Abstract
Rotavirus (RV) is the major etiological agent of acute gastroenteritis in infants worldwide. Although high-pressure processing (HPP) is a popular method to inactivate enteric pathogens in food, the sensitivity of different virus strains within same species and serotype to HPP is variable. This study aimed to compare the barosensitivities of seven RV strains derived from four serotypes (serotype G1, strains Wa, Ku, and K8; serotype G2, strain S2; serotype G3, strains SA-11 and YO; and serotype G4, strain ST3) following high-pressure treatment. RV strains showed various responses to HPP based on the initial temperature and had different inactivation profiles. Ku, K8, S2, SA-11, YO, and ST3 showed enhanced inactivation at 4°C compared to 20°C. In contrast, strain Wa was not significantly impacted by the initial treatment temperature. Within serotype G1, strain Wa was significantly (P < 0.05) more resistant to HPP than strains Ku and K8. Overall, the resistance of the human RV strains to HPP at 4°C can be ranked as Wa > Ku = K8 > S2 > YO > ST3, and in terms of serotype the ranking is G1 > G2 > G3 > G4. In addition, pressure treatment of 400 MPa for 2 min was sufficient to eliminate the Wa strain, the most pressure-resistant RV, from oyster tissues. HPP disrupted virion structure but did not degrade viral protein or RNA, providing insight into the mechanism of viral inactivation by HPP. In conclusion, HPP is capable of inactivating RV at commercially acceptable pressures, and the efficacy of inactivation is strain dependent.
INTRODUCTION
Rotavirus (RV) is the major etiological agent of acute gastroenteritis in infants worldwide (1, 2). RVs are estimated to cause nearly 500,000 deaths annually among children (3, 4). The virus is transmitted by the fecal-oral route, and contaminated water and food are common vehicles for infections (1, 5, 6). RV belongs to the genus Rotavirus, subfamily Sedoreovirinae, and family Reoviridae. There are eight species (groups) of rotavirus, referred to as A, B, C, D, E, F, G, and H. Humans are infected primarily by species A, B, and C, most commonly by species A. Rotavirus species A can be further divided into different serotypes. RV is a segmented double-stranded RNA virus with a triple-layer icosahedral capsid. The outer capsid glycoprotein (VP7) and the spike protein (VP4) differentiate RVs into 14 G (glycoprotein) serotypes and 27 different P (protease sensitivity) genotypes (1, 3, 4). Currently, five serotypes (G1 to G4 and G9) are the predominant circulating viruses, accounting for almost 95% of strains worldwide (1). Recently, commercial RV vaccines have been used in children to provide immunity against the most commonly circulating strains (4). Despite major efforts, RV outbreaks still occur worldwide due to the high genetic diversity of RVs and lack of cross-protection (2, 7–9). Therefore, alternative strategies for the prevention of RV infection must be established.
Enteric viruses are a leading cause of foodborne illnesses. Within foodborne viruses, human norovirus (NoV), rotaviruses (RVs), hepatitis A virus (HAV), and human sapovirus are the most prevalent viral pathogens associated with foodborne outbreaks (5, 10–12). In recent years, epidemiological evidence indicates that viruses cause the majority of outbreaks associated with bivalve shellfish (6, 7). RV has frequently been detected in both freshwater and marine water sources (8, 13). As a consequence, RVs are often found to contaminate bivalve shellfish (9, 14–16). In a survey of 300 shellfish (including oysters, mussels, and cockles) harvested in growing waters off the coast of Thailand, RV was detected in 8% of the samples (17). In a survey of oysters in Mexico City (n = 30), 26.9% were found to contain RV (16). Although outbreaks of RV are rare in adults and infections are typically asymptomatic, infected adults may unknowingly expose infants, the elderly, and the immunocompromised to the virus (1). Therefore, there is an urgent need to develop technologies that can inactivate RV in foods while maintaining the sensory and nutritional qualities of the product.
High-pressure processing (HPP) is a promising nonthermal technology that inactivates foodborne viruses while maintaining the organoleptic properties of processed foods (18–22). The technology applies hydrostatic pressure instantaneously and uniformly throughout foods regardless of size, shape, and geometry, thus inactivating both surface and internalized pathogens (21, 22). HPP is an increasingly popular method used by the shellfish industry to inactivate Vibrio parahaemolyticus, enteric viruses, and other pathogens (18, 22, 23). HPP levels of up to 600 MPa for several minutes are sufficient to inactivate most pathogenic microorganisms, such as bacteria and viruses (22). In addition to ensuring the safety of the shellfish, HPP treatment at between 100 and 600 MPa separates the meat of the shellfish from their shell, which minimizes labor costs (19, 22, 24). High-pressure-treated oysters are more voluminous, more juicy, and higher in moisture content than untreated oysters (25). It was reported that pressure-treated oysters are also more desirable based on sensory evaluations (22, 26).
To effectively inactivate pathogens, it is critical to optimize the conditions for pressure treatment. The effectiveness of HPP is influenced by many factors, including both processing parameters (pressure, temperature, and holding time) and nonprocessing parameters (the virus structure itself, food matrix, and pH and water activity [aw] of foods). In general, it has been established that increasing both the treatment pressure and treatment time increases viral inactivation. However, one interesting observation for HPP inactivation of viruses is that different viruses are variable in their susceptibility to high pressure. For example, enveloped viruses are less stable to environmental stresses than nonenveloped viruses. However, some enveloped viruses (e.g., vesicular stomatitis virus [VSV]) are much more stable than nonenveloped viruses during HPP treatment. In addition, viruses are more stable in cold environments than at room temperature. However, many viruses (e.g., human NoV, murine norovirus [MNV], and Tulane virus [TV]) are more easily inactivated by HPP at cold temperatures (e.g., 4°C) than at ambient temperature.
According to the International Committee on Taxonomy of Viruses (ICTV), viral classification starts at the level of order and continues as follows: family, subfamily, genus, species, and serotype. To date, only a few studies have shown that different viruses from the same family or genus have variable high-pressure susceptibilities. However, it is not known whether different viruses from the same species or serotype have different pressure susceptibilities. The genus Rotavirus has substantial genetic diversity. For instance, the amino acid homology of the capsid proteins of species A rotavirus strains can range from 70 to 95%. This high genetic diversity makes rotavirus an ideal model to study the role of strain diversity in pressure sensitivity.
This study aimed to compare the barosensitivities of different RV strains derived from four serotypes to HPP and to gain a better understanding of the correlation between strain diversity and pressure resistance. Understanding this fundamental question will help to optimize the conditions for pressure inactivation of RVs and facilitate the development of technologies to eliminate RVs from high-risk foods.
MATERIALS AND METHODS
Viruses and cell culture.
Seven RV strains were used in this study. These strains were of serotype G1 (the Wa, Ku, and K8 human strains), G2 (the S2 human strain), G3 (the SA-11 simian strain and YO human strain), and G4 (the ST3 human strain). For tissue culture adaption, RV strains S2 and ST3 were first propagated in grivet monkey kidney (BGM-70) cells before being adapted to the rhesus monkey kidney (MA-104) cell line. All other strains were grown in MA-104 cells cultured in Eagle's minimum essential medium (MEM). Briefly, confluent MA-104 cells in T75 flasks were infected by each RV strain at a multiplicity of infection (MOI) of 1.0. After 1 h of incubation, 10 ml of MEM containing 6 μg/ml of trypsin (Invitrogen) was added. At 48 to 96 h postinfection, viruses were harvested by three freeze-thaw cycles and centrifugation at 1,500 × g for 15 min. The virus titer was determined by plaque assay, and virus stocks were stored at −80°C.
RV plaque assay.
RV plaque assay was performed as described previously (27). Briefly, monolayers of MA-104 cells were grown in six-well plates (Corning Life Sciences, Wilkes-Barre, PA) at a density of 2 × 106 cells per well. Four hundred microliters of a 10-fold dilution series of RV was added to each well, and the plates were incubated for 1 h at 37°C with agitation every 10 to 15 min. The plates were overlaid with 1% agarose–MEM in the presence of 2.5 μl/ml trypsin, 1% sodium bicarbonate, 0.1 mg of kanamycin/ml, 0.05 mg of gentamicin/ml, 15 mM HEPES (pH 7.7), and 2 mM l-glutamine. Plates were incubated at 37°C and 5% CO2 for 72 h. A 10% formaldehyde solution was used to fix the plates for 2 h, and plaques were visualized by crystal violet staining. The viral titer was expressed as log10 PFU/ml.
