Abstract
Myxobacteria are well-known for their complex life cycle, including the formation of spore-filled fruiting bodies. The model organism Myxococcus xanthus exhibits a highly complex composition of neutral and phospholipids, including triacylglycerols (TAGs), diacylglycerols (DAGs), phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), cardiolipins (CLs), and sphingolipids, including ceramides (Cers) and ceramide phosphoinositols (Cer-PIs). In addition, ether lipids have been shown to be involved in development and signaling. In this work, we describe the lipid profile of M. xanthus during its entire life cycle, including spore germination. PEs, representing one of the major components of the bacterial membrane, decreased by about 85% during development from vegetative rods to round myxospores, while TAGs first accumulated up to 2-fold before they declined 48 h after the induction of sporulation. Presumably, membrane lipids are incorporated into TAG-containing lipid bodies, serving as an intermediary energy source for myxospore formation. The ceramides Cer(d-19:0/iso-17:0) and Cer(d-19:0/16:0) accumulated 6-fold and 3-fold, respectively, after 24 h of development, identifying them to be novel putative biomarkers for M. xanthus sporulation. The most abundant ether lipid, 1-iso-15:0-alkyl-2,3-di-iso-15:0-acyl glycerol (TG1), exhibited a lipid profile different from that of all TAGs during sporulation, reinforcing its signaling character. The absence of all these lipid profile changes in mutants during development supports the importance of lipids in myxobacterial development. During germination of myxospores, only the de novo biosynthesis of new cell membrane fatty acids was observed. The unexpected accumulation of TAGs also during germination might indicate a function of TAGs as intermediary storage lipids during this part of the life cycle as well.
INTRODUCTION
The analysis of small metabolites has become an important tool to acquire information on metabolism in complex biological systems. While genes and transcripts hold the master plan for an organism and proteins drive chemical reactions, a high number of small molecules, like lipids, amino acids, sugars, and nucleotides, are required to keep an organism alive. Technical progress within the last decade has enabled researchers to develop high-throughput analysis using mass spectrometry (MS) for all different subdisciplines in metabolomics (1). In lipidomics, several applications describing the analysis of single lipid classes have been published in the past (2–4). Lorenzen et al. developed an ultraperformance liquid chromatography (UPLC)-electrospray ionization (ESI)-MS method applicable for the analysis of whole-lipid extracts for their neutral and phospholipid contents, as exemplarily demonstrated for the Gram-negative deltaproteobacterium Myxococcus xanthus (5). Like most myxobacteria, M. xanthus forms spore-containing fruiting bodies upon amino acid depletion (6, 7). Accompanying the complex developmental program from rod-shaped vegetative cells to round myxospores, the lipid composition of the cells changes extensively and mostly occurs in ether lipids. Lipids containing ether-linked fatty acids accumulate during development, with a maximum amount occurring at about 48 h after the onset of starvation (8, 9). The most dominant ether lipid, 1-iso-15:0-alkyl-2,3-di-iso-15:0-acyl glycerol (TG1), has been reported to be a signal involved in myxobacterial development (10). Also, the plasmenylphosphatidylethanolamine vinyl ether lipid phosphatidylethanolamine(P-iso-15:0/iso-15:0) (VEPE) was previously described to be a biomarker for development (9). Lipids are also involved in myxobacterial chemotaxis (11). Furthermore, it has been shown that during spore maturation, M. xanthus cells reduce the surface area of their membrane while simultaneously producing lipid bodies filled predominantly with triacylglycerides (TAGs) (12). Thereby, phosphatidylethanolamines (PEs) from the cell membrane serve as a source for fatty acids incorporated into lipid bodies (13). In addition, the exchange of proteins and lipids in M. xanthus via direct cell contact has been observed (14, 15). As neutral and phospholipids are involved in a broad range of related functions and because several otherwise rare lipids have been identified in the unusually complex lipidome of myxobacteria, M. xanthus is an ideal model with which to study the function of these different lipid classes.
To generate a complete overview of the lipidome profile of M. xanthus during development and myxospore germination, we applied the previously described lipidome analysis (5) to M. xanthus at different stages of its complex life cycle (6, 7) using the lipid compound list already generated by tandem mass spectrometry (5). In addition, different mutant strains with developmental defects were analyzed. In order to deal with the large amount of data from the different samples, an automated procedure that also enabled the first study of lipids involved in myxospore germination was established.
MATERIALS AND METHODS
Bacterial strains and growth conditions. Wild-type M. xanthus DK1622 and mutant strains with an esg (Δbkd) mutation (16) and a fruA mutation (17) were grown in CTT medium (18) supplemented with 40 μg/ml kanamycin (Km) (Carl Roth, Karlsruhe, Germany) when necessary. For each sample, a total of approximately 2 × 1010 cells were used. Fruiting body and spore development was performed on TPM agar plates (diameter, 14 cm) with the same number of cells (19). For the isolation of myxospores, fruiting bodies were harvested and washed with water twice. The spores were incubated for 2 h at 60°C to inactivate the remaining vegetative cells before treatment with sonication. Germination experiments were performed in CTTYE medium, prepared by adding 0.2% yeast extract to CTT medium.
Determination of iso-15:0 fatty acid de novo biosynthesis.
An Agilent gas chromatography (GC)-MS system with a 7890A gas chromatograph with a DB-5HT column (30 m by 250 μm by 0.1 μm) coupled to a 5975C mass spectrometer was used for analysis.
The ratio of l-leucine and D10-l-leucine in the CTT medium used to grow M. xanthus was assigned by hydrolysis and subsequent GC-MS analysis of nonlabeled CTT medium. A total of 50 μl of medium was hydrolyzed by adding 800 μl of 6 M HCl and incubating the mixture at 55°C overnight. The hydrolysate was dissolved in 80 μl dichloromethane (DCM), and 20 μl N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) was added. Two microliters of the sample was measured in split mode at a rate of 50:1. The helium flow rate was set to 1 ml/min. The inlet temperature was set to 300°C. Analysis was performed with an initial oven temperature of 35°C, and the temperature was increased by 5°C/min to 70°C and then by 120°C/min to 300°C. The ionization energy was set to 70 eV.
The amount of leucine in the CTT medium was determined to be 10 mM. Therefore, 10 mM l-[2,3,3,4,5,5,5,5′,5′,5′-2H10]leucine(Sigma-Aldrich) was added to the germination medium in order to obtain an equal ratio of deuterated and nondeuterated leucine. Germination took place in CTTYE medium with purified myxospores. Samples were taken every 2 h to determine the ratio of deuterated to nondeuterated iso-15:0 fatty acid by fatty acid methyl ester (FAME) GC-MS analysis.
Extraction procedures and HPLC-MS conditions.
Sample extractions and high-performance liquid chromatography (HPLC) measurements were performed in triplicate as described previously (5). Immediately before the extraction procedure, PE(12:0/12:0) and TAG(18:0/18:0/18:0) were added to the dried cells as internal standards to a final concentration of 50 μM.
Data processing.
