Abstract
Streptomyces clavuligerus claR::aph is a claR-defective mutant, but in addition to its claR defect it also carries fewer copies of the resident linear plasmids pSCL2 and pSCL4 (on the order of 4 × 105-fold lower than the wild-type strain), as shown by qPCR. To determine the function of ClaR without potential interference due to plasmid copy number, a new strain, S. clavuligerus ΔclaR::aac, with claR deleted and carrying the wild-type level of plasmids, was constructed. Transcriptomic analyses were performed in S. clavuligerus ΔclaR::aac and S. clavuligerus ATCC 27064 as the control strain. The new ΔclaR mutant did not produce clavulanic acid (CA) and showed a partial expression of genes for the early steps of the CA biosynthesis pathway and a very poor expression (1 to 8%) of the genes for the late steps of the CA pathway. Genes for cephamycin C biosynthesis were weakly upregulated (1.7-fold at 22.5 h of culture) in the ΔclaR mutant, but genes for holomycin biosynthesis were expressed at levels from 3- to 572-fold higher than in the wild-type strain, supporting the observed overproduction of holomycin by S. clavuligerus ΔclaR::aac. Interestingly, three secondary metabolites produced by gene clusters SMCp20, SMCp22, and SMCp24, encoding still-cryptic compounds, had partially or totally downregulated their genes in the mutant, suggesting a regulatory role for ClaR wider than previously reported. In addition, the amfR gene was downregulated, and consequently, the mutant did not produce aerial mycelium. Expression levels of about 100 genes in the genome were partially up- or downregulated in the ΔclaR mutant, many of them related to the upregulation of the sigma factor-encoding rpoE gene.
INTRODUCTION
The claR gene, located in the Streptomyces clavuligerus CA gene cluster, encodes a LysR-type regulator (1, 2). LysR-type transcriptional regulators (LTTRs) follow the pattern of the model regulator controlling lysA expression in Enterobacteriaceae (3). The LTTRs act on metabolic pathways but also in quorum sensing, virulence, motility, nitrogen fixation, oxidative stress responses, and other systems (3, 4, 5). These global transcriptional regulators act mostly as activators but also as repressors and control single genes or operons. The LysR proteins are tetramers, have a helix-turn-helix (HTH) motif close to the N terminus, and bind palindromic sequences identified as T-N11-A (6). The binding affinity is usually determined by a ligand coinducer molecule (7).
The presence of LysR-type regulators in antibiotic biosynthesis gene clusters is relatively frequent (8, 9, 10). In undecylprodigiosin and actinorhodin production, the autoregulated StgR LTTR negatively controls the expression of the redD and actII-orf4 genes encoding pathway-specific activators (11).
The claR gene of S. clavuligerus is expressed as a monocistronic transcript that encodes a protein of 431 amino acids (Mr, 47,080). ClaR contains HTH motifs in its amino- and carboxyl-terminal regions. The HTH close to the C-terminal end of the protein contains, fully conserved, the S352Q, T357, and L363E positions present in all LysR-type regulators; an additional HTH motif present at the N-terminal end of ClaR (amino acids 3 to 51) is less conserved (2). Disruption of the claR gene resulted in lack of CA production and accumulation of the intermediate clavaminic acid (1). Amplification of claR in a multicopy plasmid increased 3-fold clavulanic acid production, whereas cephamycin C production was significantly reduced (2).
Northern transcriptional analysis using RNA isolated from the wild type and a claR mutant obtained in S. E. Jensen's laboratory showed that the expression of genes encoding the early steps of the pathway, ceaS2 to cas2, was normal in the mutant (1); these results and the accumulation of the intermediate clavaminic acid in the claR mutant supported a ClaR control on the expression of genes for the late steps of the clavulanic acid pathway.
Expression of claR itself is subject to control by CcaR, the cephamycin C-clavulanic acid supercluster specific regulator. Northern hybridization (2) or S1 analysis (1) showed undetectable claR transcription in ccaR-disrupted mutants. Transcriptomic studies indicated that the ccaR-disrupted mutant expressed claR at a level on the order of 16- to 24-fold lower than for the wild-type strain (12). Recombinant CcaR protein purified from Escherichia coli gave two complexes of gel mobility shift on probes carrying the claR promoter, showing that CcaR had higher specificity for the lower-mobility complex, as detected by competition studies (13).
Improving our knowledge of claR function has been hampered by the difficulty in purifying the recombinant ClaR (rClaR) in an active form. Therefore, in this work we focused our attention on the transcriptomic analysis of the strain S. clavuligerus ΔclaR::aac in comparison to the wild-type strain. The analysis revealed that ClaR has a wider effect than previously thought, affecting S. clavuligerus development and additional clusters for secondary metabolites.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this work are listed in Table 1. A preculture of these strains was grown in Trypticase soy broth (TSB) for 24 h at 28°C and 220-rpm shaking to an optical density at 600 nm (OD600) of 6.5. These seed cultures were used to inoculate (5%, vol/vol) duplicated 500-ml triple-baffled flasks containing 100 ml of SA defined medium (14), and the culture were grown for 72 h under the same conditions. ME medium (15) was used to analyze the aerial mycelium formation and sporulation of the strains. Apramycin (50 μg/ml), hygromycin (50 µg/ml), or nalidixic acid (25 μg/ml) was added to the cultures when required.
TABLE 1.
Strains used in this work
| Strain | Origin | Characteristics |
|---|---|---|
| S. clavuligerus ATCC 27064 | ATCC | Wild-type strain. Produces cephamycin C and clavulanic acid. Does not produce holomycin. Carries standard copy no. of plasmids pSCL2 and pSCL4. |
| S. clavuligerus ΔclaR::aac | This work | Clavulanic acid nonproducer, holomycin producer, apramycin-resistant strain. Standard copy no. of pSCL2 and pSCL4. |
| S. clavuligerus claR::aph | Fuente et al., 2002 (16) | Clavulanic acid nonproducer, holomycin producer strain. Low copy no. of pSCL2 and pSCL4. Obtained by transformation of protoplasts. Renamed S. clavuligerus claR::aph pSCLlow |
| S. clavuligerus oppA2::aph pSCLlow | Lorenzana et al., 2004 (32); Álvarez-Álvarez et al., 2014 (21) | Clavulanic acid nonproducer, holomycin producer strain. Kanamycin resistant. Low copy no. of pSCL2 and pSCL4. No sporulating strain. Used in qPCR as low plasmid copy no. control strain. |
| S. clavuligerus ΔclaR::aac(pMS83-claR) | This work | Clavulanic acid producer, holomycin nonproducer, apramycin- and hygromycin-resistant strain. Standard copy no. of pSCL2 and pSCL4. Sporulating strain. |
| S. clavuligerus ΔclaR::aac(pMS83) | This work | Clavulanic acid nonproducer, holomycin producer, apramycin- and hygromycin-resistant strain. Standard copy no. of pSCL2 and pSCL4. |
Strain construction.
S. clavuligerus ΔclaR::aac was obtained from S. clavuligerus ATCC 27064 using a SuperCos1 cosmid carrying the whole clavulanic acid gene cluster, oligonucleotides claR-F and claR-R (TCTTTACTGGACGCGGTGGGACACTGCGGAGACCTCATGATTCC GGGGATCCGTCGACC and CCCGCCCGGTCCGGTCCGGACCCGGTCGCGGCCCGCTCATGTAGGCTGGAGCTGCTTC, where nucleotides in bold correspond to the claR sequence) and the ReDirect Method. In the apramycin-resistant exconjugants, the claR deletion was reconfirmed by PCR, using oligonucleotides cyp-F and Am-R (ACATCGGGACCATCTCCTC and TCAGCCAATCGACTGGCGAGCGGCATCGCATTCTTCGCAT), and by sequencing of the amplicon.
To complement S. clavuligerus ΔclaR::aac, the claR gene with its own promoter (1,516 bp) was amplified by PCR using oligonucleotides claR_cF (GGGCGCGTTCCGCTTCCCG) and claR_cR (CCGCTCAGCCGGACATCCG). The amplified DNA fragment was sequenced and subcloned into the EcoRV site of the integrative vector pMS83, leading to pMS83-claR. The complemented mutant S. clavuligerus ΔclaR::aac(pMS83-claR) was obtained by conjugation and selected by apramycin and hygromycin resistance. The presence of claR in this strain was reconfirmed by PCR and sequencing of the amplicon. S. clavuligerus ΔclaR::aac(pMS83) was used as the complementation control strain.
Antibiotic assays.
Clavulanic acid and cephamycin C were quantified as indicated by Pérez-Redondo et al. (2). Holomycin was determined by bioassay against Micrococcus luteus ATCC 9341 as described by de la Fuente et al. (16). Clavaminic acid was detected as described by Paradkar et al. (1).
Nucleic acids isolation.
DNA was isolated as described by Pospiech and Neumann (17). For RNA isolation, samples from the Streptomyces cultures were stabilized with 2 volumes of RNA Protect Bacteria Reagent (Qiagen) for 5 min, and 150 μl lysozyme (30 mg/ml) in Tris-EDTA (TE) buffer was added to the stabilized mycelium pellet of 1 ml of culture samples. After 10 min, 600 μl buffer RLT (Qiagen) with β-mercaptoethanol was added to the samples, mixed by vortex, transferred to Lysing Matrix B (MP Biomedicals) microtubes, and processed in a FastPrep instrument (MP Biomedicals) with the following program: 30 s, 6.5 m/s; 1 min at 0°C; 30 s, 6.5 m/s; 1 min at 0°C. One volume of phenol-chloroform-isoamyl alcohol was added to the extracts, and the aqueous phase was applied to RNeasy minikit columns (Qiagen) according to the manufacturer's instructions. RNA preparations were incubated with DNase I (Qiagen) to eliminate DNA contamination. When used in reverse transcription-quantitative PCR (RT-qPCR) analysis, the RNA preparations were further treated with DNase I (Ambion) to eliminate all DNA contamination. The RNA was quantified spectrophotometrically in a NanoDrop ND-1000 UV-vis (Thermo Scientific), and its integrity was determined using a Bioanalyzer 2100 and the RNA 6000 Nano LabChip kit (Agilent Technologies). Only RNAs with RNA integrity values above 7.0 were used.
PCR.
PCRs were performed as described by Kieser et al. (18) with a T-gradient thermocycler (Biometra). The PCR mixture contained, in a 20-μl volume, 40 ng DNA template, 0.2 mM each deoxynucleoside triphosphate (dNTP), 0.75 mM MgCl2, 5% dimethyl sulfoxide (DMSO), and 0.1 units of GoTaq DNA Polymerase (Promega). The amplification was carried out with an initial 3-min, 95°C denaturing step. The cycles comprised a denaturing step of 30 s at 95°C, annealing for 30 s at 68°C, and extension for 3.5 min at 72°C. Successive cycles were carried out, decreasing the annealing temperature by 1°C/cycle between 68°C and 58°C. The amplification was completed with a final extension of 5 min at 72°C. The EcoRI-HindIII 1,382-bp fragment from pIJ774 was used to obtain the deletion cassette (19). Quantification and purity analysis of PCR products were performed using a NanoDrop ND-1000 UV-vis (Thermo Scientific), and the fidelity of the amplification was confirmed by sequencing.
qPCR.