Pressure inactivation of different RV strains.
RV strains were treated by HPP at levels from 200 to 450 MPa with a holding time of 2 min at an initial temperature of 4°C or 20°C. Briefly, 400 μl of each RV strain was suspended in MEM and was double packaged and sealed in sterile polyethylene stomacher pouches (Fisher Scientific International, Ontario, Canada). Samples were subjected to HPP treatment using a lab-scale HPP unit (model Avure PT-1; Avure Technologies, Kent, WA) with water as the hydrostatic fluid. The 2-min holding time did not include the pressure come-up and release time (22 MPa/s, and 4 s, respectively). The virus survival was determined by plaque assay and expressed as log10 PFU/ml.
Purification of RV.
Purification of the RV Wa and SA-11 strains was performed using the protocol described previously (28, 29). Approximately 180 ml of RV Wa or SA-11 strain stock (1.5 × 106 PFU/ml) was centrifuged at 82,000 × g through a 40% (wt/vol) sucrose cushion at 4°C for 5 h in a Ty50.2 rotor (Beckman Coulter, Fullerton, CA). The virus pellet was resuspended in 300 μl of TNC buffer (100 mM NaCl, 10 mM Tris, 1 mM CaCl2) on ice overnight. The virus suspension contained a mixture of double-layer particles (DLPs) and triple-layer particles (TLPs) of RV. A CsCl isopycnic gradient (1.37 g/ml) was used to separate the TLPs and DLPs in an SW50.1 rotor (Beckman Coulter), centrifuging at 115,000 × g at 4°C for 18 h. The upper and lower bands, containing TLPs and DLPs, respectively, were separately collected and resuspended in TNC buffer. The TLPs and DLPs were further purified by ultracentrifugation at 68,000 × g for 2 h at 4°C, and the final TLP or DLP pellets were resuspended in 300 μl of TNC buffer.
RT-PCR.
Reverse transcription-PCR (RT-PCR) was used to determine whether the VP7 gene, the RV viral capsid gene, was degraded by HPP. Total viral RNA of strain Wa or SA-11 was extracted from RV treated at 400 or 550 MPa and from untreated virus using the RNeasy minikit (Qiagen, Valencia, CA). Two primers were used to amplify the VP7 gene (5′-GAGAGAATTTCCGTCTGGCTAA-3′ and 5′-CTTGCCACCACTTTTTCCAAT-3′ for strain Wa and 5′GGTCACATCATACAATTCTAACC and 5′GGCTTTAAAAAGAGAGAATTTCC for strain SA-11). DNA bands were visualized using 1% gel electrophoresis.
In addition, the RNA of RV (Wa) was extracted from the virus particle. Either viral RNA was directly subjected to HPP treatment, or 20 μl of viral RNA was treated with 1 μl RNase-out (Invitrogen) (diluted 1:10) prior to HPP treatment. Viral RNA without RNase-out or with RNase-out was then amplified by RT-PCR. This method was used to establish whether HPP treatment directly degraded viral RNA.
Transmission electron microscopy (TEM).
Ten microliters of purified TLPs and DLPs of strain Wa or SA-11 was treated at 200, 400, and 600 MPa for 2 min at an initial temperature of 4°C; control samples were untreated. The samples were then fixed to copper grids (Electron Microscopy Sciences, Inc.) and subjected to negative staining using uranyl acetate. Virus particles were then visualized with an FEI Tecnai G2 Spirit transmission microscope at the Microscopy and Imagining Facility at the Ohio State University.
Analysis of RV proteins by SDS-PAGE.
Ten microliters of purified treated (200, 400, or 600 MPa) or untreated TLPs and DLPs of strains Wa and SA-11 was examined by SDS-PAGE. Each sample was mixed (1:4, vol/vol) with SDS-PAGE loading buffer, which consists of 1% SDS, 2.5% β-mercaptoethanol, 6.25 mM Tris-HCl (pH 6.8), and 5% glycerol. The mixture was boiled for 5 min and loaded onto a 15% polyacrylamide gel. The protein bands were visualized by Coomassie blue staining.
Bioaccumulation of RV in oyster tissues.
To mimic the natural bioaccumulation of RV in oysters, 25 live oysters (Kroger Co.) were cultivated in 4 liters of salt water containing 1 × 106 PFU/ml of Wa for 24 h under aeration conditions with phytoplankton feed. Following bioaccumulation, the contaminated oysters were shucked and the meat was packaged and sealed in sterile polyethylene stomacher pouches. The oyster meat was treated at pressure levels ranging from 200 to 500 MPa for 2 min at an initial temperature of 4°C or 20°C. Following HPP treatment, the oyster meat was homogenized in 5 ml Hanks balanced salt solution (HBSS) using a mortar and pestle. Virus survival was determined by plaque assay.
Statistical analysis.
All experiments were performed in triplicate. Virus titers were expressed as mean log10 PFU/ml ± 1 standard deviation. Statistical analysis was performed by one-way multiple comparisons using SPSS 8.0 statistical analysis software (SPSS Inc., Chicago, IL). A P value of <0.05 was considered statistically significant.
RESULTS
Effect of HPP initial temperature on different RV strains.
The initial temperature of HPP is a critical parameter influencing the effectiveness of virus inactivation, working either synergistically or antagonistically with pressure. Chilling (4°C) and ambient (20°C) temperatures were selected to avoid the thermal factor (combination of heat and pressure) in comparing HPP inactivation of different RV strains. To determine the role of temperature in HPP inactivation of RV strains, seven RV strains of G1 (Wa, Ku, and K8 human strains), G2 (S2 human strain), G3 (SA-11 simian strain and YO human strain), and G4 (ST3 human strain) were treated with different levels of HPP ranging from 200 to 450 MPa at an initial temperature of either 4 or 20°C for a holding time of 2 min. Plaque assay was used to determine virus survival.
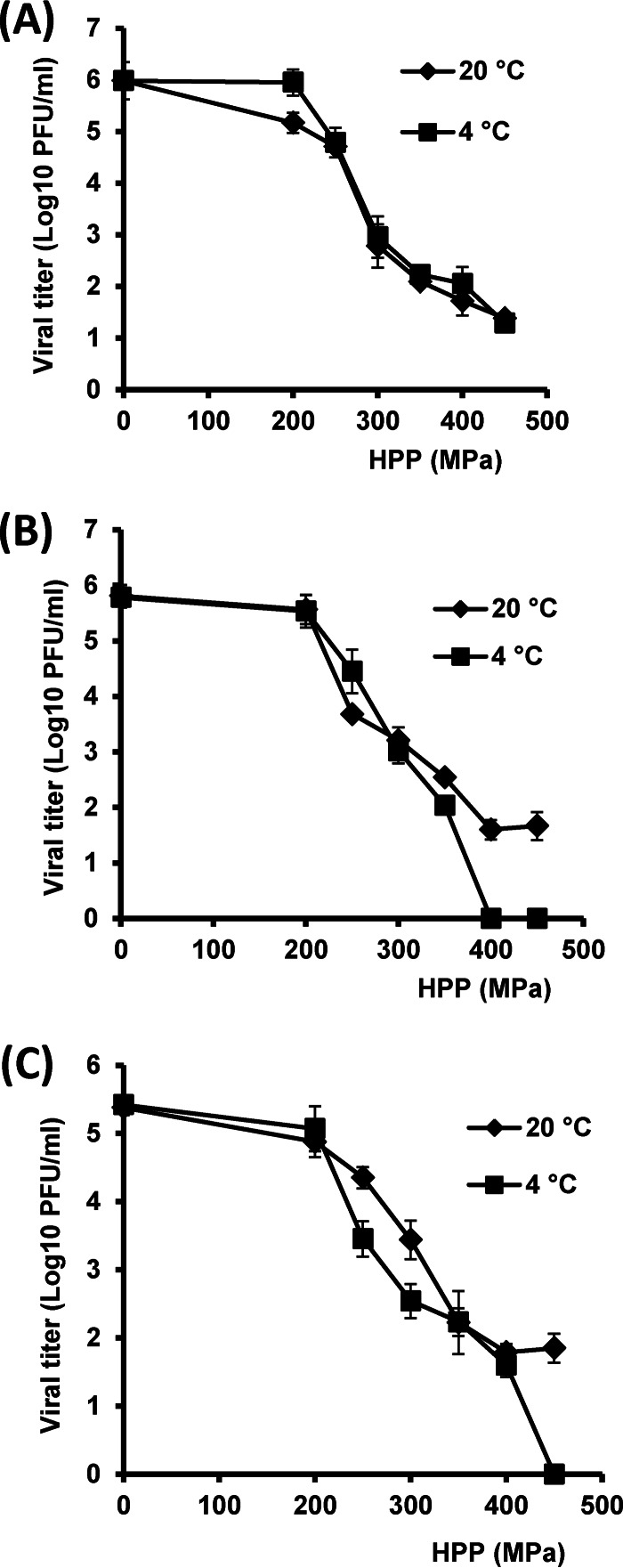
The results for the G1 strains are shown in Fig. 1. In general, increasing the pressure level enhanced virus inactivation. At the initial temperature of 20°C, a shoulder effect was observed at low HPP levels (200 MPa) and a tailing effect was observed at high HPP levels (400 and 450 MPa) for all three G1 strains (Fig. 1). There was no significant difference in the inactivation kinetics of strain Wa at either initial temperature, 4 or 20°C (P > 0.05). A pressure level of 450 MPa for 2 min was not sufficient to completely inactivate the Wa strain (5.9 log10 PFU) suspended in culture medium, and 1.4 and 1.8 log10 PFU of Wa survived following treatment with 450 MPa at either 4 or 20°C, respectively (Fig. 1A). Ku was more susceptible to HPP at the low temperature (4°C) than at an initial temperature of 20°C (Fig. 1B). At 4°C, 400-MPa treatment reduced the Ku titer to below the detection limit (Fig. 1B). However, 1.8 log10 PFU of Ku was still detected at an initial temperature of 20°C after 400- and 450-MPa treatments (Fig. 1B). Similarly, strain K8 showed the same high barosensitivity as Ku at the low initial temperature (4°C) (Fig. 1C). A 5-log reduction of K8 was achieved at 400 MPa at 4°C (Fig. 1C). Conversely, approximately 1 log10 PFU of K8 was detected at 450 MPa at 20°C (Fig. 1C).