Lipid species in the UPLC-ESI-MSn (where n represents the number of rounds of MS) raw data were quantified using an automated integration algorithm provided by TargetAnalysis software (Bruker, Germany). An in-house library including the molecular masses, sum formulae, and trivial names of >120 lipid species (subdivided into positive and negative ionized forms) was established and used as the input data. The settings of the TargetAnalysis method were as follows: (i) for detection parameters, a ±0.15-min retention time tolerance, a ±500-mDa EIC width, a ±1-min chromatogram time slice, and extracted ion chromatogram (EIC) smoothing enabled; (ii) for identification parameters: a ±500-mDa identification m/z tolerance and an mSigma threshold (i.e., the rate for the agreement of the theoretical and measured isotopic patterns of the mass peak of interest, given in milliSigma) of 1,000; (iii) for scoring parameters, a retention time tolerance of 0 to 0.5 min (narrow/wide range), a mass accuracy tolerance of 100 to 1,000 mDa (narrow/wide range), and an mSigma tolerance of 1 to 1,000 (narrow/wide range); and (iv) for compound detection parameters, algorithm version 3.0, a sensitivity of 95%, no area threshold, an absolute intensity threshold of 10,000 counts or 1,000 counts (positive or negative ionization mode, respectively), a minimum peak valley of 15%, and a line spectrum only with no background subtraction. After the first TargetAnalysis screening, results were surveyed in the DataAnalysis program (Bruker, Germany) and lipid species were validated by their specific MSn patterns and retention times. For quantification, area counts were exported and partially corrected manually. Intensities for the internal standards PE(12:0/12:0) (negative ionization group) and TAG(18:0/18:0/18:0) (positive ionization group) were used as correction factors for the extraction. The area counts of their signals were set equal to 1,000, and sample area counts were adjusted accordingly.
For three-dimensional bar graph visualization, Origin (version 9) software (OriginLab, Northampton, MA, USA) was used. Statistical analysis and two-dimensional bar graphs were illustrated with GraphPad Prism (version 6) software (GraphPad Software, San Diego, CA, USA).
Microarray analysis of genes involved in lipid biosynthesis.
Descriptions for genes involved in lipid pathways during M. xanthus development were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG). BLAST was used to find homologous genes for lipid pathways not yet elucidated in M. xanthus.
Nomenclature.
For the names and abbreviations of lipids, which are given in Table 1, we used the nomenclature of the LIPID Metabolites and Pathways Strategy lipid classification system (www.lipidmaps.org).
TABLE 1.
Lipid species nomenclature used in this work
| Abbreviation | Lipid species |
|---|---|
| TAG | Triacylglycerol |
| DAG | Diacylglycerol |
| PE | Phosphatidylethanolamine |
| PE(P) | Plasmenylphosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| CL | Cardiolipin |
| Cer | Ceramide |
| Cer-PI | Ceramide phosphoinositol |
| PE(16:1/15:0) | Phosphatidylethanolamine with two fatty acids, one with 16 carbons with one desaturated bond and one with 15 carbons without any desaturation |
| Cer(d-19:0/iso-17:0 2-OH) | Dihydroceramide consisting of acyl-sphinganine with 19 carbons and an iso-branched fatty acid with 17 carbons with a hydroxylation |
RESULTS
Analysis of lipid samples from developing M. xanthus DK1622 cultures.
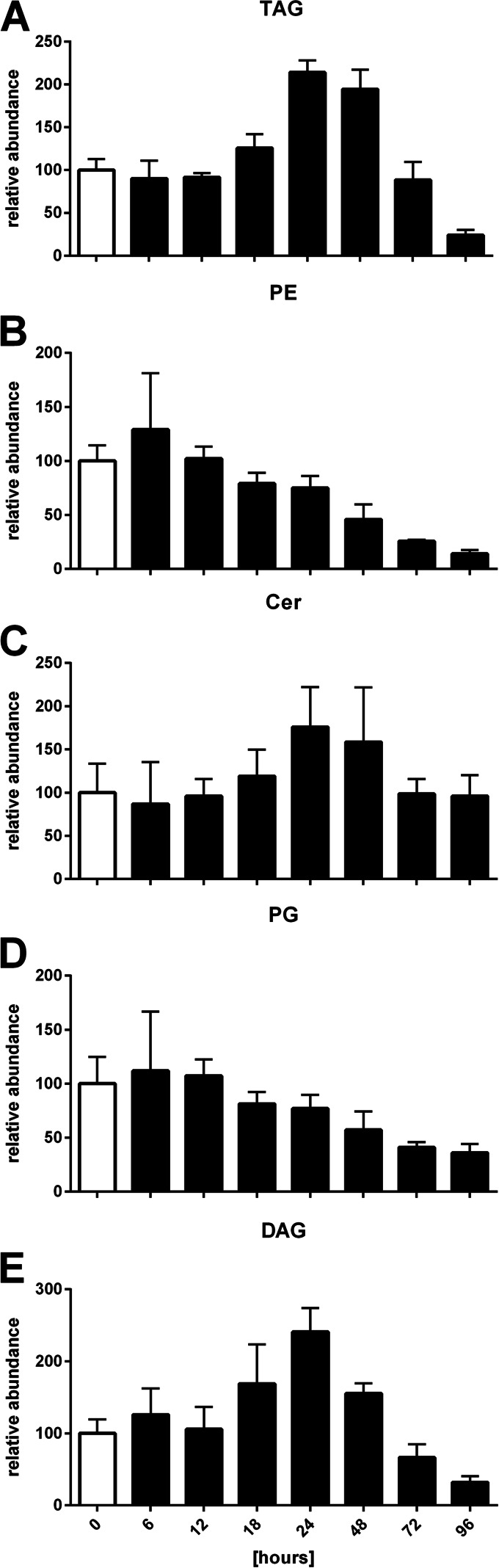
Whole lipid samples were extracted from M. xanthus cultures during vegetative growth, development, and germination. The samples were analyzed by UPLC-MS in two runs using positive and negative ionization modes, respectively. After identification of the lipid species by retention time and tandem mass spectrometry, the acquired data were separated into the different lipid classes, resulting in 43 triacylglycerols (TAGs), 7 diacylglycerols (DAGs), 32 phosphatidylethanolamines (PEs), 12 phosphatidylglycerols (PGs), 3 ceramides (Cers), and 4 ceramide phosphoinositol (Cer-PI) species (see Tables S1 and S2 in the supplemental material). In contrast to previous analyses, we could not identify cardiolipins in the lipid extracts, probably because of the minute amount of cardiolipins, which made up less than 1% of total lipids (5, 20) and which were thus present at levels below the detection limit. Internal standards for the analysis in both ionization modes allowed correction for possible deviations caused by extraction efficiencies. PE(12:0/12:0) and TAG(18:0/18:0/18:0) were chosen as internal standards for the negative and positive ionization modes, respectively, as these lipids are absent in the lipid profile of M. xanthus and PEs and TAGs represent the two most abundant lipid classes in the profile. On the basis of the MS fragmentation spectra, it was not possible to differentiate exactly between some isobaric species. Hence, they were grouped into one entry. Analysis of lipid A was not performed in this work, as the required phenol-water extraction could not be performed parallel to the whole-lipid extraction procedure, and thus, we focused on neutral and phospholipids. Figure 1 shows the profile of TAGs during the development of M. xanthus strain DK1622 from vegetative cells at 0 h to fully developed spores in fruiting bodies at 96 h. The most striking observation was a 2-fold increase of all TAGs until 24 h after development, followed by a decline until 96 h to a level about 1/4 of the level during vegetative growth. TAGs with a combined length of incorporated fatty acids of about 44 to 46 carbons were the most abundant species. Published fatty acid profiles of DK1622 indicated that iso-15:0 was the most common fatty acid at 40.8%, followed by 16:1 (9.5%), 14:0 (5.5%), and 16:2 (5.2%) (16). Consequently, TAG(iso-15:0/iso-15:0/iso-15:0) was the major TAG in the profile. DAGs were found at a much lower concentration in the lipid samples but showed a lipid profile very close to that of the TAGs (Fig. 2A and D).
FIG 1.