Detection and quantification of plasmids pSCL2 and pSCL4 were carried out using 20 ng DNA by qPCR as described by Lee et al. (20). To detect and quantify pSCL2, the parApSCL2 gene was used, and the brp, parBpSCL4, and traA genes were used to detect and quantify pSCL4. The chromosomal genes adpA and hrdB were used as controls. The oligonucleotides used in this experiment were previously described by Álvarez-Álvarez et al. (21).
RT-qPCR.
Gene expression analysis and quantification were performed by RT-qPCR as described by López-García et al. (22) using the 2−ΔΔCt method (where CT is threshold cycle) (23, 24) and the constitutive housekeeping gene hrdB gene as internal control (25). cDNAs were synthesized as described by López-García et al. (22). Negative controls were always carried out to confirm the absence of contaminating DNA.
Microarray design.
(i) Prediction of noncoding RNAs. S. clavuligerus genome sequence and annotation (5,710 chromosomal coding sequences [CDS], 1,581 pSCL4 CDS, 18 rRNA, and 66 tRNA) were downloaded from StrepDB (http://strepdb.streptomyces.org.uk/). To identify noncoding RNA (ncRNA) in the genome sequence, two procedures were used. First, 27 sequences of small RNA (sRNA) of the closely related species Streptomyces avermitilis were used to identify homologous sequences in the genome of S. clavuligerus by means of BLAST searches. These sequences were obtained from the StrepDB database (ssrA, srp, and rnpB genes) and from the fRNAdb database of predicted ncRNA (26). In this way, 20 sequences were identified (systematic names, SCLAV_mr001 to SCLAV_mr020). Second, the procedure and nocoRNAc program of Herbig and Nieselt (27) were used to predict 164 ncRNAs in the chromosome (systematic names with prefix “SCLAV_nr”) and 30 ncRNAs in the pSCL4 plasmid (SCLAV_pnr). Thus, a total of 7,589 loci were the starting target set for the probe design.
Microarray probes (45- to 60-mer) were designed with the online tool eArray (Agilent) for gene expression probes (1 to 3 probes per locus) and with the chipD program (28) for tiling probes. The eArray procedure was the “Tm matching methodology” used with the option “best probe methodology” (DSCV01 probes) or “best distribution” (DSCV02 probes, an option used to obtain 3 probes per gene only for those genes larger than 1,500 nucleotides [nt]); in both cases, 85°C was the “preferred probe Tm.” The chipD procedure was used to cover 13 genome regions for a deeper analysis, including the known or putative biosynthesis clusters for secondary metabolites. The tiling design (DSCV05, 5,559 probes) included the following parameters: Na concentration, 1.134; target Tm, 97; Tm model, 3; and interval size, 30. Finally, a total of 14,867 probes were selected, covering 98.85% of the targeted locus. Microarrays were manufactured by Agilent Technologies (Santa Clara, CA, USA) in the format 8 × 15K.
(ii) Microarray labeling and hybridization.
RNA was extracted from the culture samples at 22.5, 46.5, and 60 h. The analysis was performed for two biological replicates for each condition (two strains and three growth times). Labeling of RNA preparations with Cy3-dCTP, labeling of genomic DNA as the reference sample with Cy5-dCTP, and the purification procedures were carried out as indicated previously (21), except that 2.5 pmol was the amount of Cy5-gDNA added to 50 μl of the hybridization solution. The hybridization conditions, washing, scanning with Agilent Scanner G2565BA, and the quantification of the images were carried out as previously described (29).
Transcriptome analysis.
Microarray fluorescence data were normalized and analyzed with the Bioconductor package limma (30).
First, net fluorescence intensities were calculated as mean foreground minus background median; when this subtraction was lower than the background standard deviation, the latter value was used as the surrogate.
Second, the binary logs of Cy3/Cy5 net intensities were normalized (Mg value) with cycling loess and global median using spot weights (assigning weights of 1 for CDS probes and of 1E−6 for the rest of the probes), so the only values taken into account in the normalization were those from the chromosome coding genes, while the pSCL4 probes were practically discarded. The Mg value is proportional to the abundance of the transcript for a particular gene (31).
Third, the spot values corresponding to the same probe sequence were averaged, and then the probe values of the same locus were averaged. Tiling probe data were discarded for this analysis.
The Mg transcription values of the six experimental conditions were compared using three contrasts, mutant versus wild type, corresponding to the three studied growth times. For each gene, P values and Mc values were calculated (three sets of values, one for each comparison). Mc values are the binary log of the differential transcription between the mutant and the wild-type strain.
The Benjamini-Hochberg (BH) false-discovery rate correction was applied to the P values. For each comparison, a result was considered statistically significant if the BH-corrected P value was ≤0.05. A positive Mc value indicates upregulation, and a negative Mc value indicates downregulation.
Microarray data accession number.
The microarray data used in this work have been deposited in the National Center for Biotechnology Information-Gene Expression Omnibus database under accession number GSE66683.
RESULTS
The mycelium of the old mutant S. clavuligerus claR::aph carries a low number of copies of plasmids pSCL4 and pSCL2.
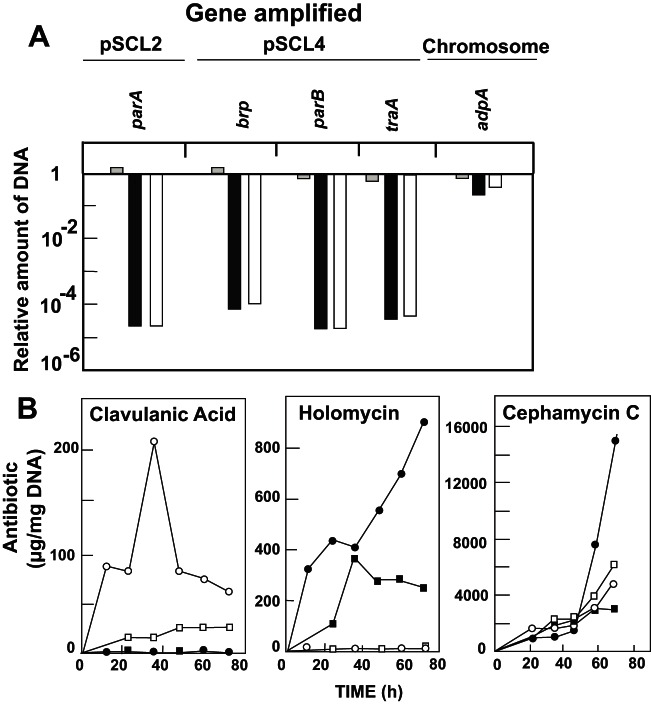
The S. clavuligerus oppA2::aph strain used previously in our laboratory (16, 32) was shown by qPCR amplification and transcriptomic analysis to carry in the mycelium 25,000-fold fewer copies of plasmid pSCL2 and 10,000-fold fewer copies of plasmid pSCL4 than the wild-type strain (21). The lack of sporulation of S. clavuligerus oppA2::aph, previously believed to be due to the oppA2 mutation (32), was shown to be dependent on the lack of pSCL4 (21). To discriminate whether the effects described for the mutations in the clavulanic acid gene cluster are real or due to the absence of plasmids in the mutant strains, the levels of plasmids pSCL2 and pSCL4 were quantified in our collection of mutant strains and compared to those of the wild-type strain. The parA gene in pSCL2 and 11 genes located in pSCL4 (SCLAV_p0032, p0126, p0353, p0528, p0713, p0828, p1090, p1250, p1328, p1452, and p1539) were quantified by qPCR. Levels of the linear plasmids similar to those of the wild-type strain were found in strains S. clavuligerus ccaR::aph, S. clavuligerus cyp::tsr, S. clavuligerus orf13::apr, S. clavuligerus orf14::aph, S. clavuligerus oppA1::acc, S. clavuligerus relA::neo, and S. clavuligerus ΔadpA (22, 32, 33, 34, 35, 36). However, the old S. clavuligerus claR::aph strain, obtained by transformation of protoplasts to disrupt the claR gene (16), was found to give a very low qPCR DNA amplification of genes located in either pSCL2 or pSCL4 (Fig. 1A), suggesting that this mutant has a low number of copies of both plasmids. A transcriptomic study performed as described by Álvarez-Álvarez et al. (12) confirmed that genes located in the pSCL2 and pSCL4 plasmids gave a very poor transcription signal in S. clavuligerus claR::aph (data not shown). Therefore, the old S. clavuligerus claR::aph strain has now been renamed S. clavuligerus claR::aph pSCLlow. To distinguish the phenotypes due to the absence of the ClaR regulator from the effects due to the lack of linear plasmids, we constructed a new mutant with claR deleted, which carried a normal number of copies of pSCL2 and pSCL4. The new strain, named S. clavuligerus ΔclaR::aac, was constructed by the ReDirect method using S. clavuligerus ATCC 27064 as the parental strain (Table 1), and its correct number of copies of plasmids was confirmed by qPCR (Fig. 1A). The old S. clavuligerus claR::aph pSCLlow mutant did not produce clavulanic acid but produced larger amounts of cephamycin C and of holomycin than the wild-type strain (16). When tested by bioassay, the new S. clavuligerus ΔclaR::aac broth cultures did not contain clavulanic acid at any time during the fermentation. These culture broths contained about 40% more cephamycin C than those of the parental strain S. clavuligerus ATCC 27064 at 72 h of fermentation and produced significant amounts of holomycin (Fig. 1B). Since the same pattern of antibiotic production was found in both claR-null strains, we conclude that these effects were due to the lack of ClaR.
FIG 1.
Plasmid copy number and antibiotic production by S. clavuligerus ΔclaR::aac. (A) Relative amount of DNA measured by qPCR of the genes indicated above. DNA samples were obtained from S. clavuligerus claR::aph pSCLlow (black columns), S. clavuligerus ΔclaR::aac (gray columns), and S. clavuligerus oppA2::aph pSCLlow (white columns). The relative amounts of DNA shown for the three mutant strains were determined by comparison to the amounts obtained with wild-type S. clavuligerus. (B) Production of clavulanic acid (left panel), holomycin (central panel), and cephamycin C (right panel) by S. clavuligerus ATCC 27064 (white circles), S. clavuligerus ΔclaR::aac (black circles), S. clavuligerus ΔclaR::aac(pMS83) (black squares), and S. clavuligerus ΔclaR::aac(pMS83-claR) (white squares).
Transcriptomic analysis of S. clavuligerus ΔclaR::aac in comparison to the parental S. clavuligerus ATCC 27064.
