Abstract
In head and neck area, neck dissection (ND) is one of the most complex and precision-needed procedure. The long cervical scar and post-operative neck discomfort have been also inevitable brands after this procedure. Heretofore, few dare to try endoscopic surgical technique to the ND mainly due to its complexity and jeopardy of complication. Although, there have been several reports about the endoscopic approaches for functional ND or ND, they had so many technical and instrumental limitations. The dexterities of the surgical robotics have advanced the techniques of endoscopic surgery, and have facilitated the most precise and delicate endoscopic surgical procedure in head and neck area. The technical feasibility and early surgical outcomes of robotic ND using the transaxillary approach for the management of metastatic thyroid cancer have already been reported as satisfactory. Robotic ND can allow complete compartment-oriented lymph node (LN) dissection without any fatal complications, or compromising oncologic principles. We previously described a novel method of robotic thyroidectomy with ND using a gasless transaxillary approach for metastatic thyroid cancer, and here, we firstly introduce a less invasive robotic procedure which has been modified from the original one, which we refer to as the transaxillary single-incision robotic ND.
Keywords: Robotic neck dissection (ND), transaxillary, single incision, thyroid cancer
Introduction
The movie director Christopher Nolan has been famous for the creative trials in his film, and the movie “Interstellar” made by him has been a box office hit in 2014. He made again the innovative concepts—transcending time and space—in this movie, and it made profound impression to the cinema audiences.
Robotic surgical system was firstly introduced in 1999, and substantially overcame optic (2-D representation) and instrumental limitations of conventional endoscopic procedures, and facilitated minimally invasive surgery (1-5). However, some of thoracic and abdominal surgeries can only be performed by the robotic surgical system at that time.
The first introduction of robotic thyroidectomy has similar impact and impression to the head and neck surgeons and patients who acutely want to remove the conspicuous scars on the neck. Because, the application of robotic surgery in head and neck achieved in a hard way due to the lack of a pre-existing working space, relatively bulky robotic arms in deep, narrow operative fields, and the hypervascularities of target organs, which are invariably surrounded by critical nerves and major vessels (6,7).
In the head and neck area, well-differentiated thyroid cancer is the most common malignancy, and the incidence of early stage cancer of the thyroid has markedly increased due to the various health-screening programs. Furthermore, the proportion of thyroid cancer in young women who are particularly sensitive to cosmesis is increasing. Accordingly, the needs and trials on robotic or minimally invasive techniques in thyroid surgery have continuously grown with the aim of avoiding prominent cervical scars, and many of early satisfactory results have already been reported for these techniques (8-11).
Even though the technical dexterity of the robotic surgical system for thyroidectomy has been proved through various routes, there are still hot debating about the necessity and suitability of robotic thyroidectomy considering the cost-effectiveness of the procedure and the monopoly of robotic company (6-11). Furthermore, although robotic thyroidectomy has excellent cosmetic benefits and little discomfort on anterior neck area, it is still widely invasive technique comparing to the conventional open procedure.
However, in terms of robotic neck dissection (ND), the situation is somewhat different from robotic thyroidectomy. Papillary carcinoma of the thyroid (PTC)—the most common type of differentiated thyroid cancer—usually has a mild biologic course, but nevertheless, it frequently metastasizes to local cervical lymph nodes (LN). In cases of metastasis to the lateral neck nodes (LNM) from PTC, bilateral total thyroidectomy with ipsilateral ND is the treatment of choice. Although conventional open ND is the safest and most efficient type of surgical treatment, extensive surgical dissection and long incision scar on the neck are inevitable. In view of the wide surgical extent and cosmetic problems of open ND, minimally invasive and remote site approaches to the ND has been facilitated. Accordingly, we have applied robotic techniques to thyroidectomy and ND procedures, and the dexterous robotic technology enables more precise and meticulous dissection during the complex procedure required for ND. Recently, the technical feasibility and safety of robotic ND and functional benefits of robotic ND over the conventional open ND have been serially reported, and the technique has been found to be capable of complete compartment-oriented dissection (12-14).
In this chapter, we firstly describe in detail of robotic ND using single incision method for the management of thyroid cancer with LNM.
Preoperative diagnosis
Well-differentiated thyroid cancer should be diagnosed in all patients by preoperative fine needle aspiration biopsy (FNAB). High-resolution staging ultrasonography (US) and computed tomography (CT) of the neck can be performed for preoperative disease staging. Comprehensive preoperative evaluation should be performed to rule out any advanced disease with thyroid cancer to avoid unexpected difficult situation.
All patients with clinically palpable lateral neck nodes or a lateral LN with a suspicious ultrasound appearance by preoperative staging US should undergo US-guided FNAB.
The presence of metastasis to a lateral neck node can be determined by US-guided FNAB histology or by measuring thyroglobulin (Tg) levels in FNAB wash out fluid (FNA-Tg >10 ng/mL, > mean + 2 SD of FNA-Tg measured in node negative patients, or > serum-Tg) from lateral neck LNs.
Indication
The eligibility criteria for robotic ND are as follows: (I) well-differentiated thyroid cancer with clinical LNM (cases with a minimum numbers of metastatic LNs in the lateral neck); (II) a primary tumor size of ≤4 cm; or (III) minimal invasion of the anterior thyroid capsule and strap muscle by the primary cancer.
