Abstract
To understand the relationship between elevation and bacterial communities in wastewater treatment plants (WWTPs), bacterial communities in 21 municipal WWTPs across China, located 9 to 3,660 m above sea level (masl), were investigated by 454 pyrosequencing. A threshold for the association of elevation with bacterial community richness and evenness was observed at approximately 1,200 masl. At lower elevations, both richness and evenness were not significantly associated with elevation. At higher elevations, significant declines with increased elevations were observed for community richness and evenness. The declining evenness trend at the phylum level was reflected by distinct trends in relative abundance for individual bacterial phyla. Betaproteobacteria, Bacteroidetes, and Firmicutes displayed significant increases, while most other phyla showed declines. Spearman correlation analysis indicated that the community richness and evenness at high elevations were more correlated with elevation than with any other single environmental variable. Redundancy analysis indicated that the contribution of elevation to community composition variances increased from 3% at lower elevations to 11% at higher elevations whereas the community composition variance at higher elevations remained much more explained by operational variables (39.2%) than by elevation. The influent total phosphorus concentration, food/microorganism ratio, and treatment process were the three shared dominant contributors to the community composition variance across the whole elevation gradient, followed by effluent ammonia nitrogen and temperature at higher elevations.
INTRODUCTION
Wastewater treatment plants (WWTPs) are broadly applied for the efficient removal of contaminants from wastewater before its discharge into the natural environment. A complex microbial community is the most dominant contributor to the removal of contaminants in WWTPs. A better understanding of the microbial community structure and its relationship to influential variables could provide important guidance for the improvement of wastewater treatment efficiency (1). Multiple studies have investigated the significant associations between WWTP microbial community structures and operational parameters or wastewater characteristics, such as dissolved oxygen concentration (2), temperature (3), treatment process (4), influent ammonia nitrogen concentration (5), biological oxygen demand (6), and total phosphorus (TP) concentration (7). However, regardless of the weakly neutral assembly of bacterial communities (8), there is still a large part of the bacterial community variance that cannot be explained by these well-known variables. Thus, it is presumed that there are still other environmental variables that potentially influence the variance of WWTP microbial communities.
In recent years, a few studies were performed to explore the relationship between microbial communities and geographical locations of WWTPs. Zhang et al. (9) found significant geographical differences in bacterial communities among 15 activated-sludge samples collected from Asian and North American WWTPs. A study by Zhao et al. (10) indicated that the bacterial community structures were geographically different among 10 WWTPs located in eastern and western China. Wang et al. (11) reported that geographical locations explained approximately 14.7% of the bacterial community variation among 14 WWTPs located in four cities in China. The studies mentioned above suggested that the geographical distribution of WWTPs might be an important and influential variable in the variance of WWTP microbial communities.
However, to date, no study has been performed to systematically assess the geographical characteristics of WWTP microbial communities. On the one hand, the previous studies collected activated-sludge samples from just several particular cities, and the small number of sampled cities might lead to a biased result that is different from the real geographical characteristics of WWTP microbial communities. On the other hand, all of the previous studies focused on only two geographical variables, longitude and latitude, and the variable of elevation was never considered. Elevation gradients are characterized by distinct changes in climate and biotic turnover over short elevation distances. Microbial community structure and metabolic processes respond significantly to elevation in natural environments (12). For example, Bryant et al. found that bacterial taxonomic richness decreased monotonically along the Rocky Mountains from 2,460 to 3,380 m above sea level (masl) (13). Yang et al. revealed that endemic stress genes were more abundant at higher elevations on the Tibetan grassland (12). However, the knowledge of the relationship between elevation and microbial communities in man-made WWTPs remains quite poor.
In recent years, multiple WWTPs were constructed in high-elevation areas, such as Bolivia and the Tibetan Plateau, which are approximately 3,000 masl. Nevertheless, many high-elevation WWTPs fail to reach the required quality for effluent contaminants (according to Bolivian environmental law) (14) or exhibit a lower efficiency for the removal of contaminants than that obtained at low elevations. The function and performance of WWTPs are closely associated with WWTP microbial communities, which have been characterized by community richness (15), evenness (16), and composition (17). To improve the performance of high-elevation WWTPs, it is necessary to reveal the relationship between WWTP microbial communities and elevation. Thus, we hypothesized that the microbial communities in WWTPs change with the elevation gradient.
Based on the hypothesis mentioned above, the relationships between WWTP microbial communities and environmental variables should change with the variance of WWTP microbial communities along an elevation gradient. Therefore, the objectives of this study were to test this hypothesis and to assess the potential linkages between WWTP microbial communities and elevation, as well as other environmental variables, along different elevation gradients. Twenty-one full-scale WWTPs distributed across China, along an elevation gradient from 9 to 3,660 masl, were chosen for the investigation of bacterial communities. The bacterial communities of 63 activated-sludge samples were screened using 454 pyrosequencing. Redundancy analysis (RDA) and Spearman correlation analysis were performed to assess the association between environmental variables and bacterial communities.
MATERIALS AND METHODS
Sample collection and DNA extraction.
In this study, 63 activated-sludge samples were collected in May 2014 from aeration tanks of 21 full-scale WWTPs treating municipal wastewater. These WWTPs are located across China, at elevations of 9 to 3,660 masl (Fig. 1). Triplicate samples taken at least 5 m apart were collected from each tank. Each sample was made by mixing five activated-sludge replicates randomly collected within a 1-m-diameter circle. Detailed characteristics of the WWTPs are shown in Tables S1 to S3 in the supplemental material. Activated-sludge samples were kept in a dry-ice box immediately following collection and stored at −80°C in the lab until DNA extraction. After thawing at room temperature, each sample was centrifuged at 14,000 × g for 8 min. The supernatant was decanted, and 5 g of the pellet was weighed out for DNA extraction.
FIG 1.
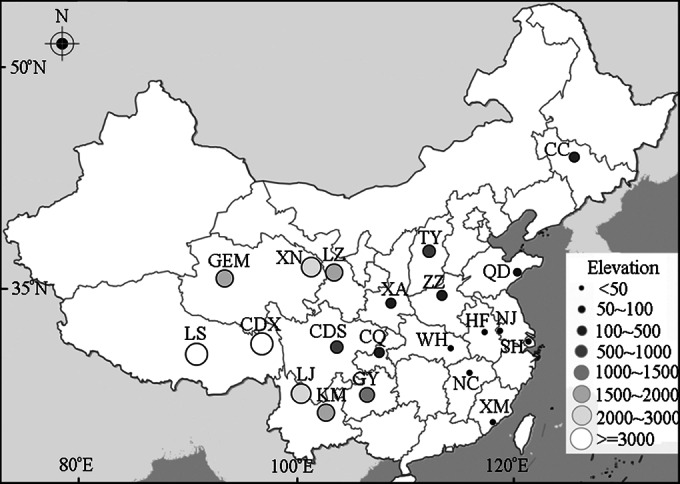
Geographical distribution of 21 wastewater treatment plants (WWTPs) in China. Elevations of these WWTPs are represented by the sizes of symbols. (The map was created using the online software Mapping-together [Map Hui].)