The differential transcriptomic analysis between the S. clavuligerus ΔclaR::aac mutant and the wild-type parental strain were performed on 22.5-, 46.5-, and 60-h SA-grown culture samples. The transcriptomic results were filtered for the three sampling times, applying the criteria of Mc ≥1.0 or Mc ≤−2.0 and a BH-corrected P value of ≤0.05, to restrict the data to only those genes that were strongly affected by the lack of ClaR. By these criteria, 103 genes were clearly downregulated and 64 genes were upregulated in the ΔclaR mutant.
Differential transcription of the clavulanic acid (CA) and clavam gene clusters.
The effect produced by the lack of ClaR on the CA biosynthesis gene expression is shown in Fig. 2. Genes for the early steps of the pathway were relatively well expressed (39 to 56% in relation to the wild-type strain). All the genes for the late steps of CA biosynthesis (gcas, orf16, oppA2, orf14, orf13, orf12, cyp-fd) had very low expression levels, between 1% (orf16) and 8% (car), in relation to those of the control strain. The fluorescence value for the claR probe, a gene that is not present in the mutant, indicated a background level of 1.3% in relation to the wild-type-strain-derived value. Therefore, we might conclude that the late genes for CA biosynthesis, as well as the two oligopeptide permease-encoding genes oppA1 and oppA2 (32) with differential transcription of 3.1% and 1.2%, respectively, are, for all practical purposes, not expressed in the ΔclaR mutant (Fig. 2A). Only car, a gene divergent from claR, which encodes the enzyme for the last step of the pathway, is partially expressed (8%). All the cephamycin C-clavulanic acid supercluster genes putatively involved in β-lactam antibiotic resistance (pbpA, pbp2, pcbR) were upregulated in the ΔclaR mutant. This finding is important and correlates with the increased production of cephamycin C. An expression increase is observed at 22.5 h of culture for all the cephamycin C biosynthesis genes in the S. clavuligerus ΔclaR::aac strain, explaining the increase of cephamycin production observed in the mutant; however, all the biosynthesis genes' expression decreases thereafter.
FIG 2.
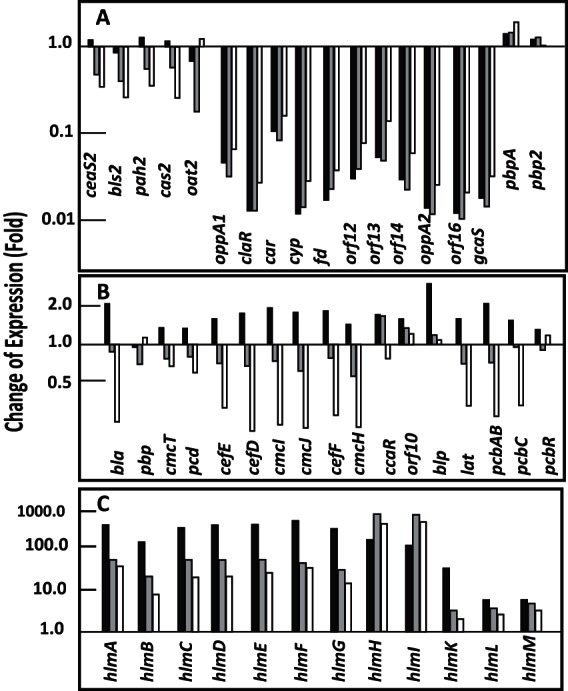
Effects of the lack of ClaR on transcription of clavulanic acid, cephamycin C, and holomycin biosynthesis genes. Transcriptomic results of genes for clavulanic acid biosynthesis (A), cephamycin C biosynthesis (B), and holomycin biosynthesis (C) in S. clavuligerus ΔclaR::aac compared to S. clavuligerus ATCC 27064. The columns represent the average of the fold change of expression at 22.5 h (black columns), 46.5 h (gray columns), and 60 h (white columns). The corresponding gene is indicated at the bottom of the columns. The values are compared to those of the control strain, S. clavuligerus ATCC 27064, taken as 1.
Interestingly, the clavam gene cluster (SCLAV_2921 to 2935), which is located far from the CA cluster (37), showed no significant differences in the expression of most genes. Only cvm5 and cvm7 were slightly upregulated (2.0-fold and 2.4-fold, respectively) and cvm3 was downregulated (2.4-fold) in the mutant, at 46.5 h of culture. In the clavam paralogous gene cluster (SCLAV_p1069 to p1082), located in the pSCL4 megaplasmid, the bls1, pah1, cvm6P, and orfB genes were upregulated (2.2- to 5.8-fold) (data not shown). The cluster SMCp25 (SCLAV_p1508 to SCLAV_p1510) (38) showed a downregulation of 21-fold on average. This cluster contains a clavaminate synthase-like oxygenase (18-fold downregulated) and a putative carbamoyltransferase.
Differential transcription of the cephamycin C and holomycin gene clusters.
All the genes in the cephamycin C gene cluster were moderately upregulated at 22.5 h of culture in the ΔclaR mutant, with an average fold change of 1.7 at this sampling time, but their expression decreased at 46.5 and 60 h of culture (Fig. 2B). The hlm genes, encoding enzymes for holomycin biosynthesis (Fig. 2C), showed diverse expression changes. The expression increases were small for hlmK, hlmL, and hlmM genes (14-fold change average at 22.5 h). The other hlm genes had a large expression increase at 22.5 h, on the order of 250.7-fold change average. These high expression differences decreased at later sampling times. Only two genes, hlmI and hlmH, showed a high and maintained upregulation at all sampling times with maximum differences in relation to the wild-type strain at 46.5 h and 60 h. The hlmI gene encodes the thioredoxin-like dithiol oxidase forming the disulfide bond that cyclizes the second ring in holomycin (39). The hlmH gene encodes an MSF-type transporter, and its role in holomycin production is still unknown (40).
The differences in the cephamycin C biosynthesis gene expression did not meet the criteria initially applied to the data (Mc ≥1.0 or Mc ≤−2.0 and BH-corrected P value of ≤0.05); however, the differences observed correlated well with the different cephamycin C production levels by S. clavuligerus ΔclaR::aac and the wild-type strain (Fig. 1B).
Other putative secondary metabolites gene clusters affected by the lack of ClaR.
Three clusters (SMCp20, SMCp22, and SMCp24) putatively involved in secondary metabolite formation (38) and located in plasmid pSCL4 were specially affected by the lack of ClaR. The block of genes SCLAV_p1334 to SCLAV_p1337 (cluster SMCp20) was strongly downregulated. These genes encode proteins with a UPF0089 domain, an acyl-coenzyme A (acyl-CoA) synthetase, a caffeoyl O-methyltransferase, and a histidine ammonia-lyase, respectively. The remaining genes in the cluster, encoding nonribosomal peptide synthetases (SCLAV_ p1339, SCLAV_p1340, SCLAV_p1341) were not affected (Fig. 3, top panel). The SMCp20 cluster appears to be involved in the biosynthesis of a nonribosomal peptide containing a caffeic acid-derived moiety. All the genes of cluster SMCp22 were downregulated, especially SCLAV_p1407, encoding a pentalenene synthase (140-fold down-expressed), SCLAV_p1410, encoding a protein with a DUF397 domain, and SCLAV_p1415, encoding a hydrolase (Fig. 3, middle panel); the cluster genes showed an average of 5-fold downregulation in the mutant strain. Also, all the genes in cluster SMCp24 were downregulated in the ΔclaR mutant (average, 4.75-fold decrease), but specially affected were (i) SCLAV_p1474 and SCLAV_p1475, encoding a putative indigoidine synthase and a transporter, and (ii) SCLAV_p1478, SCLAV_p1482, and SCLAV_p1483 for two DUF-domain-containing proteins and a hypothetical protein, respectively (Fig. 3, bottom panel). The products of clusters SMCp20, SMCp22, and SMCp24 have not yet been characterized.
FIG 3.
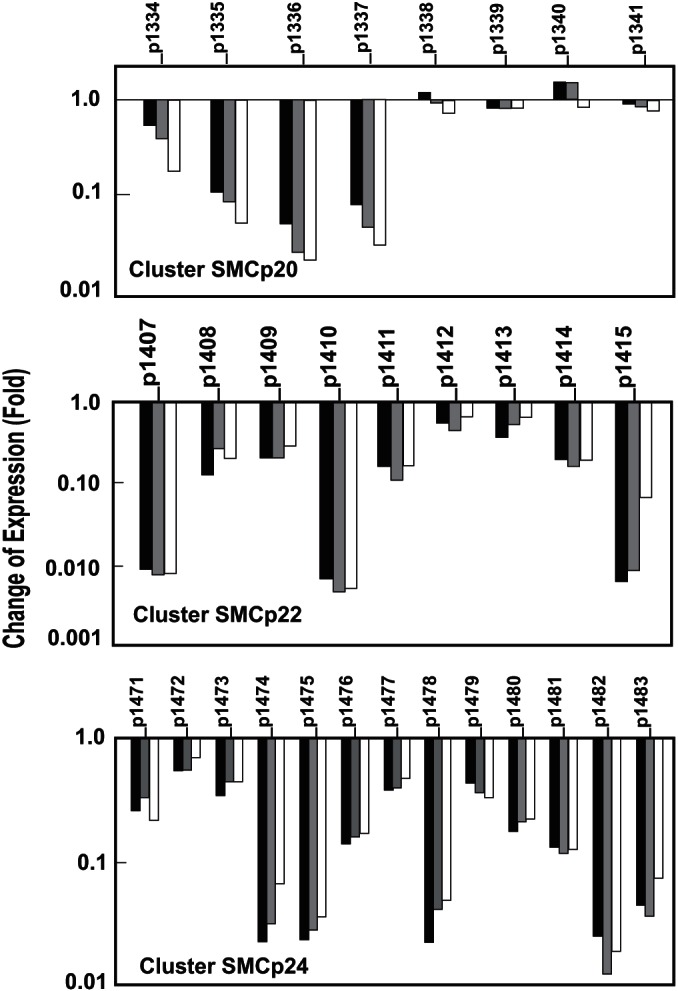
Effects of the lack of ClaR on the SMCp20, SMCp22, and SMCp24 gene cluster transcription. Transcriptomic results of genes of the SMCp20 cluster (top), the SMCp22 cluster (middle), and the SMCp24 cluster (bottom) in S. clavuligerus ΔclaR::aac compared to S. clavuligerus ATCC 27064. The columns represent the average of the fold change of expression at 22.5 h (black columns), 46.5 h (gray columns), and 60 h (white columns). The corresponding gene is indicated at the top of the columns. The values are compared to those of the control strain, S. clavuligerus ATCC 27064, taken as 1.
Other genes affected in the ΔclaR mutant. (i) Regulatory genes.