The role of the robotic procedure for the management of cancer of the thyroid with LNM remains controversial. For experienced surgeons, this approach may be well suited for cases with limited LNM from well-differentiated thyroid cancer (WDTC), but its role in cases of more locally advanced cancer is uncertain, and thus, robotic ND is clearly contraindicated in such cases.
The exclusion criteria which should be applied are: (I) definite tumor invasion to an adjacent organ [recurrent laryngeal nerve (RLN), esophagus, major vessels, or trachea]; (II) multiple LN metastases in multiple levels of the lateral neck; (III) LN metastasis on the substernal area or area below the clavicle; or (IV) peri-nodal infiltration at a metastatic LN.
Extent of dissection
The role of prophylactic central ND in PTC remains the subject of considerable debate. However, therapeutic lateral ND in PTC patients with clinically determined LNM is always necessary.
Most commonly used surgical approaches in cases with LNM from PTC are bilateral total thyroidectomy with central compartment ND and concurrent modified radical ND [type III, sparing sternocleidomastoid muscle (SCM), spinal accessory nerve, and the internal jugular vein (IJV)].
In terms of extent of the dissection, the submental, submandibular, parotid, and retroauricular nodes are virtually never dissected, and levels IIB and VA LNs are not routinely dissected either in cancer of the thyroid with LNM, because its rarely metastasizes to levels I or IIB, or VA.
However, if an enlarged or suspicious cervical LN is encountered by palpation or by preoperative US in these areas, these compartments are also included in en bloc dissection. Thus, the usual extents of surgical dissection for ND in differentiated thyroid cancer with LNM are levels IIA, III, IV, VB, and VI, which applies to robotic and open ND procedures as well (Figure 1).
Figure 1.
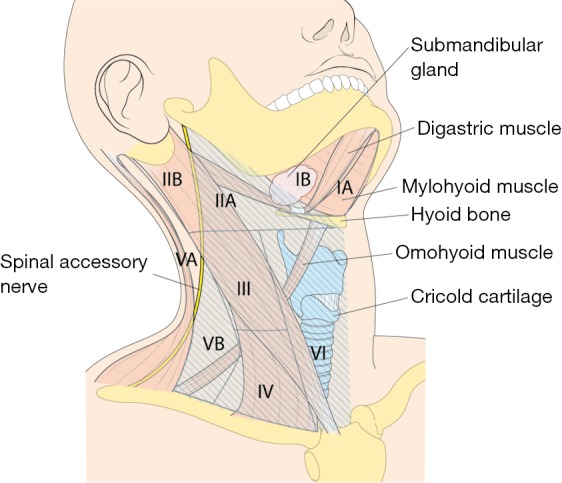
The anatomic landmarks used to divide the lateral and central LN compartments into levels I-VI; the area with a deviant crease line is where LN dissection is made during MRND. LN, lymph nodes; MRND, modified radical neck dissection.
Special equipment and preparation materials
For the patient position, arm board (lesion side) which can be attached to the operating table and soft pillow (for neck extension) should be prepared. During the development of working space, electrocautery with regular and extended-sized tip, vascular Debakey or Russian forceps (extended length), two army-navy retractors, right-angled retractors, and breast lighted retractors are used. Laparoscopic clip appliers can be used for the ligation of external jugular vein during this procedure. After placing the wide and long blade of external retractor (special set of Chung’s retractor for robotic ND) (Figure 2A,B), for maintenance of the working space, actual robotic procedures are started. For the robotic ND, da Vinci S or Si system (Intuitive, Inc., Sunnyvale, CA, USA) can be used. Three robotic instruments (5-mm Maryland dissector, 8-mm ProGrasp forceps, and 5-mm Harmonic curved shears) and dual channel camera (30 degree, used in the rotated down position) are needed. For the energy device, Harmonic curved shears is preferred (Table 1).
Figure 2.
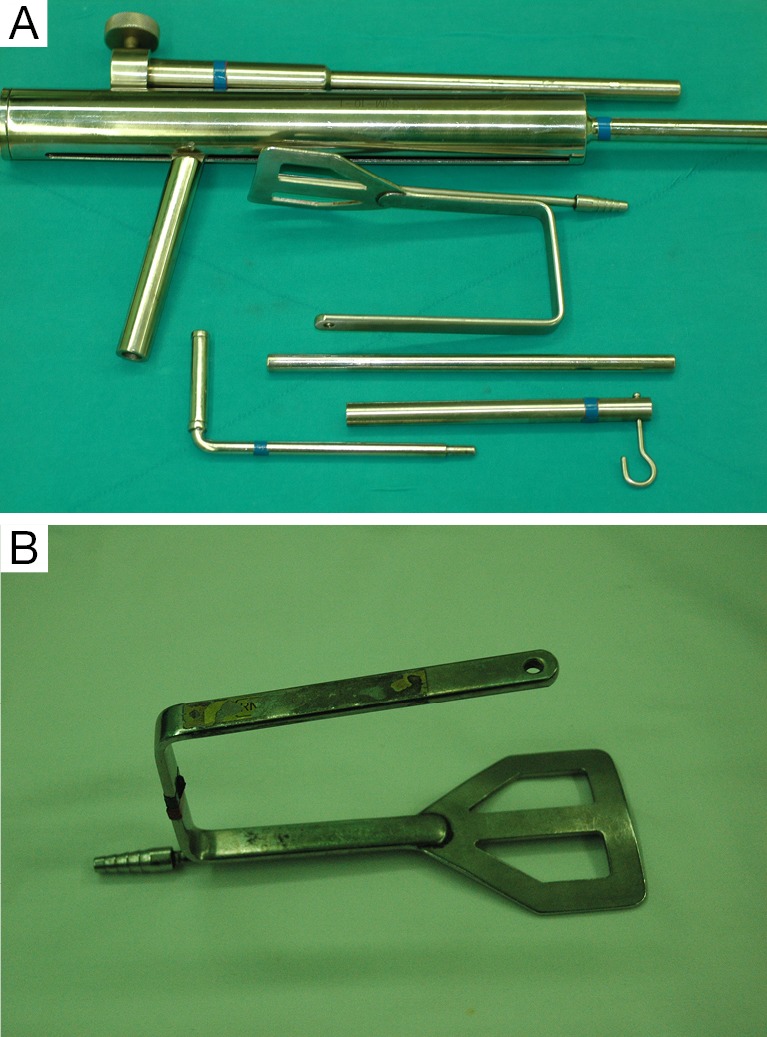
Special set of Chung’s retractor for ND. (A) Wide and long blade of external retractor; (B) table mount and suspension devices.
