Abstract
Human norovirus (NoV) is responsible for over 90% of outbreaks of acute nonbacterial gastroenteritis worldwide and accounts for 60% of cases of foodborne illness in the United States. Currently, the infectivity of human NoVs is poorly understood due to the lack of a cell culture system. In this study, we determined the survival of a human NoV genogroup II, genotype 4 (GII.4) strain in seeded oyster homogenates after high-pressure processing (HPP) using a novel receptor binding assay and a gnotobiotic pig model. Pressure conditions of 350 MPa at 0°C for 2 min led to a 3.7-log10 reduction in the number of viral RNA copies in oysters, as measured by the porcine gastric mucin-conjugated magnetic bead (PGM-MB) binding assay and real-time RT-PCR, whereas pressure conditions of 350 MPa at 35°C for 2 min achieved only a 1-log10 reduction in the number of RNA copies. Newborn gnotobiotic piglets orally fed oyster homogenate treated at 350 MPa and 0°C for 2 min did not have viral RNA shedding in feces, histologic lesions, or viral replication in the small intestine. In contrast, gnotobiotic piglets fed oysters treated at 350 MPa and 35°C for 2 min had high levels of viral shedding in feces and exhibited significant histologic lesions and viral replication in the small intestine. Collectively, these data demonstrate that (i) human NoV survival estimated by an in vitro PGM-MB virus binding assay is consistent with the infectivity determined by an in vivo gnotobiotic piglet model and (ii) HPP is capable of inactivating a human NoV GII.4 strain at commercially acceptable pressure levels.
INTRODUCTION
Human norovirus (NoV), a member of the Caliciviridae family, is responsible for over 90% of the outbreaks of acute nonbacterial gastroenteritis worldwide and accounts for more than 60% of the cases of foodborne illness in the United States (1, 2). It is estimated that 48 million individuals, or about 17% of the U.S. population, are sickened each year, leading to approximately 128,000 hospitalizations and 3,000 fatalities in the United States (2). Human NoV is transmitted primarily through the fecal-oral route either by direct person-to-person contact or by fecally contaminated food or water. Human NoV is highly contagious and stable, and only a few virus particles are thought to be sufficient to cause an infection (3, 4). Outbreaks frequently occur in restaurants, hotels, day care centers, schools, nursing homes, cruise ships, swimming pools, hospitals, and military installations. Despite the significant economic impact and high morbidity caused by human NoV, no vaccines or antiviral drugs with activity against this virus are currently available (5, 6). This is due in large part to the lack of a cell culture system and a small-animal model for human NoV (6, 7). As a consequence, the survival of human NoV is poorly understood.
A major high-risk food for human NoV contamination is seafood, particularly bivalves, such as oysters, mussels, and clams (8–11). These animals are filter feeders and can readily bioaccumulate human NoV in their tissues if the virus is present in the waters in which they grow. Epidemiological studies showed a high prevalence rate and also high titers of human NoV in shellfish and oyster tissues (9–11). Worldwide, a substantial number of human NoV outbreaks are associated with the consumption of raw or undercooked shellfish. Human NoVs can persist in oysters for several weeks and are not effectively removed from contaminated oysters during depuration. Importantly, it has been reported that multiple types of histo-blood group antigens (HBGAs), the receptors of human NoV, are expressed in the gastrointestinal tissues of oysters, mussels, and clams, which contributes to the high rate of bioaccumulation and persistence of human NoV in shellfish (9–12). The concern associated with the safety of raw oyster consumption has increased in the shellfish industry, and this must be balanced with the high demand for oysters that maintain their original texture and flavor. Therefore, alternative nonthermal processing methods are needed to improve the safety of oysters for human consumption.
High-pressure processing (HPP) has come to the forefront as a promising intervention to effectively inactivate foodborne pathogens, including bacteria (e.g., Vibrio spp.) and viruses (e.g., hepatitis A virus), in oysters (4, 13–16). Moreover, this nonthermal process has made a revolutionary change in the oyster-shucking process, as HPP treatment causes the oysters to open and has also been shown to improve the organoleptic quality of the oysters, increasing consumer acceptance (15). Unfortunately, despite the substantial health and economic impacts caused by human NoV, evaluation of the effectiveness of HPP for inactivating human NoV has been hindered due to the unsuccessful in vitro cultivation of the virus. HPP at 400 MPa and 4°C for 2 min effectively inactivates cultivable human NoV surrogates (murine norovirus type 1 [MNV-1], feline calicivirus [FCV], and Tulane virus [TV]) in aqueous medium and/or oysters (14, 17–19). However, the validity of using these surrogates has been questioned because human NoV differs from these surrogates in many aspects, such as the clinical manifestations that it causes, its pathogenesis, and its host receptors and tropisms (6, 20). In fact, it has been reported that the prototype Norwalk-like virus (a human NoV genogroup I, genotype 1 [GI.1] strain) was much more stable than surrogate viruses when subjected to HPP (21). In a human volunteer study, treatment by HPP at 600 MPa and 6°C for 5 min was found to inactivate Norwalk virus in oysters, protecting subjects from disease development following oyster consumption (21). In contrast, treatment at 400 MPa (at 6°C or 25°C) for 5 min, which can completely inactivate surrogate viruses, was insufficient to prevent Norwalk virus infection and shedding in human subjects (21). Although the study with human subjects provided valuable insight into the survival of Norwalk virus GI.1 following HPP treatment, the stability of human NoV GII.4 strains, the most prevalent genotype circulating in the population, remains unknown. In the past 10 years, more than three global pandemics of human NoV have occurred, and all of these were due to strains of GII.4 (2, 22). Therefore, there is an urgent need to develop novel approaches to determine the survival of human NoV GII.4 strains following HPP treatment.
In this study, we undertook multiple novel approaches to evaluate the survival of a human NoV GII.4 strain in oyster homogenate following HPP treatment. First, we estimated the stability of human NoV using viral receptor binding activity as an indicator of virus survival, as disruption of receptor binding activity is likely lethal to the virus. Second, we determined the infectivity of human NoV following HPP treatment in a gnotobiotic pig model. The gnotobiotic pig is an excellent model for human enteric viruses because pigs share many similarities with humans in gastrointestinal structure, physiology, immunology, and, more importantly, the HBGA phenotypes (types A and H) on enterocytes (23–27). We found that HPP treatment at 350 MPa for 2 min at an initial temperature of 0°C reduced the amount of human NoV in oysters by approximately 3.7 log10 units, as determined by a combination of a receptor binding assay and real-time reverse transcription-PCR (RT-PCR). Gnotobiotic piglets orally fed oyster homogenate treated under these HPP conditions did not have significant intestinal histologic lesions, viral antigen expression, or viral RNA shedding in feces. In contrast, HPP treatment at 350 MPa for 2 min at an initial temperature of 35°C achieved only a 1-log10 reduction in the viral RNA copy number, as determined by the receptor binding assay. Consistent with this finding, gnotobiotic piglets orally fed oyster homogenate treated under these pressure conditions exhibited significant intestinal lesions, viral antigen expression in the intestine, and viral RNA shedding in feces. Therefore, HPP treatment at 350 MPa for 2 min at an initial temperature of 0°C was sufficient to prevent human NoV infection in gnotobiotic piglets.