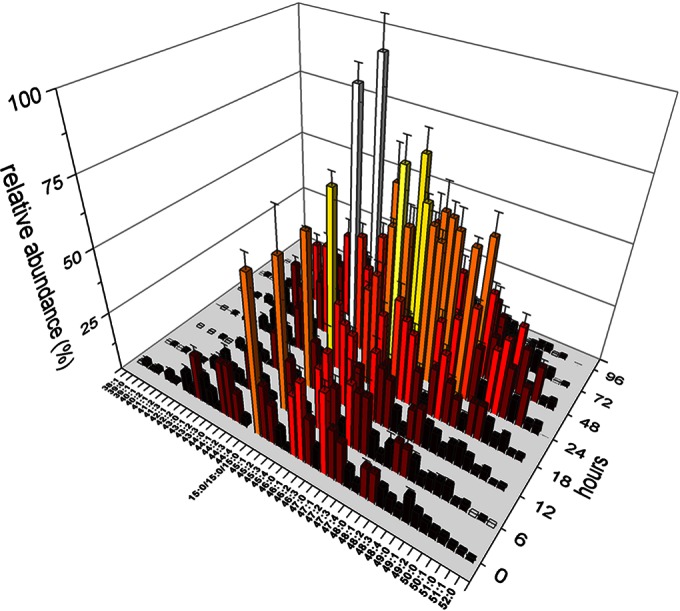
Complete profile of TAGs during development of M. xanthus. The relative amounts of TAGs with different combined fatty acid chain lengths from 0 to 96 h after starvation are shown. The abundance of the most abundant TAG (iso-15:0/iso-15:0/iso-15:0) was set equal to 100. As it was not possible to exactly differentiate between isobaric TAGs by mass spectra and retention times, they were grouped to one entry bearing the same combined length of fatty acids. Bar colors indicate intensities from low (dark red) to high (white).
FIG 2.
Relative intensities of the TAG (A), PE (B), Cer (C), PG (D), and DAG (E) lipid classes during development of M. xanthus from 0 to 96 h. The relative abundance of the individual lipid subspecies at 0 h was set equal to 100. Error bars show standard deviations from triplicate experiments.
In contrast to the amount of TAGs that accumulated, the amount of PEs detected during the development of M. xanthus decreased constantly (Fig. 2B). Also, the overall profile of PEs (see Tables S2 and S3 in the supplemental material) showed a distribution of combined carbon chains matching the fatty acid profile of DK1622 mentioned above. Like the PEs, the PGs, which were generally less abundant in the lipid extracts, also showed a constant decline during development to about 50% of the amount in vegetative cells after 96 h of development (Fig. 2D). Both species were the major membrane lipids in vegetative cells.
The lipid profiles of ether lipids are different from the TAG and PE lipid profiles during development.
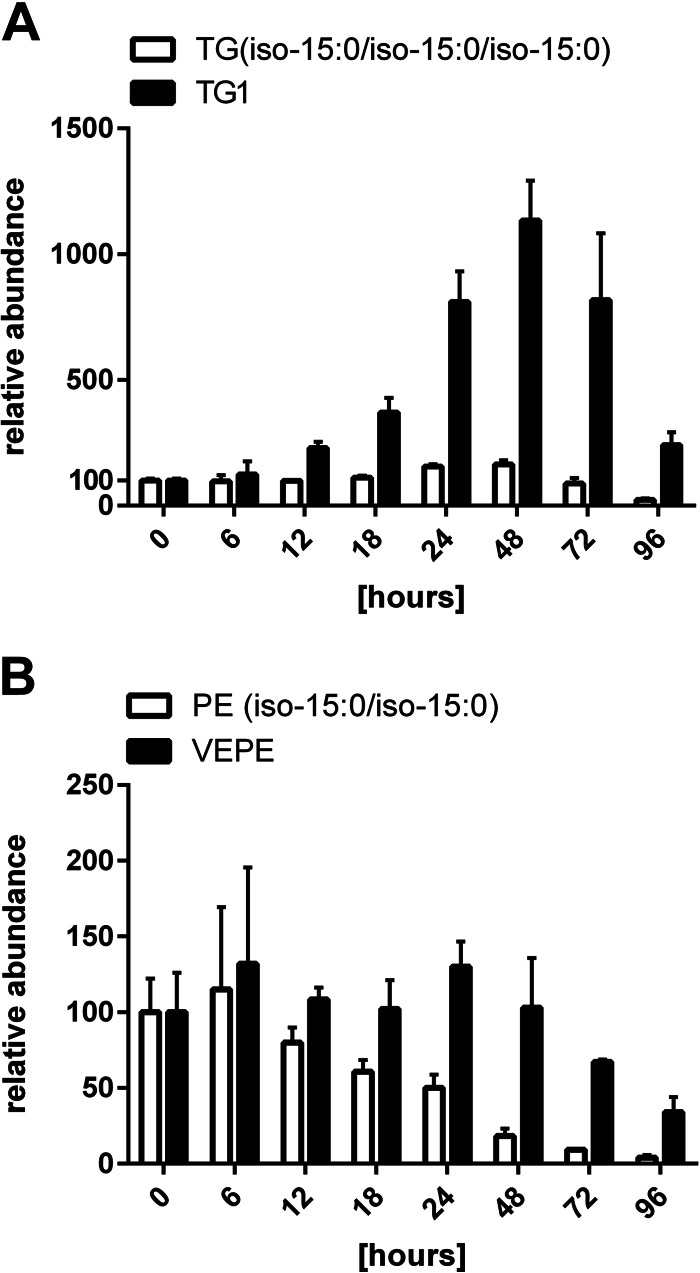
Interestingly, the most common ether lipid in M. xanthus TG1 showed a profile different from that of other TAGs (Fig. 3A). TG1 was hardly found in vegetative cells but accumulated to much higher levels than TAGs during development, reaching a maximum after 48 h. It accumulated up to a 10-fold higher level than TAGs and decreased during later development, resulting in a 1.5-fold larger amount at 96 h than the amount in vegetative cells. The amounts of all other TAGs only doubled, and their final drop in intensity was less dramatic than that of TG1. The vinyl ether lipid PE(P-iso-15:0/iso-15:0), also known as VEPE, is a possible precursor of TG1 (8) and also showed a profile different from that of standard PE. The amount of VEPE decreased more slowly and to a level of only about 40% of that of vegetative cells. In contrast, the most abundant PE, PE(iso-15:0/iso-15:0), steadily decreased to a level less than 5% of that of vegetative cells during 96 h of development (Fig. 3B).
FIG 3.
Ether lipid profiles during development of M. xanthus. (A) Abundance of ether lipid TG1 compared to that of the most abundant TAG (iso-15:0/iso-15:0/iso-15:0). (B) Abundance of VEPE compared to that of PE(iso-15:0/iso-15:0). The relative abundance of the individual lipid subspecies at 0 h was set equal to 100. Error bars show standard deviations from triplicate experiments.
Cers accumulate during development.
Ceramides (Cer), including Cer phosphatidylinositols (Cer-PIs), represented only about up to 2% of the overall lipids in a vegetative culture of M. xanthus. At first sight, the Cer profile did not show any significant changes (Fig. 2C). However, a closer look into the different ceramide species revealed that the amounts of Cer(d-19:0/16:0) and Cer(d-19:0/iso-17:0) rose significantly during spore development (Fig. 4). At their maximum at about 24 h, their intensity was increased 3- to 5-fold compared to that in the samples of vegetative cells at 0 h. In contrast, Cer-PIs did not show major alterations.
FIG 4.
