Abstract
Mouse models are widely used for studying gastrointestinal (GI) tract-related diseases. It is necessary and important to develop a new set of primers to monitor the mouse gut microbiota. In this study, 16S rRNA gene-targeted group-specific primers for Firmicutes, Actinobacteria, Bacteroidetes, Deferribacteres, “Candidatus Saccharibacteria,” Verrucomicrobia, Tenericutes, and Proteobacteria were designed and validated for quantification of the predominant bacterial species in mouse feces by real-time PCR. After confirmation of their accuracy and specificity by high-throughput sequencing technologies, these primers were applied to quantify the changes in the fecal samples from a trinitrobenzene sulfonic acid-induced colitis mouse model. Our results showed that this approach efficiently predicted the occurrence of colitis, such as spontaneous chronic inflammatory bowel disease in transgenic mice. The set of primers developed in this study provides a simple and affordable method to monitor changes in the intestinal microbiota at the phylum level.
INTRODUCTION
More than 90% of the 100 trillion cells in the human body are microbes, most of which reside in intestines and are collectively known as the intestinal microbiota (1). The intestinal microbiota plays a key role in immune system maturation, food digestion, drug metabolism, detoxification, and prevention of pathogenic bacterial adhesion (2). Disruption of the intestinal microbiota has been demonstrated in patients suffering from inflammatory bowel disease (IBD), asthma, obesity, liver disease, and diabetes (1, 3). Increasing evidence suggests that the intestinal microbiota play a role in initiating, maintaining, and determining the phenotype of IBD (3). To understand the relationship between the intestinal microbiota and IBD, it is important to develop a sensitive and accurate molecular detection method to analyze various microbial populations.
A variety of methods are available to quantify microbiota of the gut. Traditionally, culture-based techniques were used to determine the composition of the gut microbiota, but only 10 to 50% of gut bacteria are culturable (4). 16S rRNA gene-targeted oligonucleotide probes have been used with fluorescent in situ hybridization as a culture-independent method (5, 6). The 16S rRNA gene is a suitable marker gene for quantification of microbiota on taxonomic and phylogenetic levels. Quantitative real-time PCR with 16S rRNA gene-based specific primers has been utilized as a sensitive and rapid method to quantify intestinal microbiota (7–10). Now, high-throughput sequencing (HTS) technologies have been used widely to examine the complexity of the gut microbiota due to the speed, scale, and precise information it can provide (11). However, quantitative PCR (qPCR) can also be a good choice for analyzing microbial communities due to the relatively small amount of template required, high sensitivity, high-throughput processing, and affordable cost (12).
Real-time PCR has been used successfully to quantify specific bacterial groups and species from intestinal mucosa and stools. To date, phylum- and group-specific primers for Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria have been developed and applied to the analysis of the intestinal microbiota (7–9, 13). In the present study, we developed a new set of primers to detect the Actinobacteria, Bacteroidetes, Deferribacteres, “Candidatus Saccharibacteria,” Verrucomicrobia, Tenericutes, and Proteobacteria (including beta, epsilon, and gamma classes) in the gut. The microbiota from fecal samples of C57BL/6 mice were analyzed by qPCR with these primers. The accuracy of qPCR assay was validated by HTS. In order to demonstrate its applicability and effectiveness, changes in the intestinal microbiota in mouse fecal samples from mice with trinitrobenzene sulfonic acid (TNBS)-induced colitis and spontaneous colitis were also examined.
MATERIALS AND METHODS
Design of 16S rRNA gene-targeted group-specific primers.
According to the dominating bacterial microbiota of mouse feces, listed in Table S1 in the supplemental material (14), we designed a set of primers for testing bacteria, including Actinobacteria, Bacteroidetes, Deferribacteres, “Candidatus Saccharibacteria,” Verrucomicrobia, Tenericutes, and the beta, epsilon, and gamma subdivisions of Proteobacteria. In order to develop phylum- and class-specific primers, between 20 and 30 16S rRNA sequences of each dominant genus of the target taxonomic group were randomly downloaded from the Ribosomal Database Project II (RDP-II) and grouped into Fasta files (12, 15). Sequences from each taxon were clustered using ClustalX (16), and consensus sequences were obtained using BioEdit (17). The consensus sequences were aligned with the Multalin program (http://bioinfo.genopole-toulouse.prd.fr/multalin/multalin.html). The alignments of these consensus sequences (see Fig. S1 in the supplemental material) were visually inspected to design primers. It is known that nucleotide mismatches at the primer's 3′ termini are extended by DNA polymerases with lower efficiency than correctly matched. Therefore, primers were designed to possess the taxon-specific nucleotide(s) at the 3′ end (12, 18). Primers were assessed in silico using the tool “probematch” from the RDP-II and compared to available 16S rRNA gene sequences by using the NCBI BLAST database search program (http://www.ncbi.nlm.nih.gov/BLAST/). Primer pairs of each target group were developed based on in silico comparisons and reserved for further validation (Table 1).
TABLE 1.