Table 1. For the robotic total thyroidectomy and ND, the instruments and materials needed additionally are summarized below.
| Procedure | Instruments or materials |
|---|---|
| Patient position | • Arm board |
| • Soft pillow | |
| Development of working space | • Electrocautery with regular and extended-sized tip |
| • Vascular Debakey or Russian forceps (extended length) | |
| • Army-navy retractor ×2 | |
| • Right-angled retractors ×2 | |
| • Breast lighted retractor ×2 | |
| • Endoscopic clip appliers | |
| Maintenance of working space | • Chung’s retractor (special set of retractor for ND) |
| • Table mount and suspension device (Marina Medical, Sunrise, USA) | |
| Robotic procedure | • 5 mm Maryland dissector |
| • 8 mm ProGrasp forceps | |
| • 5 mm Harmonic curved shears | |
| • Dual channel 30 degree endoscope (used in the rotated down position) | |
| • Endoscopic graspers and forceps | |
| • Endoscopic suction irrigator |
ND, neck dissection.
Surgical technique
Patient preparation
With a patient in a supine position under general anesthesia, the neck is slightly extended by inserting a soft pillow under the shoulder, and turning the face away from the lesion. The lesion-side arm is stretched out laterally and abducted about 80 degrees from the body (for optimal expose of the axillar and lateral neck areas). The landmarks for dissection are; the connecting line of sternal notch and the SCM bifurcation medially, the anterior border of trapezius muscle laterally, and the submandibular gland superiorly (Figure 3).
Figure 3.
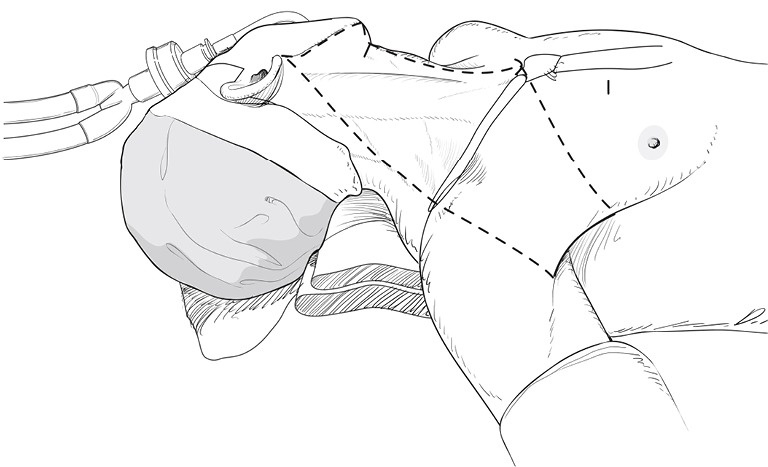
Patient position and superficial landmark for flap dissection.
Development of working space
A 10-12 cm linear skin incision is placed in the axilla along the anterior axillary fold and the lateral border of the pectoralis major. A subcutaneous skin flap is made over the anterior surface of the pectoralis muscle from axilla to the clavicle and the sternal notch. After crossing the clavicle, a subplatysmal skin flap is made. The flap is dissected medially to the anterior border of SCM clavicular head. Laterally, the trapezius muscle is identified and dissected upwards along its anterior border. The spinal accessory nerve is identified and traced carefully along its course until it passes on the undersurface of the SCM muscle. The subplatysmal skin flap is elevated upwards to the Erb’s point, and after exposure of this point, the dissection proceeds underneath of the posterior surface of the SCM muscle toward the submandibular gland superiorly. After subplatysmal flap dissection, the posterior half of clavicular head of the SCM is transected at the level of clavicle-attachment point (to completely expose the junction area between the IJV and subclavian vein). The external jugular vein is ligated where it crosses the SCM muscle, and the dissection proceeds underneath of SCM and goes upward until the submandibular gland and the posterior belly of digastric muscle are exposed. The superior belly of omohyoid muscle is divided at the level of thyroid cartilage, and IJV and lateral border of strap muscles are carefully separated. Thyroid gland is then exposed and detached from the strap muscles until contralateral lobe is fully exposed. For the Flap dissection, special retractor (breast lighted retractor) and long vascular Debakey forcep are very helpful. Especially, the blade end of breast lighted retractor is thin and slightly hooked shape, so it is extremely beneficial for lifting up strap muscle during thyroid exposure. It also can be connected with light cable, so deep area dissection from the skin incision (such as submandibular gland exposure or contralateral thyroid gland exposure) can be easily performed without head light.