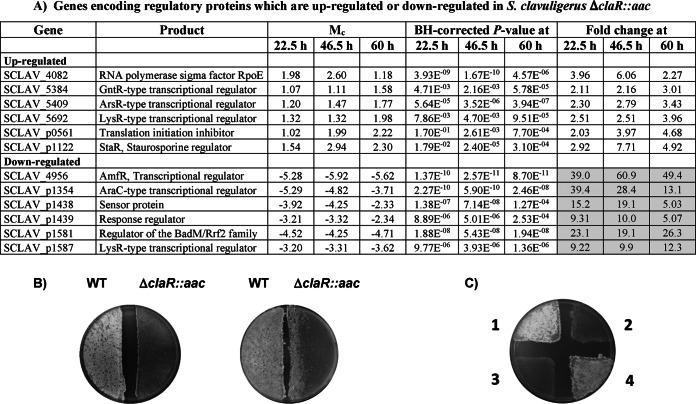
Twelve regulatory genes were up- or downregulated in the ΔclaR mutant (Fig. 4). Six genes were upregulated, with an average increase of 4.2-fold. A high change (6-fold increase) was observed in SCLAV_4082, a gene annotated as rpoE, which encodes the RNA polymerase sigma factor RpoE. The orthologous gene of S. coelicolor is sigR, encoding the SigR regulator, which, coordinated with the anti-sigma factor RsaR, controls the cellular redox homeostasis and a regulon of 160 genes (41). Strongly affected was also staR (SCLAV_p1122), encoding the staurosporine biosynthesis transcriptional activator (42). This gene showed a 7.7-fold increase in the mutant; surprisingly, the staurosporine biosynthesis genes were not affected, with the exception of SCLAV_p1123, encoding a methyltransferase, which was upregulated at all sampling times, with a maximum change of 70-fold at 46.5 h.
FIG 4.
Different transcription of genes encoding regulators. (A) Regulatory genes up- or downregulated in S. clavuligerus ΔclaR::aac. Note that the shaded data correspond to downregulated genes. (B) Plates of ME medium at 7 days (left plate) and 12 days (right plate) of culture. S. clavuligerus ATCC 27064 (WT) is grown at the left side of the plates. S. clavuligerus ΔclaR::aac is grown at the right side of each plate. Note in the 12-day culture the formation of aerial mycelium on the S. clavuligerus ΔclaR::aac lawn when grown in the proximity of the wild-type strain.(C) Aerial mycelium and sporulation by S. clavuligerus ATCC 27064 (1), S. clavuligerus ΔclaR::aac (2), S. clavuligerus ΔclaR::aac(pMS83) (3), and S. clavuligerus ΔclaR::aac(pMS83-claR) (4).
Six regulatory genes were downregulated by the lack of ClaR, with an average change of 24.6-fold. The most affected downregulated gene was SCLAV_4956, encoding the response transcriptional regulator AmfR, with a 61-fold change. This gene is orthologous to S. coelicolor ramR and S. griseus amfR (43, 44); the latter is known to control the operon amfTSBA, which is strictly required for aerial mycelium formation. This low expression of amfR supports the observation that S. clavuligerus ΔclaR::aac is unable to form aerial mycelium (Fig. 4B) (see Discussion). The S. clavuligerus chromosomal genes SCLAV_4787 and SCLAV_4788 are orthologous to the Streptomyces lividans two-component system (TCS)-encoding genes cutS and cutR, and their expression was not affected in the ΔclaR mutant. However, a putative paralogous TCS-encoding cutS-cutR (SCLAV_p1438 and SCLAV_p1439) was downregulated in the ΔclaR mutant (14.5-fold average decrease).
(ii) Genes with various functions.
In addition to the genes already mentioned, 48 genes encoding miscellaneous proteins were upregulated and 69 genes were downregulated in the ΔclaR mutant (Table 2). The proteins encoded by these genes exert different functions in the cell. The more affected genes were the ATP-dependent Clp protease-encoding gene (SCLAV_0526) and the staurosporin-related methyltransferase (SCLAV_p1123), which was upregulated 70-fold. Upregulated changes between 10- and 20-fold were observed for an ATP grasp superfamily enzyme (SCLAV_1184), for putative hydroxylase- and putative acetyltransferase-encoding genes (SCLAV_5199, SCLAV_0254), and for genes encoding a copper oxidase, a flavodoxin, and a fumarate hydratase (SCLAV_5198, SCLAV_p0560, SCLAV_3946). The most affected genes were those encoding a CnaB domain-containing protein (SCLAV_p1381) and a putative surface layer protein (SCLAV_p1431), which seemed to be not expressed at any time in the culture (decreased 100-fold compared to the wild-type strain). Sixteen genes (about 13% of these genes) encode oxidoreductases, oxidases, flavodoxins, peroxidases, or reductases, which might form part of the RpoE regulon (see Discussion) as occurs with the oxidoreductases encoded by SCLAV_1808 and SCLAV_4869. From them, SCLAV_1808, SCLAV_4869, and SCLAV_5583, encoding oxidoreductases, SCLAV_5198, encoding a copper oxidase, SCLAV_p0560, encoding a flavodoxin, and SCLAV_p0842, coding for an NADPH-dependent FMN reductase, are upregulated, while all the other 10 genes are downregulated.
TABLE 2.
Genes encoding nonregulatory proteins that are upregulated or downregulated in S. clavuligerus ΔclaR::aac in relation to S. clavuligerus ATCC 27064
| Gene | Product |
Mc |
BH-corrected P value |
Fold changea |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 22.5 h | 46.5 h | 60 h | 22.5 h | 46.5 h | 60 h | 22.5 h | 46.5 h | 60 h | ||
| Upregulated | ||||||||||
| SCLAV_0156 | Hypothetical protein | 1.60 | 2.87 | 2.71 | 4.52E−02 | 2.64E−04 | 3.90E−04 | 3.03 | 7.32 | 6.56 |
| SCLAV_0254 | Putative acetyltransferase | 4.07 | 1.51 | 2.18 | 8.61E−06 | 4.56E−02 | 3.56E−03 | 16.80 | 2.86 | 4.55 |
| SCLAV_0526 | ATP-dependent Clp protease | 1.63 | 4.45 | 3.02 | 2.50E−02 | <1E−06 | 5.64E−05 | 3.10 | 21.99 | 8.15 |
| SCLAV_1182 | Hypothetical protein | 1.69 | 2.03 | 1.61 | 1.03E−05 | <1E−06 | 1.63E−05 | 3.24 | 4.11 | 3.05 |
| SCLAV_1184 | ATP grasp superfamily enzyme | 2.99 | 3.58 | 1.34 | <1E−06 | <1E−06 | 6.86E−04 | 7.98 | 12.02 | 2.54 |
| SCLAV_1186 | Putative dephospho-CoA kinase | 1.17 | 2.12 | 1.16 | 1.01E−02 | 1.58E−05 | 4.88E−03 | 2.25 | 4.37 | 2.24 |
| SCLAV_1187 | Hypothetical protein | 1.57 | 1.74 | 1.43 | 5.77E−04 | 5.80E−05 | 3.92E−04 | 2.97 | 3.35 | 2.70 |
| SCLAV_1453 | Alkaline d-peptidase | 1.70 | 1.60 | 1.56 | 7.69E−03 | 1.21E−02 | 6.99E−03 | 3.27 | 3.04 | 2.95 |
| SCLAV_1808 | DsbA oxidoreductase | 2.17 | 2.38 | 1.48 | <1E−06 | <1E−06 | 5.70E−06 | 4.51 | 5.21 | 2.80 |
| SCLAV_1991 | N-Acetylglucosamine catabolism protein | 1.01 | 1.22 | 1.45 | 2.60E−03 | 2.69E−04 | 3.83E−05 | 2.02 | 2.33 | 2.74 |
| SCLAV_2412 | GTP cyclohydrolase I | 1.03 | 1.69 | 1.93 | 1.29E−03 | 2.99E−06 | <1E−06 | 2.04 | 3.24 | 3.82 |
| SCLAV_2900 | GCN5-related N-acetyltransferase | 1.38 | 1.74 | 1.27 | 2.36E−05 | <1E−06 | 4.66E−05 | 2.60 | 3.35 | 2.42 |
| SCLAV_2956 | Hypothetical protein | 1.51 | 1.79 | 1.36 | 6.38E−03 | 8.38E−04 | 6.51E−03 | 2.85 | 3.48 | 2.57 |
| SCLAV_3213 | Glycosyltransferase | 2.78 | 2.71 | 1.10 | <1E−06 | <1E−06 | 3.98E−05 | 6.89 | 6.57 | 2.15 |
| SCLAV_3214 | DUF2596 domain-containing protein | 1.53 | 1.40 | 1.41 | 3.75E−05 | 8.39E−05 | 6.56E−05 | 2.89 | 2.64 | 2.68 |
| SCLAV_3281 | Putative permease of the MFS superfamily | 2.73 | 2.81 | 1.50 | <1E−06 | <1E−06 | 2.05E−04 | 6.65 | 7.04 | 2.83 |
| SCLAV_3946 | Fumarate hydratase | 2.35 | 3.61 | 2.04 | <1E−06 | <1E−06 | <1E−06 | 5.13 | 12.29 | 4.13 |
| SCLAV_4021 | Hypothetical protein | 2.15 | 1.95 | 1.61 | 2.45E−06 | 7.86E−06 | 6.93E−05 | 4.46 | 3.88 | 3.07 |
| SCLAV_4151 | Hypothetical protein | 1.29 | 3.08 | 1.31 | 7.78E−04 | <1E−06 | 3.80E−04 | 2.45 | 8.50 | 2.48 |