FIG 1.
Effect of temperature on inactivation of RV serotype G1 strains. RV stock (106 PFU/ml) in cell culture medium (MEM) was processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at either 4°C or 20°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations. (A) Effect of temperature on strain Wa; there was no significant difference in viral reduction between 4 and 20°C (P > 0.05). (B) Effect of temperature on strain Ku; there was a significant difference in reduction between 4 and 20°C (P < 0.05). (C) Effect of temperature on strain K8; there was a significant difference in reduction between 4 and 20°C (P < 0.05).
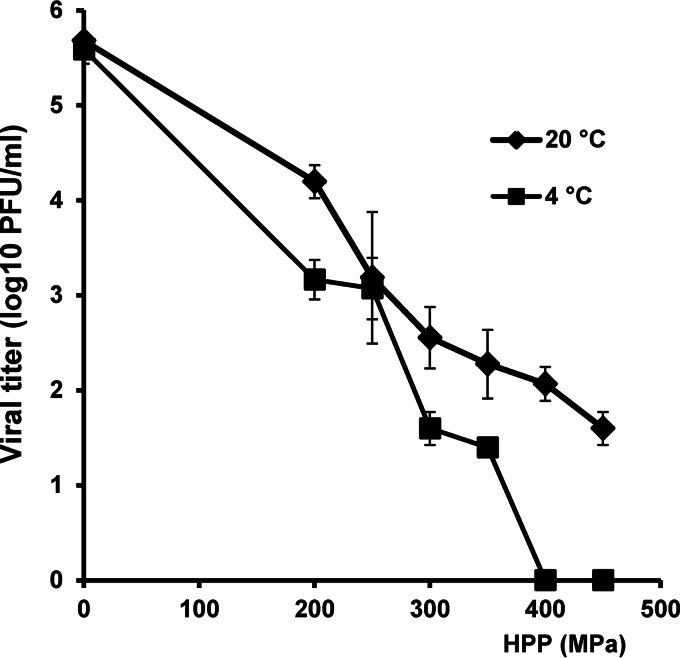
The HPP inactivation profile of the G2 virus S2 strain is presented in Fig. 2. S2 was more susceptible to HPP at a low initial temperature than at a high initial temperature. Treatment with 450 MPa at 4°C for 2 min reduced the S2 titer below the detection limit (Fig. 2). In contrast, 1.5 log10 PFU of S2 remained after the treatment with 450 MPa at 20°C for 2 min (Fig. 2).
FIG 2.
Effect of temperature on inactivation of RV serotype G2 strain S2. RV stock (106 PFU/ml) in cell culture medium (MEM) was processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at either 4°C or 20°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations. Viral reduction was significantly different between the initial temperatures of 4°C and 20°C (P < 0.05).
Viruses in genotype G3 (SA-11 and YO) had differing inactivation patterns in response to high-pressure treatment depending on the initial treatment temperature (Fig. 3). YO was more susceptible to pressure treatment at the low initial temperature. At 4°C, a 5.3-log reduction was achieved at 400 MPa (below the detection limit); however, at 20°C, a tailing effect was observed, with 1 log10 PFU detected following 450-MPa HPP treatment (Fig. 3B). On the other hand, there was no significant effect of the initial temperature on SA-11 inactivation. Approximately 1 log10 PFU of SA-11 was still viable after 450 MPa of pressure treatment at both 4°C and 20°C (Fig. 3A).
FIG 3.
Effect of temperature on inactivation of RV serotype G3 strains. RV strains SA-11 (7.5 log10 PFU/ml) and YO (5.5 log10 PFU/ml) in cell culture medium (MEM) were processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at either 4°C or 20°C. The virus survival was determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations. (A) Effect of temperature on strain SA-11. (B) Effect temperature on strain YO. Viral reduction was significantly different between the initial temperature of 4°C and 20°C (P < 0.05).
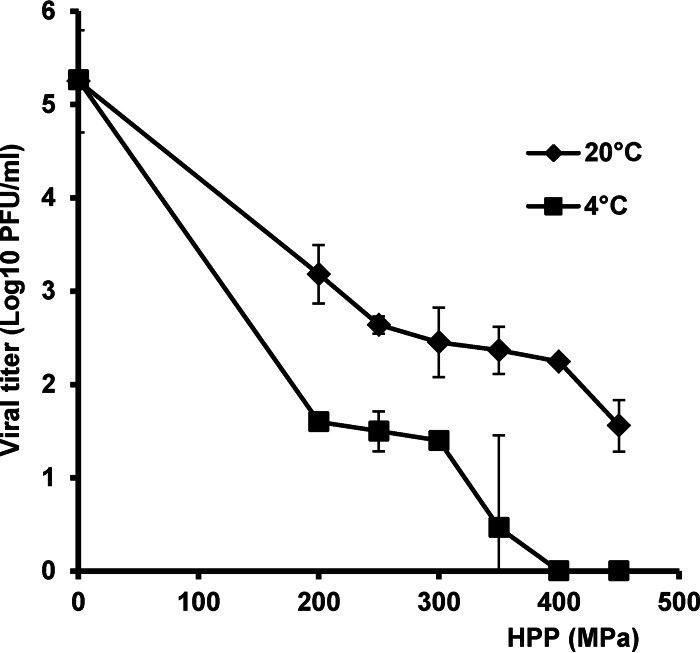
For the G4 strain, ST3, decreasing the initial temperature to 4°C resulted in a significant enhancement in viral inactivation compared to that at 20°C. Treatment with 400 MPa at 4°C reduced the level of ST3 below the detection limit (Fig. 4). At 20°C, >1 log10 PFU remained after treatment with 450 MPa (Fig. 4).
FIG 4.
Effect of temperature on inactivation of RV serotype G4 strain ST3. RV stock (106 PFU/ml) in cell culture medium (MEM) was processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at either 4°C or 20°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations. Viral reduction was significantly different between the initial temperatures of 4°C and 20°C (P < 0.05).
Comparing the barosensitivities of different human RV strains.
