective proof-of-concept study in humans. World J Surg 2011;35:543-51. [DOI] [PubMed] [Google Scholar]
- 9.Nakajo A, Arima H, Hirata M, et al. Trans-Oral Video-Assisted Neck Surgery (TOVANS). A new transoral technique of endoscopic thyroidectomy with gasless premandible approach. Surg Endosc 2013;27:1105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo SH. Endoscope-assisted transoral thyroidectomy using a frenotomy incision. J Laparoendosc Adv Surg Tech A 2014;24:345-9. [DOI] [PubMed] [Google Scholar]
- 11.Richmon JD, Holsinger FC, Kandil E, et al. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg 2011;5:279-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HY, Hwang SB, Ahn KM, et al. The safety of transoral periosteal thyroidectomy: results of Swine models. J Laparoendosc Adv Surg Tech A 2014;24:312-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee HY, You JY, Woo SU, et al. Transoral periosteal thyroidectomy: cadaver to human. Surg Endosc 2015;29:898-904. [DOI] [PubMed] [Google Scholar]