| SCLAV_4287 | NAD-dependent epimerase/dehydratase | 1.07 | 1.46 | 1.31 | 3.56E−02 | 2.30E−03 | 4.35E−03 | 2.10 | 2.76 | 2.49 |
| SCLAV_4768 | 3′-OH-methylcephem-O-carbamoyltransferase | 1.12 | 2.50 | 1.34 | 3.81E−02 | 1.49E−05 | 5.53E−03 | 2.17 | 5.69 | 2.54 |
| SCLAV_4869 | Oxidoreductase | 1.12 | 2.57 | 1.13 | 6.63E−05 | <1E−06 | 4.42E−05 | 2.19 | 5.96 | 2.20 |
| SCLAV_4922 | Ribosome-associated GTPase | 1.19 | 2.52 | 1.28 | 5.71E−04 | <1E−06 | 1.71E−04 | 2.30 | 5.77 | 2.43 |
| SCLAV_5145 | Potassium-transporting ATPase subunit B | 1.94 | 2.21 | 1.97 | <1E−06 | <1E−06 | <1E−06 | 3.85 | 4.64 | 3.94 |
| SCLAV_5146 | Potassium-transporting ATPase C chain | 2.98 | 2.68 | 2.58 | <1E−06 | <1E−06 | <1E−06 | 7.93 | 6.43 | 5.99 |
| SCLAV_5150 | TrkABC domain-containing protein | 1.18 | 1.08 | 1.39 | 2.89E−05 | 6.59E−05 | 3.11E−06 | 2.27 | 2.11 | 2.63 |
| SCLAV_5161 | Hypothetical protein | 1.85 | 1.16 | 1.03 | 1.49E−05 | 1.72E−03 | 3.79E−03 | 3.62 | 2.25 | 2.04 |
| SCLAV_5162 | NocE-like protein | 1.36 | 1.26 | 2.33 | 1.98E−02 | 1.91E−02 | 9.08E−05 | 2.58 | 2.40 | 5.03 |
| SCLAV_5163 | Hypothetical protein | 1.18 | 1.77 | 1.20 | 8.26E−05 | <1E−06 | 4.86E−05 | 2.27 | 3.43 | 2.30 |
| SCLAV_5198 | Copper oxidase | 1.35 | 2.91 | 3.80 | 1.28E−04 | <1E−06 | <1E−06 | 2.56 | 7.52 | 14.00 |
| SCLAV_5199 | Putative hydroxylase | 1.15 | 2.86 | 3.77 | 6.01E−04 | <1E−06 | <1E−06 | 2.22 | 7.30 | 13.66 |
| SCLAV_5222 | Hypothetical protein | 1.79 | 2.14 | 1.16 | 8.85E−05 | 8.10E−06 | 3.47E−03 | 3.47 | 4.42 | 2.24 |
| SCLAV_5248 | Zn-dependent hydrolase | 1.44 | 1.55 | 1.08 | 2.63E−04 | 8.20E−05 | 2.30E−03 | 2.72 | 2.94 | 2.11 |
| SCLAV_5256 | Putative ABC transporter permease | 1.33 | 1.43 | 1.05 | 5.92E−03 | 1.91E−03 | 1.54E−02 | 2.52 | 2.70 | 2.07 |
| SCLAV_5350 | Acyltransferase 3 | 1.03 | 1.80 | 1.27 | 1.34E−05 | <1E−06 | <1E−06 | 2.05 | 3.49 | 2.41 |
| SCLAV_5511 | Hypothetical protein | 1.23 | 1.62 | 1.37 | 2.69E−02 | 2.12E−03 | 6.19E−03 | 2.35 | 3.08 | 2.60 |
| SCLAV_5534 | Selenocysteine lyase | 1.26 | 1.22 | 2.62 | 3.76E−02 | 2.70E−02 | 1.39E−05 | 2.40 | 2.34 | 6.16 |
| SCLAV_5547 | YD repeat protein | 1.02 | 1.66 | 1.19 | 1.01E−02 | 5.78E−05 | 1.31E−03 | 2.03 | 3.17 | 2.29 |
| SCLAV_5583 | Putative oxidoreductase | 1.44 | 1.97 | 1.19 | 1.95E−02 | 9.78E−04 | 2.95E−02 | 2.73 | 3.93 | 2.29 |
| SCLAV_5617 | Putative dehydrogenase | 1.12 | 1.40 | 1.28 | 1.28E−02 | 1.26E−03 | 2.54E−03 | 2.18 | 2.64 | 2.43 |
| SCLAV_5688 | Hypothetical protein | 1.58 | 1.43 | 1.14 | 4.15E−05 | 1.10E−04 | 8.55E−04 | 3.01 | 2.69 | 2.21 |
| SCLAV_5714 | Cysteine desulfurase | 1.40 | 1.51 | 1.22 | 2.01E−02 | 7.02E−03 | 2.25E−02 | 2.65 | 2.85 | 2.34 |
| SCLAV_p0020 | Hypothetical protein | 1.37 | 1.33 | 1.25 | 1.89E−02 | 1.31E−02 | 1.60E−02 | 2.59 | 2.52 | 2.38 |
| SCLAV_p0560 | Multimeric flavodoxin WrbA | 3.40 | 2.97 | 1.37 | <1E−06 | <1E−06 | 1.61E−03 | 10.63 | 7.85 | 2.59 |
| SCLAV_p0693 | Luciferase domain-containing protein | 1.85 | 1.63 | 1.61 | 9.22E−04 | 1.95E−03 | 1.76E−03 | 3.62 | 3.11 | 3.06 |
| SCLAV_p0703 | Carbonic anhydrase | 1.28 | 1.17 | 1.77 | 2.86E−02 | 2.89E−02 | 1.18E−03 | 2.44 | 2.26 | 3.43 |
| SCLAV_p0842 | NADPH-dependent FMN reductase | 3.15 | 3.14 | 1.18 | <1E−06 | <1E−06 | 9.18E−03 | 8.91 | 8.85 | 2.28 |
| SCLAV_p1123 | Putative methyltransferase | 4.57 | 6.13 | 6.06 | <1E−06 | <1E−06 | <1E−06 | 23.83 | 70.03 | 66.95 |
| Downregulated | ||||||||||
| SCLAV_3892 | DUF946 domain-containing protein | −2.09 | −4.38 | −2.63 | 1.23E−03 | <1E−06 | 6.82E−05 | 4.26 | 20.8 | 6.19 |
| SCLAV_4953 | AmfT: putative AmfS-modifying enzyme | −5.99 | −6.68 | −4.86 | <1E−06 | <1E−06 | <1E−06 | 63.6 | 103 | 29.1 |
| SCLAV_4954 | AmfB, membrane translocator | −1.98 | −2.56 | −1.68 | 2.10E−03 | 9.78E−05 | 4.03E−03 | 3.95 | 5.93 | 3.22 |
| SCLAV_4955 | AmfA, membrane translocator | −2.45 | −2.85 | −1.15 | 3.01E−05 | 3.68E−06 | 1.74E−02 | 5.47 | 7.21 | 2.23 |
| SCLAV_4957 | Hypothetical protein | −3.72 | −4.68 | −4.31 | <1E−06 | <1E−06 | <1E−06 | 13.3 | 25.8 | 19.9 |
| SCLAV_4968 | CbbY/CbbZ/GpH/YieH family hydrolase | −5.40 | −6.38 | −5.60 | <1E−06 | <1E−06 | <1E−06 | 42.4 | 83.8 | 48.6 |
| SCLAV_4971 | ATPase with various cellular activities | −6.24 | −6.26 | −5.55 | <1E−06 | <1E−06 | <1E−06 | 76.0 | 77.0 | 47.0 |
| SCLAV_4973 | Hypothetical protein | −3.15 | −3.14 | −2.11 | <1E−06 | <1E−06 | <1E−06 | 8.89 | 8.82 | 4.34 |
| SCLAV_p1192 | Peptidoglycan-binding LysM | −2.00 | −3.67 | −2.97 | 4.11E−03 | 3.56E−06 | 4.60E−05 | 4.00 | 12.8 | 7.85 |
| SCLAV_p1196 | Secreted protein | −2.45 | −4.70 | −3.51 | 9.78E−04 | <1E−06 | 1.21E−05 | 5.50 | 26.1 | 11.5 |
| SCLAV_p1197 | Phage tail protein | −2.42 | −3.93 | −3.21 | 4.78E−03 | 2.06E−05 | 1.74E−04 | 5.37 | 15.2 | 9.31 |
| SCLAV_p1198 | Tail sheath protein | −2.77 | −5.20 | −3.75 | 2.12E−03 | 1.03E−06 | 5.69E−05 | 6.86 | 36.8 | 13.5 |
| SCLAV_p1200 | Hydrolytic protein | −2.85 | −5.19 | −3.71 | 1.07E−03 | <1E−06 | 3.98E−05 | 7.22 | 36.5 | 13.2 |
| SCLAV_p1328 | SAM-dependent O-methyltransferase | −2.80 | −2.63 | −3.42 | <1E−06 | <1E−06 | <1E−06 | 6.99 | 6.20 | 10.7 |
| SCLAV_p1329 | Peptidases S1 and S6; chymotrypsin/Hap | −5.41 | −6.13 | −6.47 | <1E−06 | <1E−06 | <1E−06 | 42.5 | 70.0 | 89.2 |
| SCLAV_p1330 | Oxidoreductase UcpA | −4.85 | −5.25 | −5.29 | <1E−06 | <1E−06 | <1E−06 | 29.0 | 38.1 | 39.1 |
| SCLAV_p1342 | Hypothetical protein | −3.89 | −4.46 | −3.91 | <1E−06 | <1E−06 | <1E−06 | 14.9 | 22.1 | 15.0 |
| SCLAV_p1352 | Flavin reductase domain protein | −2.50 | −2.40 | −2.99 | <1E−06 | <1E−06 | <1E−06 | 5.69 | 5.28 | 7.97 |
| SCLAV_p1355 | Hypothetical protein | −6.24 | −5.70 | −5.27 | <1E−06 | <1E−06 | <1E−06 | 75.7 | 52.3 | 38.6 |
| SCLAV_p1370 | Acyl-CoA dehydrogenase | −5.00 | −4.97 | −3.79 | 1.18E−06 | 1.19E−06 | 3.47E−05 | 32.1 | 31.5 | 13.9 |
| SCLAV_p1378 | Dyp-type peroxidase family protein | −3.42 | −3.36 | −2.09 | <1E−06 | <1E−06 | 2.93E−05 | 10.7 | 10.3 | 4.27 |
| SCLAV_p1379 | Plastocyanin | −6.14 | −6.33 | −4.73 | <1E−06 | <1E−06 | <1E−06 | 71.0 | 80.6 | 26.6 |
| SCLAV_p1381 | CnaB domain-containing protein | −7.44 | −7.81 | −7.59 | <1E−06 | <1E−06 | <1E−06 | 174.0 | 225.0 | 193.0 |
| SCLAV_p1382 | Putative peptidase inhibitor | −3.03 | −3.95 | −3.83 | <1E−06 | <1E−06 | <1E−06 | 8.18 | 15.5 | 14.3 |
| SCLAV_p1383 | YVTN family beta-propeller repeat protein | −4.90 | −5.06 | −3.48 | <1E−06 | <1E−06 | 7.61E−06 | 29.9 | 33.5 | 11.2 |
| SCLAV_p1388 | Putative secreted metalloprotease | −5.43 | −5.92 | −6.17 | <1E−06 | <1E−06 | <1E−06 | 43.2 | 61.0 | 72.2 |
| SCLAV_p1389 | Putative secreted protein | −5.88 | −6.60 | −7.19 | <1E−06 | <1E−06 | <1E−06 | 59.2 | 97.3 | 147 |