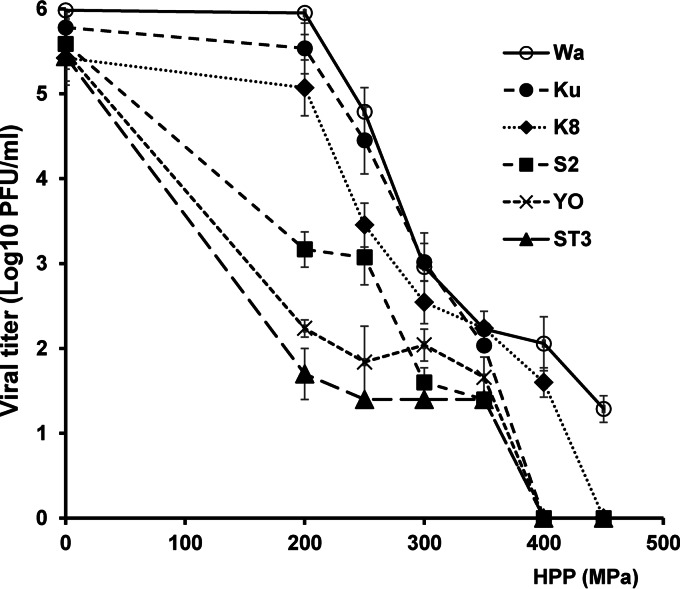
Since all RV strains tested were more susceptible to HPP at 4°C, we directly compared the sensitivities of all RV strains at this temperature. As shown in Fig. 5, RV strains showed different inactivation profiles due to HPP treatment, even within strains of the same serotype. G1 strain Wa was significantly (P < 0.05) more resistant to HPP inactivation than Ku and K8. Overall, the levels of resistance of G1 strains to HPP at an initial temperature of 4°C can be ranked as Wa > Ku = K8. The G2 strain, S2, was more significantly (P < 0.05) inactivated by HPP than the G1 strains (Wa, Ku, and K8) at the low initial temperature (4°C). The G3 strain, YO, had significantly (P < 0.05) increased inactivation by HPP than the G2 and G1 strains at an initial temperature of 4°C. ST3, the G4 strain, was the most sensitive strain to HPP treatment at an initial temperature of 4°C. Overall, the resistance of the six strains to HPP treatment at an initial temperature of 4°C can be ranked as Wa > Ku = K8 > S2 > YO > ST3, and in terms of serotypes the ranking is G1 > G2 > G3 > G4.
FIG 5.
Direct comparison of pressure inactivation of six human RVs derived from four serotypes. RV stock (106 PFU/ml) in cell culture medium (MEM) was processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at 4°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations. Strain Wa was significantly (P < 0.05) more resistant to HPP inactivation than strains Ku and K8. Strain S2 was more significantly (P < 0.05) inactivated by HPP than the G1 strains (Wa, Ku, and K8). YO had significantly (P < 0.05) increased inactivation by HPP compared to the G2 and G1 strains. ST3 was the most sensitive strain (P < 0.05).
Effect of HPP on the RV capsid.
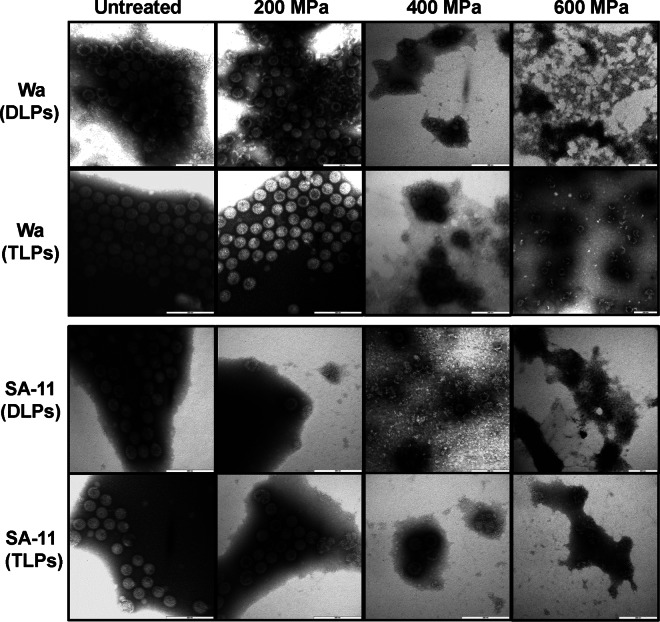
To understand the mechanism of RV inactivation by HPP, we determined the effects of HPP on the viral capsid, proteins, and genomic RNA using two RV strains, a human strain (Wa) and a simian strain (SA-11). Briefly, purified TLPs and DLPs of strains Wa and SA-11 suspended in TNC buffer were pressure treated at levels between 200 and 600 MPa at 4°C for a 2-min holding time. The pressured particles were negatively stained with ammonium molybdate and examined by TEM.
At the lowest pressure level applied, 200 MPa, the TLPs were intact and the appearance was similar to that of untreated samples (Fig. 6). At the same pressure level, the DLPs were damaged and high levels of debris were observed. By increasing the pressure level to 600 MPa, the TLPs and DLPs were completely disrupted, and no intact particles were observed (Fig. 6). These results indicate that the RV capsid can be completely disrupted at 600 MPa for 2 min at 4°C. Also, it was observed that TLPs (complete viral particle) were more resistant to HPP treatment than DLPs, which lack the outermost layer of the complete virion, which is comprised of VP4 and VP7.
FIG 6.
HPP disrupts the integrity of RV particles. Purified TLPs and DLPs of RV strains Wa and SA-11 were treated with HPP at 200, 400, and 600 MPa at 4°C for 2 min. HPP-treated and untreated samples were negatively stained with 1% ammonium molybdate and visualized by transmission electron microscopy.
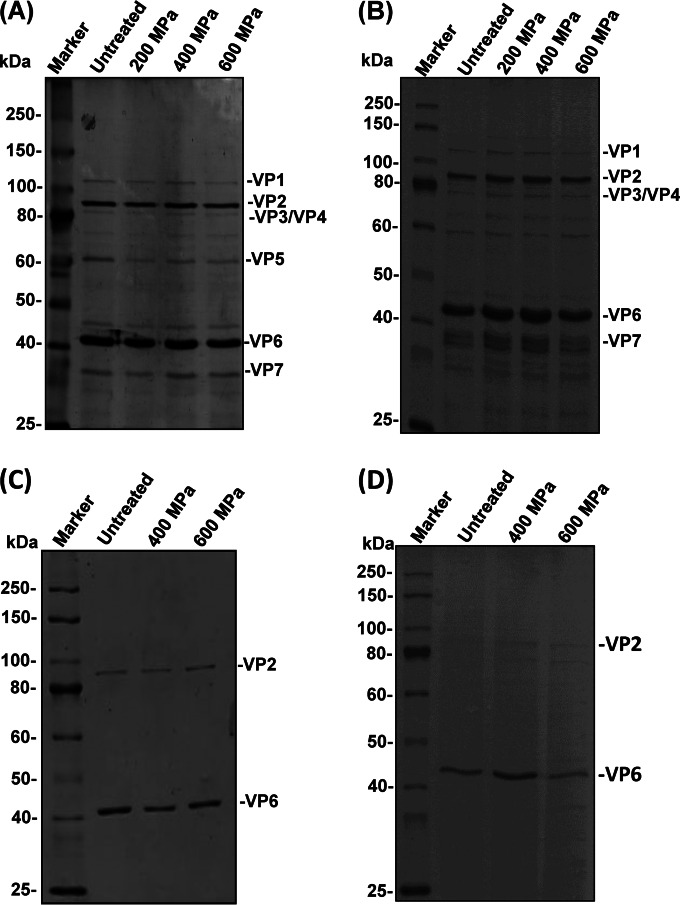
HPP effect on the viral proteins.
To investigate the effect of HPP on viral proteins of different RV strains, we compared the most HPP-resistant strain (Wa) and the simian strain (SA-11). Ten microliters of purified TLPs of Wa or SA-11 was treated at 200, 400, and 600 MPa for 2 min at 4°C, and viral proteins were analyzed by SDS-PAGE. For both strains Wa and SA-11, six structural proteins (VP1, VP2, VP3, VP4, VP6, and VP7) were observed in untreated and treated TLPs. HPP treatment of 200, 400, and 600 MPa did not alter the abundance of viral proteins (Fig. 7A and B). Similarly, the density of the proteins of DLPs was not significantly altered by HPP treatment for both the Wa and SA-11 strains (Fig. 7C and D). The data suggest that the primary structure of the viral proteins remained intact although the virion structure was completely disrupted at a pressure level of 600 MPa.
FIG 7.
Effect of HPP on RV proteins. Purified RV TLPs and DLPs were pressurized at 200, 400, and 600 MPa at 4°C for 2 min. The structural proteins of untreated and treated RVs were analyzed by 15% SDS-PAGE followed by Coomassie blue staining. (A) SDS-PAGE of 2 μg of total RV Wa TLPs. (B) SDS-PAGE of 2 μg of total RV SA-11 TLPs. (C) SDS-PAGE of 1.0 μg of total RV Wa DLPs. (D) SDS-PAGE of 1.0 μg of total RV SA-11 DLPs.