After flap dissection, the patient’s face is returned to the front direction for bilateral total thyroidectomy. A long and wide retractor blade (Chung’s retractor) designed for ND is inserted through the axillary incision and lift up the skin flap and SCM & strap muscles (Figure 4). The entire thyroid gland and levels IIA, III, IV, VB, VI area are fully exposed by elevating the two heads of the SCM muscle and the strap muscles.
Figure 4.
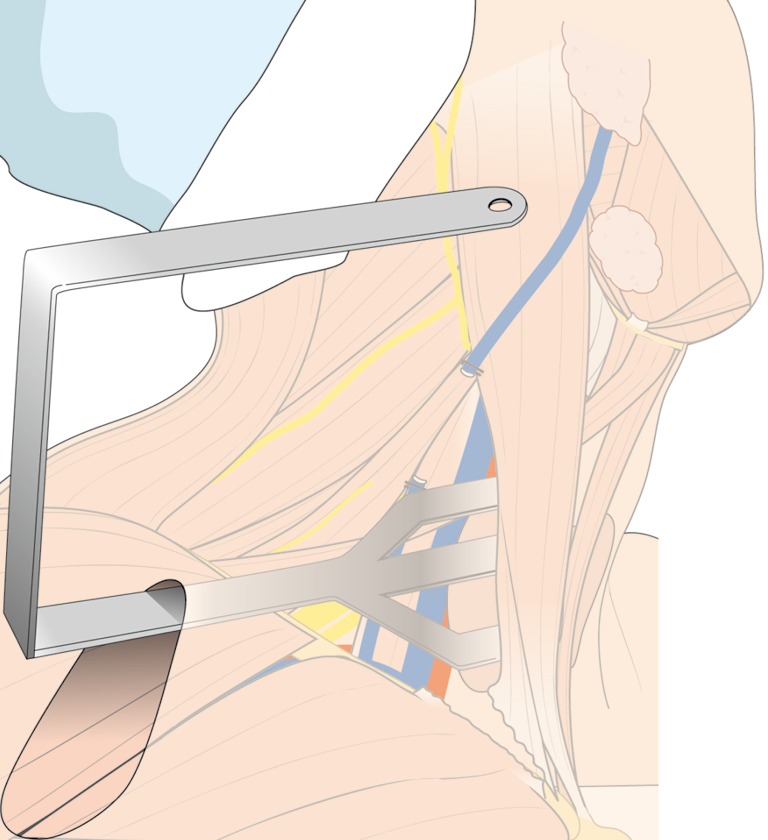
External retractor insertion; initial position of retractor for thyroidectomy and neck dissection of levels III, IV, VB.
Docking and instrumentation
The patient cart is placed on the lateral side of the patient (opposite side to the skin incision). The operating table should be positioned slightly oblique with respect to the direction of the robotic column to allow the straight line between the robotic column and the surgical approach route (direction of retractor insertion).
Previously, we described a novel method of robotic ND using a gasless, transaxillary approach, which requires two skin incisions; an axillary incision for camera, 1st and 2nd robotic arm access and an anterior chest wall incision for the 3rd robotic arm (12,13). However, according to the procedure described here, all four robotic arms are inserted through an axillary single incision. To prevent interference between robotic arms, we offer several tips and rules as to where to place the ProGrasp forceps and how to introduce the robotic arms at appropriate angles and inter-arm distances.
For the Rt. Side approach, a 12 mm trocar for the camera and a 30° dual channel endoscope are located in the center of the axillary incision. The camera is inserted in the upward direction (the external 3rd joint should be placed in the lowest part (floor) of the incision entrance, and the camera tip should be directed upward). An 8 mm trocar for the ProGrasp forceps is then positioned at the right of camera, parallel with the suction tube of the retractor blade. At this point, the ProGrasp forceps must be located as close as possible to the ceiling of the working space (the retractor blade) (Figure 5A,B). The 5 mm trocar of a Maryland dissector is then positioned on the left of the camera (at the left edge of the incision), and the 5 mm trocar for the Harmonic curved shears at the right side of the camera (at the right edge of the incision). Instruments should be as far apart as possible, and inserted upward direction (the same way of camera insertion) (Figure 6A,B).
Figure 5.
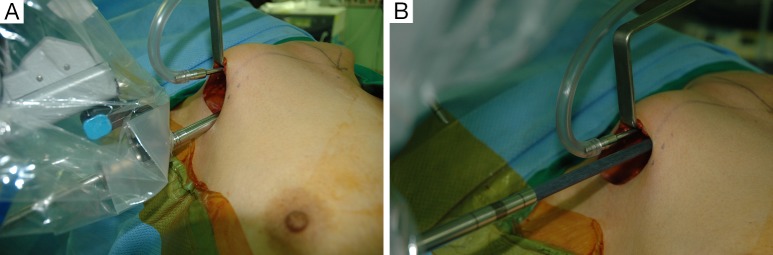
(A) Position of the camera: located in the center of the axillary incision and the external third joint should be placed in the lowest part of the incision and the camera tip should be directed upward; (B) position of the ProGrasp forceps: located as close as possible to the ceiling of the working space.