16S rRNA gene-targeted group-specific primers used in this study
| Target group | Primera | Sequence (5′–3′)b | Amplicon length (bp) | Source or reference |
|---|---|---|---|---|
| Bacteroidetes | Bac960F | GTTTAATTCGATGATACGCGAG | 122 | This study |
| Bac1100R | TTAASCCGACACCTCACGG | 122 | This study | |
| Firmicutes | Firm934F | GGAGYATGTGGTTTAATTCGAAGCA | 126 | 9 |
| Firm1060R | AGCTGACGACAACCATGCAC | 126 | 9 | |
| Actinobacteria | Act664F | TGTAGCGGTGGAATGCGC | 277 | This study |
| Act941R | AATTAAGCCACATGCTCCGCT | 277 | This study | |
| “Candidatus Saccharibacteria” | Sac1031F | AAGAGAACTGTGCCTTCGG | 187 | This study |
| Sac1218R | GCGTAAGGGAAATACTGACC | 187 | This study | |
| Deferribacteres | Defer1115F | CTATTTCCAGTTGCTAACGG | 150 | This study |
| Defer1265R | GAGHTGCTTCCCTCTGATTATG | 150 | This study | |
| Verrucomicrobia | Ver1165F | TCAKGTCAGTATGGCCCTTAT | 97 | This study |
| Ver1263R | CAGTTTTYAGGATTTCCTCCGCC | 97 | This study | |
| Tenericutes | Ten662F | ATGTGTAGCGGTAAAATGCGTAA | 200 | This study |
| Ten862R | CMTACTTGCGTACGTACTACT | 200 | This study | |
| Betaproteobacteria | Beta979F | AACGCGAAAAACCTTACCTACC | 174 | This study |
| Beta1130R | TGCCCTTTCGTAGCAACTAGTG | 174 | This study | |
| Epsilonproteobacteria | Epsilon940F | TAGGCTTGACATTGATAGAATC | 189 | This study |
| Epsilon1129R | CTTACGAAGGCAGTCTCCTTA | 189 | This study | |
| Delta- and Gammaproteobacteria | Gamma877F | GCTAACGCATTAAGTRYCCCG | 189 | This study |
| Gamma1066R | GCCATGCRGCACCTGTCT | 189 | This study | |
| Universal | 926F | AAACTCAAAKGAATTGACGG | 136 | 12 |
| 1062R | CTCACRRCACGAGCTGAC | 136 | 12 | |
| Bacterial 16S rRNA | 27F | AGAGTTTGATCCTGGCTCAG | 18 | |
| 1525R | AAGGAGGTGWTCCARCC | 18 |
Numbers within the primer name indicate the nucleotide position based on the Escherichia coli ATCC 11775T 16S rRNA gene.
Nucleotide symbols: R = A or G; Y = C or T; N = any nucleotide; W = A or T; M = A or C; K = T or G; S = C or G; and H = A/C/T.
Primer specificity.
16S rRNA gene sequences amplified from total fecal DNA using the primers 27F and 1525R (19) were cloned into the pMD18-T vector. After genotyping by partial 16S rRNA sequencing, 38 clones (see Table S2 in the supplemental material) with 16S rRNA gene sequences belonging to different taxa (EMBL accession numbers KP713718 to KP713755) were used as the templates to test the specificity of the group target primers. The annealing temperature that maximized primer specificity in vitro was determined by using the target and nontarget clones described above as the templates, with annealing temperatures ranging from 55 to 65°C (12). The PCR conditions were one initial denaturing step of 3 min at 94°C, 30 cycles of 95°C for 20 s, gradient annealing for 20 s and 72°C for 20 s, and a final elongation step at 72°C for 5 min. Every PCR mixture contained 0.1 U/μl of Taq polymerase (TaKaRa, Dalian, China), 0.25 mM concentrations of each deoxynucleoside triphosphate, 0.5 μM concentrations of each primer, 1× buffer, ∼50 ng of DNA, and water to 50 μl. A minimum of two target and the remaining nontarget clones were used to test each primer pair, and the specificity was claimed when only target DNA was amplified (Table 2); the PCR products were determined by standard gel electrophoresis. Through optimization of the PCR annealing temperature, it was found that 60°C maximized specificity of all pairs (Table 2). The specificity of each primer pair was also verified by qPCR and inferred from the shift of the threshold cycle (CT), obtained by amplifying target and nontarget sequences for comparison. Real-time PCRs were carried out in 96-well optical plates on an ABI StepOne plus real-time PCR system sequence detector with 2×FastStart SYBR green mix (Vazyme, Nanjing, China). All qPCR mixtures contained 10 μl of 2×FastStart SYBR green with dye1, 0.5 μl of each forward and reverse primer (final concentration, 0.4 μM), and 9 μl of the DNA template (equilibrated to 10 ng).The PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Melting-curve analysis was performed after amplification. The CT values and baseline settings were determined by automatic analysis settings (9). qPCR products were selected at random and cloned into the pMD18-Tvector (TaKaRa) for sequencing confirmation.
TABLE 2.