Effect of HPP on viral RNA.
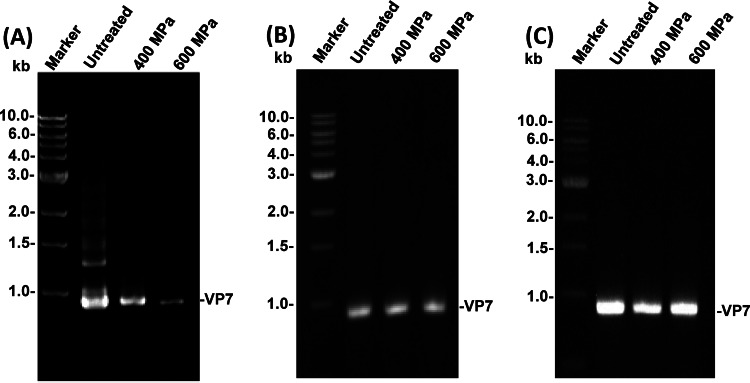
To evaluate the effect of HPP on the virus genomic RNA, purified RV strain Wa with or without RNase inhibitor was treated with HPP ranging from 200 to 600 MPa at 4°C for 2 min. After treatment, total RNA was extracted and subjected to RT-PCR targeting the outer capsid gene VP7, and the amplified DNA bands were visualized by 1% agarose gel electrophoresis. For RV without RNase inhibitor, the density for the VP7 gene decreased as the pressure increased (Fig. 8A). This suggests that exogenous RNase degraded the genomic RNA after the viral capsid was disrupted by HPP. However, no significant decrease in the density of the VP7 gene was observed between the pressurized particle and the control at any pressure level in an RNase-free environment (Fig. 8B). To determine whether HPP directly degraded viral RNA, total viral RNA was extracted from RV strain Wa (without HPP) and subjected to HPP treatment, and the VP7 gene was amplified by RT-PCR. As shown in Fig. 8C, there was no significant difference in VP7 gene detection by RT-PCR between treated and untreated samples. This suggests that HPP does not directly degrade RNA. This observation is consistent with the fact that HPP does not break covalent bonds.
FIG 8.
Effect of HPP on viral genomic RNA. (A) Effect of HPP on RV genomic RNA in the absence of RNase inhibitor. Purified RV strain Wa was treated at 400 and 600 MPa for 2 min at 4°C. After treatment, total viral RNA was extracted, and the VP7 gene of RV was amplified by one-step RT-PCR and visualized by 1% agarose gel electrophoresis. (B) Effect of HPP on RV genomic RNA in the presence of RNase inhibitor. One unit of RNase inhibitor (RNase-out; Invitrogen) was added to the purified RV Wa strain. The samples were treated at 400 and 600 MPa for 2 min at 4°C. After treatment, the VP7 gene of RV was amplified by one-step RT-PCR. (C) Effect of HPP on naked RV genomic RNA. Total viral RNA was extracted from RV strain Wa and treated at 400 and 600 MPa for 2 min at 4°C, and the VP7 gene of RV was amplified by one-step RT-PCR.
Inactivation of RV in oyster tissues.
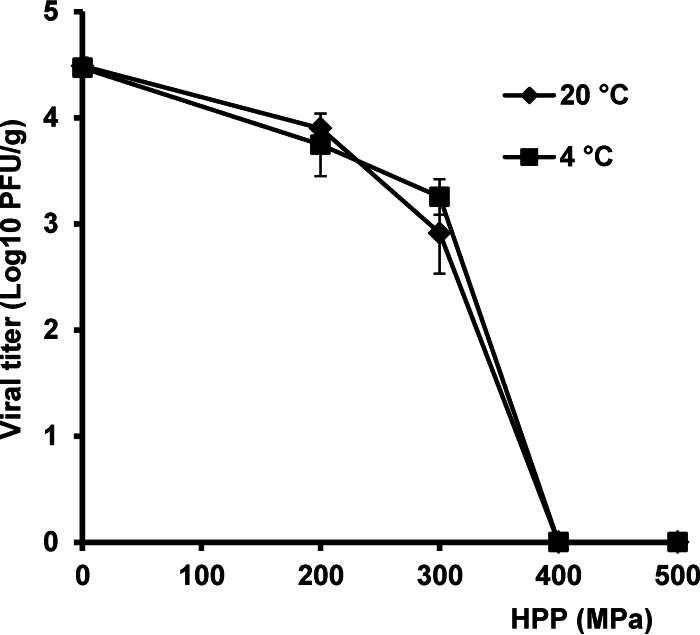
Finally, we determined whether HPP is capable of inactivating the RV Wa strain in oysters, one of the high-risk foods often contaminated by foodborne viruses. To mimic the natural contamination route, strain Wa was bioaccumulated in oysters by adding virus to feed water during oyster growth. After bioaccumulation, oysters were harvested and treated by HPP at pressure levels ranging between 200 and 500 MPa at either 4 or 20°C with a holding time of 2 min. After HPP treatment, oysters were homogenized and virus survival was determined by plaque assay. In oysters, the inactivation curve showed a shoulder effect at low pressure levels (200 and 300 MPa) (Fig. 9), which was similar to the effect observed when the virus was suspended in culture medium. Following treatment of oysters at 400 MPa, a 4.5 log10 PFU virus reduction was achieved, and virus levels were below the detection limit at either initial temperature used (Fig. 9). There was no significant difference (P > 0.05) caused by initial temperature in the virus inactivation in oyster tissues, which is consistent with the results obtained for strain Wa HPP inactivation in culture medium.
FIG 9.
Inactivation of RV strain Wa in oyster tissues by HPP. RV strain Wa (106 PFU) was added to the feed water during oyster growth. At day 3 postinoculation, oysters were harvested and treated with HPP at 200, 300, 400, and 500 MPa at 4°C for 2 min. After treatment, five oysters in each treatment were homogenized, and the surviving RV was quantified by plaque assay. Data are the means of three replicates. Error bars represent standard deviations.
DISCUSSION
RV is a major cause of infant gastroenteritis and death worldwide (1, 2). Although outbreaks in the United States are less frequent due to vaccination, RV remains a major public health concern in the developing world. The genus Rotavirus is highly diverse, and vaccination is insufficient to protect against all strains of RV (1, 2). In addition, many susceptible populations, such as immunocompromised individuals, cannot receive this vaccine. RV outbreaks have been associated with the consumption of contaminated foods and water; therefore, the development of control measures to eliminate RV from food and water sources is critical to prevent outbreaks.
HPP is a nonthermal process that can be used to eliminate foodborne pathogens while maintaining the organoleptic and nutritional properties of foods. To date, the HPP sensitivity of different virus strains in the same genus or family has not been well studied. RVs are genetically diverse, which makes RV a good model to study the role of strain diversity in HPP inactivation of viruses (1, 2). In this study, we found that different RV strains in same genus and genotype responded to HPP differently. Within same genus, the sensitivity of RV strains to HPP can be ranked G1 > G2 > G3 > G4. Interestingly, HPP inactivation of the Wa strain was not dramatically different in culture medium at 4°C and 20°C. Likewise, temperature differences were not apparent for Wa strain inactivation in oyster tissue. In contrast, all other RV strains were more easily inactivated at 4°C than at 20°C. Within the same genotype G1, strain Wa was significantly more stable than strains Ku and K8. The different sensitivities of different RV strains to HPP also raise the possibility that these strains may have different stabilities in the environment and in response to other treatments. It is possible that the difference in the degree of resistance to environmental stress may correlate with the difference in prevalence between those serotypes, although direct evidence is lacking.
Role of initial temperature in the inactivation of seven RV strains.
Temperature is a critical factor influencing virus inactivation by HPP. The optimal initial temperature during HPP varies greatly between different viruses (27, 28, 30–34). In this study, we choose to compare two different initial temperatures, 4°C and 20°C, for their influence on HPP inactivation of the seven RV strains. These temperatures were selected in order to avoid thermal effects caused by the combination of high temperature and HPP. RV strains Ku, K8, S2, SA-11, YO, and ST3 showed enhanced inactivation at an initial temperature of 4°C compared to 20°C. Interestingly, HPP inactivation of the Wa strain was not significantly impacted by the initial temperature during treatment in culture medium or oyster tissues. This is consistent with our previous observation of an approximately a 5-log virus reduction for the Wa strain under 400 MPa at either 4 or 20°C (27). Overall, our results suggest that a low initial temperature increases the inactivation of a majority of the RV strains by HPP. Also, it was previously reported that treatment of RV strain Wa with 300 MPa for 2 min at 25°C inactivated approximately 8 log10 50% tissue culture infective doses (TCID50)/ml of the virus, although different temperature conditions were not compared in that study (35). This is a much more dramatic reduction in the titer of strain Wa than what we observed in this study. It is possible that the initial viral titer used for treatment and/or the quantification methods (PFU versus TCID50) contributed to this difference. Collectively, these data demonstrate that HPP is capable of effectively inactivating RV.