MATERIALS AND METHODS
Preparation of human norovirus inoculum.
The human NoV GII.4 strain 765 was originally obtained from stool samples collected from an outbreak of acute gastroenteritis in Ohio. Stool samples were diluted 1:2 in minimal essential medium (MEM; Gibco-Invitrogen, Carlsbad, CA) and further processed by vortexing, centrifugation at 3,500 × g for 20 min, and filtration through a 0.8-μm-pore-size filter followed by a 0.2-μm-pore-size filter. The possibility of the presence of other enteric viral pathogens, such as human rotavirus, human sapovirus, and human astrovirus, was excluded by RT-PCR analysis prior to initiation of the study. The amount of RNA copies in the human NoV strain 765 filtrate was quantified by real-time RT-PCR, and the level of RNA was 1.3 × 108 RNA copies/ml. Viruses were aliquoted and stored at −80°C until used.
Oyster inoculation and high-pressure treatment.
The human NoV stock was shipped overnight to the collaborative laboratory at the University of Delaware for oyster inoculation and HPP treatment. Live oysters (Crassostrea virginica) were purchased from local seafood markets, shucked, and blended for 30 s using a blender (model 36BL23; Waring Commercial, New Hartford, CT). Before inoculation, the oyster homogenate was treated at 600 MPa for 5 min at 0°C to inactivate human NoV or other pathogens that could be naturally present in the oysters. A total of 1.2 ml of the human NoV GII.4 stock was used to inoculate 12 g HPP-pretreated oyster homogenate to reach a final concentration of 107 RNA copies/g. Human NoV-inoculated oyster samples (3 g) were then transferred into sterile plastic polyethylene pouches (Fisher Scientific, Fair Lawn, NJ), which were double sealed and double bagged, followed by treatment at pressures ranging from 300 MPa to 450 MPa for 2 min at initial temperatures of 0, 25, and 35°C. Four pouches containing 3 g of human NoV-inoculated oyster samples were prepared for each treatment condition, and each sample was separately processed in the HPP unit. The pressure treatments were applied using a high-pressure unit (model Avure PT-1; Avure Technologies, Kent, WA) with water as the hydrostatic medium. Processing temperatures and pressures were monitored and recorded by use of a personal computer-based data acquisition and control system (Dasytec USA, Bedford, NH). The 2-min pressurization holding time reported in this study does not include the pressure come-up time (ca. 22 MPa/s) or release time (<4 s).
Estimation of human NoV survival by PGM-MB binding assay.
Type III porcine gastric mucin (PGM; Sigma, St. Louis, MO) was cross-linked to MagnaBind carboxyl-derivatized magnetic beads (MBs; Pierce Biotechnology, Rockford, IL) following the manufacturer's instructions. Briefly, 1 ml of beads was washed 3 times with phosphate-buffered saline (PBS) and separated using a magnetic separation rack (New England BioLabs, Ipswich, MA). Following the washing, 1 ml of 10 mg/ml type III PGM in MES (N-morpholinoethanesulfonic acid) buffer and 0.1 ml of 10 mg/ml EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride] in MES buffer were added to the beads, and the mixture was incubated for 30 min at room temperature on a Labquake shaker rotisserie (Thermo Scientific, Waltham, MA) rotating at 8 rpm. After incubation, the beads were separated from the PGM solution using a magnetic attracter, followed by 3 washes with PBS. The PGM-MBs were finally resuspended in 1 ml of PBS containing 0.05% sodium azide and stored at 4°C until use. The level of PGM incorporation was evaluated by measuring the absorbance of the supernatant at 280 nm.
After HPP at 300 MPa to 450 MPa at an initial temperature of 0°C, 25°C, or 35°C, oyster homogenates (0.2 g) were blended with glycine buffer (pH 9.5; 0.1 M glycine, 0.3 M NaCl) at room temperature (∼21°C) at a 1:9 ratio. The mixture was centrifuged at 15,000 × g for 15 min at room temperature. Viral particles were precipitated from the supernatant by an equal volume of 16% polyethylene glycol 8000 (PEG 8000; Sigma, St. Louis, MO) with 0.525 M NaCl. After a 1-h precipitation on ice, the mixture was centrifuged at 10,000 × g for 5 min at room temperature. The pellet was suspended in 2 ml PBS by vigorous vortex mixing and pipetting and incubated with 5 μl of RNase (20 U/μl; Life Technologies) for 30 min at 37°C. All samples were transferred to 15-ml centrifuge tubes, and the volume was brought up to 5 ml with PBS before being subjected to PGM-MB binding assays. PGM-MBs (500 μl) were added to the tube containing the oyster homogenate sample. The tubes were incubated for 15 min at room temperature on a Labquake shaker rotisserie rotating at 8 rpm. The PGM-MBs were separated from the liquid after incubation using a magnetic separator (Qiagen, Valencia, CA), washed 3 times with PBS, and resuspended in 140 μl of PBS. Human NoV RNA was extracted by use of an RNeasy kit (Qiagen) and quantified by real-time RT-PCR.
Delivery of gnotobiotic piglets.
The animal protocol used in this study was approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University (IACUC-OSU; approval number IACUC-OSU-2013A00000098), and all animals were also handled in accordance with the guidelines of the IACUC-OSU. A date-mated pregnant adult multiparous sow was purchased from a commercial pork production unit (Shoup Brothers, Smithville, OH) and transported to the Goss Laboratory at The Ohio State University. A litter of 12 gnotobiotic piglets was delivered by cesarean section following the procedures described previously (27, 28). Neonatal piglets were transferred and maintained in sterile isolator units containing six partitions and an exterior heat source. The piglets were fed a liquid milk replacement diet (Parmalat) three times daily (100 to 150 ml per feeding).
Inoculation of human NoV-seeded oyster homogenate into gnotobiotic piglets.
Twelve 2-day-old gnotobiotic piglets from the same litter were randomly assigned to three groups: piglets in group 1 received human NoV-inoculated oyster homogenate without pressure treatment (n = 4), piglets in group 2 received human NoV-inoculated oyster homogenate treated at 350 MPa and 35°C for 2 min (n = 4), and piglets in group 3 received human NoV-inoculated oyster homogenate treated at 350 MPa and 0°C for 2 min (n = 4). To neutralize stomach acids prior to inoculation, piglets received 8 ml of 100 mM sodium bicarbonate orally. Each gnotobiotic piglet in group 1 received one oral dose of 3 ml of untreated oyster homogenate containing 107 genomic RNA copies of human NoV GII.4. Piglets in group 2 were orally inoculated with 3 ml of human NoV-seeded oyster homogenate treated at 350 MPa for 2 min at 35°C. Piglets in group 3 were orally inoculated with 3 ml of human NoV-seeded oyster homogenate treated at 350 MPa for 2 min at 0°C. After inoculation, fecal swabs were collected daily from each piglet for virus detection, and a diarrhea/fecal consistency score was assigned using a subjective scale (0, normal; 1, creamy; 2, pasty; 3, watery). All the piglets were euthanized on postinfection day (PID) 7, and adjacent intestinal tissue segments (duodenum, jejunum, ileum, and colon) were collected from each piglet for the examination of gross pathological and histopathological changes.