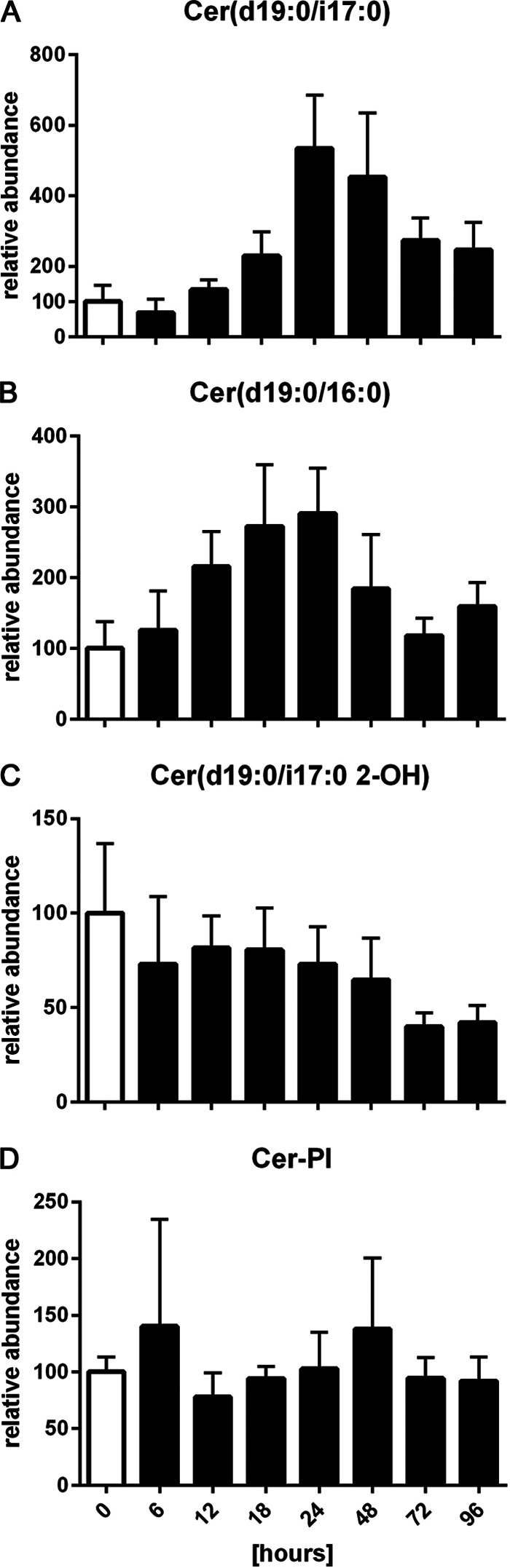
Accumulation of ceramides during development of M. xanthus. The relative amounts of the individual lipid subspecies at 0 h were set equal to 100. Error bars show standard deviations from triplicate experiments. (A) Cer(d-19:0/iso-17:0); (B) Cer(d-19:0/16:0); (C) Cer(d-19:0/iso-17:0 2-OH); (D) Cer-PIs.
Lipid metabolism during germination.
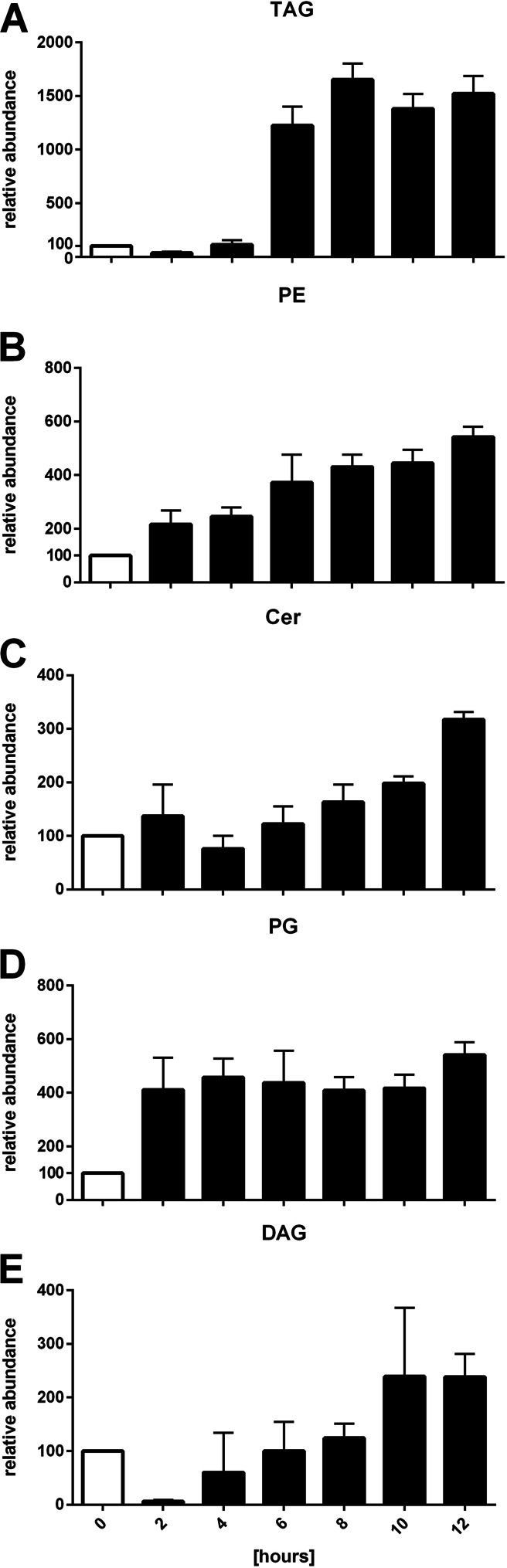
For the investigation of the lipid composition during germination, wild-type spores were generated by the induction of fruiting body development through starvation, and spores were subsequently isolated. Germination was induced by incubation in CTTYE medium. In general, that amounts of all lipid classes investigated increased within the time period of observation (Fig. 5). The PG intensity remained stable after 2 h of germination. While the amount of PEs in the lipid profile during spore formation steadily declined to 1/7 of the level during vegetative growth (Fig. 2B), the PE content during germination increased 5-fold in a similar manner (Fig. 5B). The TAG intensity did not change significantly within the first 4 h after the induction of germination and stayed at the level found in spores. After 6 h, the amount of TAGs increased 12-fold compared to that at 0 h of germination, followed by a moderate increase until the end of the experiment, after 12 h. The amount of DAGs also increased but to a lower level of about 2-fold at the end of the experiment, after 10 and 12 h. Ceramides increased steadily over the whole experiment to a final 5-fold higher level than that at 0 h of germination.
FIG 5.
Relative intensities of the TAG (A), PE (B), Cer (C), PG (D), and DAG (E) lipid classes during germination of M. xanthus from 0 to 12 h. The relative abundance of the individual lipid subspecies at 0 h was set equal to 100. Error bars show standard deviations from triplicate experiments.
The lipid profiles of mutants with developmental mutations deviate strongly from those of the wild type.