The mechanism behind the increase in the inactivation of the RV strains at the lower initial temperature is not clear; however, this effect has also been observed for many other viruses. For example, viruses in the family Caliciviridae, including human NoV, feline calicivirus (FCV), Tulane virus (TV), and murine norovirus (MNV), were all found to be more sensitive to HPP at low treatment temperatures than at higher initial temperatures (30, 31, 36–40). In contrast, pressure inactivation of HAV, a nonenveloped virus in the family Picornaviridae, is enhanced at ambient temperature and above compared to at lower temperatures (36, 41, 42). It has been suggested that at lower initial temperatures the viscosity of the hydrostatic fluid is altered, leading to increased inactivation of nonenveloped viruses (28). However, this does not explain the increased sensitivity of HAV to HPP at elevated temperatures. Also, a decrease in temperature in combination with HPP may also influence the stability of the viral capsid, making the particle disrupted more easily at low temperatures than at higher temperatures (28). More research is needed in order to establish the mechanism behind this phenomenon.
Role of strain diversity in HPP inactivation.
It has been established that different viruses have differing optimal conditions for inactivation by HPP (27, 29, 32, 36, 38, 39, 41, 43–46). However, the role of strain diversity within the same genus, species, or serotype in inactivation by HPP is still poorly understood. In this study, we examined six human RV strains that belong to the most prevalent genotypes (G1 to G4) for sensitivity to HPP. We found that RV strains showed different inactivation profiles even within the same genotype. For instance, G1 strains Ku and K8 were more susceptible to HPP than the Wa strain. Overall, the ranking of the stability of RV genotypes to HPP is G1 > G2 > G3 > G4. These results indicate that the response of different RV strains to HPP is widely different even though they are closely genetically related and have a similar capsid composition. Sequence analysis found that the amino acid homology of capsid proteins among the species A RV strains can range from 70 to 95%. Perhaps the nucleotide and amino acid diversity could impact both protein-protein and RNA-protein stabilities, which contribute to the differences in stability under pressure treatment. Also, trypsin is required for rotavirus infectivity. It is possible that trypsin can bind to virions and can be copurified with the virions, which may affect the stability of the virus during HPP treatment. These results suggest that there are some distinct molecular or biological differences between the strains that led to the differences in stability to HPP.
Previously, it has been documented that viruses within the same family or genus are highly diverse in profiles of inactivation by HPP treatment. Coxsackie B5 virus (species Enterovirus B, genus Enterovirus) and poliovirus (species Enterovirus B, genus Enterovirus), which belong to the family Picornaviridae, are extremely stable at high levels of HPP. Both Coxsackie B5 virus and poliovirus had less than a 1 log reduction in viral titer after 600-MPa treatments for 5 min at room temperature (33, 38). In contrast, HAV, a virus in species Enterovirus C in genus Enterovirus, was found to be highly susceptible to HPP compared to coxsackie B5 virus and poliovirus (26). A 7-log reduction in HAV was achieved after 450-MPa treatment at room temperature for 5 min in culture medium (24, 26). Human NoV can potentially serve as a good model to study the role of genotype differences in pressure sensitivity, because it is highly diverse both genetically and antigenically. However, it has been a challenge to study human NoV, as it cannot be grown in cell culture. Recently, a surrogate assay (viral receptor binding assay) was developed to discriminate noninfectious and infectious human NoV particles. Using this assay, it was found that the human NoV GI.1 strain was more resistant to HPP than the GII.4 strain at 450 MPa at 1°C (34). In addition, recent evidence suggests that different species in the genus Norovirus with the family Caliciviridae have different pressure sensitivities. Pressure treatment at 400 MPa at 6°C for 5 min completely inactivated murine norovirus, a member of genogroup V within the genus Norovirus. However, this pressure condition was insufficient to prevent Norwalk virus (genogroup 1, genotype 1 [G.1.1.] within genus Norovirus) infection and shedding in human subjects. Interestingly, HPP at 600 MPa and 6°C for 5 min was required to completely inactivate Norwalk virus in seeded oysters, based on the lack of infection and virus shedding in the challenged volunteers (47). Overall, the response of different viruses to HPP appears not to be correlated with virus size, virus shape, presence of an envelope, family, genus, or genotype. The large disparity in the resistance of the different viruses may be attributed to the nature of the virus itself, the size and shape of the virus particle, its high thermodynamic stability, differences in viral receptor binding properties, or differences in protein structure, amino acid composition, and isoelectric point. Understanding the continuity or variability of inactivation associated with different viral strains following HPP can aid in the design of appropriate treatment parameters to inactivate diverse viral populations.
Mechanism underlying HPP inactivation of viruses.
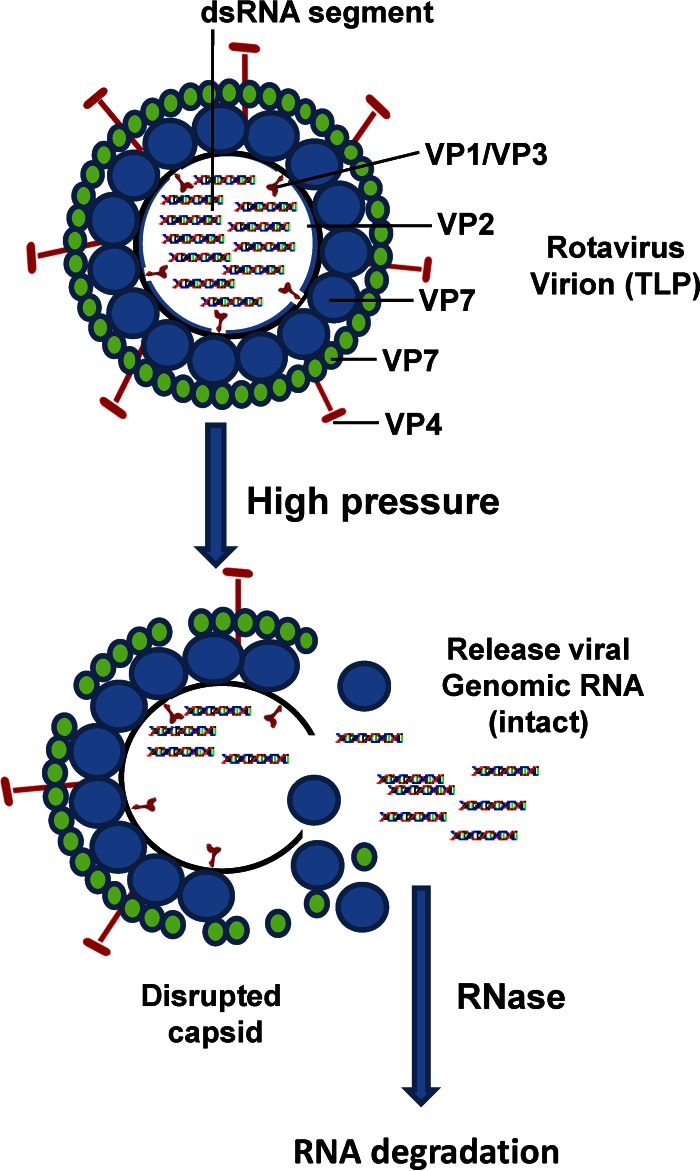
In this study, we found that disruption of the integrity of the viral capsid but not degradation of viral protein or genomic RNA is the primary mechanism of viral inactivation by HPP. The size of the complete TLP of RV is between 70 to 80 nm, with a round shape. The TLP is composed of three structural proteins: VP7, the outer capsid protein; VP6, the medial capsid protein; and VP2, the inner capsid protein. VP4, the outer spike protein, is cleaved by proteases into VP5 and VP8. The DLPs lack VP7 and VP4 and consist of only VP6 and VP2 (1). We found that at a low pressure level (200 MPa) the DLPs of both the Wa and SA-11 strains were disrupted, whereas the TLPs remained intact. This indicates that the outer capsid proteins (VP7 and VP4) stabilize the capsid structure of RV upon HPP treatment. Elevating the pressure level to 600 MPa resulted in disruption of DLPs and TLPs of both strains, and no intact particles were observed.