Quantification of human NoV shedding in feces by real-time RT-PCR.
Fecal samples from the rectal swabs collected daily were eluted in 300 μl of PBS at a dilution of 1:10. All samples were vortexed and centrifuged at 6,000 × g for 15 min. The supernatants were collected for RNA extraction using an RNeasy minikit (Qiagen, Valencia, CA), followed by real-time RT-PCR. Primers for cDNA synthesis and real-time PCR were designed on the basis of the published sequence for human NoV GII.4 strain 5M (GenBank accession number JQ798158). First-strand cDNA was synthesized by the use of SuperScript III reverse transcriptase (Invitrogen) following the manufacturer's protocol, using the primer VP1-P1 (5′-TTATAATACACGTCTGCGCCC-3′), which targets the VP1 gene of human NoV GII.4. The VP1 gene was then quantified by real-time PCR using custom TaqMan primers and probes (forward primer, 5′-CACCGCCGGGAAAATCA-3′; reverse primer, 5′-GCCTTCAGTTGGGAAATTTGG-3′; reporter, 5′-FAM-ATTTGCAGCAGTCCC-NFQ-3′, where FAM is 6-carboxyfluorescein) on a StepOne real-time PCR machine (Applied Biosystems, Foster City, CA). Three technical replicates were run for each sample, and the PCR and cycling parameters were those described in the manufacturer's protocol (Applied Biosystems). TaqMan Fast Universal master mix was used for all reactions. For cycling parameters, a holding stage at 95°C was maintained for 2 min, prior to 40 cycles of 94°C for 15 s for denaturation, 55°C for 30 s for annealing, and 72°C for 15 s for extension. A standard plasmid carrying human NoV was constructed by inserting the sequence of the entire open reading frame 2 (ORF2; encoding viral protein VP1) into the pGEM-T Easy vector (Promega, Madison, WI). The plasmid of known concentration was 10-fold serially diluted to generate a standard curve for real-time PCR. StepOne software (v2.1) was used to quantify the genomic RNA copies. Viral RNA was expressed as the mean log10 number of genomic RNA copies per milliliter ± standard deviation.
Detection of human NoV antigen in intestine by IFA.
An indirect immunofluorescence assay (IFA) was performed on whole-mount sections of duodenum, jejunum, and ileum collected from the inoculated pigs at PID 7. The fresh intestinal tissues were sectioned into small pieces and fixed with 4% (vol/vol) paraformaldehyde–0.2% (vol/vol) glutaraldehyde in 0.1 M potassium phosphate buffer (PPB), pH 7.4, for 2 h at room temperature. After being washed 4 times with PPB, the sections were quenched with PPB containing 50 mM glycine overnight at 4°C. The tissues were permeabilized with 0.1% Triton X-100 in PBS for 1 h at room temperature, washed twice with PBS, blocked with PBS containing 2% bovine serum albumin–5% normal goat serum for 30 min at room temperature, and incubated with 1:5,000-diluted human NoV VP1-specific polyclonal antibody overnight at 4°C in incubation buffer (10 mM potassium phosphate buffer containing 150 mM NaCl, 10 mM sodium azide, and 0.2% bovine serum albumin). After 6 washes with PBS, the tissues were incubated with a 1:1,200 dilution of secondary antibody (goat anti-guinea pig IgG labeled with Alexa Fluor 488) in incubation buffer overnight at 4°C. Nuclei and actin were counterstained with SYTOX orange and Alexa Fluor 633-labeled phalloidin, respectively. The tissue samples were then trimmed into small pieces, mounted onto slides, and examined using an Olympus FV1000 confocal microscopy imaging system at The Ohio State University.
Histologic examination.
Tissues from the small intestine (duodenum, jejunum, and ileum) and large intestine (colon) were examined histologically. Sections of tissues collected from the inoculated pigs at PID 7 were fixed in 10% (vol/vol) phosphate-buffered formalin. Fixed tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) for the examination of histological changes by light microscopy. The severity of the intestinal histological change was scored on the basis of the following criteria: grade 3, severe; grade 2, moderate; grade 1, mild; and grade 0, no lesions.
Statistical analysis.
All values are expressed as the means ± standard deviations. Samples in which RNA was undetectable (concentration, <2 log10 RNA copies/ml) during the shedding period were assigned a value of 0 for statistical analysis. Statistical analysis by one-way analysis of variance was performed by using Minitab statistical analysis software (Minitab, Inc., State College, PA). A P value of <0.05 was considered statistically significant.
RESULTS
Estimation of survival of a human NoV GII.4 strain in oyster homogenate determined by a PGM-MB binding assay and real-time RT-PCR.
Since human NoV cannot be cultivated, an in vitro assay with porcine gastric mucin (PGM)-conjugated magnetic beads (MBs) was developed to estimate the infectivity of human NoV. This assay is based on the virus-receptor interaction. PGM contains mixed type A, type H1, and Lewis B histo-blood group antigens (HBGAs), which are the functional receptors of human NoVs (29, 30). Intact viral particles that possess a receptor binding capability bind to the PGM-MBs, and viral particles that have disrupted capsids or alterations to the receptor binding protein domain of the capsid are unable to bind to the PGM-MBs. After binding to PGM-MBs and subsequent washing, only viral particles capable of receptor binding are collected, and their genomic RNA is then quantified by real-time RT-PCR. Fecal filtrate containing human NoV GII.4 was seeded into oyster homogenate to reach a final concentration of 7 log10 RNA copies/ml. The samples were treated by HPP at 350 MPa for 2 min at an initial temperature of 0, 20, or 35°C. After treatment, the inactivation of human NoV in oyster homogenate was first estimated using the PGM-MB capture assay and real-time RT-PCR. The effect of the initial temperature on the effectiveness of human NoV inactivation during HPP is shown in Table 1. The efficiency of the inactivation of human NoV GII.4 was enhanced as the pressure level was increased. For example, at an initial temperature of 25°C, a 3.0-log10 reduction in the number of viral genomic RNA copies was achieved at 350 MPa, whereas only a 0.9-log10 reduction was achieved at 300 MPa. At an initial temperature of 0°C, a 3.7-log10 reduction in the number of viral genomic RNA copies was achieved at 350 MPa, whereas only a 2.8-log10 reduction was achieved at 300 MPa. Additionally, the initial temperature significantly impacted the effectiveness of human NoV inactivation by HPP treatment. Specifically, the efficiency of inactivation was dramatically enhanced at lower temperatures. For instance, at 300 MPa, a 2.8-log10 reduction was observed at 0°C. That treatment was significantly more efficient than treatment with 300 MPa at 25°C (0.9-log10 reduction) and 35°C (0.5-log10 reduction). At 350 MPa, a 3.7-log10 reduction was observed at 0°C, whereas only a 1.0-log10 reduction was achieved with 350 MPa at an initial temperature of 35°C. In addition, there was a significantly greater (P < 0.05) reduction in RNA levels by HPP at 350 MPa at an initial temperature of 25°C (3.0-log10 reduction) than at an initial temperature of 35°C (1.0-log10 reduction). Therefore, the survival of human NoV GII.4 in oyster homogenate upon HPP can be estimated by the PGM-MB binding assay and real-time RT-PCR.