Genomic DNA was extracted from each sample by using an EZNA soil DNA kit (Omega Bio-Tek Inc.) following the manufacturer's protocol (18). DNA quality was analyzed by agarose gel electrophoresis. The absorption ratios of DNA at 260/280 nm and 260/230 nm were assessed using an ND-2000 spectrophotometer (NanoDrop Inc., Wilmington, DE). The DNA extracts were stored at −80°C until subsequent analysis.
PCR amplification and purification.
The DNA samples were amplified using the universal bacterial primer set 27F and 518R to amplify the V1 to V3 regions of the bacterial 16S rRNA gene, as previously described (19). Bar codes that allow sample multiplexing during pyrosequencing were incorporated between the 454 adapter and the forward primers.
Pyrosequencing and sequence analysis.
The DNA samples were sequenced by use of a Roche 454 FLX Titanium sequencer (Roche, Nutley, NJ) at the Chinese National Human Genome Center (SinoGenoMax). The obtained sequence reads were processed using mothur (20). Raw sequences were removed from consideration if they were shorter than 150 bp or if they had even a single base bias. PCR chimeras were checked and removed using the chimera.uchime command implemented in mothur. The remaining sequences were aligned to the RDP Classifier database to determine their phylogenetic assignments, with a confidence threshold of 80%.
Data analysis.
The alpha-diversity indices (Shannon H index and evenness index) for each sample were calculated using the vegan package in R (v. 3.12; http://www.r-project.org/). Comparisons of the relative abundances of the most abundant operational taxonomic units (OTUs) among samples were performed using the pheatmap package in R. Detrended correspondence analysis showed that the data set used in this study followed a linear relationship (21). Thus, RDA assuming a linear relationship was performed to evaluate the relationships between bacterial community compositions and environmental variables, with 999 permutations, by use of the vegan package in R. The contributions of wastewater characteristics, operational parameters, and geographical locations to the variation in bacterial communities were calculated by variance partitioning analysis using RDA. The treatment process was numbered according to the number of separate process tanks across the treatment process (see Table S4 in the supplemental material) (4).
All of the environmental parameters were log transformed for the analysis. Correlation coefficients among environmental variables and bacterial communities were measured by the Spearman method, using SPSS 20.0, as previously described (3). Clustering analysis of the bacterial communities of samples was performed based on the Bray-Curtis similarity method, using PAST 3.0, as previously described (9). The significance index (i.e., P value) was calculated with two sides, and a P value of <0.05 was considered significant.
Nucleotide sequence accession number.
The raw sequence reads obtained in this study were deposited in the NCBI Sequence Read Archive database under accession number SRX958005.
RESULTS AND DISCUSSION
In this study, 454 pyrosequencing of 16S rRNA genes generated 503,366 quality-filtered sequence reads from 63 samples, with an average of 7,990 sequences for each sample. These sequences were grouped into a total of 19,345 OTUs, with a 3% sequence dissimilarity cutoff. The abundant OTUs (with >100 reads in the total samples) accounted for only 4.6% of the total number of OTUs but represented 63.1% of all reads (see Table S5 in the supplemental material). Rarefaction curves revealed that samples from the LS and CDX sites presented the lowest OTU richness values and appeared to reach an asymptote, whereas the samples from the SH and QD sites appeared to be the most diverse and required greater sequence sampling to cover the full extent of taxonomic diversity (see Fig. S1 in the supplemental material).
Diversity of bacterial communities in activated sludge.
RDP Classifier was used to assign sequences to different taxonomic levels, with an 80% threshold. A large portion of the sequences were grouped as unclassified sequences in this study. The size of the unclassified fraction increased from the phylum (0.24%) to the genus (42.8%) level.
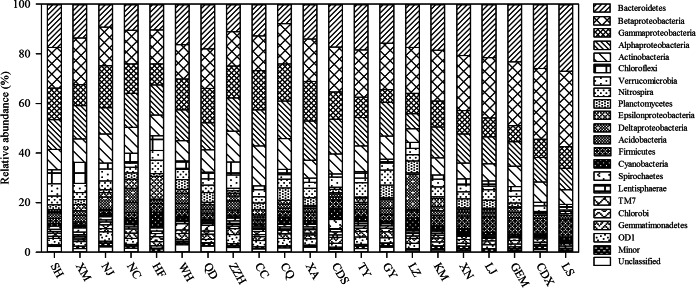
Figure 2 summarizes the relative abundances of the bacterial community at the phylum level for each WWTP. Proteobacteria represented the most abundant phylum, accounting for an average of 46.1% of the total sequences. Bacteroidetes was the second most abundant phylum (16.7%), followed by Actinobacteria (9.1%), Firmicutes (3.5%), and Chloroflexi (2.9%). This result is similar to those observed in many previous studies focusing on municipal WWTPs (9, 22) but is also different from the results of a few other studies. For example, it was found previously that Acidobacteria comprised 1 to 27% of bacteria detected in membrane bioreactor (MBR) wastewater treatment systems (23), but in the present study, Acidobacteria accounted for only 0.5 to 4.1% of bacteria detected in the wastewater treatment systems. On average, 2.5% of the total sequences belonged to minor phyla (<0.3% of total reads), and their abundances are displayed as the sum of the abundances of the minor groups (Fig. 2).
FIG 2.
Relative abundances of bacterial phyla and classes of Proteobacteria in 21 WWTPs. “Minor” refers to phyla with a maximum abundance of 1% in any sample. The relative abundances of bacterial phyla in each WWTP were calculated by their average percentages in the total sequences of the triplicate samples.
Within the Proteobacteria, Epsilonproteobacteria was present only at a very low level, with an average abundance of 1.6% of the total sequences. Except for the NC and CC WWTPs, which had Alpha- and Gammaproteobacteria, respectively, as the most dominant subdivisions, Betaproteobacteria was the predominant subdivision (averaging 18.9% abundance), followed by Alpha-, Gamma-, and Deltaproteobacteria (12.1%, 10.9%, and 2.5%, respectively). The abundance of betaproteobacteria observed in this study agrees with the results revealed by other researchers (23).