Testing the specificity of the group target primers and optimization of PCR annealing temperature
| Target group | Primer | Tm (°C) | Template(s) | PCR resulta |
|---|---|---|---|---|
| Bacteroidetes | Bac960F | 60 | Clones 29, 33, 34, and 35 | + |
| Bac1100R | The remaining clones | – | ||
| Firmicutes | Firm934F | 60 | Clones 1, 2, 3, 23, 28, 31, and 32 | + |
| Firm1060R | The remaining clones | – | ||
| Actinobacteria | Act664F | 60 | Clones 26 and 27 | + |
| Act941R | The remaining clones | – | ||
| “Candidatus Saccharibacteria” | Sac1031F | 60 | Clones 24 and 25 | + |
| Sac1218R | The remaining clones | – | ||
| Deferribacteres | Defer1115F | 60 | Clones 7, 8, and 9 | + |
| Defer1265R | The remaining clones | – | ||
| Verrucomicrobia | Ver1165F | 60 | Clones 21 and 22 | + |
| Ver1263R | The remaining clones | – | ||
| Tenericutes | Ten662F | 60 | Clones 16, 17, and 36 | + |
| Ten862R | The remaining clones | – | ||
| Betaproteobacteria | Beta979F | 60 | Clones 4, 5, 6, 18, 19, and 20 | + |
| Beta1130R | The remaining clones | – | ||
| Epsilonproteobacteria | Epsilon940F | 60 | Clones 13, 14, 15, 30, 37, and 38 | + |
| Epsilon1129R | The remaining clones | – | ||
| Delta- and Gammaproteobacteria | Gamma877F | 60 | Clones 10, 11, and 12 | + |
| Gamma1066R | The remaining clones | – | ||
| Universal | 926F | 60 | All of the clones | + |
| 1062R |
+, positive; –, negative.
Primer amplification efficiencies.
The primer amplification efficiencies were determined by standard procedure: making dilution series of fecal total DNA, calculating a linear regression based on the CT data points, and inferring the efficiency from the slope of the line. Serial dilutions of 1, 1:4, 1:16, 1:64, and 1:256 were used (12). Each dilution point and primer pair was tested three times. In order to confirm that the CT value generated by the lowest concentrated DNA was not an artifact, a nontemplate control was included in each assay. Lastly, CT data were uploaded to an Excel spreadsheet, and the resulting efficiency graphs are shown in Fig. S2 in the supplemental material.
Quantitative analysis of synthetic communities of 16S rRNA genes.
The real-time PCR protocol was validated by constructing an artificial mixture of 16S rRNA genes in a similar way to published approaches (12, 20). Briefly, equal amounts of plasmid containing the 16S rRNA gene sequence for each taxon investigated were mixed to simulate the artificial communities of 16S rRNA genes, which were then used as the template for qPCR validation experiments. The plasmids were selected from these 38 clones. The average CT value obtained from each primer pair was transformed into a percentage using the following formula (12):
where Eff.Univ is the calculated efficiency of the universal primers (2 = 100% and 1 = 0%), and Eff.Spec refers to the efficiency of the taxon-specific primers. CTuniv and CTspec are the CT values registered by the thermocycler. “X” represents the percentage of 16S taxon-specific copy number existing in a sample (12). All of the fecal samples were analyzed with the qPCR assay, and the CT values were used to calculate the proportion of higher bacterial taxa in the feces.
Fecal sample collection and DNA extraction.
All animal care procedures were approved by the Institutional Animal Care and Use Committee of Nanjing University prior to initiation of the experiment. C57BL/6 mice aged 8 to 10 weeks were housed in a standard animal laboratory with a 12-h light-dark cycle and were fed a standard diet. The feces samples were collected within 2 h when mice were transferred to fresh sterilized cages. After the fecal samples were thoroughly frozen in liquid nitrogen, they were then stored at −80°C until DNA extraction (14). The total genomic DNA from each fecal sample (100 mg) was extracted (14) using the QIAamp DNA stool minikit (Qiagen, Germany) according to the manufacturer's instructions. Absorbance ratios at 260/280 nm and at 260/230 nm were determined to quantify and assess the purity of DNA samples.
Quantitative analysis of natural bacterial communities.
Six wild-type C57BL/6 mice were housed separately for feces collection. The total genomic DNA from each fecal sample (100 mg) was extracted using a QIAamp DNA stool minikit according to the manufacturer's instructions, resuspended in 100 μl of TE buffer (pH 8.0), and quantified with an Eppendorf biophotometer. These samples were analyzed with the qPCR assay, and the CTs were used to calculate the proportion of higher bacterial taxa in the feces, as described above.
Deep sequencing of 16S rRNA genes.