Figure 6.
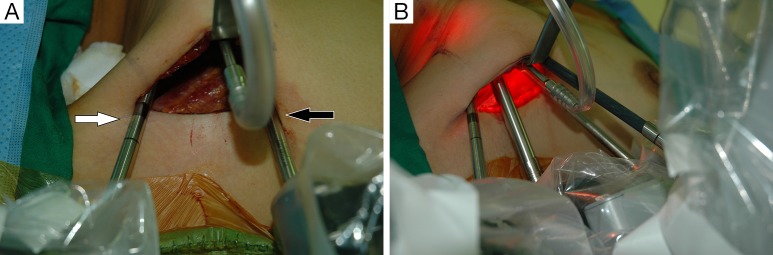
(A) External view of the Maryland dissector and harmonic curved shears: Maryland dissector (white arrow) and harmonic curved shears (black arrow) should be as far apart as possible; (B) all the robotic instruments are introduced through the axillary skin incision.
Robotic total thyroidectomy with central compartment ND
The procedure used is same with that of two-incision robotic thyroidectomy & ND, except for the use of the ProGrasp forceps. Previously, we used movements of the endowrist and the external joint of the ProGrasp forceps to achieve tissue traction (Figure 7). However, during the single-incision approach, tissue should be drawn mostly using endowrist motion of the forceps and its external joint should be moved as little as possible. Because all four robotic arms are inserted through the same incision, minimizing the movements of the external joints of the robotic arms is best for prevention of collisions. We use a Harmonic curved shears for vessel ligation and dissection during the entire procedure. After applying traction to the thyroid upper pole in the medio-inferior direction with the ProGrasp forceps, the superior thyroidal vessel is identified and individually divided Harmonic curved shears. The ProGrasp forceps is used to pull the upper pole steadily, and is repeatedly repositioned in accord with gradual upper pole dissection. The superior parathyroid gland is identified and preserved by detaching the thyroid gland from the cricothyroid muscle. The entire dissection is performed carefully so as not to injure the RLN insertion site. Central compartment node dissection (CCND) is performed after thyroid superior pole dissection. The RLN should be identified before central LN dissection. After tracing the whole running course of RLN, the thyroid can be detached from the trachea completely. In the Berry ligament region, great caution is required to prevent direct or indirect thermal injury of the RLN by the Harmonic curved shears. After Rt. Lobectomy of the thyroid gland, contralateral lobectomy of the thyroid is performed by subcapsular dissection manner, while safely preserving the parathyroid glands and the RLN. In some cases, to achieve better exposure of the contralateral tracheo-esophageal groove, the operating table can be tilted to 10-15 degrees (left side up). After specimen extraction, ND procedure can be followed.
Figure 7.
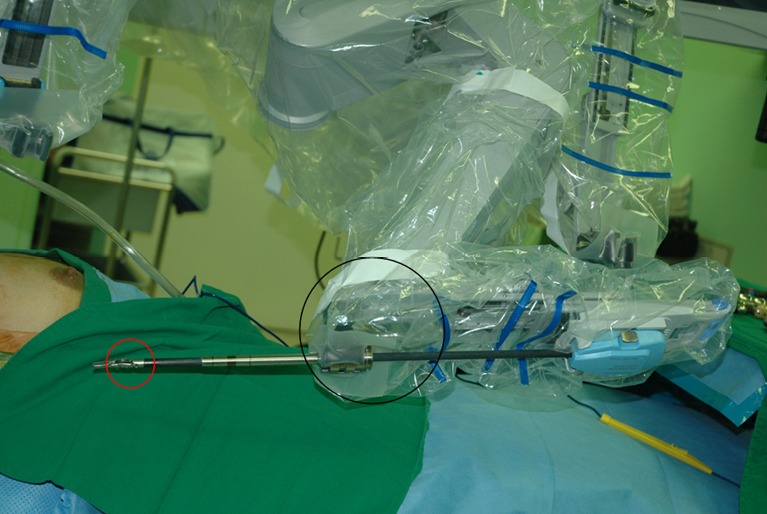
Parts of the ProGrasp forceps are showing; red circle indicates endowrist and black circle is external 3rd joint of the ProGrasp forceps.