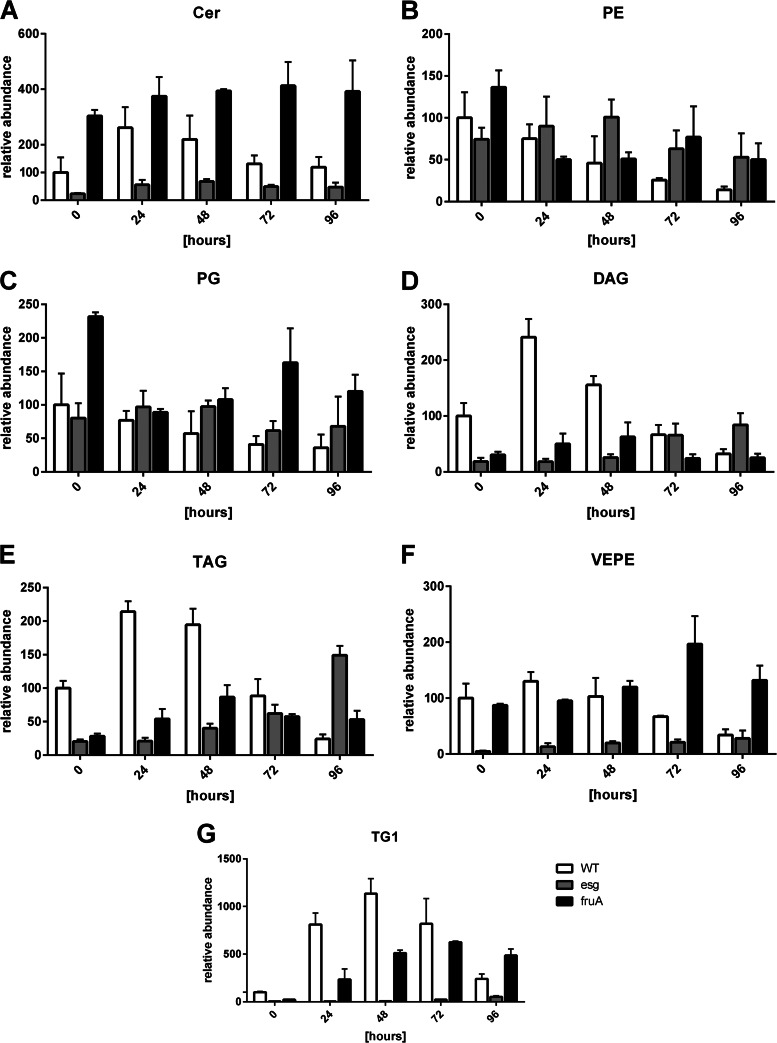
The esg mutant was defective in producing a subunit of the BKD complex (9, 21), an enzyme key to the production of iso-fatty acids in lipid metabolism. Thus, this mutant showed a much smaller amount of iso-fatty acids, as was also clearly visible in the lipid pattern during development (Fig. 6). In the mutant, the levels of PEs and PGs did not decrease compared to those in the wild type and stayed almost constant. DAGs and TAGs accumulated in the mutants during the entire duration of the experiment and failed to decrease at the end, which was observed during wild-type cell development. The same observation applied to the ether lipids TG1 and VEPE. Ceramides were detected in a much smaller amount in the mutants and showed a lipid profile different from that in the wild type, as they accumulated only slightly after 48 h. The growth of the fruA mutant was blocked in a later stage of development, and the mutant did not form fruiting bodies or spores. The profile of each lipid subgroup was similar to that of the wild type, but the trend was less clear. The most striking difference in comparison to the wild type was the ether lipid profile, which especially showed a delayed and constant accumulation of TG1.
FIG 6.
Lipid development in wild-type and developmental mutants of M. xanthus. All lipid subspecies of the wild type and the esg and fruA mutants during development were investigated, and their abundances were compared. White bars, lipid profiles in wild-type cells; gray and black bars, lipid profiles in esg and fruA mutant cells, respectively. The relative amounts of the individual lipid subspecies at 0 h were set equal to 100. Error bars show standard deviations from triplicate experiments. (A) Ceramides; (B) phosphatidylethanolamines; (C) phosphatidylglycerols; (D) diacylglycerols; (E) triacylglycerols; (F) PE(P-iso-15:0/iso-15:0); (G) 1-iso-15:0-alkyl-2,3-di-iso-15:0-acyl glycerol.
Fatty acids are synthesized de novo during germination of M. xanthus.
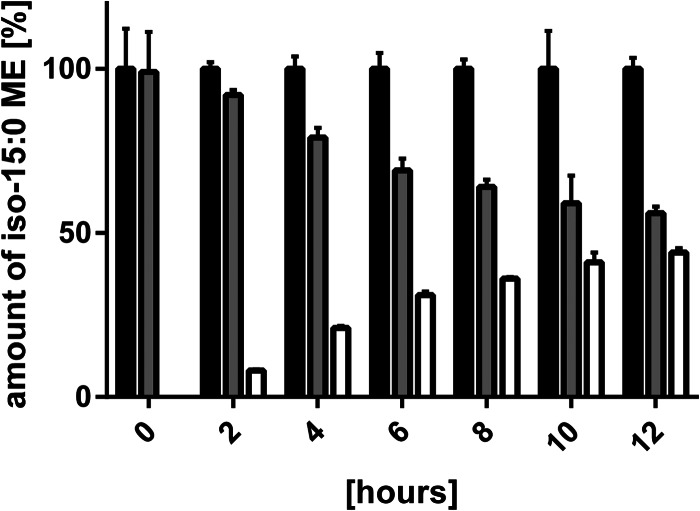
The most abundant fatty acid in M. xanthus, iso-15:0, is synthesized using isovaleryl coenzyme A (CoA) derived from leucine as a starter unit or an alternative biosynthesis pathway (9, 9, 16, 21–23). In order to differentiate between fatty acids which are recycled from existing spore material and those synthesized de novo, cells were germinated in medium with equal amounts of leucine and D10-leucine. Prior to the induction of germination, no de novo synthesized D9-iso-15:0 methyl ester (ME) could be detected (Fig. 7). Within the first 6 h of germination, the percentage of labeled iso-15:0 ME rose to more than 30% and finally increased to 44% of total iso-15:0 ME at the end of the experiment, after 12 h.
FIG 7.
Amount of iso-15:0 methyl esters obtained by whole-cell FAME analysis during germination of M. xanthus. Germination was performed in CTTYE medium containing 10 mM D10-leucine. Black bars, the total amount of iso-15:0 MEs, which was set equal to 100%; gray bars, nonlabeled iso-15:0 MEs; white bars, D9-iso-15:0 MEs synthesized de novo by incorporation of D10-leucine from germination medium. Error bars show standard deviations from triplicate experiments.
Whole-cell FAME GC-MS analysis of lipid samples supports the HPLC-MS data for ether lipids.
The decline in the amount of ether lipids, in particular, TG1, at the end of development (Fig. 3) could be caused by their possible connection to the spore coat or spore coat components (proteins, sugars), resulting in a more rigid spore envelope but preventing their extraction using standard solvents. Thus, we determined by HPLC-MS the amount of all ether lipid-derived moieties in the hydrolysate of the samples which were used in the lipid analysis. Therefore, whole-cell hydrolysis was performed by the FAME reaction. Hydrolysis converts iso-15:0 fatty acids or lipids containing them into iso-15:0 MEs, VEPE into iso-15:0 dimethylacetal (DMA), and alkyl ether lipids like TG1 into iso-15:0 O-alkylglycerol (OAG). The amount of 18:0 ME cleaved off the internal standard TAG(18:0/18:0/18:0) by hydrolysis was used as the internal standard in this analysis. The amount of iso-15:0 ME showed no significant decrease within the first 48 h of development, but during late development their level dropped to about 10% of the level found during vegetative growth (Fig. 8). The profile of the overall ether lipid contents is very similar to the profiles achieved for VEPE and TG1 in the lipid analyses (compare Fig. 3 and 8). In both analyses, iso-15:0 DMA accumulated slightly from about 24 to 48 h, and its amount decreased at later stages of development. The results for TG1 and iso-15:0 OAG matched very closely. In both analyses, the maximum amount was achieved at 48 h of development, even though the difference from the level during vegetative growth was considerably higher in the whole-lipid analysis by HPLC-MS.
FIG 8.