TABLE 1.
Reduction of human NoV GII.4 strain 765 in oyster homogenate determined by PGM-MB binding assay and real-time RT-PCR
| Pressure (MPa)a | Mean reduction of log10 no. of RNA copies ± SD at initial temp ofb: |
||
|---|---|---|---|
| 0°C | 25°C | 35°C | |
| 300 | 2.8 ± 0.1A | 0.9 ± 0.1A | 0.5 ± 0.1A |
| 350 | 3.7 ± 0.3B | 3.0 ± 0.1B | 1.0 ± 0.3B |
| 400 | 4.0 ± 0.3B | 3.8 ± 0.2C | ND |
| 450 | 4.2 ± 0.2B | 4.0 ± 0.1C | ND |
Pressure treatment was held for 2 min.
ND, not determined. Values within a column followed by different capital letters (A, B, and C) are significantly different (P < 0.05).
Diarrhea scores for gnotobiotic piglets orally fed oyster homogenate seeded with GII.4 and treated by HPP.
The oyster homogenates treated with HPP at 350 MPa and 0°C and 35°C for 2 min, which achieved 3.7- and 1.0-log10 reductions of a human NoV GII.4 strain, respectively, were selected to feed gnotobiotic piglets. The goal was to determine whether viral survival estimated by an in vitro PGM-MB virus binding assay correlated with the in vivo results obtained with a gnotobiotic pig model. To do this, 12 2-day-old gnotobiotic piglets were randomly divided into three groups (n = 4 in each group) and inoculated orally with human NoV in oyster homogenate that had been treated with 350 MPa for 2 min at 0°C or 35°C or that did not undergo HPP treatment. Viral pathogenesis was evaluated by measuring the development of diarrhea in the piglets and the histopathological changes in the intestine. The diarrhea/fecal consistency score of stool samples obtained from each pig daily was assigned using a subjective scale, where 0 is normal, 1 is creamy, 2 is pasty, and 3 is watery. Only mild (score = 1) to moderate (score = 2) diarrhea was observed in 3 out of 4 gnotobiotic piglets inoculated with untreated oyster homogenate, and none of the pigs scored a 3, suggesting that the diarrhea caused by human NoV GII.4 strain 765 was limited in the piglets during the 7-day observation period. HPP at 350 MPa and 35°C did not significantly affect the development of diarrhea in pigs, and 3 out 4 piglets developed creamy feces (score = 1). Among the four piglets inoculated with human NoV treated at 350 MPa and 0°C, 2 piglets had normal stools (score = 0), and the other 2 piglets had creamy feces (score = 1). Overall, there was no significant difference in the development of diarrhea in piglets fed oyster homogenate treated at 350 MPa for 2 min at an initial temperature of either 0°C or 35°C.
Histologic lesions in the small intestine of gnotobiotic piglets caused by human NoV GII.4-seeded oyster homogenate treated by HPP.
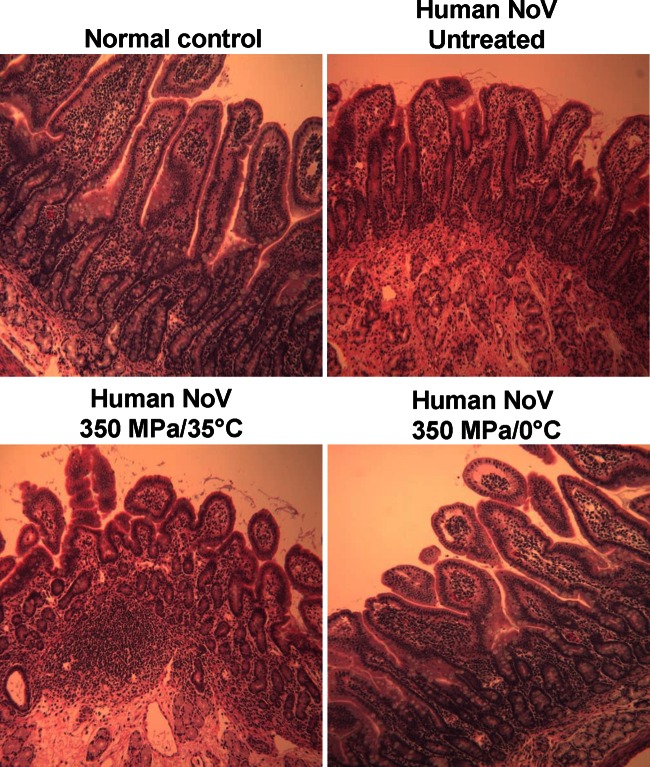
The histological changes observed in the intestines of gnotobiotic piglets due to inoculation with oyster homogenate seeded with HPP-treated and untreated human NoV GII.4 strain 765 were evaluated and are presented in Table 2. At day 7 postinoculation, all piglets were sacrificed and intestinal segments, including the duodenum, jejunum, ileum, and colon, were collected for the examination of gross pathological and histopathological changes. No significant gross pathological changes were found in the intestines of any of the gnotobiotic piglets. Mild to moderate histological changes were observed in the duodenum (4/4 piglets), jejunum (4/4), and ileum (1/4) of piglets infected with untreated oyster homogenate. The highest mean histological score for piglets infected with untreated oyster homogenate was 1.3 ± 0.5 for duodenum tissue (Table 2). These lesions included an increase in inflammatory cell infiltrates within the lamina propria of the duodenum and jejunum and epithelial loss (necrosis or apoptosis) of villous tips (Fig. 1). All four piglets inoculated with oyster homogenate treated at 350 MPa and 35°C showed histological changes in the duodenum and jejunum similar to those for the control piglets inoculated with untreated oyster homogenate, with the mean lesion scores being 1.4 and 0.9, respectively, and with no lesions being found in the ileum or colon. Interestingly, significantly lower (P < 0.05) mean histologic lesion scores for the duodenum and jejunum tissues of piglets fed oyster homogenate treated at 350 MPa and 0°C for 2 min than for the duodenum and jejunum tissues of the other piglets were observed, although no significant difference for ileum tissues was observed (P > 0.05). Therefore, gnotobiotic piglets inoculated with human NoV-seeded oyster homogenate treated at 350 MPa and 0°C for 2 min had significantly lower mean lesion scores in duodenum and jejunum tissue than piglets fed untreated oyster homogenate or those fed oyster homogenate treated at 350 MPa and 35°C.