| SCLAV_p1402 | Actinohivin | −2.33 | −2.91 | −3.45 | 2.95E−03 | 2.11E−04 | 3.02E−05 | 5.04 | 7.53 | 10.9 |
| SCLAV_p1403 | DNA-binding protein | −5.48 | −5.25 | −4.43 | <1E−06 | <1E−06 | <1E−06 | 44.9 | 38.3 | 21.6 |
| SCLAV_p1404 | Multicopper oxidase, type 2 | −2.56 | −2.82 | −2.55 | <1E−06 | <1E−06 | <1E−06 | 5.93 | 7.10 | 5.87 |
| SCLAV_p1417 | Hypothetical protein | −3.94 | −4.30 | −5.75 | <1E−06 | <1E−06 | <1E−06 | 15.4 | 19.8 | 53.9 |
| SCLAV_p1420 | Short-chain dehydrogenase/reductase SDR | −3.40 | −3.34 | −3.59 | <1E−06 | <1E−06 | <1E−06 | 10.6 | 10.2 | 12.1 |
| SCLAV_p1421 | Hypothetical protein | −3.29 | −3.68 | −4.52 | <1E−06 | <1E−06 | <1E−06 | 9.8 | 12.8 | 23.0 |
| SCLAV_p1424 | Hypothetical protein | −2.03 | −2.36 | −2.23 | 5.62E−04 | 8.00E−05 | 1.29E−04 | 4.10 | 5.13 | 4.71 |
| SCLAV_p1425 | Hypothetical protein | −6.05 | −6.15 | −5.91 | <1E−06 | <1E−06 | <1E−06 | 66.5 | 71.5 | 60.5 |
| SCLAV_p1428 | Prenyltransferase/squalene oxidase | −4.07 | −4.16 | −3.34 | <1E−06 | <1E−06 | 2.85E−06 | 16.9 | 18.0 | 10.2 |
| SCLAV_p1431 | Putative surface layer protein | −6.92 | −8.23 | −7.18 | <1E−06 | <1E−06 | <1E−06 | 121 | 302 | 146 |
| SCLAV_p1434 | Hypothetical protein | −5.19 | −5.27 | −4.83 | <1E−06 | <1E−06 | <1E−06 | 36.6 | 38.8 | 28.6 |
| SCLAV_p1442 | Hypothetical protein | −2.94 | −4.27 | −4.42 | 5.55E−05 | <1E−06 | <1E−06 | 7.70 | 19.4 | 21.5 |
| SCLAV_p1443 | Integrin alpha-2 domain-containing protein | −5.89 | −6.30 | −4.17 | <1E−06 | <1E−06 | <1E−06 | 59.6 | 79.0 | 18.1 |
| SCLAV_p1445 | Calcium binding protein | −4.72 | −5.43 | −3.07 | <1E−06 | <1E−06 | 5.30E−06 | 26.5 | 43.4 | 8.42 |
| SCLAV_p1448 | Methyltransferase family protein | −5.26 | −4.40 | −2.11 | <1E−06 | <1E−06 | 4.97E−06 | 38.4 | 21.2 | 4.33 |
| SCLAV_p1449 | Hypothetical protein | −5.86 | −6.38 | −5.81 | <1E−06 | <1E−06 | <1E−06 | 58.1 | 83.3 | 56.4 |
| SCLAV_p1451 | Cytochrome P450 | −4.12 | −4.91 | −5.35 | <1E−06 | <1E−06 | <1E−06 | 17.4 | 30.1 | 41.0 |
| SCLAV_p1453 | Trypsin | −4.07 | −5.48 | −4.76 | 1.43E−05 | <1E−06 | 1.78E−06 | 16.8 | 44.8 | 27.3 |
| SCLAV_p1455 | Hypothetical protein | −3.66 | −3.41 | −3.50 | <1E−06 | <1E−06 | <1E−06 | 12.7 | 10.7 | 11.3 |
| SCLAV_p1459 | Mg2+ transporter C | −3.50 | −3.60 | −6.28 | 2.70E−04 | 1.04E−04 | <1E−06 | 11.4 | 12.2 | 78.2 |
| SCLAV_p1469 | Lipoprotein | −6.34 | −5.34 | −3.96 | <1E−06 | <1E−06 | <1E−06 | 81.4 | 40.8 | 15.6 |
| SCLAV_p1484 | Oxidoreductase | −2.53 | −3.01 | −2.25 | 5.14E−05 | 5.02E−06 | 1.36E−04 | 5.81 | 8.06 | 4.78 |
| SCLAV_p1485 | ATP-dependent RNA helicase | −4.07 | −4.12 | −4.31 | <1E−06 | <1E−06 | <1E−06 | 16.9 | 17.5 | 19.9 |
| SCLAV_p1491 | Hypothetical protein | −2.33 | −2.22 | −2.81 | 1.38E−05 | 2.15E−05 | 1.19E−06 | 5.06 | 4.67 | 7.05 |
| SCLAV_p1497 | Inhibitor I36 domain-containing protein | −3.28 | −3.28 | −3.01 | <1E−06 | <1E−06 | <1E−06 | 9.8 | 9.7 | 8.06 |
| SCLAV_p1506 | WD40 domain-containing protein | −3.50 | −4.20 | −3.22 | <1E−06 | <1E−06 | 1.08E−06 | 11.4 | 18.4 | 9.37 |
| SCLAV_p1511 | Putative integron gene cassette protein | −4.39 | −4.91 | −5.31 | <1E−06 | <1E−06 | <1E−06 | 21.0 | 30.1 | 39.9 |
| SCLAV_p1515 | NAD-dependent epimerase/dehydratase | −5.50 | −5.45 | −5.24 | <1E−06 | <1E−06 | <1E−06 | 45.5 | 44.0 | 37.9 |
| SCLAV_p1526 | Hypothetical protein | −4.56 | −4.06 | −3.67 | <1E−06 | <1E−06 | <1E−06 | 23.6 | 16.7 | 12.8 |
| SCLAV_p1527 | Secreted protein | −3.50 | −3.20 | −3.34 | <1E−06 | <1E−06 | <1E−06 | 11.4 | 9.21 | 10.1 |
| SCLAV_p1528 | DUF574 domain-containing protein | −3.87 | −4.07 | −4.73 | <1E−06 | <1E−06 | <1E−06 | 14.7 | 16.8 | 26.7 |
| SCLAV_p1539 | Folylpolyglutamate synthetase | −2.24 | −4.19 | −4.55 | 1.17E−03 | <1E−06 | <1E−06 | 4.72 | 18.3 | 23.5 |
| SCLAV_p1556 | Adenosylcobinamide amidohydrolase (putative) | −5.29 | −4.92 | −3.04 | <1E−06 | <1E−06 | 2.80E−05 | 39.2 | 30.4 | 8.23 |
| SCLAV_p1558 | Beta-xylosidase | −2.97 | −3.97 | −4.40 | 5.36E−04 | 1.42E−05 | 4.10E−06 | 7.85 | 15.7 | 21.2 |
| SCLAV_p1566 | Phenolic acid decarboxylase | −4.82 | −4.69 | −4.40 | <1E−06 | <1E−06 | <1E−06 | 28.3 | 25.8 | 21.3 |
| SCLAV_p1580 | Short-chain dehydrogenase/reductase SDR | −3.28 | −2.92 | −2.18 | 4.44E−06 | 1.70E−05 | 3.57E−04 | 9.7 | 7.57 | 4.55 |
| SCLAV_p1582 | Putative DNA-binding protein | −2.40 | −2.21 | −2.46 | 1.18E−06 | 3.21E−06 | <1E−06 | 5.28 | 4.65 | 5.53 |
| SCLAV_p1585 | Hypothetical protein | −2.11 | −2.28 | −2.06 | 4.78E−04 | 1.10E−04 | 2.81E−04 | 4.33 | 4.88 | 4.17 |
| SCLAV_p1586 | Oxidoreductase | −3.24 | −3.51 | −4.06 | <1E−06 | <1E−06 | <1E−06 | 9.5 | 11.4 | 16.8 |
| SCLAV_p1588 | Reductase | −2.79 | −2.99 | −2.81 | 1.24E−05 | 4.30E−06 | 9.27E−06 | 6.94 | 7.99 | 7.06 |
| SCLAV_pnr026 | Predicted ncRNA | −4.67 | −3.66 | −3.43 | 6.25E−06 | 9.90E−05 | 1.75E−04 | 25.5 | 12.7 | 10.8 |
| SCLAV_pnr029 | Predicted ncRNA | −2.73 | −2.54 | −2.54 | 9.73E−06 | 2.03E−05 | 2.02E−05 | 6.68 | 5.85 | 5.82 |
Boldface data correspond to downregulated fold changes.
Complementation of S. clavuligerus ΔclaR::aac.
To confirm the ClaR effect on antibiotic production and the lack of aerial mycelium formation, the deleted ΔclaR mutant was complemented with plasmid pMS83-claR. S. clavuligerus ΔclaR::aac, S. clavuligerus ΔclaR::aac(pMS83-claR), and the control strains S. clavuligerus ΔclaR::aac(pMS83) and S. clavuligerus ATCC 27064 were grown in SA medium to compare the antibiotic production levels. The complementation did not affect appreciably cephamycin C production (Fig. 1B, right panel); however, a clear effect on clavulanic acid and holomycin production was observed. Clavulanic acid was produced by the complemented strain, although to a lower level than by S. clavuligerus ATCC 27064 (Fig. 1B, left panel). No holomycin production was observed in the complemented claR mutant (Fig. 1B, central panel), confirming that the lack of ClaR was responsible for the holomycin production phenotype. Aerial mycelium formation and sporulation were fully restored in the complemented claR strain (Fig. 4C).
Validation of the transcriptomic data.
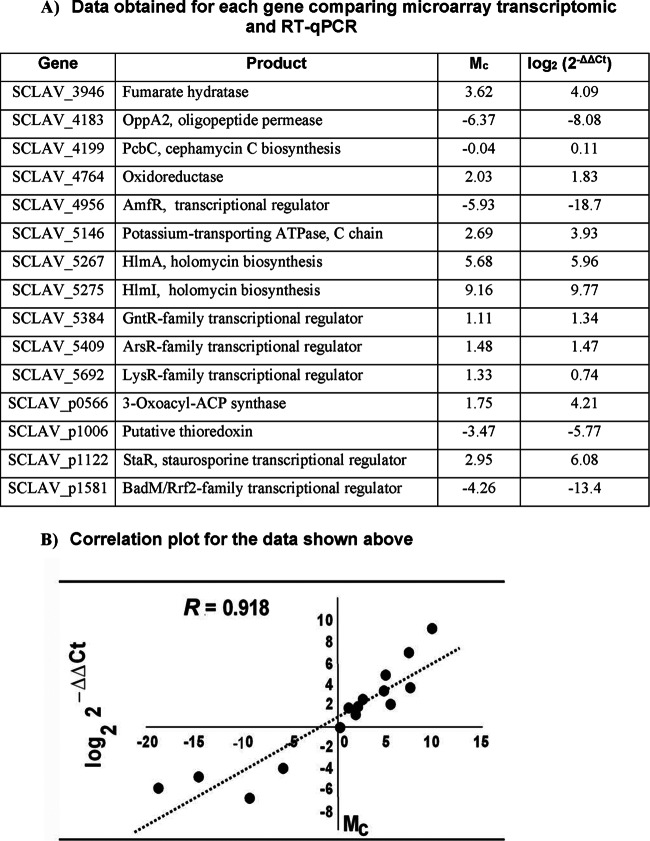
The transcriptomic data have been validated using RT-qPCR on the same RNA samples as those used for the transcriptomic studies at 46.5 h. Fifteen genes were validated (Fig. 5), including genes for the biosynthesis of clavulanic acid (SCLAV_4183), cephamycin C (SCLAV_4199), holomycin (SCLAV_5267, SCLAV_5275), and staurosporine (SCLAV_p1122) and several genes encoding regulatory proteins (SCLAV_4956, SCLAV_5384, SCLAV_5409, SCLAV_5692, SCLAV_p1581), as well as five genes for miscellaneous proteins. The RT-qPCR values consistently confirmed those obtained in the microarrays; a Pearson's correlation of 0.918 between the data obtained by the two techniques was obtained (Fig. 5B).