Total genomic DNA from fecal samples (100 mg) was extracted using the QIAamp DNA stool minikit according to the manufacturer's instructions. The forward primer (5′-CCTACGGGNGGCWGCAG-3′) and the reverse primer (5′-TACNVGGGTATCTAATCC-3′) containing the A and B sequencing adaptors (454 Life Sciences) were used to amplify a region covering the V3-V4 region of the 16S rRNA gene (14). PCRs were carried out in triplicate using 25-μl reaction mixtures with 0.5 μM concentrations of each primer, 20 to 50 ng of template DNA, 5 μl of the PCR buffer, and 2.5 U of DNA polymerase. The amplification program consisted of an initial denaturation step at 94°C for 5 min, followed by 25 cycles of 94°C for 10 s (denaturation), 55°C for 10 s (annealing), and 72°C for 15 s (extension), and then a final extension of 72°C for 10 min (14). Replicated PCR products of the same sample were mixed and purified with a DNA gel extraction kit. Prior to sequencing, the DNA concentration of each PCR product was determined using a Quant-iTPicoGreen double-stranded DNA assay (Invitrogen, USA) and was quality controlled on an Agilent 2100 bioanalyzer (Agilent, USA). A master DNA pool was generated from the purified products in equimolar ratios. The pooled products were sequenced using a Roche 454 Titanium pyrosequencer at BGI Shenzhen Science and Technology Co., Ltd., Shenzhen, China.
Sequence processing and bioinformatics analysis.
Pyrosequencing reads with more than one ambiguous nucleotide within correct barcodes or the length of the reads outside the range of 200 bp and 1,000 bp were removed and excluded from further analysis. Sequences that passed quality filtering using the default parameters in QIIME were checked for chimeras (21) and assigned to operational taxonomic units (OTU) using mothur (22). Sets of sequences with ≥97% identity were defined as an OTU. OTU were assigned to a taxonomy using an RDP naïve Bayesian classifier (23). Species analysis of every OTU was based on the reads classified by the RDP. If >51% of the reads in an OTU belonged to the same taxon (such as a genus), then that OTU was considered to belong to that taxon. The number of effective reads in every OTU was counted, and the effective reads of every species were generalized. These analyses were used to reveal the changes of bacterial communities in fecal samples from mice with IBD compared to those from wild-type mice. Most of the sequence analysis was completed by BGI.
Colitis model in mice.
TNBS-induced colitis is a classical model widely used in the research of IBD (24). A total of 24 male mice aged 8 weeks were divided into four groups and fasted but were allowed free access to water overnight prior to the induction of colitis. The animals were anesthetized with a 50% (vol/vol) mixture of ketamine and xylazine (100 mg/ml) diluted in saline (0.9% [wt/vol] NaCl) at the dose of 1 ml/kg. TNBS (Sigma-Aldrich, USA) diluted in 50% (vol/vol) ethanol was injected via a polyethylene catheter inserted at 4 cm from the anus at a dose of 100 mg/kg in 25 μl to induce an experimental colitis (13). When the colitis was the most serious, at day 3, the feces were collected according to the method described above. The mice were then sacrificed, and their colons were prepared for hematoxylin and eosin (H&E) staining. Spontaneous chronic colitis that occurred in FADD (Fas-associated death domain-containing protein)-deficient mice was described by Welz et al. (25). We constructed a series of FADD mutant mice. The feces were collected from these transgenic mice at about 8 weeks of age. By using a qPCR assay to screen their feces samples, it was determined that one of them, designated TgD, showed a significant alteration in intestinal microbiota. To verify the presence of chronic IBD, the mouse was sacrificed, and its colon was assessed for microscopic examination and H&E staining (26).
Microarray data accession numbers.
Newly determined sequence data were deposited in the Sequence Read Archive (SRA) under accession numbers SRX862855 and SRX863943.
RESULTS
Newly designed qPCR assay targeting bacterial taxa.
To analyze the predominant bacteria in mouse feces, we designed a set of phylum- and class-specific primers for testing Actinobacteria, Bacteroidetes, Deferribacteres, “Candidatus Saccharibacteria,” Verrucomicrobia, Tenericutes, and the beta, epsilon, and gamma subdivisions of Proteobacteria. The primers were optimized by in silico analyses in the RDP database to maximize the theoretical specificity and universality of the newly designed primers. The primers were modified to function at the same annealing temperatures (60°C), allowing us to perform all PCRs simultaneously in the same thermocycler. The specificity of each primer set was validated with plasmids containing target or nontarget 16S rRNA sequences as the template, and the primer pairs were specific for their target taxa at the appropriate annealing temperatures and yielded the PCR products of the expected size only from their targets (Table 2). In real-time PCR analyses, all of the assays were specific for the target group, and the CT values obtained with nontarget 16S rRNA sequences were close to the CT values of the no-template control (see Fig. S3 in the supplemental material). Further testing was carried out using total stool DNA as the template, and qPCR products of each primer pairs were determined by gel electrophoresis (Fig. 1). Each specific primer yielded the expected PCR product for the corresponding target bacteria 16S rRNA without any unexpected results, and sequencing of the PCR products confirmed the identity of the corresponding target bacteria. qPCR products were cloned into pMD18-T vector, and 20 clones were picked randomly for sequencing. Amplification products belonging to the target group defined as targeted rate are listed in Table 3. Most of the primer pairs had a very high targeting efficiency; the lower targeted rate with the primer pairs Act664F/Act941R and Gamma877F/Gamma1066R also reached 90%. After we confirmed the specificity, we validated the qPCR assay in artificial mixes of 16S rRNA sequences (data not shown). The amplification efficiency of each primer pair was determined by using the ΔCT method (Table 3), which provided an estimate of the proportion of 16S rRNA copies belonging to each phylum, as described above. After optimization and evaluation, a real-time PCR was used to detect the microbiota composition in mouse feces.