TABLE 2.
Histological changes in the small intestines of gnotobiotic piglets fed human NoV GII.4-contaminated oysters with or without HPP
| Treatmenta | Mean severity of intestinal histological changes ± SD inb: |
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| None | 1.3 ± 0.5 (4/4)A | 1.0 ± 0.0 (4/4)A | 0.3 ± 0.5 (1/4) |
| 350 MPa, 35°C | 1.4 ± 0.8 (4/4)A | 0.9 ± 0.3 (4/4)A | 0.0 ± 0.0 (0/4) |
| 350 MPa, 0°C | 0.6 ± 0.5 (3/4)B | 0.5 ± 0.3 (4/4)B | 0.0 ± 0.0 (0/4) |
Pressure treatment was held for 2 min.
All piglets were terminated at PID 7. The severity of the intestinal histological change was scored as follows: 3, severe; 2, moderate; 1, mild; 0, no lesions. Values in parentheses are the number of piglets with histological changes/total number of piglets tested. Values within a column followed by different letters (A and B) are significantly different (P < 0.05).
FIG 1.
Histological changes in the duodenum of gnotobiotic pigs. Duodenum sections were embedded in paraffin, sectioned at 5 μm, and stained with H&E for histological examination by light microscopy. Duodenum tissue from an uninfected piglet is shown at the upper left (Normal control).
Viral shedding in feces of gnotobiotic piglets fed human NoV GII.4-seeded oyster homogenate treated by HPP.
After the gnotobiotic piglets were fed human NoV-contaminated oysters, fecal samples were collected from each piglet until PID 7. Viral RNA shedding in feces was quantified by quantitative real-time RT-PCR. The presence and the average titer of viral RNA detected in pig feces at each PID are summarized in Table 3. When the piglets were fed untreated human NoV-inoculated oyster homogenate, viral RNA shedding in feces was observed in 3 out of 4 pigs at PID 1 and all 4 pigs from PID 2 through PID 7. The average human NoV RNA level in piglet feces gradually increased from 3.5 log10 copies of RNA/g feces at PID 1 to a peak value of 6.0 log10 copies of RNA/g at PID 4. Similar levels of viral RNA shedding were observed in gnotobiotic piglets orally inoculated with human NoV-contaminated oysters treated at 350 MPa and 35°C, suggesting that HPP treatment at 350 MPa and 35°C for 2 min did not affect the replication of human NoV in a gnotobiotic pig model. Specifically, at PID 1, 2 out of 4 pigs shed viral RNA at an average level of 2.0 log10 copies of RNA/g feces, and viral RNA was detected in all 4 pigs from PID 2 to PID 7, with titers ranging from 5.1 to 5.9 log10 copies of RNA/g feces. In contrast, when gnotobiotic piglets were fed human NoV-inoculated oyster homogenates processed at 350 MPa and 0°C, no viral RNA was detected in any of the pig feces at PIDs 2, 4, 5, and 7, and only 1 or 2 pigs were positive for viral shedding at PIDs 1, 3, and 6, with the average RNA level ranging from 1.2 to 2.7 log10 copies of RNA/g feces. Thus, the gnotobiotic piglets had minimal viral RNA shedding in feces when contaminated oyster homogenate was treated by 350-MPa HPP at an initial temperature of 0°C, demonstrating that this pressure condition substantially inactivates human NoV GII.4 strain 765, limiting infection of gnotobiotic piglets.
TABLE 3.
Quantification of human NoV RNA in feces of gnotobiotic piglets fed human NoV GII.4-contaminated oysters with or without HPP
| Treatmenta | Mean log10 no. of RNA copies/g feces ± SD on PIDb: |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| None | 3.5 ± 2.4 (3/4) | 5.3 ± 0.6 (4/4) | 5.8 ± 0.4 (4/4) | 6.0 ± 0.3 (4/4) | 5.7 ± 0.2 (4/4) | 5.2 ± 0.4 (4/4) | 5.2 ± 0.5 (4/4) |
| 350 MPa, 35°C | 2.0 ± 2.5 (2/4) | 5.6 ± 0.7 (4/4) | 5.9 ± 0.4 (4/4) | 5.8 ± 0.3 (4/4) | 5.1 ± 0.8 (4/4) | 5.2 ± 1.1 (4/4) | 5.1 ± 0.2 (4/4) |
| 350 MPa, 0°C | 1.2 ± 2.4 (1/4) | 0.0 ± 0.0 (0/4) | 1.3 ± 2.7 (1/4) | 0.0 ± 0.0 (0/4) | 0.0 ± 0.0 (0/4) | 2.7 ± 3.1 (2/4) | 0.0 ± 0.0 (0/4) |
Pressure treatment was held for 2 min.
Fecal samples collected daily were examined by RT-qPCR. Values in parentheses are the number of virus-positive piglets/total number of piglets tested.
Detection of human NoV antigen in small intestines of gnotobiotic piglets following oral inoculation with human NoV GII.4-seeded oyster homogenate treated by HPP.