Robotic ND
After total thyroidectomy with central compartment ND, lateral ND is started at the level III/IV area around the IJV. The IJV is drawn medially using the ProGrasp forceps, soft tissues and LNs are pulled in the lateral direction using the Maryland dissector and detached from the anterior surface of the IJV to the posterior aspect of IJV until the common carotid artery and vagus nerve are identified. Smooth, sweeping lateral movements of the Harmonic curved shears can establish a proper plane and delineate vascular structures from specimen tissues (Figure 8). Skeletonization of the IJV progresses upward from the level IV to the upper level III area. During this procedure, the superior belly of omohyoid muscle is cut at the level of thyroid cartilage. Packets of LNs are then drawn superiorly using the ProGrasp forceps, and LNs are meticulously detached from the junction of the IJV and subclavian vein. Careful dissection is performed to avoid injury to the thoracic duct. Difficulty may be experienced in this point. Especially for the right side ND, reaching the straight Harmonic curved shears to the deepest point of level IV can be hindered by the prominent clavicle. In these cases, the remote center in the shears’ robotic arm should be re-positioned higher than previous status, and the introduction angle of the Harmonic curved shears should be increased. However, two instruments (ProGrasp forceps and Harmonic curved shears) are concurrently inserted at the same side (right side of the camera), so, there is too little space to raise the remote center of shears’ arm up. The best solution is changing the position of Harmonic curved shears with Maryland dissector, and using the straight harmonic instrument on the left side to the camera enables it to reach the deepest area of level IV without any disturbance. The second-best choice is shifting the 3rd robotic arm for ProGrasp forceps to the left side of camera during this procedure, and re-positioning of the robotic instrument (Maryland in 3rd arm, ProGrasp in the 2nd arm, and Harmonic in the 1st arm) can make some space for elevating the remote center of shears’ arm. For the left side ND, these problems are seldom encountered. In general, the transverse cervical artery (a branch of the thyrocervical trunk) courses laterally across the anterior scalene muscle, anterior to the phrenic nerve. Using this anatomic landmark, the phrenic nerve and transverse cervical artery can be preserved without injury or ligation (Figure 9). Further dissection is followed along the subclavian vein in a lateral direction. After clearing level IV area, the inferior belly of omohyoid muscle is cut at the point where it meets trapezius muscle. The distal external jugular vein (which can join the IJV or subclavian vein) is ligated with Weck® Hem-o-lok® Clips at the inlet to the subclavian vein. Dissection then proceeds upward along the anterior border of trapezius muscle while preserving the spinal accessory nerve (Figure 10). After finishing levels III, IV, and VB node dissections, re-docking is needed to improve the operation view for the dissection of the level II LN. The external retractor is firstly removed, and patient face should be turned the opposite side of ND for the better exposure of lateral neck. After then, the retractor is re-inserted to the direction of submandibular gland (Figure 11). The second docking procedure is performed in the same manner as the first docking, and thus, the operative table should be repositioned more obliquely with respect to the direction of the robotic column to allow alignment between the axis of the robotic camera arm and the direction of retractor blade insertion. Drawing the specimen tissue inferolaterally, the soft tissues and LNs are detached from the lateral border of the sternohyoid muscle, submandibular gland, anterior surfaces of carotid arteries, and the IJV. Level IIA dissection proceeds to the posterior belly of digastric muscle and the submandibular gland superiorly (Figure 12). After removal of the specimen, the operating field is irrigated, and fibrin glue is sprayed around the area of the thoracic duct and minor lymphatics. A closed suction drain (3 mm in size) is inserted just under the axillary skin incision, and the wound is closed cosmetically. The incision scar in the axilla is completely covered when the arm is in its natural position.
Figure 8.
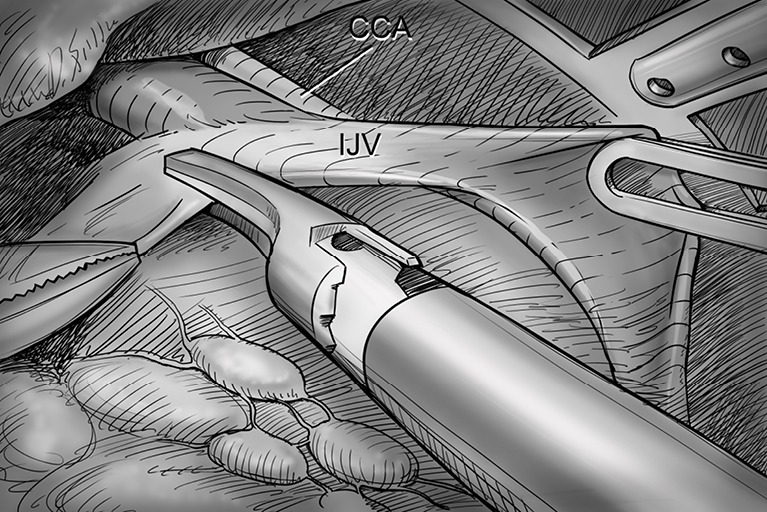
Level III/ IV dissection. The IJV is drawn medially using the ProGrasp forceps, soft tissues and lymph nodes are pulled lateral direction by Maryland dissector and detached from the anterior surface of the IJV to the posterior aspect of IJV until the CCA and vagus nerve are identified. CCA, common carotid artery; IJV, internal jugular vein.
Figure 9.