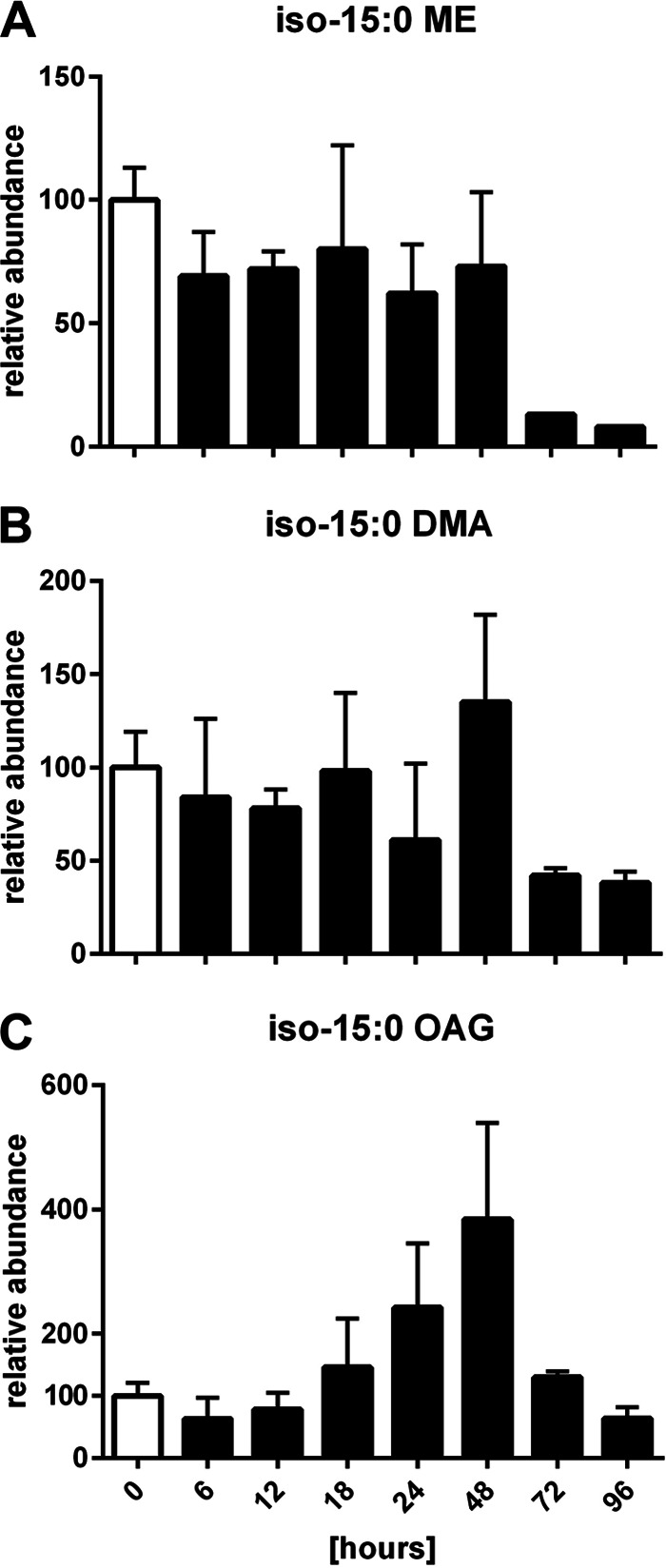
Relative amounts of iso-15:0 MEs (A), iso-15:0 DMA (B), and iso-15:0 OAG (C) from whole-cell FAME GC-MS analysis of lipid samples from M. xanthus during development. The relative amounts at 0 h were set equal to 100. Error bars show standard deviations from triplicate experiments.
Genes for ceramide and TAG biosynthesis are upregulated during development.
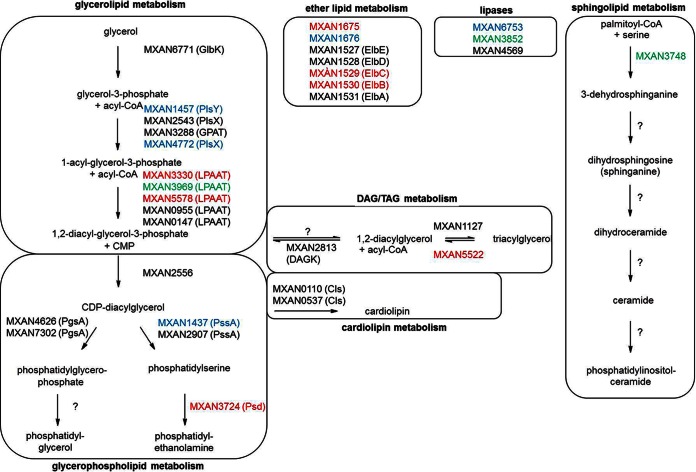
Published microarray data for developing cells for the first 24 h of development (24) were used to shed some light on the regulation of the different lipid biosynthesis pathways. The results shown in Fig. 9 and Table S3 in the supplemental material indicate that the overall glycerolipid metabolism, which provides precursors for the biosynthesis of phospholipids and TAGs, was not significantly influenced. While genes involved in the biosynthesis of PGs are not affected by regulation, MXAN_3724, encoding a phosphatidylserine decarboxylase (25) that catalyzes the last step in PE formation, was downregulated throughout the first 24 h of development. Similarly, MXAN_5522, encoding a lipase homolog involved in DAG and TAG degradation, was also downregulated. This corresponds to the decreasing PE level (Fig. 2B) and the accumulation of TAGs (Fig. 2A) during development. Sphingolipid metabolism is largely unknown in myxobacteria. Only the initial step, which is catalyzed by a serine palmitoyltransferase, has been assigned to MXAN_3748 (26). While the gene is downregulated after 6 h of development, it is overexpressed at 15 and 18 h. Accordingly, the amount of ceramides in the lipid analysis increased after 18 and 24 h (Fig. 2C). We could not find other clear homologs for the sphingolipid pathway using KEGG and BLAST analysis. The cardiolipin and ether lipid biosynthesis genes were not clearly up- or downregulated. In addition to genes involved in lipid biosynthesis, we also investigated the expression levels of different lipases, which could play a role in lipid degradation (27). However, phospholipase D (MXAN_6753) was not included in the microarray, and the lipases MXAN_3852 (up to 3-fold upregulation) and MXAN_4569 (no regulation) were differently affected by regulation.
FIG 9.
Postulated lipid metabolism pathways during development of M. xanthus with incorporated microarray data (24). Gene information and pathways were obtained from KEGG. Gene upregulation (green) and downregulation (red) are indicated if they are ≥1.5. Black, genes that are not regulated; blue, genes not present in the microarray. Biosynthesis steps without a clear homolog in M. xanthus are indicated by a question mark. See Table S3 in the supplemental material for more information.
DISCUSSION
Membrane lipids are metabolized to storage lipids.
Our lipidome analysis strongly supports our hypothesis regarding the incorporation of membrane lipids into lipid bodies during myxobacterial development. As TAGs are not widespread among prokaryotes (28), most existing studies concerning bacterial lipids were performed on phospholipids (29). Our method enabled the identification of a range of phospholipids in myxobacteria broader than that described previously (20, 30) and made it possible to compare them to neutral lipids. During the morphological change from a vegetative rod to a round myxospore, the membrane surface area of the myxospore decreases to about 1/8 that of the rod (13). The amount of PEs in myxospores decreases to about 14% (≈1/7) of that in vegetative cells (Fig. 2B), closely reflecting this reduction. Developmental changes in the phospholipid profile were already described in myxobacteria (30) and also in other bacteria, like Pseudomonas aeruginosa (31). At the same time, the amount of TAGs first increased and subsequently dropped toward the end of development (Fig. 2A). DAGs, which are intermediates of TAGs, showed a similar profile (Fig. 2E). The increase in the amount of TAGs strongly suggests that they are first synthesized in order to serve as an intracellular carbon source in lipid bodies. The subsequent drop in TAG amounts indicates that lipid bodies are also released into the developing fruiting body (12) and might provide energy to developing cells. Accordingly, genes for fatty acid degradation are upregulated during sporulation (13). Further microarray data (Fig. 9; see also Table S3 in the supplemental material) support our analytical findings. Downregulation of MXAN_3724 might prevent the de novo production of PEs during development. In TAG metabolism, the downregulation of MXAN_5522, encoding a triacylglycerol lipase, might prevent the degradation of already formed TAGs. Another unsolved step is TAG biosynthesis. Here the best candidate for a diacylglycerol acyltransferase (DGAT) is MXAN_1127, which shows no phenotype regarding development or a lipid profile (12). However, it is already known that important pathways in myxobacteria have backup pathways, as shown for the formation of isovaleryl-CoA as an example (32). FAME analysis of the lipid samples confirms the conclusions drawn from whole-lipid analysis. The overall amount of fatty acids, represented by iso-15:0 MEs (Fig. 8), changes only during later phases of development. This strongly argues for a subsequent conversion of PEs and PGs into TAGs before fatty acids are used as an energy source for spore formation.