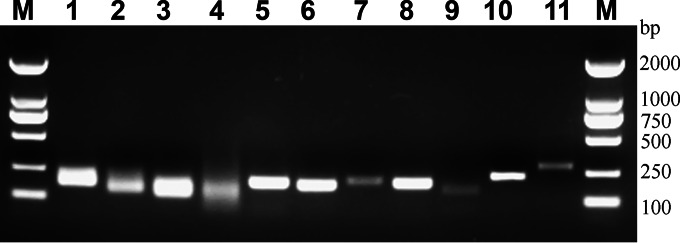
FIG 1.
Specificity of qPCR amplification with new sets of primer pairs. QPCR products amplified from the mouse fecal DNA with group-target primers as follows: universal (lane 1), Bacteroidetes (lane 2), Firmicutes (lane 3), Verrucomicrobia (lane 4), “Candidatus Saccharibacteria” (lane 5), Betaproteobacteria (lane 6), Deltaproteobacteria and Gammaproteobacteria (lane 7), Epsilonproteobacteria (lane 8), Deferribacteres (lane 9), Tenericutes (lane 10), Actinobacteria (lane 11); DNA markers indicated in base pairs (lane M).
TABLE 3.
Specificity test of phylum- and class-specific primers by qPCR
| Target group | Primer pair | Specificitya (%) | Efficiencyb (%) | Taxon-specific nucleotide position(s)c | Amplicon sequences belonging to the target groupd (%) |
|---|---|---|---|---|---|
| Bacteroidetes | Bac960F/Bac1100R | 99.2 | 98.5 | 0 and 8/* | 100 |
| Firmicutes | Firm934F/Firm1060R | 99.6 | 94.6 | 0/* | 100 |
| Actinobacteria | Act664F/Act941R | 99.4 | 92.7 | 0 and 7/0 and 2 | 90 |
| “Candidatus Saccharibacteria” | Sac1031F/Sac1218R | 99.1 | 94.6 | 0–3/* | 100 |
| Deferribacteres | Defer1115F/Defer1265R | 99.4 | 92.6 | */* | 100 |
| Verrucomicrobia | Ver1165F/Ver1263R | 98.7 | 93.2 | */* | 100 |
| Tenericutes | Ten662F/Ten862R | 95.0 | 96.5 | 0 and 10/0 and 2 | 100 |
| Betaproteobacteria | Beta979F/Beta1130R | 98.4 | 94.2 | 0–2/* | 95 |
| Epsilonproteobacteria | Epslion940F/Epsloin1129R | 99.7 | 92.1 | */* | 100 |
| Delta- and Gammaproteobacteria | Gamma877F/Gamma1066R | 76.4 | 96.1 | 0 and 3/* | 90 |
| Universal | 926F/1062R | 99.1 |
The percentage matches within target groups obtained by using the online tool Probe Match within the RDP-II database.
The amplification efficiency of the primer uses the fecal DNA as the template.
The distance of a specific nucleotide(s) from the 3′ end of the primer is reported, with 0 being the nucleotide at the 3′ termini. *, the specificity is given by a combination rather than a single nucleotide.
That is, the percentage of qPCR products belonging to the target group based on sequencing and classified by using the RDP-II database.
Quantification of bacterial groups in intestinal microbiota.
To evaluate the ability of the qPCR assay to resolve the composition of complex bacterial communities, fecal samples from healthy mice were collected to detect the distribution of the predominant bacterial groups in fecal microbiota. The same sample was divided into two parts: one for the qPCR assay described here and another for HTS (Sequence Read Archive accession number SRX862855). Upon comparison of the qPCR assay to 16S rRNA gene HTS in detection of fecal samples, the relative proportions of bacteria were found to be largely similar, as shown in Table 4. Since there was a certain deviation between the qPCR assay and HTS, we included data from an earlier study (27). The proportion of Bacteroidetes was slightly lower with HTS, whereas the percentage of Deferribacteres was higher with the qPCR method. Overall, this qPCR assay can be successfully used to determine the relative abundance of fecal microbiota at the phylum level.
TABLE 4.
Comparison of bacterial populations in fecal samples from C57BL/6 mice as determined by qPCR and HTSa
| Target group | Mean ± SD |
Pd |
|||
|---|---|---|---|---|---|
| qPCRa (%) | HTSb (%) | HTSc (%) | qPCR vs HTS | HTS vs HTS | |
| Bacteroidetes | 77.26 ± 6.64 | 81.50 ± 2.7 | 62.92 ± 2.85 | 0.33 | * |
| Actinobacteria | 0.13 ± 0.11 | 0.20 ± 0.20 | 0.11 ± 0.03 | 0.51 | 0.28 |
| Firmicutes | 13.95 ± 6.02 | 13.97 ± 2.8 | 20.73 ± 1.21 | 0.99 | * |
| Deferribacteres | 0.44 ± 0.54 | 0.01 ± 0.01 | 0.48 ± 0.20 | 0.22 | 0.06 |
| “Candidatus Saccharibacteria” | 0.42 ± 0.24 | 0.27 ± 0.11 | 0.13 ± 0.06 | 0.35 | * |
| Tenericutes | 0.78 ± 0.44 | 0.87 ± 0.15 | 6.77 ± 2.45 | 0.74 | * |
| Verrucomicrobia | 0.37 ± 0.34 | 0.03 ± 0.05 | 0.04 ± 0.01 | 0.14 | 0.63 |
| Proteobacteria | 2.78 ± 0.41 | 2.43 ± 0.06 | 2.83 ± 0.60 | 0.19 | 0.14 |
| Other | 3.63 ± 2.16 | 0.97 ± 0.40 | 6.00 ± 0.61 | 0.13 | * |
Wild-type C57BL/6 mouse fecal samples were collected from 8-week-old mice in our study (n = 6).