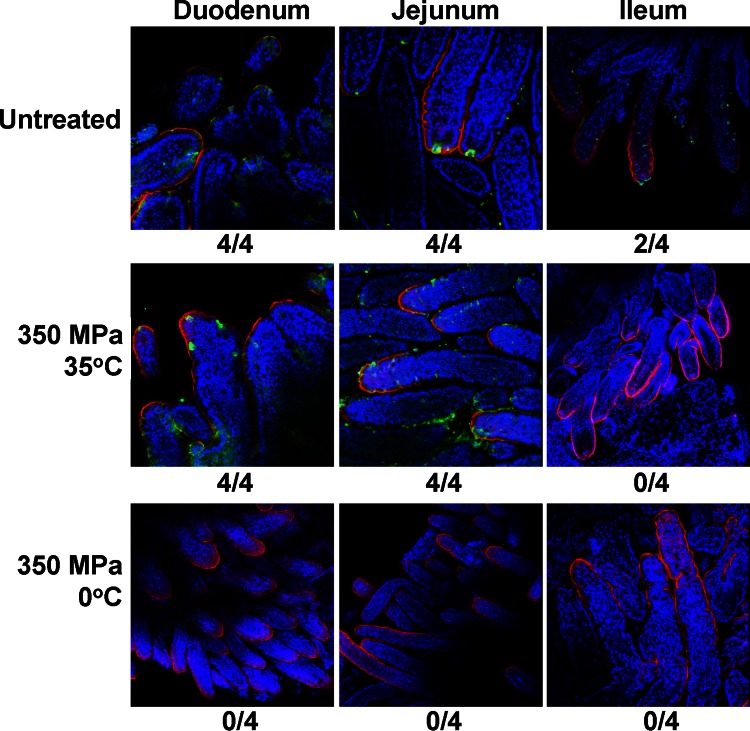
Finally, we determined whether human NoV antigens could be found in intestinal tissues. To do this, a whole-mount tissue indirect immunofluorescence assay (IFA) was performed on fresh duodenum, jejunum, and ileum tissues at PID 7 using a polyclonal antibody against the VP1 protein of human NoV. The presence of human NoV VP1 antigens was visualized by confocal fluorescence microscopy. As shown in Fig. 2, all duodenum and jejunum tissues from gnotobiotic piglets fed untreated virus-contaminated oyster homogenate were IFA positive. Human NoV-positive staining (green) of cells at villous tips and the adjacent sides of individual villi indicated the presence of human NoV antigen in enterocytes and the replication of human NoV in these cells. An IFA-positive signal was also detected in the ileum of 2 pigs in the untreated group (Fig. 2). When piglets were fed the virus-contaminated oyster homogenate treated at 350 MPa and 35°C for 2 min, a large number of human NoV-positive cells were observed in all of the duodenum and jejunum sections, indicating that human NoV survived this treatment and was able to replicate in the gnotobiotic piglets. However, no IFA-positive cells were detected in any intestinal segments from piglets orally fed oyster homogenate treated at 350 MPa and 0°C for 2 min. Collectively, HPP treatment at 350 MPa and an initial temperature of 0°C for 2 min prevented human NoV replication in gnotobiotic piglets, as demonstrated by the lack of a viral structural protein in intestinal epithelial cells of the piglets.
FIG 2.
Detection of human NoV antigen-positive cells in the intestine by IFA. Fresh intestinal tissues collected from inoculated piglets at PID 7 were sectioned into small pieces, fixed, quenched, and permeabilized. The tissues were incubated with NoV-specific polyclonal antibody, followed by incubation with goat anti-guinea pig IgG labeled with Alexa Fluor 488. Nuclei and actin were counterstained with SYTOX orange and phalloidin labeled with Alexa Fluor 633. The stained tissues were mounted onto slides and examined using an Olympus FV1000 confocal microscopy imaging system. The number of IFA-positive samples/total number of samples tested is indicated beneath each panel. A green signal represents human NoV antigen, a red signal is actin, and a blue signal is the nucleus.
DISCUSSION
In this study, we determined the susceptibility of a human NoV GII.4 strain to high pressure using a receptor binding assay and a gnotobiotic pig model. We have shown that treatment of oyster homogenate inoculated with human NoV GII.4 strain 765 at 350 MPa and an initial temperature of 0°C for 2 min resulted in a 3.7-log10 reduction in the number of RNA copies using an in vitro PGM-MB binding assay. Gnotobiotic piglets fed this oyster homogenate had minimal viral shedding in feces and no viral replication in intestinal cells and did not have significant histologic lesions in duodenum tissues. These results suggest that pressure conditions of 350 MPa at 0°C for 2 min are sufficient to inactivate human NoV and, thus, prevent infection in gnotobiotic piglets. In contrast, pressure conditions of 350 MPa at an initial temperature of 35°C for 2 min, which achieved only a 1-log10 reduction in the number of viral RNA copies, as determined by the PGM-MB binding assay, were not sufficient to prevent human NoV infection in piglets. These data suggest that the rate of human NoV survival following HPP treatment is consistent when it is estimated by the PGM-MB binding assay and by use of the gnotobiotic pig model.
Currently, the infectivity/survival of human NoV is poorly understood because it cannot be grown in cell culture. Traditional methods of human NoV detection, such as RT-PCR and quantitative real-time RT-PCR, are widely used to detect the presence of human NoV (29, 31). However, a major disadvantage of these nucleic acid-based methods is that they cannot discriminate between the RNAs from infectious viruses and those from noninfectious viruses. For example, it was recently reported that a human NoV GII.4 strain (isolated from clinical samples from an outbreak) was inactivated by cold atmospheric pressure plasma (CAPP), as determined by real-time RT-PCR (32). The initial starting quantity was 2.36 ×104 genomic equivalents/ml, and sample exposure to CAPP reduced the RNA level by 1.23 log10 and 1.69 log10 genomic equivalents/ml after 10 and 15 min exposure, respectively (32). However, a major concern is that the results of real-time RT-PCR do not accurately reflect the level of viral infectivity. We previously found that the mechanism of viral inactivation by HPP is the disruption of the viral capsid and not the degradation of genomic RNA, and this capsid disruption would directly affect the receptor binding ability (14, 33). Thus, intact virus particles that possess receptor binding activity should be able to bind to PGM-MBs and subsequently be detected by real-time RT-PCR. Using this novel assay, we found that human NoV GII.4 strain 765 in oyster homogenate was readily inactivated at a lower initial temperature. The effectiveness of temperature on human NoV inactivation by HPP can be ranked 0°C > 25°C > 35°C. For instance, HPP at 350 MPa and 0°C for 2 min led to a 3.7-log10 reduction in the human NoV RNA titer, whereas only a 1.0-log10 reduction was achieved when the initial temperature was 35°C. This temperature-dependent inactivation of virus by HPP was also observed with other cultivable animal caliciviruses, including MNV-1, FCV, and TV, all of which were more easily inactivated at 0°C than at 25°C (14, 17, 19, 34). Clearly, a combination of the PGM-MB virus binding assay and real-time RT-PCR is an improved strategy to estimate the survival of human NoVs. In our subsequent study with piglets, we chose HPP treatment levels based on the level of human NoV survival/inactivation determined by the PGM-MB binding assay.
It is generally believed that HBGAs are functional receptors for human NoV and determine host specificity. Swine have HBGA phenotypes similar to those of humans, making gnotobiotic piglets an excellent model with which to study human NoVs (23–25, 35, 36). Previous studies have shown that gnotobiotic piglets are susceptible to oral infection with several human NoV GII.4 strains, and this animal model has been used for evaluation of the efficacy of vaccine candidates and antiviral therapies against human NoVs (23–25, 37). We found that gnotobiotic piglets inoculated with human NoV GII.4 strain 765 exhibited mild diarrhea, viral RNA shedding in feces, histologic lesions in the intestines, and viral replication in the proximal small intestine. Viral shedding in pig feces was detected by real-time RT-PCR to PID 7. The average viral RNA level gradually increased from 3.5 log10 copies of RNA/g feces at PID 1 to a peak level of approximately 6.0 log10 copies of RNA/g feces at PID 4. On average, each piglet produced more than 100 g of feces per day. Thus, the total amount of virus that was shed was substantially more than the original amount of virus that was input (7 log10 RNA copies). In addition, all of the piglets inoculated with human NoV-seeded oyster homogenate that had not been treated with HPP had detectable norovirus antigens in the duodenal and jejunal enterocytes, as determined by an indirect immunofluorescence assay. Also, histologic lesions were found in the intestinal segments from infected piglets, and these were characterized by the epithelial loss of villous tips, villous shortening, atrophy, and edema in the lamina propria. These data provide compelling evidence that human NoV GII.4 strain 765 replicated in the gnotobiotic piglets.