The population of Bacteroidetes was mainly composed of two classes: Sphingobacteria and Bacteroidia. Consistent with other studies on activated sludge (23), Sphingobacteria was the most predominant class of Bacteroidetes and was commonly obtained from all samples (averaging 8.8% abundance). Bacteroidia was the subdominant class (averaging 4.2% abundance), following Sphingobacteria; however, the abundance of Bacteroidia was much higher than that of Sphingobacteria in the LS, CDX, GEM, and JL WWTPs, which are located in western China. This result agrees with those of Zhao et al., who showed that Bacteroidia, Clostridia, and Betaproteobacteria were significantly abundant in WWTPs located in northwestern China (10).
Shared families/genera among activated-sludge samples.
Using RDP Classifier, a total of 201 families were observed. Sixty-three families, representing 79 to 91% of the classified sequences, including Rhodocyclaceae, Xanthomonadaceae, Verrucomicrobiaceae, Nitrosomonadaceae, Alcaligenaceae, and Cytophagaceae, were commonly shared by all samples. Most of the shared families were also shared in many other studies (3, 24).
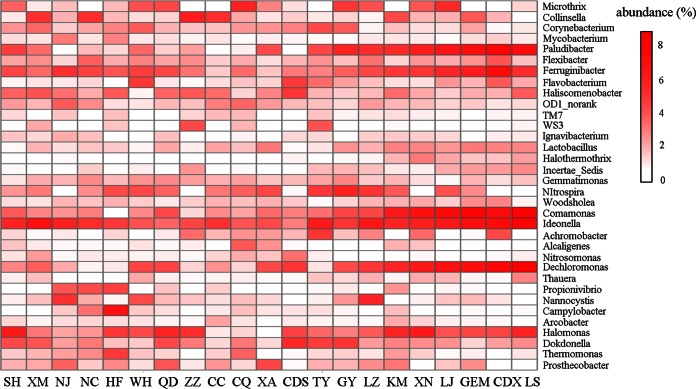
At the genus level, a total of 687 genera were obtained from the 63 samples. Among them, 65 genera were shared by all samples, accounting for 57 to 82% of the classified sequences and 32.5 to 46.7% of the total sequences. The 10 most abundant genera in each WWTP were selected (a total of 34 genera for 21 WWTPs), and their relative abundances were compared to those in other WWTPs by heat map analysis (Fig. 3). Thirteen genera were abundant (>1%) in at least 15 WWTPs.
FIG 3.
Heat map of the top 10 abundant genera in each WWTP. The relative abundances of bacterial genera in each WWTP were calculated by their average percentages in the total sequences of the triplicate samples.
Most of these genera were commonly found in other WWTPs, as described in previous studies (9). For instance, Dechloromonas is frequently reported as a phosphate-accumulating organism in enhanced biological phosphorus removal reactors (9). Zoogloea is the main agent for the flocculation of activated sludge by forming extracellular gelatinous matrices (25, 26). Achromobacter can efficiently degrade different types of antibiotics (27) and polycyclic aromatic hydrocarbons (28). Nitrospira is the dominant N-cycle genus (29).
Relationships among bacterial community compositions of activated-sludge samples.
Principle component analysis (PCoA) was performed to examine the overall variance of bacterial communities among the 63 samples (see Fig. S2 in the supplemental material). The triplicate samples from the same WWTPs exhibited high similarity to each other (Simpson similarity values of >0.96). PCoA showed that the samples collected from the CDX, LS, GEM, XN, and LJ WWTPs grouped together and deviated from the other samples. This result was further demonstrated by clustering analysis of the OTU compositions among the 21 WWTPs, based on the Bray-Curtis similarity method (see Fig. S3). On the clustering tree, a single cluster comprised the LZ and KM WWTPs and the five WWTPs mentioned above. The above results suggested that the bacterial community compositions in WWTPs displayed clear geographical or elevation-based differences. On the one hand, these seven WWTPs are located in western China, while most of the other WWTPs are in eastern China. This pattern is consistent with the results revealed for other WWTPs in China (10). Geographical differences among bacterial communities of activated-sludge samples were also revealed in Asia and America (9). On the other hand, the elevations of the seven WWTPs are above 1,500 masl, which is higher than the others. This observation suggested a potential relationship between elevation and WWTP bacterial community compositions.
Elevation-dependent patterns in bacterial community richness and evenness.
To assess the complexity of individual bacterial communities, the sequences in all samples were randomly normalized to the smallest number of sequences (6,540, in the GEM3 sample). The OTU richness, Shannon H index, and evenness index values of the individual communities were then calculated at the OTU level (see Table S6 in the supplemental material). Based on the bacterial communities of all samples, the OTU richness and Shannon H index values were significantly and positively associated with each other (correlation coefficient = 0.86; P < 0.001), while the correlation coefficients between evenness and richness as well as the Shannon H index were <0.6 (P < 0.01). Thus, OTU richness and evenness were selected for subsequent analysis in this study.
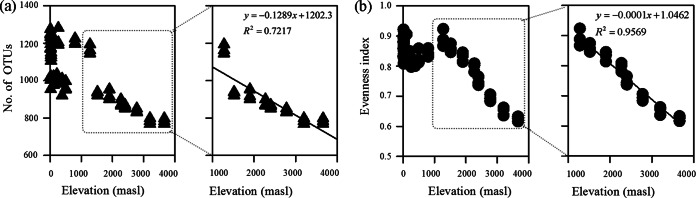
The results showed that there was no trend in either OTU richness or evenness with elevation for the samples collected from WWTPs below 1,200 masl (Fig. 4). At elevations below 1,200 masl, the values for OTU richness and evenness did not change significantly, ranging from 921 to 1,281 and from 0.79 to 0.92, respectively. The two indices were not significantly associated with elevation. Similar results were presented in some studies on soil communities (30, 31) that mixed different climate zones, latitudes, and geologies. In the present study, among all the detected environmental variables, only chemical oxygen demand (COD) was positively associated with bacterial evenness (correlation coefficient = 0.57; P < 0.01), and none of the variables was singly and significantly associated with taxonomic richness (see Table S7 in the supplemental material). It is plausible that the influence of elevation on the taxonomic richness and evenness of WWTP microbial communities was disguised by combinations of the environmental variables rather than by single variables.
FIG 4.