Wild-type C57BL/6 mouse fecal samples were collected from 8-week-old mice in our study (n = 3).
These results were obtained from Carvalho et al. (27). The mice were 12 weeks old (n = 6).
P value analysis was done using ANOVA. Statistical significance (P < 0.05) is denoted by an asterisk (*).
Microbiota composition analysis of the TNBS model in mice.
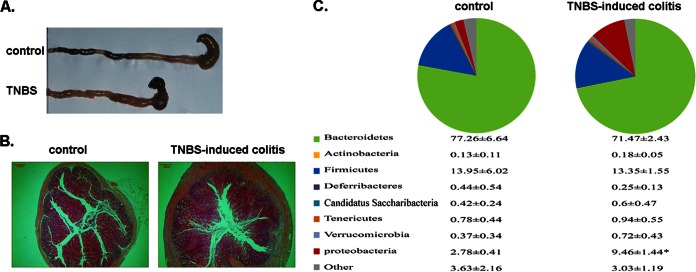
Disruption of the intestinal microbiota has been demonstrated to be associated with the occurrence of enteritis. To prove that this qPCR method can be effectively applied to analysis of changes of microbial populations under pathological conditions, we established a classical model of TNBS-induced colitis in mice. Model mice were examined for various inflammatory indices, such as the typical features of acute enteritis, including a shortened but thickened colon (Fig. 2A). Figure 2B shows a representative H&E-stained colon with mucosal ulceration, submucosal edema, and inflammatory cell infiltration. Fecal samples from TNBS model mice or control mice were collected, and the relative abundance of bacteria was calculated from the CT values as described above. The composition ratios of the target bacteria are reported as means ± the standard deviations (SD) (Fig. 2C). To more clearly and directly reflect the change of predominate bacteria, the characterization of communities was also shown in a pie chart (Fig. 2C). Compared to that in control mice, the proportion of Bacteroidetes was stable, whereas the subdivisions of Proteobacteria in the TNBS model increased significantly from 2.78% up to 9.46% (P < 0.05). This increase in the relative abundance of proteobacteria was in agreement with previous findings that the presence of Enterobacteriaceae correlates with disease activity in mice (27, 28). The results suggest that the qPCR assay can be used to analyze changes of gut microbiota.
FIG 2.
Microbiota composition analysis of a TNBS model in mice. (A) Gross picture of colons from two groups of model mice. (B) Histopathologic changes in individual colons (magnification, ×40) were shown in representative H&E-stained sections. Epithelial damage and leukocyte infiltration was observed in the TNBS model group. (C) Fecal microbiota detected by qPCR. The proportions of predominant bacterial groups are presented as means ± SDs (n = 6), analysis of variance (ANOVA) was performed, and statistical significance is denoted by an asterisk (*, P < 0.05). The relative abundances of phyla in each of the sample groups are indicated in the upper panel.
Application on the screening of chronic enteritis.
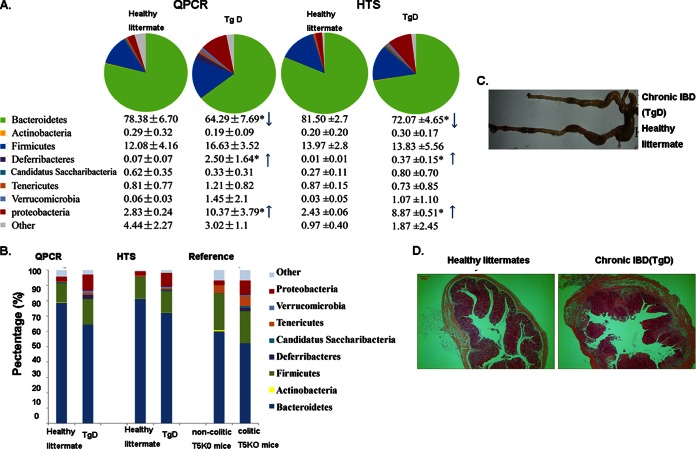
Since changes in microbe populations are closely related to IBD, the qPCR assay was a simple and quick method to assess the enteritis sample by analyzing the shifts in the predominant microbiota community. We applied our qPCR to predict chronic inflammation in transgenic mice. Fecal samples were collected from different transgenic mice prepared by our lab. Compared to samples from healthy mice, the fecal samples from TgD mice exhibited a distinct shift in microbiota composition, as presented in Fig. 3. The proportion of Bacteroidetes was significantly decreased (P < 0.05), whereas the proportions of Deferribacteres and the subdivisions of Proteobacteria were significantly increased (P < 0.05) (Fig. 3A) in TgD mice compared to those in control mice. We further performed HTS and obtained results similar to those obtained by using qPCR (SRA accession number SRX863943) with regards to the proportions of Bacteroidetes, Deferribacteres, and Proteobacteria (Fig. 3A). This distribution of intestinal microbiota was observed in IL-10−/− or TLR-5−/− mice (27, 29, 30), and a comparison of data is presented in Fig. 3B, implying the existence of spontaneous enteritis in TgD mice. We next determined whether TgD mice develop colitis by assessing several well-defined indicators of this disease state. Compared to healthy C57/BL6 mice, TgD mice displayed gross features of severe colitis, including contracted ceca, swelling, and the absence of well-formed feces (Fig. 3C). Moreover, mouse colonic specimens assessed by H&E staining demonstrated moderate mucosal and submucosal inflammation (Fig. 3D). Therefore, real-time PCR detection with our designed primer set can be applied successfully on the screening, tracking, and evaluation for IBD and related diseases.