Using this unique model, we found that the pressure condition of 350 MPa at 35°C for 2 min was not sufficient to inactivate human NoV GII.4 strain 765. Viral RNA was detected in all the inoculated pigs from PID 2 to PID 7 at levels ranging from 5.1 to 5.9 log10 copies of RNA per gram of fecal matter, which was not significantly different from the RNA level in piglets fed untreated human NoV-contaminated oyster homogenate. In addition, these piglets exhibited histologic lesions similar to those in the untreated control piglets, and antigen was detected in the small intestines of these piglets and untreated control piglets. Interestingly, the pressure condition of 350 MPa at 0°C for 2 min sufficiently blocked human NoV GII.4 infection in gnotobiotic piglets. Under this pressure condition, no viral RNA was detected in any of the inoculated pig feces at PID 2, 4, 5, or 7. No or minimal histologic lesions were found in the duodenum tissue, and no antigen expression was found in the small intestine. These data suggest that HPP at 350 MPa and 0°C for 2 min is capable of effectively inactivating a human NoV GII.4 strain to a level which does not cause infection in the gnotobiotic pig model. Although there was no significant difference in the rates of development of diarrhea between piglets fed oyster homogenate treated with pressure at the two temperatures (0°C and 35°C), viral shedding in feces, histologic lesions in the intestine, and the presence of viral antigen in enterocytes can be used as evidence of human NoV replication in gnotobiotic piglets. Overall, the infectivity of human NoV determined in the gnotobiotic pig model was consistent with that estimated by the PGM-MB binding assay and real-time RT-PCR. Therefore, the loss of HBGA binding of human NoV detected by the in vitro PGM-MB binding assay accurately reflects the inability of the virus to cause infection in vivo.
Recently, Richards suggested that the priority of research on human NoVs needs to be shifted from human NoV surrogates to human volunteer studies in order to identify practical processing approaches that may be used to eliminate this pathogen (38). This argument is based on the fact that the ability of surrogates to validly represent the inactivation profile of human NoV has been seriously questioned. To date, only one human challenge study to determine the effectiveness of HPP to reduce human NoV levels in oysters has been performed (21). In that study, the infection caused by human NoV GI.1 (Norwalk virus) was judged by the symptoms of volunteers and the presence of viral RNA in stool, as determined by traditional RT-PCR (21). It was shown that the higher pressure condition (600 MPa, 6°C, 5 min) but not the lower pressure condition (400 MPa, 6 or 25°C, 5 min) completely inactivated human NoV GI.1 in oysters and prevented human NoV infection among all of the subjects challenged with HPP-treated oysters (21). The inactivation of Norwalk virus by HPP has also been evaluated using the PGM-MB binding assay (29). It was found that HPP treatment of Norwalk virus RNA at 600 MPa reduced the number of RNA copies by 4.7 log10 units using the PGM-MB assay, which was comparable to the results obtained in the human volunteer study (29).
Although the results obtained from the human clinical trials are of great value, there are many obstacles associated with studies with human subjects. It is estimated that the overall expense of even a limited volunteer study may approach $500,000, including compensation for participants, hospital costs, labor, etc. (38). Also, there are potential health risks to human subjects in these types of studies. Although human NoVs cause self-limiting infections, lethal cases, particularly in developing countries, have been reported (39). In addition, it could be difficult to rule out the possibility that unknown and, thus, undetected pathogens are present in challenge materials to be administered to human volunteers, posing another potential risk. Thus, volunteer studies need to be performed under strictly controlled conditions to avoid any unforeseen complications. As a consequence, studies with human subjects take years to be approved and executed. However, the gnotobiotic pig model is not without limitations. Cost is also a factor that may limit the use of the gnotobiotic pig model for human NoV research, with the estimated cost of a study with one litter of piglets reaching $25,000. Another limitation is the sample size, with the capacity of the experimental facility and also the number of piglets delivered by the sow dictating the number of piglets available for a particular study. However, despite the limitations of the gnotobiotic pig model, studies with gnotobiotic pigs are easier to perform, more cost-effective, and more convenient to execute than studies with human subjects. As demonstrated in this study, the gnotobiotic pig model provides valuable information about the infectivity of human NoVs following HPP treatment.
We used oysters as a food matrix because human NoV is frequently associated with the consumption of shellfish, especially oysters. The practical application of HPP to human NoV-contaminated shellfish will require the process to be economical, viable for current commercial units, and, most importantly, acceptable to consumers. The 2-min HPP treatments ranging from 300 MPa to 450 MPa at 0°C, 25°C, and 35°C used in this study are economical for high-throughput operations. In fact, pressures of approximately 300 MPa have been used in the shellfish industry to facilitate fresh shell oyster shucking, extend the shelf life, and reduce total bacterial counts, including those of pathogenic Vibrio species (15). Moreover, it has been shown that HPP causes minimal changes to the appearance of the oyster and the pressure-treated oysters were acceptable to consumers (15, 21). In addition, our data demonstrate that a lower initial temperature significantly favors human NoV inactivation in oysters by HPP. The processing of oysters at lower temperatures will likely enhance the freshness and quality of the oysters. Therefore, the use of HPP to safely process oysters in a manner that would meet industry requirements and consumer needs is economically and practically feasible.
In summary, our study addresses a major gap in our understanding of whether HPP can effectively inactivate a strain of human NoV GII.4, the most prevalent human NoV genotype worldwide. Using a novel receptor binding assay and a unique gnotobiotic pig model, we found that HPP is capable of inactivating a human NoV GII.4 strain at a commercially acceptable pressure level. Application of HPP in the oyster industry would significantly improve food safety and public health.
ACKNOWLEDGMENTS
This study was supported by a food safety grant (2011-68003-30005) and the NoroCORE project (grant 2011-68003-30395) from the USDA Agriculture and Food Research Initiative (AFRI). Erin DiCaprio is supported by a NoroCORE graduate fellowship.
We thank Xi Jiang at Cincinnati Children's Hospital for providing human NoV antibody for this study.