Variance in bacterial OTU richness (a) and evenness (b) across the elevation gradient. At elevations above 1,200 masl, bacterial community richness and evenness were negatively correlated with elevation. Model choice was based on the Akaike information criteria. Taxon richness is represented by the OTU richness. Evenness represents the similarity of the population sizes of the taxa present. The two indices were calculated based on normalized sequences from across the 63 samples.
Compared with the values for sites below 1,200 masl, there was a shift at the midelevation, at approximately 1,200 masl, with linear declines in both the richness and evenness of the bacterial community (R2 = 0.7217 and 0.959, respectively) toward the highest elevation of 3,660 masl (Fig. 4). At the high elevations, above 1,200 masl, minimal richness and evenness values were observed for the LS samples (3,660 masl), which reached approximately 69.8% and 67.3%, respectively, of those observed for the GY samples (1,280 masl). Therefore, our hypothesis was partially supported by the linear patterns of decline in the richness and evenness of WWTP bacterial communities along the elevation gradient from 1,200 to 3,660 masl. It is noteworthy that all samples investigated in this study were collected in May 2014 and that the fluctuation of WWTP microbial communities in different seasons was not considered (6, 8). Thus, the general elevation-dependent patterns of WWTP microbial community richness and evenness should be confirmed further by examining more samples from other seasons, such as winter, and comparisons of the influences of geographical and seasonal variances on the WWTP microbial communities will be an interesting issue in future studies. In the following study, the low and high elevations were separated by a threshold of 1,200 masl.
It is well known that the taxonomic richness and evenness of WWTP microbial communities are closely correlated with the functional characteristics of these communities. Johnson et al. empirically demonstrated the significantly positive association between functional and taxonomic richness (15). Cardinale indicated that communities with more taxa displayed higher nitrogen uptake rates and took greater advantage of capturing biologically available resources (32). Community evenness is considered a key factor in preserving the functional stability of an ecosystem. Wittebolle et al. demonstrated that communities with higher levels of evenness presented more functional resistance to environmental stress (16). Werner et al. reported that methanogenic activity and substrate removal efficiency were correlated with community evenness in full-scale bioenergy systems (33). In all, the data indicate that community richness and evenness are important factors in determining the general performance of WWTPs.
In this study, both the WWTP microbial community richness and evenness at high elevations were almost 2 to 3 times lower than those at low elevations. As mentioned above, the lower richness and evenness values for high elevations might suggest less functional richness and a more susceptible functional stability of WWTP microbial communities than those observed at low elevations. Furthermore, at high elevations, the significantly declining community richness and evenness with increasing elevation might imply a decreased functional resistance of WWTP microbial communities to the extreme environmental stress on the Tibetan Plateau. Though an empirical test of the functional richness and stability of high-elevation WWTPs is lacking, these speculations are partly supported by the real performances of WWTPs at high elevations across the world. In Bolivia (approximately 4,000 masl), many WWTPs have difficulty reaching the required quality for effluent biochemical oxygen demand (BOD) or COD and enteric virus levels according to Bolivian environmental law (14, 34). On the Tibetan Plateau (>3,000 masl), the removal efficiency for ammonia nitrogen in WWTPs, such as the Lhasa and Changdu WWTPs, is generally lower than that in low-elevation WWTPs (see Table S3 in the supplemental material).
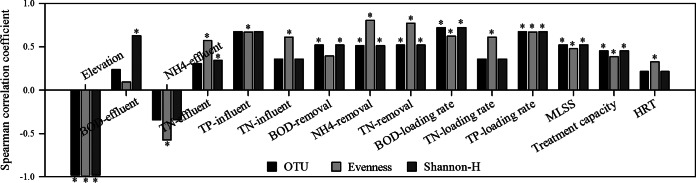
In order to determine whether the observed community changes in richness and evenness at high elevations were the result of elevation or other factors, Spearman analysis was performed to assess the correlations between the community richness and evenness and environmental variables (see Tables S1 to S3 in the supplemental material). The results showed that the community richness and evenness were significantly correlated with 15 environmental variables, such as elevation, influent organic concentrations, and organic loading rates (Fig. 5; see Fig. S4). Among the 15 variables, both of the community indices were most correlated with elevation (correlation coefficients of <−0.97; P < 0.001). Following elevation, the OTU richness was next correlated with BOD and total phosphorus (TP) loading rates (correlation coefficients of >0.67; P < 0.001); the OTU evenness was significantly correlated with ammonia nitrogen (NH4-N) and total nitrogen (TN) removal efficiencies (correlation coefficients of >0.77; P < 0.001). However, based on the results observed above, it remained difficult to identify the real influence of a factor(s) on the changed community richness and evenness with elevation.
FIG 5.
Spearman correlation coefficients for correlations between bacterial diversity indices and environmental variables for high-elevation WWTPs. An asterisk indicates that the variable is significantly correlated with the diversity index (P < 0.001).
On the one hand, the two community indices were indeed most correlated with elevation. This suggested that the varied richness and evenness of bacterial communities might be influenced more by elevation than by any other single variable in high-elevation WWTPs. Focusing just on high elevations, above 1,200 masl, the declining trend in WWTP microbial community richness with increasing elevation agrees with that in natural environments, such as for bacterial communities from mountain soil (35, 36). Thus, the declining trend in WWTP microbial community richness might also be influenced by the climatic conditions at high elevations, which are characterized by more extreme temperature fluctuations, low air pressure, and stronger UV radiation. It has been reported that the amounts of endemic stress genes, such as σ24 genes responsive to extreme temperature stress and the obgE gene for the response to radiation stress, are enriched in the microbial community on the Tibetan grassland (12). This implies that fewer and fewer bacteria can adapt to the extreme physiological challenges on the Tibetan Plateau. However, the main flaw here is that the direct influence of elevation on WWTP microbial community richness and evenness, which needs to be assessed only when all other conditions are identical, has never been assessed in any previous studies as well as in this study.
On the other hand, though the correlations between community richness and evenness and any other single variable were smaller than those for elevation, the effect of the combination of the operational variables on community richness and evenness is still unknown and should not be underestimated. As discussed above, community richness and evenness are two important factors influencing the functional stability and general performances of WWTPs (15). However, although the community richness declined greatly with increased elevation, from an average of 1,234 OTUs in GY samples to 807 OTUs in LS samples, the efficiencies of removal of BOD and TN did not decrease significantly with increasing elevation (see Table S3 in the supplemental material). The nonsignificant declines of organic removal efficiencies might result from the efficient regulation of the combination of the operational variables, which might greatly lessen the potential influence of elevation.
Elevation-dependent patterns in individual bacterial phyla.