FIG 3.
Abnormal stool microbiota composition correlates with chronic colitis development in mice. (A) Community composition of chronic inflammation in transgenic mice (TgD). Samples were collected from mouse feces for qPCR and HTS detection. For all 8-week-old mice (qPCR [n = 6] and HTS [n = 3]), ANOVA was performed, and the statistical significance is denoted by an asterisk (*, P < 0.05). (B) Characterization of communities at the phylum level. The similar shift of microbial communities observed in qPCR and HTS detection was consistent with data reported by Carvalho et al. (27). (C) Gross picture of the colons. (D) Representative H&E-stained colons (magnification, ×40). Histologic evaluation of the extent of epithelial damage, submucosal edema, and inflammatory cell infiltration showed that the mice developed worsening, extensive colitis that was relatively limited in control mice.
DISCUSSION
Gut microbiota plays an important role in the pathogenesis of IBD (31–33). Mice are the most commonly used animals for studying gut microbiota-related disease. Here, we developed phylum- and class-specific primers to assess the population structure of the predominant bacteria in mouse intestinal microbiota. In comparison to previously published taxon-specific quantitative analysis, these new primers possess similar specificity but largely increase the coverage of the taxon they target. Furthermore, an important advantage of the assay presented here lies in the ability to use all primer pairs with the same highly specific annealing temperature, which significantly facilitates data acquisition. However, due to the variability in rRNA operon copy number in different bacteria, the proportion of 16S rRNA gene copies that the qPCR assay provides cannot be directly transformed into the number of cells (34). Nevertheless, qPCR is an accurate method to determine the abundance of DNA sequences that can be used to estimate the distribution of bacteria (12). The real-time PCR system with universal primers and specific probes presented here provides an accurate and stable method to assess bacterial concentrations in mouse feces. We want to emphasize that the efficiencies of the primers presented here need to be recalculated in individual laboratories, since their values are known to be influenced by the presence of PCR inhibitors or instrumental factors.
High-throughput 16S rRNA gene sequencing is one of the most effective means widely used to examine the complexity of the gut microbiota. It is important to compare and contrast qPCR with HTS to validate this method for genetic fecal screening. It is clear that there is a striking degree of similarity among the Actinobacteria, Firmicutes, Tenericutes, and Proteobacteria proportions identified by qPCR and HTS, as shown in Table 4. Although some deviation in the proportions of Bacteroidetes, Deferribacteres, and Verrucomicrobia was detected in the two analytical methods, the changes in intestinal microbiota in the inflammatory state relative to the normal state remained highly consistent (Fig. 3) between these two detection methods. We found the targeted qPCR assay to have greater values in low-abundance bacterial groups than did the HTS method, such as 0.42% versus 0.27% (“Candidatus Saccharibacteria”) or 0.37% versus 0.03% (Verrucomicrobia), respectively, in the qPCR-versus-HTS detection rates. Taken together, the results of the qPCR and HTS approaches tested here demonstrate that the two methods are capable of generating high-fidelity data sets with no statistical difference between them. Although HTS technologies might give a more precise and a deeper scale at the genus level, our qPCR assay provides a relatively inexpensive platform for high-throughput detection and analysis.