FIG 5.
Validation of the transcriptomic results. (A) Comparison of the data obtained for each gene analyzed in the transcriptomic experiment and by RT-qPCR. (B) Representation of the correlation between the results shown in panel A.
DISCUSSION
The detection of a low copy number of plasmids pSCL2 and pSCL4 in the mycelium of the old S. clavuligerus claR::aph indicates that our previous results on the loss of large plasmids in Streptomyces mutants (21) is a rather frequent event, probably associated to protoplast preparation and regeneration. Control of the cellular levels of these large plasmids is essential to avoid errors in the interpretation of the phenotypes caused by mutants, especially those obtained by classical mutagenesis and methods that utilize protoplasts.
The construction of a new strain, S. clavuligerus ΔclaR::aac, carrying a wild-type dosage of pSCL2 and pSCL4, allowed us to confirm by transcriptomic methods the results obtained previously by Northern and S1 high-resolution transcriptional analyses (1). These authors described that ClaR controls genes encoding enzymes for the late steps of CA biosynthesis (oppA1, car, and cyp). In addition, the transcriptomic approach showed that also gcaS, oppA2, and the orf12, orf13, orf14, and orf16 genes of the CA gene cluster were downregulated in the ΔclaR mutant with an average transcription of 2.3% in relation to the control strain. It was previously described that the genes for the early steps of the CA pathway were not affected by the claR mutation (1); in this study, we found that they are also downregulated, although to a lower extent (39 to 56%). This low expression allows a partial flux of the early part of the pathway to clavaminic acid and explains that this intermediate is accumulated in the S. clavuligerus ΔclaR::aac mutant grown in SA medium barely above the levels found for the wild-type strain (data not shown). The flux of the clavam pathway, starting with clavaminic acid, is probably weak and unable to use all the clavaminic acid accumulated in the ΔclaR mutant cells.
Pérez-Redondo et al. (2) described that increasing the copy number of claR resulted in lower cephamycin C production. This transcriptomic study confirms that the lack of ClaR upregulates the expression of the cephamycin C genes (up to 1.7-fold) at early times of the fermentation, which results in a 3-fold increase of cephamycin C production at 72 h of culture. The effect of the claR deletion is much stronger on the upregulation of the holomycin biosynthesis gene cluster (3- to 572-fold increase, depending on the gene), a metabolite that was overproduced in the deleted ΔclaR mutant and in S. clavuligerus claR::aph (16). In this work, we demonstrate that lack of ClaR upregulated all the genes of the hlmABCDEFG cluster (289-fold change on average) at the 22.5-h culture sample, and then their expression decreased gradually at 46.5 and 60 h. Two genes, hlmI and hlmH, showed a continuous upregulation along the entire fermentation with a maximum at 46.5 h (555-fold change on average). HlmI is a flavin-dependent dithiol oxidase using oxygen as a cosubstrate, which might be part of the RpoE regulon. HlmH is an MFS-type transporter; the S-methylation of holomycin has been described as the mechanism of holomycin resistance in S. clavuligerus (45); however, the high expression of hlmH when the strain is actively producing holomycin suggests that the pumping out of holomycin might be an alternative resistance mechanism.
While ClaR has been described as involved in clavulanic acid biosynthesis regulation and related to overproduction of holomycin, in this work we demonstrate that additional blocks of genes not directly related to antibiotic production are also controlled by ClaR, including genes encoding other regulators. The most affected regulator in the S. clavuligerus ΔclaR mutant is that encoded by SCLAV_4956, a gene that is almost completely silent in the mutant, especially at 46.5 h of growth. AmfR is a NarL/FixJ-type regulator, orthologous to S. griseus AmfR and to S. coelicolor RamR. AmfR activates the expression of the amfTSBA operon in S. griseus, and the same occurs with RamR in the ramCSAB operon in S. coelicolor or S. lividans (44, 46, 47). The lack of amfR expression in S. clavuligerus ΔclaR strongly downregulates amfT (100-fold), which encodes a putative protein kinase orthologous to RamC. The effect of the low amfR expression on amfA and amfB, encoding subunits of an ABC transporter, is lesser, and their transcription decreased only 6- to 7-fold in relation to the wild-type strain (Fig. 4). The amfTBAR cluster of S. clavuligerus appears to be similar to the ramCSABR cluster of S. coelicolor, but no S. clavuligerus amfS gene can be found in public databases (see Addendum in Proof). The ramS gene encodes the 42-amino-acid peptide that, after posttranslational modification, produces SapB, a “lantibiotic-like” peptide required for aerial mycelium formation in S. coelicolor (48). RamC, initially described as a protein kinase, is now considered to be the putative enzyme that dehydrates and cyclizes the RamS peptide (49, 50) to produce the pro-SapB peptide. While an amfS (ramS) annotated gene is not present in the S. clavuligerus genome, a RamS-like peptide must be produced by S. clavuligerus ATCC 27064 since it induces aerial mycelium formation in S. clavuligerus ΔclaR::aac when the two strains are grown close together (Fig. 4B, right), suggesting that a diffusible compound is produced by the wild-type strain. This extracellular complementation phenomenon, a cross-feeding experiment between the wild-type strain and the amfS mutant, was also observed in S. griseus cocultures (51) of the wild type and the amfS mutant. A similar phenomenon occurs in S. coelicolor when the RamS peptide is added to solid cultures of a ramS mutant (52). The observed effect on amfT appears to be characteristic of ClaR and not of AdpA or BldD, which have been described to control amfT expression in S. griseus (44, 53), since expression of the gene SCLAV_0719 (bldD) was not affected in S. clavuligerus ΔclaR::aac and SCLAV_1957 (adpA) was upregulated (3.7-fold) only at 60 h of culture.
The SCLAV_4082 and SCLAV_4083 genes are orthologous to the sigR-rsrA system of S. coelicolor and encode, respectively, an RNA polymerase sigma factor (RpoE), and an anti-sigma factor (RsrA). Both genes are upregulated in the ΔclaR mutant (6- and 4-fold above their level in the control strain, respectively). The SigR regulon controls oxidative stress, allowing thiol homeostasis, correct protein folding, and flavin- or Fe-S protein-dependent redox reactions (41). Oxidative stress leads to RsrA inactivation, releasing SigR for the expression activation of genes in the SigR regulon (54). The anti-sigma factor RsrA detects thiol oxidation and modulates the SigR activity in Streptomyces coelicolor (54, 55, 56). The SigR regulon is formed by at least 160 genes (41). In S. clavuligerus ΔclaR, 73 of 117 genes orthologous to those of the S. coelicolor SigR regulon are upregulated with a level equal to or greater than 1.5-fold at some of the three sampling times, 31 of them being upregulated at all the sampling times (see Table S1 in the supplemental material). S. clavuligerus ΔclaR does not produce clavulanic acid, and this compound might be a sink for cellular oxygen, since several steps of the pathway (as those involving the clavaminate synthase) consume oxygen and, therefore, CA production might help in controlling the oxidative stress. The activation of the RpoE regulon in the ΔclaR mutant would account for the upregulation of the thioredoxin reductase (SCLAV_5275) for holomycin biosynthesis and also for the differential transcription of many oxidases, oxidoreductases, and reductases genes (see Table S1 in the supplemental material).
In summary, deletion of the claR gene affects all the genes for clavulanic acid biosynthesis, especially those encoding the late steps of the pathway. It affects also all the genes for holomycin biosynthesis but also results in downregulation of several clusters for cryptic metabolites. The downregulation observed for amfR confirms the concomitant loss of aerial mycelium formation. Genes involved in oxidative stress are also affected, which would account for the differential transcription of many genes of the RpoE regulon.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant BIO2013-34723 from the Spanish Ministry of Economy and Competitiveness. Y. Martínez-Burgo and R. Álvarez-Álvarez received PFU fellowships from the Spanish Ministry of Science and Innovation.
The collaboration of R. Pérez Redondo and Juan F. Martín in different steps of this work is appreciated.
ADDENDUM IN PROOF
An ORF previously unidentified in public databases has been located in the S. clavuligerus genome between amfT and amfB (nt 5822261 to 5822392). It encodes a 43-amino-acid peptide (56% identical to SapB) which contains the RamS-conserved positions S24, S27, C31, S35, S38, and C42. The ORF should be named amfS.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00916-15.