The relative abundances of individual bacterial phyla among the total sequences ranged from 0.56 to 18.90% at 1,280 masl and from 0.08 to 30.31% at 3,660 masl (Fig. 5). Among the phyla, only three (Betaproteobacteria, Bacteroidetes, and Firmicutes) presented significantly positive correlations of relative abundance with elevation (correlation coefficients of >0.929; P < 0.001), while the proportions of most remaining phyla decreased (Fig. 6). This significantly polarized trend should be the direct reason for the pattern of decline in community evenness at the phylum level with increases in elevation (see Fig. S5 in the supplemental material). The changes in the proportions of bacterial phyla might result from the selection of bacteria in WWTPs by elevation-dependent environmental variables (37).
FIG 6.
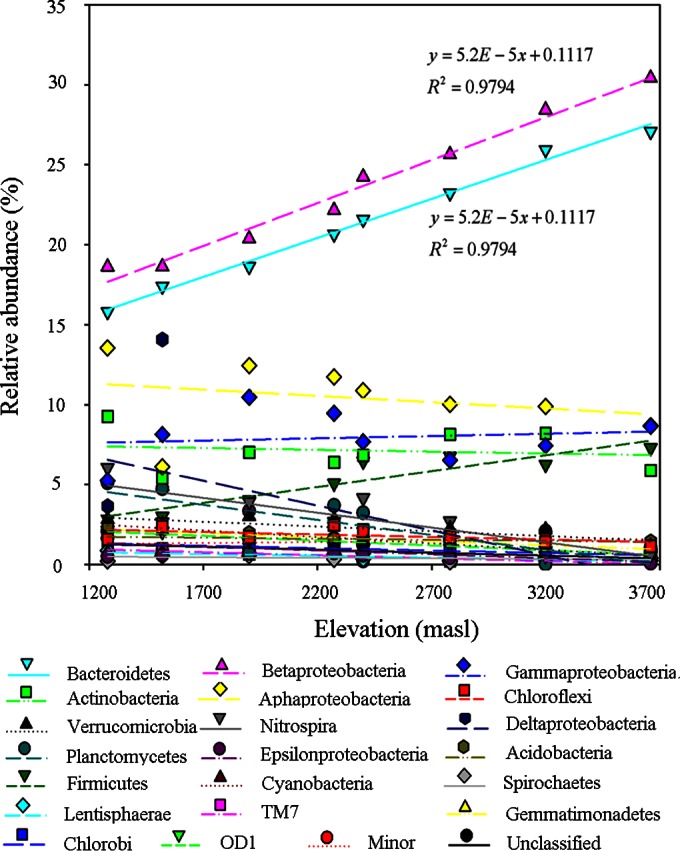
Variance in the relative abundance of each bacterial phylum with elevation for elevations above 1,200 m. The proportions of Betaproteobacteria, Bacteroidetes, and Firmicutes in the total bacterial community increased linearly with increased elevations (regression analysis; R2 > 0.97; P < 0.01). Model choice was based on the Akaike information criteria.
The increasing relative abundances of Betaproteobacteria, Bacteroidetes, and Firmicutes with increasing elevation occurred not only in high-elevation WWTPs but also in mountain soil (35). This suggested that these organisms can better resist the high-elevation environment than other groups. Yang et al. found that Bacteroidetes accumulated the largest amount of endemic stress genes on the Tibetan grassland to adapt to the extreme environment (12). This might be an important reason for the significant increase of the relative abundance of Bacteroidetes in activated sludge with increased elevation. However, Spearman correlation analysis showed that the relative abundances of Betaproteobacteria and Bacteroidetes were also significantly and negatively correlated with the influent total phosphorus concentration (correlation coefficient = −0.905; P < 0.001) (see Table S8 in the supplemental material). Thus, it remains difficult to determine the dominant factor affecting the relative abundances of these phyla in high-elevation WWTPs. The abundances of Chloroflexi and Actinobacteria also increased in the soil bacterial community with increasing elevation, as reported by Singh et al. (35), but these phyla maintained relatively low abundances across all samples in the present study. In summary, these results suggest that some bacterial phyla in high-elevation WWTPs strongly respond to the varied environments at increased elevations.
Linkages between environmental variables and bacterial community compositions.
Based on the results described above, the community richness and evenness of high-elevation WWTPs displayed significant correlations with elevation. Besides the community richness and evenness, the community composition was also an important determining factor in the functional performance of WWTPs. Therefore, redundancy analysis (RDA) was performed to further discern the possible linkages between community compositions and elevation as well as other environmental variables, including 16 wastewater characteristic parameters, 14 operational parameters, and 3 geographical parameters at the two elevation gradients (see Tables S1 to S3 in the supplemental material). Only significant parameters (P < 0.01) were included in further variance partitioning analyses based on automatic forward selection and variance inflation factors, with 999 Monte Carlo permutations. Figure 7 indicates that the majority of variances of bacterial community compositions among the total samples were explained by the three parameter groups.
FIG 7.