In general, HPP does not break covalent bonds at the level applied for food processing (up to 800 MPa) (29, 39). Consistent with this, we found that the structural proteins of both TLPs and DLPs were intact after HPP when they were analyzed by SDS-PAGE. In addition, viral genomic RNA was not physically degraded under RNase free conditions when purified RV was treated with a lethal level of HPP. However, the abundance of viral RNA was significantly decreased when RV was not treated with an RNase inhibitor. Based on these observations, a model for a mechanism of viral inactivation by HPP is illustrated in Fig. 10. After pressure treatment, the structure of the viral capsid was disrupted, and the naked viral RNA genome was released from the capsid and subsequently degraded by exogenous RNase present in the food and the environment. Previously, using human NoV virus-like particles (VLPs) as a model, we found that HPP disrupted the capability for binding to histo-blood group antigens (HBGAs), the functional receptor for human NoV (48). Collectively, HPP-induced virus inactivation may include disruption of the integrity of the viral capsid and viral receptor binding activity.
FIG 10.
Models of viral inactivation by HPP. Rotavirus is a small round particle approximately 60 to 80 nm in diameter. The native infectious rotavirus virions, termed triple-layer particles (TLPs), are composed of three concentric layers of proteins and 11 segments of double-stranded RNA. The outermost layer of the virion is comprised of two proteins, VP4 and VP7. VP4 forms dimeric spikes that project from the surface of the virion. The middle layer is comprised of VP6. The innermost layer is composed of three proteins, VP1, VP2, and VP3. After pressure treatment, the structure of the viral capsids was disrupted, whereas the viral capsid proteins and genomic RNA were not degraded by HPP. The naked viral RNA genome was released from the capsid and subsequently degraded by exogenous RNase present in food and the environment.
Inactivation of RV (Wa strain) in oyster tissues.
The consumption of raw oysters is associated with a large number of foodborne virus outbreaks. Shellfish are filter feeders; for instance, oysters can circulate around 16 gallons of water per oyster/day, and the viral and bacterial concentrations in shellfish meat can reach 400 times higher than the levels in the growing water (49). HPP can be used to eliminate foodborne pathogens, such as bacteria and viruses, from oysters to enhance safety, reduce labor costs of shucking, and increase shelf life (17, 18, 21, 43). Here, we found that HPP treatment at 400 MPa at an initial temperature of either 4 or 20°C was capable of eliminating 4.5 log10 of RV strain Wa (the most stable RV strain) from the oyster tissues. The initial temperature during treatment did not have a significant impact on the inactivation of the Wa strain in oyster tissues, which was similar to the results obtained when the virus was suspended in medium. This observation, coupled with the fact that both HAV and a human NoV G1.1 strain can be effectively inactivated by HPP at commercially acceptable pressure levels (≤600 MPa), suggests that HPP is a highly promising technology to ensure the safety of oysters and other seafood.
The complexity of the food matrix has been shown to provide a baroprotective effect to viruses during HPP (36, 39, 43, 44). Specifically, carbohydrates, fats, salts, proteins, ions, and other food constituents can protect viruses from inactivation (36, 43, 50–52). Pressure treatment of bovine enterovirus (a surrogate for hepatitis A virus) and feline calicivirus (a surrogate for human norovirus) in shellfish, seawater, and culture medium showed that the viruses were most resistant when treatment was in oysters and mussels (53). Similarly, HAV was more resistant to HPP in oyster homogenates than in 0.3% NaCl solutions at a similar pH (36). In this study, it was found that a 4.9-log reduction of the Wa strain was achieved in aqueous medium following treatment with 400 MPa for 2 min, whereas only a 4.2-log reduction was achieved at the same pressure level in oyster tissues. At 300 MPa, an approximately 3.0-log reduction of strain Wa in medium was observed, whereas only 2.0-log reduction was achieved in oysters. This result suggests that the food matrix confers protection to RV during HPP treatment. Also, it should be noted that either the Wa strain in oysters was completely inactivated at 400 MPa at 4° and 20°C or else some natural inhibitors to the infectious Wa strain were present/released from the oyster meat matrix after 400-MPa treatment. Thus, it is necessary to optimize the processing parameters for each product, since the efficiency of viral inactivation varies with the food matrix.
In conclusion, we demonstrated that (i) RV inactivation by HPP is favored at 4°C compared to 20°C (with the exception of strain Wa), (ii) RV strains in different genotypes have different susceptibilities to high pressure, and (iii) HPP treatment disrupted the RV virion structure but did not degrade viral protein or RNA.
ACKNOWLEDGMENT
This project was supported by the Agriculture and Food Research Initiative Competitive Grants Program of the USDA National Institute of Food and Agriculture (NIFA award no. 2011-68003-30005).
REFERENCES
- 1.Desselberger U. 2014. Rotaviruses. Virus Res 190:75–96. doi: 10.1016/j.virusres.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Gibson CJ, Bresee JS, Glass RI. 2006. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mameli C, Fabiano V, Zuccotti GV. 2012. New insights into rotavirus vaccines. Hum Vaccin Immunother 8:1022–1028. doi: 10.4161/hv.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollaritsch H, Kundi M, Giaquinto C, Paulke-Korinek M. 2015. Rotavirus vaccines: a story of success. Clin Microbiol Infect 21:735–43. doi: 10.1016/j.cmi.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Koopmans M, Duizer E. 2004. Foodborne viruses: an emerging problem. Int J Food Microbiol 90:23–41. doi: 10.1016/S0168-1605(03)00169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potasman I, Paz A, Odeh M. 2002. Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clin Infect Dis 35:921–928. doi: 10.1086/342330. [DOI] [PubMed] [Google Scholar]
- 7.Lees D. 2000. Viruses and bivalve shellfish. Int J Food Microbiol 59:81–116. doi: 10.1016/S0168-1605(00)00248-8. [DOI] [PubMed] [Google Scholar]
- 8.Lodder WJ, de Roda Husman AM. 2005. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl Environ Microbiol 71:1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leguyader F, Dubois E, Menard D, Pommepuy M. 1994. Detection of hepatitis A virus, rotavirus, and enterovirus in naturally contaminated shellfish and sediment by reverse transcription-seminested PCR. Appl Environ Microbiol 60:3665–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. 2013. Surveillance for foodborne disease outbreaks—United States, 2009-2010. MMWR Morb Mortal Wkly Rep 62:41–47. [PMC free article] [PubMed] [Google Scholar]
- 11.FAO/WHO. 2008. Viruses in food: scientific advice to support risk management activities: meeting report, p 151 Microbiological hazards in fresh leafy vegetables and herbs; FAO/WHO, Rome, Italy. [Google Scholar]
- 12.Jaykus LA, Escudero-Abarca B. 2010. Human pathogenic viruses in food, p 218–232. In Juneja VK, Sofos JN (ed), Pathogens and toxins in foods: challenges and interventions. ASM Press, Washington, DC. [Google Scholar]
- 13.Prevost B, Lucas FS, Goncalves A, Richard F, Moulin L, Wurtzer S. 2015. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ Int 79:42–50. doi: 10.1016/j.envint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Gabrieli R, Macaluso A, Lanni L, Saccares S, Di Giamberardino F, Cencioni B, Petrinca AR, Divizia M. 2007. Enteric viruses in molluscan shellfish. New Microbiol 30:471–475. [PubMed] [Google Scholar]
- 15.Loisy F, Atmar RL, Le Saux JC, Cohen J, Caprais MP, Pommepuy M, Le Guyader FS. 2005. Use of rotavirus virus-like particles as surrogates to evaluate virus persistence in shellfish. Appl Environ Microbiol 71:6049–6053. doi: 10.1128/AEM.71.10.6049-6053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quiroz-Santiago C, Vazquez-Salinas C, Natividad-Bonifacio I, Barron-Romero BL, Quinones-Ramirez EI. 2014. Rotavirus G2P[4] detection in fresh vegetables and oysters in Mexico City. J Food Prot 77:1953–1959. doi: 10.4315/0362-028X.JFP-13-426. [DOI] [PubMed] [Google Scholar]