It is not surprising that during development the lipid profile of the esg mutant differed dramatically from that of the wild type, as it was severely disturbed in its lipid metabolism (Fig. 6). The mutation in the bkd locus prevents the formation of isovaleryl-CoA as a precursor for the biosynthesis of iso-fatty acids (9). Even though M. xanthus exhibits an alternative pathway via 3-hydroxy-3-methylglutaryl-CoA (22, 32, 33), which is upregulated during development, the total amount of iso-15:0 fatty acids is strongly decreased. In addition, ceramides with iso-17:0 fatty acids were less abundant in the esg mutant, as one would expect from the reduction in the level of starting units for the biosynthesis of iso-fatty acids in an esg mutant. The developmental fruA mutant also failed to degrade PEs and, later, TAGs to the wild-type level, indicating that the cells were not capable of lipid shortening or lipid body production, as was recently shown (13). From these data, it can be assumed that lipid body formation is indeed crucial for the proper development of myxospores in M. xanthus.
As previous studies have shown that cardiolipins account for only 1% of M. xanthus phospholipids, their amount in our samples was probably below the detection limit (20). However, the microarray data did not indicate any regulation of cardiolipin biosynthesis.
Ether lipid metabolism differs from general lipid metabolism in regulation and biosynthesis.
Analysis of the lipid body composition indicated that ether lipids, especially TG1, are the most abundant lipid species, followed by TAGs (12). TG1 is produced only during the sporulation of M. xanthus (8), has been shown to have signaling functions, and promotes the expression of a developmentally regulated gene (10). Therefore, it is possible that ether lipids, incorporated into lipid bodies and also released into the fruiting body, are used as communication signals. In principle, it could also be possible that the acyl moieties of these ether lipids are used as a carbon source to support sporulation and fruiting body formation and that the ether lipid moiety serves as the signal. The development of TG1 during sporulation clearly differs from that of all other TAGs (Fig. 3A), strongly supporting the independent biosynthesis of ether lipids, as has indeed been shown before (8). Surprisingly, MXAN_1528, encoding ElbD, the central enzyme in ether lipid biosynthesis (8), is not upregulated during development, while other genes from the elb operon are negatively regulated. Nevertheless, ether lipids are specifically produced during development, and therefore, an additional layer of regulation beyond transcription can be assumed for this important biosynthesis pathway highly conserved in myxobacteria. The homolog MXAN_1676, encoding alkyl dihydroxyacetone phosphate synthase, known from eukaryotic ether lipid biosynthesis, was not included in the microarray assay.
Preliminary studies have shown a constant increase in the level of ether lipids as a proportion of the overall amount of lipids in FAME analysis of samples during development (9). This is probably because the overall amount within the samples is decreasing even more. The new analysis with internal standards used in this work makes it possible to have a more precise view of the changing amounts of a single lipid species during development. As TG1 from the whole-lipid analysis (Fig. 3A) and iso-15:0 OAG from the FAME analysis showed nearly the same profile, ether lipids are presumably not incorporated into the myxospore wall in large amounts but are degraded in a so far unknown manner during spore formation. Additionally, this confirms that no covalent bonds between ether lipids and spore coat components (proteins or sugars) are present, which could have been the case in order to make a more robust spore coat.
Lipids are synthesized de novo during germination.
Previous studies (13), as well as our data, suggest that PEs are degraded during spore development, in accordance with a reduction of the membrane area. The germinating spores have to synthesize new PEs in order to regain the full membrane area of a vegetative cell. In contrast to the steadily increasing amounts of PEs, which are used for membrane synthesis, TAG biosynthesis started, between 4 and 6 h after the start of germination (Fig. 5). This is the time when the majority of myxospores have germinated in rich medium (34). Thus, the cells synthesized TAGs only after most spores had germinated, and these are probably used as intermediary storage for lipids or carbon, similar to the role of TAGs during fruiting body formation. Feeding experiments showed that fatty acids are synthesized de novo during germination (Fig. 7). As the available leucine in the germination medium consists of 50% fully deuterated l-leucine, the increase in the amount of D9-iso-15:0 MEs to nearly 50% of all iso-15:0 MEs after 12 h of germination indicates that branched-chain fatty acids are almost exclusively synthesized de novo and no recycling of spore lipid material takes place. Although this seems to be a waste of energy and carbon, germination in general takes place only under nutrient-rich conditions when no growth limitations are expected, and thus, this waste might be acceptable.
Ceramides are novel biomarkers for M. xanthus development.
Sphingolipids in myxobacteria have hardly been investigated. They are highly diverse in eukaryotes and play important roles in the survival and development of cells. Moreover, alterations in sphingolipid metabolism are the cause of several human diseases (35). Sphingolipids have also been shown to be involved in development in fungi (36), as an example of lower eukaryotic organisms. In myxobacteria, they have been described to be major lipids in the outer membrane of Sorangium cellulosum So ce56 (37) and as part of the lipidome of M. xanthus (5, 26). However, no specific function has yet been assigned to them. Serine palmitoyltransferases catalyze the initial step in sphingosine biosynthesis, and inactivation of the homologues in M. xanthus and Stigmatella aurantiaca did indeed show the expected loss of sphingolipids but no effect on vegetative growth or development (26). Microarray analysis indicated the upregulation of the M. xanthus homologue MXAN_3748, thus defining ceramides to be novel biomarkers in myxobacterial fruiting body formation. As their developmental profile (Fig. 4), especially that of Cer(d-19:0/iso-17:0), is very similar to the developmental profile of the TAGs (Fig. 2A), it is possible that their production is somehow connected to the formation of lipid bodies. However, more work regarding the biosynthesis of Cer-PIs and the biological function of Cers and Cer-PIs is required to elucidate the function of these typically eukaryotic lipids in myxobacteria in the future.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01537-15.