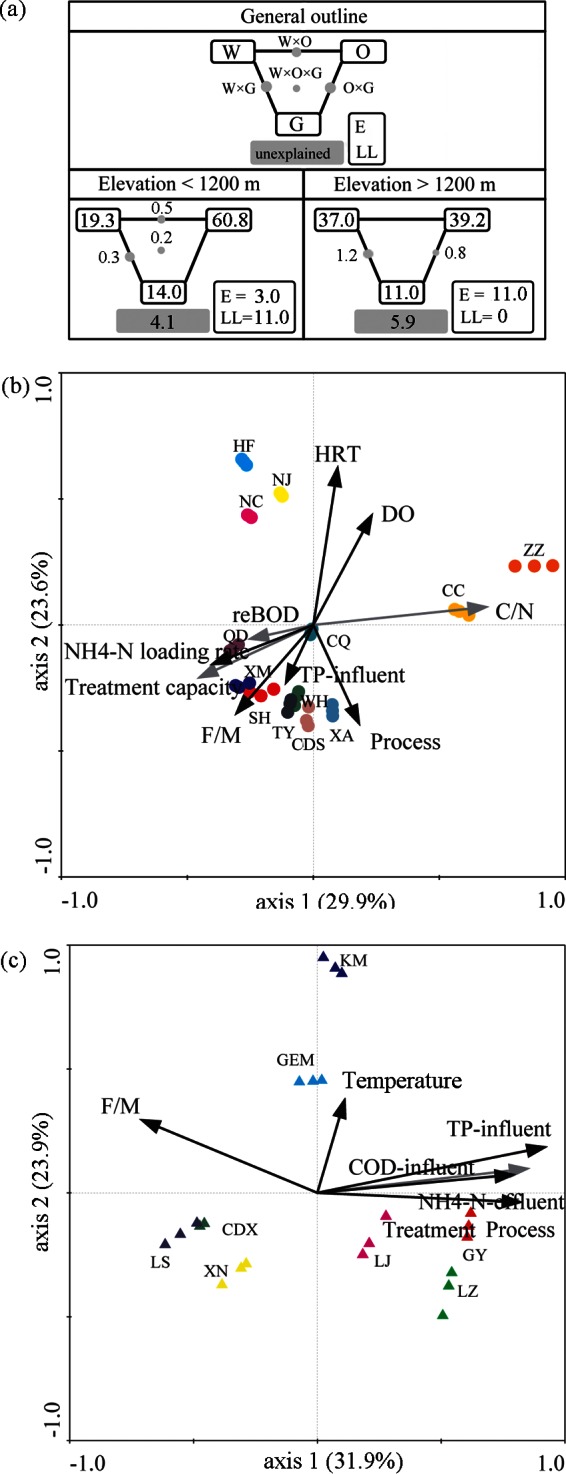
Redundancy analysis of bacterial communities and environmental variables. (a) Variation partitioning analysis was applied to calculate the contributions of wastewater characteristic parameters (W), operational parameters (O), and geographical distributions (G) to the variance of bacterial community structures. RDA ordinations corresponding to bacterial communities were used for the WWTPs at elevations of 9 to 1,200 masl (b) and 1,200 to 3,660 masl (c). E, elevation; LL, longitude and latitude; HRT, hydraulic residence time; DO, dissolved oxygen; BOD, biological oxygen demand; COD, chemical oxygen demand; TP, total phosphorus; F/M, food/microorganism ratio; C/N, COD/TN ratio.
The results showed that elevation statistically accounted for approximately 11% of the community composition variance at high elevations, while it accounted for just 3% of this variance at low elevations (Fig. 7a). On the one hand, the higher contribution of elevation to community composition variance at high elevations suggested an increasing correlation between elevation and community composition with increasing elevation. On the other hand, the different contributions partially agreed with the significant and nonsignificant associations between elevation and WWTP microbial community richness and evenness at high and low elevations, respectively. In addition, the three geographical variables explained approximately 14% and 11% of the variances of WWTP microbial community composition at low and high elevation gradients, respectively. Two geographical variables, longitude and latitude, explained approximately 11% of the variance of WWTP microbial communities at elevations below 1,200 masl, which agreed with the result (14.7%) observed previously (11). This result further suggested that bacterial community compositions in WWTPs are affected by geographical variables.
Besides geographical parameters, RDA was further conducted to assess the associations between the bacterial community composition and the wastewater and operational parameters at low and high elevations separately (Fig. 7b and c). Axes 1 and 2 in RDA ordinations displayed high levels of species-environment correlation (all values were >0.939; P < 0.001), indicating strong correlations between WWTP microbial community composition and wastewater characteristics or operational parameters. The strengths of the relationships between WWTP microbial community compositions and environmental variables are indicated by the lengths of the parameter arrows in the ordination plot of RDA biplots.
At low elevations, 9 of 30 wastewater and operational parameters were significantly associated with community composition variance and explained 80.5% of the total variance. The five most dominant variables were influent C/N ratio (15%), hydraulic retention time (HRT) (12%), food/microorganism ratio (F/M ratio) (9%), NH4-N loading rate (9%), and influent TP (8%) (Fig. 7b). These results agreed with many previous studies that focused on WWTPs at low elevations (3).
At high elevations, six variables among the 30 wastewater and operational parameters were significantly associated with community composition variances, and they statistically accounted for 83.2% of the total variance, with influent TP (19%), F/M ratio (17%), treatment process (13%), effluent NH4-N (11%), and temperature (9%) being the most dominant variables (Fig. 7c). The great contribution of the five variables to WWTP microbial community composition variance indicated that the variance of bacterial community compositions among WWTPs was strongly associated with these variables at high elevations. It is worth noting that the explanatory power of the six variables for the variance of community compositions among high-elevation WWTPs was much higher (83.2%) than that for elevation (11%), i.e., the effect of elevation on the community composition was minor. In summary, though the variances of community richness and evenness were more significantly associated with elevation, the variance of community composition was much more explained by wastewater and operational variables than by elevation. These results suggest that the WWTP microbial community can remain controlled to alleviate the influences of elevation on the performances of high-elevation WWTPs by the regulation and control of operational parameters.
At high elevations, influent TP, F/M ratio, and treatment process remained the most dominant variables but explained much more of the variance of community compositions than that at low elevations. Municipal wastewater is characterized by relatively high and stable contents of nitrogen and phosphorus. Wong et al. (7) reported that a high positive correlation between the influent TP concentration and polyphosphate-stained cells (belonging to the Betaproteobacteria) was observed for 13 activated-sludge samples from nine Japanese WWTPs. In the present study, the relative abundances of multiple bacterial phyla were significantly associated with the TP concentration, such as division OD1, TM7, Firmicutes, and Betaproteobacteria (see Table S8 in the supplemental material). However, it was noted that the relative abundance of betaproteobacteria was negatively associated with the TP concentration and positively associated with elevation. A plausible explanation for this observation may be that polyphosphate-staining cells account for a very small portion of the betaproteobacterial group in WWTPs and that the increasing abundance of betaproteobacteria is driven mainly by the increasing elevation. Further detailed investigations of the characteristics of phosphorus-metabolizing bacteria and betaproteobacteria in WWTPs are needed.
The F/M ratio is an important parameter for evaluating the acclimatization ability of bacterial communities in bioreactors. Some previous studies confirmed that the F/M ratio shaped the microbial abundance and composition in sludge bioreactors (38). In the present study, the F/M ratio was maintained at a low level of 0.05 to 0.13 kg BOD kg−1 mixed liquor suspended solids (MLSS) day−1, which would hinder the growth of bacterial communities in high-elevation WWTPs. In addition, the wastewater treatment process was also considered to be an important variable influencing microbial communities in some previous studies. Jaranowska et al. (4) suggested that the number of separated biological processes in the treatment line was the key factor in shaping the structures of ammonia-oxidizing bacteria in WWTPs. Hu et al. (23) reported that the microbial community structures differed significantly between two different treatment processes operated in parallel in the same WWTP.