- 17.Kittigul L, Singhaboot Y, Chavalitshewinkoon-Petmitr P, Pombubpa K, Hirunpetcharat C. 2015. A comparison of virus concentration methods for molecular detection and characterization of rotavirus in bivalve shellfish species. Food Microbiol 46:161–167. doi: 10.1016/j.fm.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi NK, Raghavarao KS, Balasubramaniam VM, Niranjan K, Knorr D. 2007. Opportunities and challenges in high pressure processing of foods. Crit Rev Food Sci Nutr 47:69–112. doi: 10.1080/10408390600626420. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Romero M, Smiddy M, Hill C, Kerry JP, Kelly AL. 2004. Effects of high pressure treatment on physicochemical characteristics of fresh oysters (Crassostrea gigas). Innov Food Sci Emerg 5:161–169. doi: 10.1016/j.ifset.2004.01.002. [DOI] [Google Scholar]
- 20.He H, Adamas RM, Farkas DF, Morrissey MT. 2002. Use of high-pressure processing for oyster shucking and shelf life extension. J Food Sci 67:640–645. doi: 10.1111/j.1365-2621.2002.tb10652.x. [DOI] [Google Scholar]
- 21.Hoover DG, Metrick C, Papineau AM, Farkas DF, Knorr D. 1989. Biological effects of high hydrostatic pressure on food microorganisms. Food Technol 43:99. [Google Scholar]
- 22.Lou F, Neetoo H, Chen H, Li J. 2015. High hydrostatic pressure processing: a promising nonthermal technology to inactivate viruses in high-risk foods. Annu Rev Food Sci Technol 6:389–409. doi: 10.1146/annurev-food-072514-104609. [DOI] [PubMed] [Google Scholar]
- 23.Abdel Karim P. 2011. High pressure processing as an alternative food preservation technology and its applications for fruits and vegetables. Kansas State University, Manhattan, KS. [Google Scholar]
- 24.Lopez-Caballero ME, Perez-Mateos M, Montero P, Borderias AJ. 2000. Oyster preservation by high-pressure treatment. J Food Prot 63:196–201. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley DH. 2014. High pressure processing of bivalve shellfish and HPP's use as a virus intervention. Foods 3:336–350. doi: 10.3390/foods3020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye M, Lingham T, Huang Y, Ozbay G, Ji L, Karwe M, Chen H. 2015. Effects of high-hydrostatic pressure on inactivation of human norovirus and physical and sensory characteristics of oysters. J Food Sci 80:M1330–M1335. doi: 10.1111/1750-3841.12899. [DOI] [PubMed] [Google Scholar]
- 27.Lou F, Neetoo H, Li J, Chen H. 2011. Lack of correlation between virus barosensitivity and the presence of a viral envelope during inactivation of human rotavirus, vesicular stomatitis virus, and avian metapneumovirus by high-pressure processing. Appl Environ Microbiol 77:8538–8547. doi: 10.1128/AEM.06711-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan D, Kniel K, Calci KR, Hicks DT, Pivarnik LF, Hoover DG. 2006. Response of four types of coliphages to high hydrostatic pressure. Food Microbiol 23:546–551. doi: 10.1016/j.fm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q, Li D, Xu J, Wang J, Zhao Y, Li Z, Xue C. 2010. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int J Food Microbiol 137:186–189. doi: 10.1016/j.ijfoodmicro.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Hoover DG, Kingsley DH. 2005. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J Food Prot 68:2389–2394. [DOI] [PubMed] [Google Scholar]
- 31.Cromeans T, Park GW, Costantini V, Lee D, Wang Q, Farkas T, Lee A, Vinje J. 2014. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl Environ Microbiol 80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grove SF, Lewis T, Ross T, Forsyth S, Wan J, Coventry J, Lee A, Cole M, Stewart CM. 2008. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innov Food Sci Emerg 9:206–210. doi: 10.1016/j.ifset.2007.07.006. [DOI] [Google Scholar]
- 33.Kingsley DH, Chen H, Hoover DG. 2004. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res 102:221–224. doi: 10.1016/j.virusres.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Chen H, Kingsley DH. 2013. The influence of temperature, pH, and water immersion on the high hydrostatic pressure inactivation of GI.1 and GII.4 human noroviruses. Int J Food Microbiol 167:138–143. doi: 10.1016/j.ijfoodmicro.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Khadre MA, Yousef AE. 2002. Susceptibility of human rotavirus to ozone, high pressure, and pulsed electric field. J Food Prot 65:1441–1446. [DOI] [PubMed] [Google Scholar]
- 36.Kingsley DH, Chen H. 2009. Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. Int J Food Microbiol 130:61–64. doi: 10.1016/j.ijfoodmicro.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Dancho BA, Chen H, Kingsley DH. 2012. Discrimination between infectious and non-infectious human norovirus using porcine gastric mucin. Int J Food Microbiol 155:222–226. doi: 10.1016/j.ijfoodmicro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Ye M, Neetoo H, Golovan S, Chen H. 2013. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int J Food Microbiol 162:37–42. doi: 10.1016/j.ijfoodmicro.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Lou F, Neetoo H, Chen H, Li J. 2011. Inactivation of a human norovirus surrogate by high-pressure processing: effectiveness, mechanism, and potential application in the fresh produce industry. Appl Environ Microbiol 77:1862–1871. doi: 10.1128/AEM.01918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Chen H. 2015. Evaluation of the porcine gastric mucin binding assay for high-pressure-inactivation studies using murine norovirus and tulane virus. Appl Environ Microbiol 81:515–521. doi: 10.1128/AEM.02971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingsley DH, Guan D, Hoover DG, Chen H. 2006. Inactivation of hepatitis A virus by high-pressure processing: the role of temperature and pressure oscillation. J Food Prot 69:2454–2459. [DOI] [PubMed] [Google Scholar]
- 42.Kingsley DH, Li X, Chen H. 23 November 2013. Temperature effects for high-pressure processing of picornaviruses. Food Environ Virol doi: 10.1007/s12560-013-9131-3. [DOI] [PubMed] [Google Scholar]
- 43.Kingsley DH, Chen H. 2008. Aqueous matrix compositions and pH influence feline calicivirus inactivation by high pressure processing. J Food Prot 71:1598–1603. [DOI] [PubMed] [Google Scholar]
- 44.Kingsley DH, Guan D, Hoover DG. 2005. Pressure inactivation of hepatitis A virus in strawberry puree and sliced green onions. J Food Prot 68:1748–1751. [DOI] [PubMed] [Google Scholar]
- 45.Kingsley DH, Holliman DR, Calci KR, Chen H, Flick GJ. 2007. Inactivation of a norovirus by high-pressure processing. Appl Environ Microbiol 73:581–585. doi: 10.1128/AEM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kingsley DH, Hoover DG, Papafragkou E, Richards GP. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J Food Prot 65:1605–1609. [DOI] [PubMed] [Google Scholar]
- 47.Leon JS, Kingsley DH, Montes JS, Richards GP, Lyon GM, Abdulhafid GM, Seitz SR, Fernandez ML, Teunis PF, Flick GJ, Moe CL. 2011. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol 77:5476–5482. doi: 10.1128/AEM.02801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lou F, Huang P, Neetoo H, Gurtler JB, Niemira BA, Chen H, Jiang X, Li J. 2012. High-pressure inactivation of human norovirus virus-like particles provides evidence that the capsid of human norovirus is highly pressure resistant. Appl Environ Microbiol 78:5320–5327. doi: 10.1128/AEM.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller R, Justino JF, Cassini ST. 2013. Assessment of water and seafood microbiology quality in a mangrove region in Vitoria, Brazil. J Water Health 11:573–580. doi: 10.2166/wh.2013.245. [DOI] [PubMed] [Google Scholar]
- 50.Baert L, Debevere J, Uyttendaele M. 2009. The efficacy of preservation methods to inactivate foodborne viruses. Int J Food Microbiol 131:83–94. doi: 10.1016/j.ijfoodmicro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Balny C, Masson P, Heremans K. 2002. High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim Biophys Acta 1595:3–10. doi: 10.1016/S0167-4838(01)00331-4. [DOI] [PubMed] [Google Scholar]
- 52.Gross M, Jaenicke R. 1994. Proteins under pressure. The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur J Biochem 221:617–630. [DOI] [PubMed] [Google Scholar]
- 53.Murchie LW, Kelly AL, Wiley M, Adair BM, Patterson M. 2007. Inactivation of a calicivirus and enterovirus in shellfish by high pressure. Innov Food Sci Emerg 8:213–217. doi: 10.1016/j.ifset.2006.11.003. [DOI] [Google Scholar]