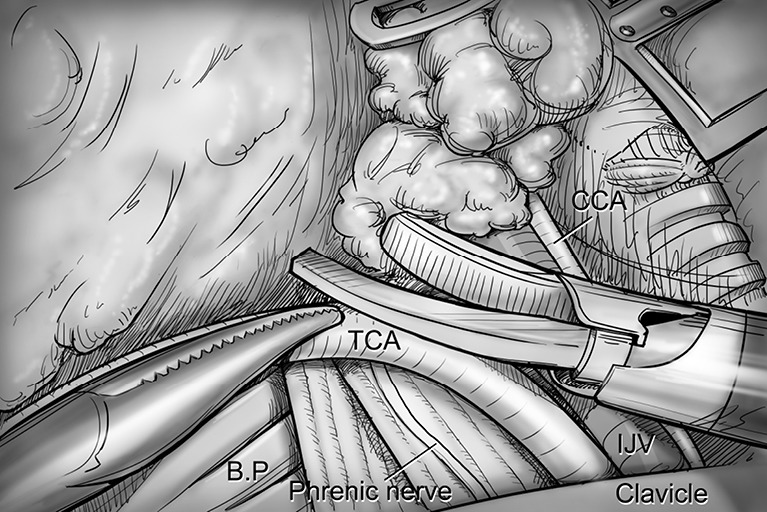
Levels IV, V dissection. Transverse cervical artery (a branch of the thyrocervical trunk) is skeletonized by detaching levels IV, V lymph nodes identifying anterior scalene muscle, phrenic nerve and brachial plexus. CCA, common carotid artery; IJV, internal jugular vein; TCA, transverse cervical artery; BP, brachial plexus.
Figure 10.
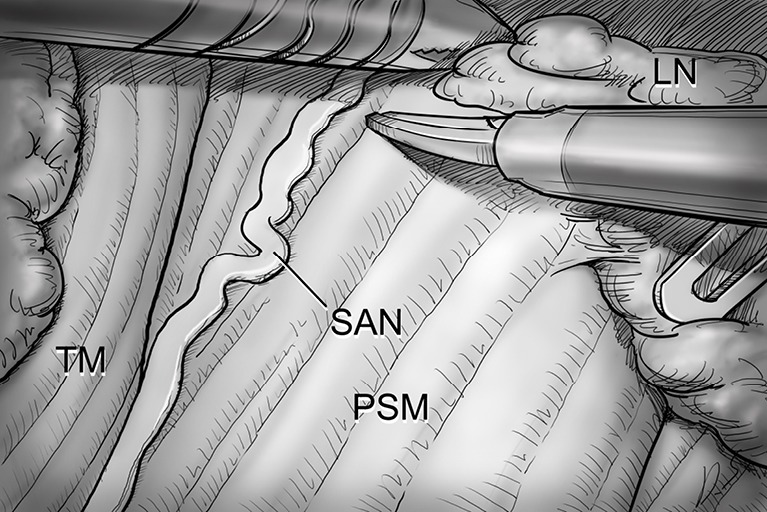
Level V dissection. After clearing level IV area, the dissection proceeds upward along the anterior border of TM while preserving the SAN. PSM, posterior scalene muscle; SAN, spinal accessory nerve; TM, trapezius muscle; LN, lymph nodes.
Figure 11.
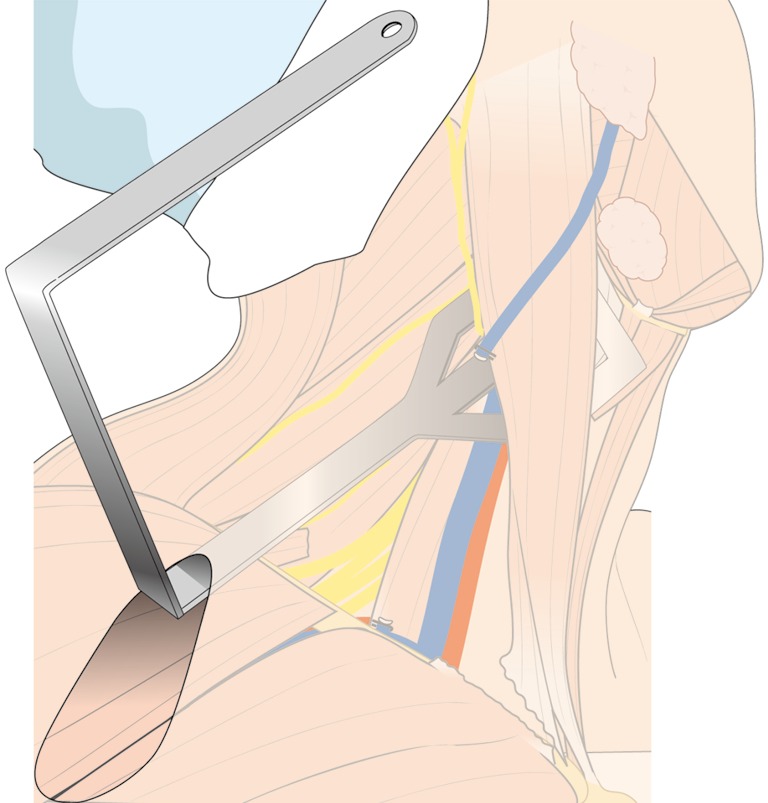
Repositioned external retractor for level II dissection.
Figure 12.