REFERENCES
- 1.Paradkar AS, Aidoo KA, Jensen SE. 1998. A pathway-specific transcriptional activator regulates late steps of clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol Microbiol 27:831–843. doi: 10.1046/j.1365-2958.1998.00731.x. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Redondo R, Rodríguez-García A, Martín JF, Liras P. 1998. The claR gene of Streptomyces clavuligerus, encoding a LysR-type regulatory protein controlling clavulanic acid biosynthesis, is linked to the clavulanate-9-aldehyde reductase (car) gene. Gene 211:311–321. doi: 10.1016/S0378-1119(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 3.Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 4.Schlaman HR, Okker RJ, Lugtenberg BJ. 1992. Regulation of nodulation gene expression by NodD in rhizobia. J Bacteriol 174:5177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis in Escherichia coli. Mol Microbiol 45:521–532. doi: 10.1046/j.1365-2958.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 6.Parsek MR, Ye RW, Pun P, Chakrabarty AM. 1994. Critical nucleotides in the interaction of a LysR-type regulator with its target promoter region. catBC promoter activation by CatR. J Biol Chem 269:11279–11284. [PubMed] [Google Scholar]
- 7.Tropel D, van der Meer JR. 2004. Bacterial transcriptional regulators for degradation pathways of aromatic compounds. Microbiol Mol Biol Rev 68:474–500. doi: 10.1128/MMBR.68.3.474-500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Costa OH, Martín-Triana AJ, Martínez E, Fernández-Moreno MA, Malpartida F. 1999. An additional regulatory gene for actinorhodin production in Streptomyces lividans involves a LysR-type transcriptional regulator. J Bacteriol 181:4353–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez M, Núñez LE, Braña AF, Méndez C, Salas JA, Blanco G. 2008. Identification of transcriptional activators for thienamycin and cephamycin C biosynthetic genes within the thienamycin gene cluster from Streptomyces cattleya. Mol Microbiol 69:633–645. doi: 10.1111/j.1365-2958.2008.06312.x. [DOI] [PubMed] [Google Scholar]
- 10.Martín JF, Liras P. 2010. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr Opin Microbiol 13:263–273. doi: 10.1016/j.mib.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Mao XM, Sun ZH, Liang BR, Wang ZB, Feng WH, Huang FL, Li YQ. 2013. Positive feedback regulation of stgR expression for secondary metabolism in Streptomyces coelicolor. J Bacteriol 195:2072–2078. doi: 10.1128/JB.00040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Álvarez-Álvarez R, Rodríguez-García A, Santamarta I, Pérez-Redondo R, Prieto-Domínguez A, Martínez-Burgo Y, Liras P. 2014. Transcriptomic analysis of Streptomyces clavuligerus ΔccaR::tsr: effects of the cephamycin C-clavulanic acid cluster regulator CcaR on global regulation. Microb Biotechnol 7:221–231. doi: 10.1111/1751-7915.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santamarta I, López-García MT, Kurt A, Nárdiz N, Alvarez-Álvarez R, Pérez-Redondo R, Martín JF, Liras P. 2011. Characterization of DNA-binding sequences for CcaR in the cephamycin-clavulanic acid supercluster of Streptomyces clavuligerus. Mol Microbiol 81:968–981. doi: 10.1111/j.1365-2958.2011.07743.x. [DOI] [PubMed] [Google Scholar]
- 14.Aidoo KA, Wong A, Alexander DC, Rittammer RA, Jensen SE. 1994. Cloning, sequencing and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene 147:41–46. doi: 10.1016/0378-1119(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez L, Braña A. 1996. Cell density influences antibiotic biosynthesis in Streptomyces clavuligerus. Microbiology 142:1209–1220. doi: 10.1099/13500872-142-5-1209. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente A, Lorenzana LM, Martín JF, Liras P. 2002. Mutants of Streptomyces clavuligerus with disruptions in different genes for clavulanic acid biosynthesis produce large amounts of holomycin: possible cross-regulation of two unrelated secondary metabolic pathways. J Bacteriol 184:6559–6565. doi: 10.1128/JB.184.23.6559-6565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pospiech A, Neumann B. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet 11:217–218. doi: 10.1016/S0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 18.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, England. [Google Scholar]
- 19.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C, Kim J, Shin SG, Hwang S. 2006. Absolute and relative qPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol 123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Álvarez-Álvarez R, Rodríguez-García A, Martínez-Burgo Y, Robles-Reglero V, Santamarta I, Pérez-Redondo R, Martín JF, Liras P. 2014. A 1.8-Mb-reduced Streptomyces clavuligerus genome: relevance for secondary metabolism and differentiation. Appl Microbiol Biotechnol 98:2183–2195. doi: 10.1007/s00253-013-5382-z. [DOI] [PubMed] [Google Scholar]
- 22.López-García MT, Santamarta I, Liras P. 2010. Morphological differentiation and clavulanic acid formation are affected in an S. clavuligerus ΔadpA-deleted mutant. Microbiology 156:2354–2365. doi: 10.1099/mic.0.035956-0. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Zakrajsek BA. 2000. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46:69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 25.Aigle B, Wietzorrek A, Takano E, Bibb MJ. 2000. A single amino acid substitution in region 1.2 of the principal sigma factor of Streptomyces coelicolorA3(2) results in pleiotropic loss of antibiotic production. Mol Microbiol 37:995–1004. doi: 10.1046/j.1365-2958.2000.02022.x. [DOI] [PubMed] [Google Scholar]
- 26.Kin T, Yamada K, Terai G, Okida H, Yoshinari Y, Ono Y, Kojima A, Kimura Y, Komori T, Asai K. 2007. fRNAdb: a platform for mining/annotating functional RNA candidates from non-coding RNA sequences. Nucleic Acids Res 35:D145–D148. doi: 10.1093/nar/gkl837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbig A, Nieselt K. 2011. nocoRNAc: characterization of non-coding RNAs in prokaryotes. BMC Bioinformatics 12:40. doi: 10.1186/1471-2105-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dufour YS, Wesenberg GE, Tritt AJ, Glasner JD, Perna NT, Mitchell JC, Donohue TJ. 2010. chipD: a web tool to design oligonucleotide probes for high-density tiling arrays. Nucleic Acids Res 38:W321–W325. doi: 10.1093/nar/gkq517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagüe P, Rodríguez-García A, López-García MT, Rioseras B, Martín JF, Sánchez J, Manteca A. 2014. Transcriptomic analysis of liquid non-sporulating Streptomyces coelicolor cultures demonstrates the existence of a complex differentiation comparable to that occurring in solid sporulating cultures. PLoS One 9:e86296. doi: 10.1371/journal.pone.0086296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth G. 2005. limma: linear models for microarray data, p 397–420. In Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S (ed), Statistics for biology and health. Springer, New York, NY. [Google Scholar]
- 31.Mehra S, Lian W, Jayapal KP, Charaniya SP, Sherman DH, Hu W-S. 2006. A framework to analyze multiple time series data: a case study with Streptomyces coelicolor. J Ind Microbiol Biotechnol 33:159–172. doi: 10.1007/s10295-005-0034-7. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzana LM, Pérez-Redondo R, Santamarta I, Martín JF, Liras PP. 2004. Two oligopeptide-permease-encoding genes in the clavulanic acid cluster of Streptomyces clavuligerus are essential for production of the β-lactamase inhibitor. J Bacteriol 186:3431–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-Llarena FJ, Liras P, Rodríguez-García A, Martín JF. 1997. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both β-lactam compounds. J Bacteriol 179:2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R, Khaleeli N, Townsend CA. 2000. Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J Bacteriol 182:4087–4095. doi: 10.1128/JB.182.14.4087-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellado E, Lorenzana LM, Rodríguez-Sáiz M, Díez BB, Liras P, Barredo JL. 2002. The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: genetic organization of the region upstream of the car gene. Microbiology 148:1427–1438. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Escribano JP, Martín JF, Hesketh A, Bibb MJ, Liras P. 2008. Streptomyces clavuligerus relA-null mutants overproduce clavulanic acid and cephamycin C; negative regulation of secondary metabolism by (p)ppGpp. Microbiology 154:744–755. doi: 10.1099/mic.0.2007/011890-0. [DOI] [PubMed] [Google Scholar]
- 37.Tahlan K, Anders C, Wong A, Mosher RH, Beatty PH, Brumlik MJ, Griffin A, Hughes C, Griffin J, Barton B, Jensen SE. 2007. 5S clavam biosynthetic genes are located in both the clavam and paralog gene clusters in Streptomyces clavuligerus. Chem Biol 14:131–142. doi: 10.1016/j.chembiol.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Medema MH, Trefzer A, Kovalchuk A, van den Berg M, Müller U, Heijne W, Wu L, Alam MT, Ronning CM, Nierman WC, Bovenberg RAL, Breitling R, Takano E. 2010. The sequence of a 1.8-Mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol 2:212–224. doi: 10.1093/gbe/evq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Walsh CT. 2011. Streptomyces clavuligerus HlmI is an intramolecular disulfide-forming dithiol oxidase in holomycin biosynthesis. Biochemistry 50:4615–4622. doi: 10.1021/bi200321c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liras P. 2014. Holomycin, a dithiolopyrrolone compound produced by Streptomyces clavuligerus. Appl Microbiol Biotechnol 98:1023–1030. doi: 10.1007/s00253-013-5410-z. [DOI] [PubMed] [Google Scholar]
- 41.Kim MS, Dufour YS, Yoo JS, Cho YB, Park JH, Nam GB, Kim HM, Lee KL, Donohue TJ, Roe JH. 2012. Conservation of thiol-oxidative stress responses regulated by SigR orthologues in actinomycetes. Mol Microbiol 85:326–434. doi: 10.1111/j.1365-2958.2012.08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onaka H, Taniguchi S, Igarashi Y, Fumurai T. 2002. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J Antibiot 55:1063–1071. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor TJ, Kanellis P, Nodwell JR. 2002. The ramC gene is required for morphogenesis in Streptomyces coelicolor and expressed in a cell type-specific manner under the direct control of RamR. Mol Microbiol 45:45–57. doi: 10.1046/j.1365-2958.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki H, Takano Y, Ohnishi Y, Horinouchi S. 2003. amfR, an essential gene for aerial mycelium formation, is a member of the AdpA regulon in the A-factor regulatory cascade in Streptomyces griseus. Mol Microbiol 50:1173–1187. doi: 10.1046/j.1365-2958.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Forseth RR, Bowers AA, Schroeder FC, Walsh CT. 2012. A backup plan for self-protection: S-methylation of holomycin biosynthetic intermediates in Streptomyces clavuligerus. Chembiochem 13:2521–2526. doi: 10.1002/cbic.201200536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keijser B, van Wezel JG, Canters PGW, Vijgenboom EJ. 2002. Developmental regulation of the Streptomyces lividans ram genes: involvement of RamR in regulation of the ramCSAB operon. J Bacteriol 184:4420–4429. doi: 10.1128/JB.184.16.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connor TJ, Nodwell JR. 2005. Pivotal roles for the receiver domain in the mechanism of action of the response regulator RamR of Streptomyces coelicolor. J Mol Biol 351:1030–1047. doi: 10.1016/j.jmb.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 48.Flärdh K, Buttner MJ. 2009. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7:36–49. doi: 10.1038/nrmicro1968. [DOI] [PubMed] [Google Scholar]
- 49.Kodani S, Hudson ME, Durrant MC, Buttner MJ, Nodwell JR, Willey JM. 2004. The SapB morphogen is a lantibiotic-like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc Natl Acad Sci U S A 101:11448–11453. doi: 10.1073/pnas.0404220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto Y, Li B, Claesen J, Shi Y, Bibb MJ, van der Donk WA. 2010. Discovery of unique lanthionine synthetases reveals new mechanistic and evolutionary insights. PLoS Biol 8:e1000339. doi: 10.1371/journal.pbio.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda K, Oinuma KG, Ikeda K, Hosono K, Ohnishi Y, Horinouchi S, Beppu T. 2002. AmfS, an extracellular peptidic morphogen in Streptomyces griseus. J Bacteriol 184:1488–1492. doi: 10.1128/JB.184.5.1488-1492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willey J, Santamaría R, Guijarro J, Geistlich M, Losick R. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell 65:641–650. doi: 10.1016/0092-8674(91)90096-H. [DOI] [PubMed] [Google Scholar]
- 53.Ueda K, Hideaki T, Nishimoto M, Inaba H, Beppu T. 2005. Dual transcriptional control of amfTSBA, which regulates the onset of cellular differentiation in Streptomyces griseus. J Bacteriol 187:135–142. doi: 10.1128/JB.187.1.135-142.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antelmann H, Helmann JD. 2011. Thiol-based redox switches and gene regulation. Antioxid Redox Signal 14:1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paget MS, Buttner MJ. 2003. Thiol-based regulatory switches. Annu Rev Genet 37:91–121. doi: 10.1146/annurev.genet.37.110801.142538. [DOI] [PubMed] [Google Scholar]
- 56.D'Autréaux B, Toledano MB. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.