Several previous studies have observed that NH4-N is one of the variables with the most influence on the variance of bacterial community compositions in activated-sludge processes. Johnson et al. (15) found significantly negative associations between taxonomic richness and effluent NH4-N concentrations and BOD among WWTPs. In the present study, Nitrosomonas was the dominant ammonia-oxidizing bacterial genus in WWTPs, accounting, on average, for 0.51% of the total sequences in each sample. However, at both low and high elevations, no significant relationship via Spearman correlation analysis was observed between Nitrosomonas and influent and effluent NH4-N concentrations, as well as the NH4-N removal efficiency and loading rate. The abundance of the nitrite-oxidizing organism Nitrospira was also not correlated with any variables associated with NH4-N. Regardless of the N-utilizing organisms, a significant and positive association was observed between NH4-N and Chloroflexi at high elevations in this study, and the latter was frequently reported within other WWTPs (39). Temperature is closely related to the survival and metabolism of bacteria. It has been evaluated as one of the most important explanatory variables associated with the long-term temporal dynamics of bacterial communities in full-scale WWTPs (3). At high elevations, extreme climate characteristics, such as extreme temperature fluctuations and low air pressure, might further enhance the sensitivity of the bacterial community to the environment (12).
There was still a minor part of the WWTP microbial community variance that could not be explained in this study. One possible reason is that limited types of deterministic variables were employed to explain the variance in microbial communities. In this study, elevation change was first identified to be a factor affecting the community compositions of high-elevation WWTPs, explaining approximately 11% of the variance. Besides the key role of deterministic variables, a minimal contribution of the neutral assembly to the variations of the microbial community might exist. A study by Ofiteru et al. indicated that the relative abundances of microbial communities in full-scale WWTPs were determined primarily by stochastic components, such as immigration, random reproduction, and deaths (40). However, Lee et al. observed that activated-sludge communities were assembled mostly by niche-based species sorting processes rather than by neutral mechanisms (8). Thus, the power of neutral processes to explain the community variances is still undetermined.
In summary, the threshold of elevation associated with community richness and evenness in WWTPs was revealed to be approximately 1,200 masl. At low elevations, community richness and evenness were not significantly correlated with elevation. At high elevations, significant declining trends with increasing elevation were observed for OTU richness and evenness.
Spearman analysis indicated that the community richness and evenness were more correlated with elevation than with any other single environmental variable at high elevations. Redundancy analysis indicated that the contribution of elevation to the variance of community composition increased with increasing elevation; however, disagreeing with the results on community richness and evenness, the community composition variance at higher elevations remained more correlated with operational variables than with elevation. The influent total phosphorus concentration, food/microorganism ratio, and treatment process were the three shared dominant contributors to the community composition variance across the whole elevation gradient, followed by effluent ammonia nitrogen and temperature at higher elevations.
Therefore, despite the potential influence of elevation on high-elevation WWTP microbial communities, the bacterial community might remain controlled to achieve desired performance objectives by regulation of the operational parameters. This study provides new insights into the relationship between elevation and activated-sludge bacterial communities along a 3,600-masl elevation gradient. The results observed in this study provide an important theoretical basis for the regulation of the performance of high-elevation WWTPs (above 1,200 masl).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grants 51322901 and 51479066), the National Science Funds for Creative Research Groups of China (grant 51421006), the China Postdoctoral Science Foundation (grant 2015M570403), Jiangsu Planned Projects for Postdoctoral Research funds (grant 1401008A), Priority Academic Program Development of Jiangsu Higher Education Institutions, Fundamental Research Funds for the Central Universities (grant 2014B07614), and the Open Research Fund of the Ministry of Education Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes (grant 2014001).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01842-15.
REFERENCES
- 1.Oerther DB, De los Reyes FL, De los Reyes MF, Raskin L. 2001. Quantifying filamentous microorganisms in activated sludge before, during, and after an incident of foaming by oligonucleotide probe hybridizations and antibody staining. Water Res 35:3325–3336. doi: 10.1016/S0043-1354(01)00057-4. [DOI] [PubMed] [Google Scholar]
- 2.Gaval G, Pernelle JJ. 2003. Impact of the repetition of oxygen deficiencies on the filamentous bacteria proliferation in activated sludge. Water Res 37:1991–2000. doi: 10.1016/S0043-1354(02)00421-9. [DOI] [PubMed] [Google Scholar]
- 3.Wells GF, Park H-D, Eggleston B, Francis CA, Criddle CS. 2011. Fine-scale bacterial community dynamics and the taxa-time relationship within a full-scale activated sludge bioreactor. Water Res 45:5476–5488. doi: 10.1016/j.watres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Jaranowska P, Cydzik-Kwiatkowska A, Zielinska M. 2013. Configuration of biological wastewater treatment line and influent composition as the main factors driving bacterial community structure of activated sludge. World J Microbiol Biotechnol 29:1145–1153. doi: 10.1007/s11274-013-1273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lydmark P, Almstrand R, Samuelsson K, Mattsson A, Sorensson F, Lindgren PE, Hermansson M. 2007. Effects of environmental conditions on the nitrifying population dynamics in a pilot wastewater treatment plant. Environ Microbiol 9:2220–2233. doi: 10.1111/j.1462-2920.2007.01336.x. [DOI] [PubMed] [Google Scholar]
