Abstract
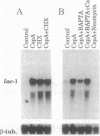
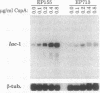
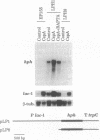
Exposure to cyclosporin A (CspA) increased laccase (lac-1) transcript accumulation in the chestnut blight fungus Cryphonectria parasitica. This response was suppressed by compounds that interfere with calcium-dependent signal transduction and by the presence of a virulence-attenuating mycovirus. CspA stimulated the accumulation of mRNA from a nonhomologous reporter fused to the lac-1 promoter, indicating that the increased transcript levels resulted from an increase in promoter activity. Based on the current model for the regulation of lac-1 transcription, these results suggest that CspA interferes with a negative regulatory pathway that normally constrains lac-1 promoter activity. Significantly, CspA did not stimulate lac-1 transcription in mutant strains deficient in CspA binding activity, directly demonstrating a requirement for the interaction of CspA and cyclophilin in the modulation of lac-1 transcription. Our results establish that CspA treatment can stimulate gene transcription and that cyclophilin is the cellular receptor that mediates this activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C. T., Macchia G., Heguy A., Melli M., Telford J. L. Cyclosporin A blocks calcium-dependent pathways of gene activation. J Biol Chem. 1991 Oct 5;266(28):19103–19108. [PubMed] [Google Scholar]
- Benigni A., Chiabrando C., Piccinelli A., Perico N., Gavinelli M., Furci L., Patino O., Abbate M., Bertani T., Remuzzi G. Increased urinary excretion of thromboxane B2 and 2,3-dinor-TxB2 in cyclosporin A nephrotoxicity. Kidney Int. 1988 Aug;34(2):164–174. doi: 10.1038/ki.1988.162. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Somers P. K., Wandless T. J., Burakoff S. J., Schreiber S. L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science. 1990 Oct 26;250(4980):556–559. doi: 10.1126/science.1700475. [DOI] [PubMed] [Google Scholar]
- Borel J. F. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology. 1976 Oct;31(4):631–641. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius G., Gebauer G., Techel D. Inositol trisphosphate induces calcium release from Neurospora crassa vacuoles. Biochem Biophys Res Commun. 1989 Jul 31;162(2):852–856. doi: 10.1016/0006-291x(89)92388-7. [DOI] [PubMed] [Google Scholar]
- Cullen D., Leong S. A., Wilson L. J., Henner D. J. Transformation of Aspergillus nidulans with the hygromycin-resistance gene, hph. Gene. 1987;57(1):21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Signal transduction. Immunosuppressants at work. Nature. 1991 Aug 29;352(6338):754–755. doi: 10.1038/352754a0. [DOI] [PubMed] [Google Scholar]
- Dietmeier K., Tropschug M. Nucleotide sequence of a full-length cDNA coding for cyclophilin (peptidyl-prolyl cis-trans isomerase) of Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Jan 25;18(2):373–373. doi: 10.1093/nar/18.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hiestand P. C., Hall M. N. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Hultsch T., Albers M. W., Schreiber S. L., Hohman R. J. Immunophilin ligands demonstrate common features of signal transduction leading to exocytosis or transcription. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6229–6233. doi: 10.1073/pnas.88.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes H. D., Jackson N. M., O'Connor R. P., Hunt D. A., White M. D. Pathogenetic mechanisms of nephrotoxicity: insights into cyclosporine nephrotoxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):51–62. [PubMed] [Google Scholar]
- Janssens P. M. The evolutionary origin of eukaryotic transmembrane signal transduction. Comp Biochem Physiol A Comp Physiol. 1988;90(2):209–223. doi: 10.1016/0300-9629(88)91106-1. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine: the agent and its actions. Transplant Proc. 1985 Aug;17(4 Suppl 1):5–18. [PubMed] [Google Scholar]
- Kino T., Hatanaka H., Hashimoto M., Nishiyama M., Goto T., Okuhara M., Kohsaka M., Aoki H., Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1987 Sep;40(9):1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Choi G. H., Nuss D. L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992 Dec;11(12):4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Sehajpal P. K., Khanna A., Vlassara H., Cerami A., Stenzel K. H., Suthanthiran M. Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med. 1991 Nov 1;174(5):1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Martel R. R., Klicius J., Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977 Feb;55(1):48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. When worlds collide: immunosuppressants meet protein phosphatases. Cell. 1991 Sep 6;66(5):823–826. doi: 10.1016/0092-8674(91)90426-y. [DOI] [PubMed] [Google Scholar]
- Nambi P., Pullen M., Contino L. C., Brooks D. P. Upregulation of renal endothelin receptors in rats with cyclosporine A-induced nephrotoxicity. Eur J Pharmacol. 1990 Oct 2;187(1):113–116. doi: 10.1016/0014-2999(90)90346-8. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Gu X. H., Casley D. J., Panagiotopoulos S., Liu J., Mottram P. L. Cyclosporine increases endothelin-1 binding site density in cardiac cell membranes. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1270–1274. doi: 10.1016/0006-291x(89)91115-7. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F., Durette P., Siekierka J. J., Peterson L., Rich D. H., Dunlap B. E., Staruch M. J., Melino M. R., Koprak S. L. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J Exp Med. 1991 Mar 1;173(3):619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Janssens P. M., Erneux C. Sensory transduction in eukaryotes. A comparison between Dictyostelium and vertebrate cells. Eur J Biochem. 1991 Jan 30;195(2):289–303. doi: 10.1111/j.1432-1033.1991.tb15706.x. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]