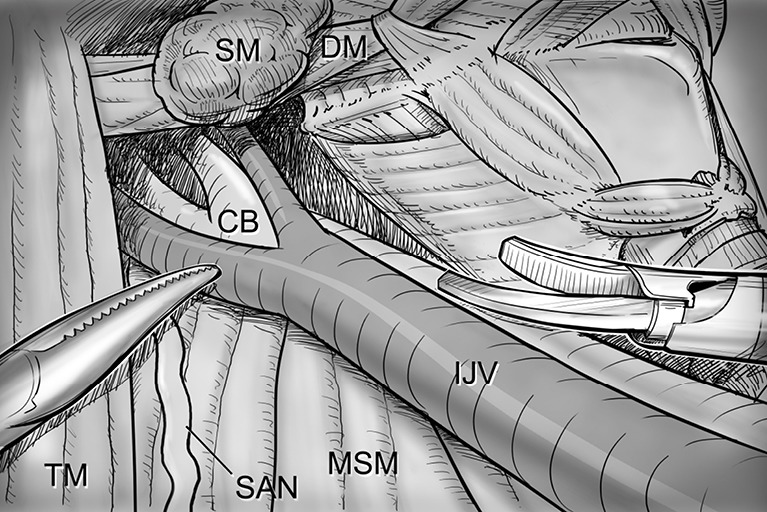
Level II dissection. The level IIA dissection proceeds to the posterior belly of digastric muscle and the submandibular gland superiorly. SM, submandibular gland; DM, digastric muscle (posterior belly); CB, bifurcation of common carotid artery; IJV, internal jugular vein; MSM, middle scalene muscle; SAN, spinal accessory nerve; TM, trapezius muscle.
Postoperative management
Post-operative pain can be controlled by the usual medication regimen for pain control.
The routine period of drain placement after the operation is usually different from each surgeon according to their own experience and preference. However, if the drainage amount is less than 50 mL per day, the drain can be safely removed without any risk of post operative seroma.
Complications
The complications after robotic ND are similar to those after conventional open ND.
Hypoparathyroidism (transient/permanent), recurrent (inferior) laryngeal nerve injury, superior laryngeal nerve injury can occur after central compartment ND, and chyle leakage, nerve injuries [spinal accessory, ramus mandibularis, sympathetic (Horner’s syndrome), phrenic, brachial plexus], hemorrhage/seroma, and wound infection can occur after lateral ND.
Through the 3-D camera in magnified view, critical nerves and thoracic ducts are more vividly identified and preserved during robotic ND than during open methods. Furthermore, multi-articulated instruments and a stable robotic platform reduce the risks of major vessel or thoracic duct injury.
If the surgeon is experienced with the manipulation of robotic instruments and with the open ND procedure, robotic ND has no technique-specific complication.
Conclusions
The dexterities of cutting-edge robotics have markedly advanced endoscopic and minimally invasive surgery. Using this technology, the most exacting procedures in the head and neck area can be managed using an endoscopic approach with excellent cosmesis. Already, satisfactory early surgical outcomes and excellent technical feasibilities have been reported for the management of thyroid cancer with LNM by robotic ND. Furthermore, robotic ND using the transaxillary approach can allow complete compartment-oriented LN dissection without injury to any major vessel or nerve and without compromising surgical oncologic principles.
With advances in instrumentation and more experience, robotic ND is sure to become as accepted alternative means of surgery in low risk thyroid cancer patients with LNM.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gutt CN, Oniu T, Mehrabi A, et al. Robot-assisted abdominal surgery. Br J Surg 2004;91:1390-7. [DOI] [PubMed] [Google Scholar]
- 2.Lobe TE, Wright SK, Irish MS. Novel uses of surgical robotics in head and neck surgery. J Laparoendosc Adv Surg Tech A 2005;15:647-52. [DOI] [PubMed] [Google Scholar]
- 3.Miyano G, Lobe TE, Wright SK. Bilateral transaxillary endoscopic total thyroidectomy. J Pediatr Surg 2008;43:299-303. [DOI] [PubMed] [Google Scholar]
- 4.Savitt MA, Gao G, Furnary AP, et al. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann Thorac Surg 2005;79:450-5; discussion 455. [DOI] [PubMed] [Google Scholar]
- 5.Link RE, Bhayani SB, Kavoussi LR. A prospective comparison of robotic and laparoscopic pyeloplasty. Ann Surg 2006;243:486-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [DOI] [PubMed] [Google Scholar]
- 7.Kang SW, Jeong JJ, Nam KH, et al. Robot-assisted endoscopic thyroidectomy for thyroid malignancies using a gasless transaxillary approach. J Am Coll Surg 2009;209:e1-7. [DOI] [PubMed] [Google Scholar]
- 8.Ryu HR, Kang SW, Lee SH, et al. Feasibility and safety of a new robotic thyroidectomy through a gasless, transaxillary single-incision approach. J Am Coll Surg 2010;211:e13-9. [DOI] [PubMed] [Google Scholar]
- 9.Holsinger FC, Sweeney AD, Jantharapattana K, et al. The emergence of endoscopic head and neck surgery. Curr Oncol Rep 2010;12:216-22. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Ryu HR, Park JH, et al. Excellence in Robotic Thyroid Surgery: A Comparative Study of Robot-Assisted versus Conventional Endoscopic Thyroidectomy in Papillary Thyroid Microcarcinoma Patients. Ann Surg 2011;253:1060-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Yun JH, Nam KH, et al. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc 2011;25:906-12. [DOI] [PubMed] [Google Scholar]
- 12.Kang SW, Lee SH, Ryu HR, et al. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010;148:1214-21. [DOI] [PubMed] [Google Scholar]
- 13.Kang SW, Lee SH, Park JH, et al. A comparative study of the surgical outcomes of robotic and conventional open modified radical neck dissection for papillary thyroid carcinoma with lateral neck node metastasis. Surg Endosc 2012;26:3251-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Kwon IS, Bae EH, et al. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab 2013;98:2701-8. [DOI] [PubMed] [Google Scholar]