REFERENCES
- 1.Milne SB, Mathews TP, Myers DS, Ivanova PT, Brown HA. 2013. Sum of the parts: mass spectrometry-based metabolomics. Biochemistry 52:3829–3840. doi: 10.1021/bi400060e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers DS, Ivanova PT, Milne SB, Brown HA. 2011. Quantitative analysis of glycerophospholipids by LC-MS: acquisition, data handling, and interpretation. Biochim Biophys Acta 1811:748–757. doi: 10.1016/j.bbalip.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krank J, Murphy RC, Barkley RM, Duchoslav E, McAnoy A. 2007. Qualitative analysis and quantitative assessment of changes in neutral glycerol lipid molecular species within cells. Methods Enzymol 432:1–20. doi: 10.1016/S0076-6879(07)32001-6. [DOI] [PubMed] [Google Scholar]
- 4.Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, Chen Y, Merrill AH. 2007. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: “inside-out” sphingolipidomics. Methods Enzymol 432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzen W, Bozhüyük Kenan AJ, Cortina NS, Bode HB. 2014. A comprehensive insight into the lipid composition of Myxococcus xanthus by UPLC-ESI-MS. J Lipid Res 55:2620–2633. doi: 10.1194/jlr.M054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zusman DR, Scott AE, Yang Z, Kirby JR. 2007. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol 5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 7.Kuner JM, Kaiser D. 1982. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol 151:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzen W, Ahrendt T, Bozhüyük Kenan AJ, Bode HB. 2014. A multifunctional enzyme is involved in bacterial ether lipid biosynthesis. Nat Chem Biol 10:425–427. doi: 10.1038/nchembio.1526. [DOI] [PubMed] [Google Scholar]
- 9.Ring MW, Schwär G, Thiel V, Dickschat JS, Kroppenstedt RM, Schulz S, Bode HB. 2006. Novel iso-branched ether lipids as specific markers of developmental sporulation in the myxobacterium Myxococcus xanthus. J Biol Chem 281:36691–36700. doi: 10.1074/jbc.M607616200. [DOI] [PubMed] [Google Scholar]
- 10.Bhat S, Ahrendt T, Dauth C, Bode HB, Shimkets LJ. 2014. Two lipid signals guide fruiting body development of Myxococcus xanthus. mBio 5(1):e00939-13. doi: 10.1128/mBio.00939-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearns DB, Shimkets LJ. 2001. Lipid chemotaxis and signal transduction in Myxococcus xanthus. Trends Microbiol 9:126–129. doi: 10.1016/S0966-842X(01)01948-5. [DOI] [PubMed] [Google Scholar]
- 12.Hoiczyk E, Ring MW, McHugh CA, Schwär G, Bode E, Krug D, Altmeyer MO, Lu JZ, Bode HB. 2009. Lipid body formation plays a central role in cell fate determination during developmental differentiation of Myxococcus xanthus. Mol Microbiol 74:497–517. doi: 10.1111/j.1365-2958.2009.06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhat S, Boynton TO, Pham D, Shimkets LJ. 2014. Fatty acids from membrane lipids become incorporated into lipid bodies during Myxococcus xanthus differentiation. PLoS One 9:e99622. doi: 10.1371/journal.pone.0099622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nudleman E, Wall D, Kaiser D. 2005. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science 309:125–127. doi: 10.1126/science.1112440. [DOI] [PubMed] [Google Scholar]
- 15.Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, Wall D. 2012. Cell contact-dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet 8:e1002626. doi: 10.1371/journal.pgen.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bode HB, Ring MW, Kaiser D, David AC, Kroppenstedt RM, Schwär G. 2006. Straight-chain fatty acids are dispensable in the myxobacterium Myxococcus xanthus for vegetative growth and fruiting body formation. J Bacteriol 188:5632–5634. doi: 10.1128/JB.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Søgaard-Andersen L, Slack FJ, Kimsey H, Kaiser D. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev 10:740–754. doi: 10.1101/gad.10.6.740. [DOI] [PubMed] [Google Scholar]
- 18.Kroos L, Kuspa A, Kaiser D. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol 117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 19.Bretscher AP, Kaiser D. 1978. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol 133:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orndorff PE, Dworkin M. 1980. Separation and properties of the cytoplasmic and outer membranes of vegetative cells of Myxococcus xanthus. J Bacteriol 141:914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downard J, Toal D. 1995. Branched-chain fatty acids: the case for a novel form of cell-cell signalling during Myxococcus xanthus development. Mol Microbiol 16:171–175. doi: 10.1111/j.1365-2958.1995.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Luxenburger E, Müller R. 2013. An alternative isovaleryl CoA biosynthetic pathway involving a previously unknown 3-methylglutaconyl CoA decarboxylase. Angew Chem Int Ed Engl 52:1304–1308. doi: 10.1002/anie.201207984. [DOI] [PubMed] [Google Scholar]
- 23.Mahmud T, Bode HB, Silakowski B, Kroppenstedt RM, Xu M, Nordhoff S, Höfle G, Müller R. 2002. A novel biosynthetic pathway providing precursors for fatty acid biosynthesis and secondary metabolite formation in myxobacteria. J Biol Chem 277:32768–32774. doi: 10.1074/jbc.M205222200. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Wegener-Feldbrügge S, Huntley S, Hamann N, Hedderich R, Søgaard-Andersen L. 2008. Bioinformatics and experimental analysis of proteins of two-component systems in Myxococcus xanthus. J Bacteriol 190:613–624. doi: 10.1128/JB.01502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Eisen J, Ronning CM, Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS, Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M, Wiegand R, Kaplan HB. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci U S A 103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ring MW, Schwär G, Bode HB. 2009. Biosynthesis of 2-hydroxy and iso-even fatty acids is connected to sphingolipid formation in myxobacteria. Chembiochem 10:2003–2010. doi: 10.1002/cbic.200900164. [DOI] [PubMed] [Google Scholar]
- 27.Moraleda-Muñoz A, Shimkets LJ. 2007. Lipolytic enzymes in Myxococcus xanthus. J Bacteriol 189:3072–3080. doi: 10.1128/JB.01772-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez HM, Steinbüchel A. 2002. Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376. doi: 10.1007/s00253-002-1135-0. [DOI] [PubMed] [Google Scholar]
- 29.Sohlenkamp C, Geiger O. 10 April 2015. Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol Rev. doi: 10.1093/femsre/fuv008. [DOI] [PubMed] [Google Scholar]
- 30.Curtis PD, Geyer R, White DC, Shimkets LJ. 2006. Novel lipids in Myxococcus xanthus and their role in chemotaxis. Environ Microbiol 8:1935–1949. doi: 10.1111/j.1462-2920.2006.01073.x. [DOI] [PubMed] [Google Scholar]
- 31.Benamara H, Rihouey C, Abbes I, Ben Mlouka MA, Hardouin J, Jouenne T, Alexandre S. 2014. Characterization of membrane lipidome changes in Pseudomonas aeruginosa during biofilm growth on glass wool. PLoS One 9:e108478. doi: 10.1371/journal.pone.0108478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bode HB, Ring MW, Schwär G, Altmeyer MO, Kegler C, Jose IR, Singer M, Müller R. 2009. Identification of additional players in the alternative biosynthesis pathway to isovaleryl-CoA in the myxobacterium Myxococcus xanthus. Chembiochem 10:128–140. doi: 10.1002/cbic.200800219. [DOI] [PubMed] [Google Scholar]
- 33.Dickschat JS, Bode HB, Kroppenstedt RM, Müller R, Schulz S. 2005. Biosynthesis of iso-fatty acids in myxobacteria. Org Biomol Chem 3:2824–2831. doi: 10.1039/b504889c. [DOI] [PubMed] [Google Scholar]
- 34.Otani M, Inouye M, Inouye S. 1995. Germination of myxospores from the fruiting bodies of Myxococcus xanthus. J Bacteriol 177:4261–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolter T. 2011. A view on sphingolipids and disease. Chem Phys Lipids 164:590–606. doi: 10.1016/j.chemphyslip.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Oura T, Kajiwara S. 2010. Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology 156:1234–1243. doi: 10.1099/mic.0.033985-0. [DOI] [PubMed] [Google Scholar]
- 37.Keck M, Gisch N, Moll H, Vorhölter F-J, Gerth K, Kahmann U, Lissel M, Lindner B, Niehaus K, Holst O. 2011. Unusual outer membrane lipid composition of the gram-negative, lipopolysaccharide-lacking myxobacterium Sorangium cellulosum So ce56. J Biol Chem 286:12850–12859. doi: 10.1074/jbc.M110.194209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.