- 6.Hai R, Wang Y, Wang X, Li Y, Du Z. 2014. Bacterial community dynamics and taxa-time relationships within two activated sludge bioreactors. PLoS One 9:e90175. doi: 10.1371/journal.pone.0090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong MT, Mino T, Seviour RJ, Onuki M, Liu WT. 2005. In situ identification and characterization of the microbial community structure of full-scale enhanced biological phosphorous removal plants in Japan. Water Res 39:2901–2914. doi: 10.1016/j.watres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Kang HJ, Park HD. 2015. Influence of influent wastewater communities on temporal variation of activated sludge communities. Water Res 73:132–144. doi: 10.1016/j.watres.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Shao MF, Ye L. 2012. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6:1137–1147. doi: 10.1038/ismej.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D, Huang R, Zeng J, Yu Z, Liu P, Cheng S, Wu QL. 2014. Pyrosequencing analysis of bacterial community and assembly in activated sludge samples from different geographic regions in China. Appl Microbiol Biotechnol 98:9119–9128. doi: 10.1007/s00253-014-5920-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Hu M, Xia Y, Wen X, Ding K. 2012. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl Environ Microbiol 78:7042–7047. doi: 10.1128/AEM.01617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Gao Y, Wang S, Xu D, Yu H, Wu L, Lin Q, Hu Y, Li X, He Z, Deng Y, Zhou J. 2014. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J 8:430–440. doi: 10.1038/ismej.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL. 2008. Colloquium paper: microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci U S A 105:11505–11511. doi: 10.1073/pnas.0801920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabalaga J, Amy G, von Munch E. 2007. Evaluation of agricultural reuse practices and relevant guidelines for the Alba Rancho WWTP (primary and secondary facultative ponds) in Cochabamba, Bolivia. Water Sci Technol 55:469–475. doi: 10.2166/wst.2007.045. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DR, Lee TK, Park J, Fenner K, Helbling DE. 19 February 2014. The functional and taxonomic richness of wastewater treatment plant microbial communities are associated with each other and with ambient nitrogen and carbon availability. Environ Microbiol doi: 10.1111/1462-2920.12429. [DOI] [PubMed] [Google Scholar]
- 16.Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N. 2009. Initial community evenness favours functionality under selective stress. Nature 458:623–626. doi: 10.1038/nature07840. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Xia Y, Wen X, Yang Y, Zhou J. 2014. Microbial community functional structures in wastewater treatment plants as characterized by GeoChip. PLoS One 9:e93422. doi: 10.1371/journal.pone.0093422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jie W, Peng Y, Ren N, Li B. 2014. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour Technol 152:124–129. doi: 10.1016/j.biortech.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Sickinger M, Ciobota V, Herrmann M, Rasch H, Rosch P, Popp J, Kusel K. 2014. Revealing the microbial community structure of clogging materials in dewatering wells differing in physico-chemical parameters in an open-cast mining area. Water Res 63:222–233. doi: 10.1016/j.watres.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TS, Jeong JY, Wells GF, Park HD. 2013. General and rare bacterial taxa demonstrating different temporal dynamic patterns in an activated sludge bioreactor. Appl Microbiol Biotechnol 97:1755–1765. doi: 10.1007/s00253-012-4002-7. [DOI] [PubMed] [Google Scholar]
- 22.Xia S, Duan L, Song Y, Li J, Piceno YM, Andersen GL, Alvarez-Cohen L, Moreno-Andrade I, Huang C-L, Hermanowicz SW. 2010. Bacterial community structure in geographically distributed biological wastewater treatment reactors. Environ Sci Technol 44:7391–7396. doi: 10.1021/es101554m. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Wang X, Wen X, Xia Y. 2012. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour Technol 117:72–79. doi: 10.1016/j.biortech.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 24.Valentin-Vargas A, Toro-Labrador G, Massol-Deya AA. 2012. Bacterial community dynamics in full-scale activated sludge bioreactors: operational and ecological factors driving community assembly and performance. PLoS One 7:e42524. doi: 10.1371/journal.pone.0042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Y, Chung BS, Lee SS, Park W, Lee SS, Jeon CO. 2009. Zoogloea caeni sp. nov., a floc-forming bacterium isolated from activated sludge. Int J Syst Evol Microbiol 59:526–530. doi: 10.1099/ijs.0.65670-0. [DOI] [PubMed] [Google Scholar]
- 26.Lim DJ, Kim JD, Kim MY, Yoo SH, Kong JY. 2007. Physicochemical properties of the exopolysaccharides produced by marine bacterium Zoogloea sp. KCCM10036. J Microbiol Biotechnol 17:979–984. [PubMed] [Google Scholar]
- 27.Reis PJ, Reis AC, Ricken B, Kolvenbach BA, Manaia CM, Corvini PF, Nunes OC. 2014. Biodegradation of sulfamethoxazole and other sulfonamides by Achromobacter denitrificans PR1. J Hazard Mater 280:741–749. doi: 10.1016/j.jhazmat.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 28.Deng MC, Li J, Liang FR, Yi M, Xu XM, Yuan JP, Peng J, Wu CF, Wang JH. 2014. Isolation and characterization of a novel hydrocarbon-degrading bacterium Achromobacter sp. HZ01 from the crude oil-contaminated seawater at the Daya Bay, southern China. Mar Pollut Bull 83:79–86. doi: 10.1016/j.marpolbul.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Chouari R, Le Paslier D, Daegelen P, Ginestet P, Weissenbach J, Sghir A. 2003. Molecular evidence for novel planctomycete diversity in a municipal wastewater treatment plant. Appl Environ Microbiol 69:7354–7363. doi: 10.1128/AEM.69.12.7354-7363.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fierer N, Jackson RB. 2006. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauber CL, Hamady M, Knight R, Fierer N. 2009. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120. doi: 10.1128/AEM.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardinale BJ. 2011. Biodiversity improves water quality through niche partitioning. Nature 472:86–89. doi: 10.1038/nature09904. [DOI] [PubMed] [Google Scholar]
- 33.Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, Yarasheski K, Cummings TA, Beers AR, Knight R, Angenent LT. 2011. Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc Natl Acad Sci U S A 108:4158–4163. doi: 10.1073/pnas.1015676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Symonds EM, Verbyla ME, Lukasik JO, Kafle RC, Breitbart M, Mihelcic JR. 2014. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res 65:257–270. doi: 10.1016/j.watres.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Singh D, Takahashi K, Kim M, Chun J, Adams JM. 2012. A hump-backed trend in bacterial diversity with elevation on Mount Fuji, Japan. Microb Ecol 63:429–437. doi: 10.1007/s00248-011-9900-1. [DOI] [PubMed] [Google Scholar]
- 36.Meng H, Li K, Nie M, Wan JR, Quan ZX, Fang CM, Chen JK, Gu JD, Li B. 2013. Responses of bacterial and fungal communities to an elevation gradient in a subtropical montane forest of China. Appl Microbiol Biotechnol 97:2219–2230. doi: 10.1007/s00253-012-4063-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LM, Wang M, Prosser JI, Zheng YM, He JZ. 2009. Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:52–61. doi: 10.1111/j.1574-6941.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 38.Cardinali-Rezende J, Araujo JC, Almeida PG, Chernicharo CA, Sanz JL, Chartone-Souza E, Nascimento AM. 2013. Organic loading rate and food-to-microorganism ratio shape prokaryotic diversity in a demo-scale up-flow anaerobic sludge blanket reactor treating domestic wastewater. Antonie Van Leeuwenhoek 104:993–1003. doi: 10.1007/s10482-013-0018-y. [DOI] [PubMed] [Google Scholar]
- 39.Peng X, Zhang Z, Zhao Z, Jia X. 2012. 16S ribosomal DNA clone libraries to reveal bacterial diversity in anaerobic reactor-degraded tetrabromobisphenol A. Bioresour Technol 112:75–82. doi: 10.1016/j.biortech.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 40.Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA, Sloan WT. 2010. Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci U S A 107:15345–15350. doi: 10.1073/pnas.1000604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.