Although the DNA stool kit for stool DNA isolation used in our study may give an under-representation of Gram-positive bacteria, the overall taxonomic groups represented within the mouse feces were similar to previous findings. The bacterial phyla Bacteroidetes and Proteobacteria could be specifically detected by our designed primers in both physiological and pathological states in the gut. It should be remembered that the microbiota shifts are subtle and generally only visible at the genus level in some cases, such as in dietary interventions; the primers cannot detect changes in microbiota at the genus level. However, microbiota differences can be observed at the phylum level in many intestinal diseases, such as IBD. The qPCR method developed in our study effectively detected the dynamic changes of gut microbiota at the phylum level. We used this method for rapid screening of the fecal sample from different transgenic mice. It successfully predicted the sample with potential IBD. Next, we plan to track the changes of intestinal microbiota using qPCR detection in the process of growth in TgD mice in order to investigate the relationship between the microbiota and the immune system in IBD. Of course, qPCR can be a powerful tool used in the research of gut-related diseases. Many environmental factors, including diet and drugs, may influence the microbiota and shape gut physiology and disease pathogenesis. qPCR would be important for assessing changes in the gut microbiota and evaluating its impact on IBD susceptibility at a greatly reduced cost compared to that of HTS. Further studies are also needed to determine whether changes in particular microbiota species induced by inflammation may impact progression to colorectal cancer. Perhaps the qPCR assay can be applied to the early detection of colorectal cancer.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Doctoral Station Science Foundation from the Chinese Ministry of Education (grant 20130091130003), the Chinese National Nature Sciences Foundation (grant 81421091), the Jiangsu Provincial Nature Science Foundation (grant BE2013630), and the Bureau of Science and Technology of Changzhou, Jiangsu, China (grants CZ20130011, CE20135013, CZ20120004, CM20122003, and WF201207).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01906-15.
REFERENCES
- 1.Yaung SJ, Church GM, Wang HH. 2014. Recent progress in engineering human-associated microbiomes. Methods Mol Biol 1151:3–25. doi: 10.1007/978-1-4939-0554-6_1. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira CM, Vieira AT, Vinolo MA, Oliveira FA, Curi R, Martins Fdos S. 2014. The central role of the gut microbiota in chronic inflammatory diseases. J Immunol Res 2014:689492. doi: 10.1155/2014/689492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartor RB. 2014. The intestinal microbiota in inflammatory bowel diseases. Nestle Nutr Inst Workshop Ser 79:29–39. doi: 10.1159/000360674. [DOI] [PubMed] [Google Scholar]
- 4.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 64:3336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmsen HJ, Raangs GC, He T, Degener JE, Welling GW. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol 68:2982–2990. doi: 10.1128/AEM.68.6.2982-2990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. 2004. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 70:7220–7228. doi: 10.1128/AEM.70.12.7220-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott SJ, Musfeldt M, Ullmann U, Hampe J, Schreiber S. 2004. Quantification of intestinal bacterial populations by real-time PCR with a universal primer set and minor groove binder probes: a global approach to the enteric flora. J Clin Microbiol 42:2566–2572. doi: 10.1128/JCM.42.6.2566-2572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 10.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. 2006. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinane CM, Cotter PD. 2013. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol 6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. 2011. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods 86:351–356. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Ettreiki C, Gadonna-Widehem P, Mangin I, Coeffier M, Delayre-Orthez C, Anton PM. 2012. Juvenile ferric iron prevents microbiota dysbiosis and colitis in adult rodents. World J Gastroenterol 18:2619–2629. doi: 10.3748/wjg.v18.i21.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, Nie Y, Wu XL. 2013. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 8:e74957. doi: 10.1371/journal.pone.0074957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maidak BL, Cole JR, Lilburn TG, Parker CT Jr, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res 29:173–174. doi: 10.1093/nar/29.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser NO 41:95–98. [Google Scholar]
- 18.Petruska J, Goodman MF, Boosalis MS, Sowers LC, Cheong C, Tinoco I Jr. 1988. Comparison between DNA melting thermodynamics and DNA polymerase fidelity. Proc Natl Acad Sci U S A 85:6252–6256. doi: 10.1073/pnas.85.17.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker S, Boger P, Oehlmann R, Ernst A. 2000. PCR bias in ecological analysis: a case study for quantitative Taq nuclease assays in analyses of microbial communities. Appl Environ Microbiol 66:4945–4953. doi: 10.1128/AEM.66.11.4945-4953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurjus AR, Khoury NN, Reimund JM. 2004. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. 2011. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 26.Cardiff RD, Miller CH, Munn RJ. 2014. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, Gewirtz AT. 2012. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Baumler AJ. 2013. Host-derived nitrate boosts growth of Escherichia coli in the inflamed gut. Science 339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchler G, Wos-Oxley ML, Smoczek A, Zschemisch NH, Neumann D, Pieper DH, Hedrich HJ, Bleich A. 2012. Strain-specific colitis susceptibility in IL10-deficient mice depends on complex gut microbiota-host interactions. Inflamm Bowel Dis 18:943–954. doi: 10.1002/ibd.21895. [DOI] [PubMed] [Google Scholar]
- 30.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. 2012. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med 209:1445–1456. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakansson A, Molin G. 2011. Gut microbiota and inflammation. Nutrients 3:637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Videla S, Vilaseca J, Guarner F, Salas A, Treserra F, Crespo E, Antolin M, Malagelada JR. 1994. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut 35:1090–1097. doi: 10.1136/gut.35.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian S, Campbell BJ, Rhodes JM. 2006. Bacteria in the pathogenesis of inflammatory bowel disease. Curr Opin Infect Dis 19:475–484. doi: 10.1097/01.qco.0000244054.69253.f3. [DOI] [PubMed] [Google Scholar]
- 34.Smith CJ, Osborn AM. 2009. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol 67:6–20. doi: 10.1111/j.1574-6941.2008.00629.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.