REFERENCES
- 1.Hedberg CW. 2011. Foodborne illness acquired in the United States. Emerg Infect Dis 17:1338. doi: 10.3201/eid1707.110019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baert L, Debevere J, Uyttendaele M. 2009. The efficacy of preservation methods to inactivate foodborne viruses. Int J Food Microbiol 131:83–94. doi: 10.1016/j.ijfoodmicro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Atmar RL, Estes MK. 2012. Norovirus vaccine development: next steps. Expert Rev Vaccines 11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan M, Jiang X. 2010. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog 6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK. 2004. Laboratory efforts to cultivate noroviruses. J Gen Virol 85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 8.Grohmann GS, Murphy AM, Christopher PJ, Auty E, Greenberg HB. 1981. Norwalk virus gastroenteritis in volunteers consuming depurated oysters. Aust J Exp Biol Med Sci 59:219–228. doi: 10.1038/icb.1981.17. [DOI] [PubMed] [Google Scholar]
- 9.Provost K, Dancho BA, Ozbay G, Anderson RS, Richards GP, Kingsley DH. 2011. Hemocytes are sites of enteric virus persistence within oysters. Appl Environ Microbiol 77:8360–8369. doi: 10.1128/AEM.06887-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian P, Engelbrektson AL, Jiang X, Zhong W, Mandrell RE. 2007. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: a possible mechanism of bioaccumulation. J Food Prot 70:2140–2147. [DOI] [PubMed] [Google Scholar]
- 11.Ueki Y, Shoji M, Suto A, Tanabe T, Okimura Y, Kikuchi Y, Saito N, Sano D, Omura T. 2007. Persistence of caliciviruses in artificially contaminated oysters during depuration. Appl Environ Microbiol 73:5698–5701. doi: 10.1128/AEM.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Caballero ME, Perez-Mateos M, Montero P, Borderias AJ. 2000. Oyster preservation by high-pressure treatment. J Food Prot 63:196–201. [DOI] [PubMed] [Google Scholar]
- 13.Kingsley DH, Hoover DG, Papafragkou E, Richards GP. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J Food Prot 65:1605–1609. [DOI] [PubMed] [Google Scholar]
- 14.Lou F, Neetoo H, Chen H, Li J. 2011. Inactivation of a human norovirus surrogate by high-pressure processing: effectiveness, mechanism, and potential application in the fresh produce industry. Appl Environ Microbiol 77:1862–1871. doi: 10.1128/AEM.01918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye M, Huang Y, Chen H. 2012. Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in oysters by high-hydrostatic pressure and mild heat. Food Microbiol 32:179–184. doi: 10.1016/j.fm.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Grove SF, Lee A, Lewis T, Stewart CM, Chen H, Hoover DG. 2006. Inactivation of foodborne viruses of significance by high pressure and other processes. J Food Prot 69:957–968. [DOI] [PubMed] [Google Scholar]
- 17.Kingsley DH, Holliman DR, Calci KR, Chen H, Flick GJ. 2007. Inactivation of a norovirus by high-pressure processing. Appl Environ Microbiol 73:581–585. doi: 10.1128/AEM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Chen H, Kingsley DH. 2013. The influence of temperature, pH, and water immersion on the high hydrostatic pressure inactivation of GI.1 and GII.4 human noroviruses. Int J Food Microbiol 167:138–143. doi: 10.1016/j.ijfoodmicro.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Ye M, Neetoo H, Golovan S, Chen H. 2013. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int J Food Microbiol 162:37–42. doi: 10.1016/j.ijfoodmicro.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 21.Leon JS, Kingsley DH, Montes JS, Richards GP, Lyon GM, Abdulhafid GM, Seitz SR, Fernandez ML, Teunis PF, Flick GJ, Moe CL. 2011. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol 77:5476–5482. doi: 10.1128/AEM.02801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes MK, Prasad BV, Atmar RL. 2006. Noroviruses everywhere: has something changed? Curr Opin Infect Dis 19:467–474. doi: 10.1097/01.qco.0000244053.69253.3d. [DOI] [PubMed] [Google Scholar]
- 23.Cheetham S, Souza M, McGregor R, Meulia T, Wang Q, Saif LJ. 2007. Binding patterns of human norovirus-like particles to buccal and intestinal tissues of gnotobiotic pigs in relation to A/H histo-blood group antigen expression. J Virol 81:3535–3544. doi: 10.1128/JVI.01306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J Virol 80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang KO, Saif LJ. 2012. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One 7:e41619. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. 1996. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl 12:153–161. [DOI] [PubMed] [Google Scholar]
- 27.Krakowka S, Eaton KA, Leunk RD. 1998. Antimicrobial therapies for Helicobacter pylori infection in gnotobiotic piglets. Antimicrob Agents Chemother 42:1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton KA, Ringler SS, Krakowka S. 1998. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis 178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 29.Dancho BA, Chen H, Kingsley DH. 2012. Discrimination between infectious and non-infectious human norovirus using porcine gastric mucin. Int J Food Microbiol 155:222–226. doi: 10.1016/j.ijfoodmicro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Tian P, Brandl M, Mandrell R. 2005. Porcine gastric mucin binds to recombinant norovirus particles and competitively inhibits their binding to histo-blood group antigens and Caco-2 cells. Lett Appl Microbiol 41:315–320. doi: 10.1111/j.1472-765X.2005.01775.x. [DOI] [PubMed] [Google Scholar]
- 31.Dicaprio E, Ma Y, Hughes J, Li J. 2013. Epidemiology, prevention, and control of the number one foodborne illness: human norovirus. Infect Dis Clin North Am 27:651–674. doi: 10.1016/j.idc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlfeld B, Li Y, Boulaaba A, Binder A, Schotte U, Zimmermann JL, Morfill G, Klein G. 2015. Inactivation of a foodborne norovirus outbreak strain with nonthermal atmospheric pressure plasma. mBio 6(1):e02300-14. doi: 10.1128/mBio.02300-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lou F, Huang P, Neetoo H, Gurtler JB, Niemira BA, Chen H, Jiang X, Li J. 2012. High-pressure inactivation of human norovirus virus-like particles provides evidence that the capsid of human norovirus is highly pressure resistant. Appl Environ Microbiol 78:5320–5327. doi: 10.1128/AEM.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cromeans T, Park GW, Costantini V, Lee D, Wang Q, Farkas T, Lee A, Vinje J. 2014. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl Environ Microbiol 80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. 2007. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain). J Virol 81:9183–9192. doi: 10.1128/JVI.00558-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bui T, Kocher J, Li Y, Wen K, Li G, Liu F, Yang X, LeRoith T, Tan M, Xia M, Zhong W, Jiang X, Yuan L. 2013. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J Gen Virol 94:2005–2016. doi: 10.1099/vir.0.054080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souza M, Costantini V, Azevedo MS, Saif LJ. 2007. A human norovirus-like particle vaccine adjuvanted with ISCOM or mLT induces cytokine and antibody responses and protection to the homologous GII.4 human norovirus in a gnotobiotic pig disease model. Vaccine 25:8448–8459. doi: 10.1016/j.vaccine.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards GP. 2012. Critical review of norovirus surrogates in food safety research: rationale for considering volunteer studies. Food Environ Virol 4:6–13. doi: 10.1007/s12560-011-9072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. 2011. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recommend Rep 60(RR-3):1–18. [PubMed] [Google Scholar]