Abstract
The cells of origin of pancreatic gastrinomas remain an enigma, since no gastrin-expressing cells are found in the normal adult pancreas. It was proposed that the cellular origin of pancreatic gastrinomas may come from either the pancreatic cells themselves or gastrin-expressing cells which have migrated from the duodenum. In the current study, we further characterized previously described transient pancreatic gastrin-expressing cells using cell lineage tracing in a pan-pancreatic progenitor and a pancreatic endocrine progenitor model. We provide evidence showing that pancreatic gastrin-expressing cells, found from embryonic day 12.5 until postnatal day 7, are derived from pancreatic Ptf1a+ and neurogenin 3-expressing (Ngn3+) progenitors. Importantly, the majority of them coexpress glucagon, with 4% coexpressing insulin, indicating that they are a temporary subpopulation of both alpha and beta cells. Interestingly, Men1 disruption in both Ngn3 progenitors and beta and alpha cells resulted in the development of pancreatic gastrin-expressing tumors, suggesting that the latter developed from islet cells. Finally, we detected gastrin expression using three human cohorts with pancreatic endocrine tumors (pNETs) that have not been diagnosed as gastrinomas (in 9/34 pNETs from 6/14 patients with multiple endocrine neoplasia type 1, in 5/35 sporadic nonfunctioning pNETs, and in 2/20 sporadic insulinomas), consistent with observations made in mouse models. Our work provides insight into the histogenesis of pancreatic gastrin-expressing tumors.
INTRODUCTION
Gastrinomas are endocrine tumors which secrete the peptide hormone gastrin. They cause Zollinger-Ellison syndrome (ZES), characterized by multiple duodenal-jejunal ulcers, diarrhea, and gastroesophageal reflux (1). Most gastrin-producing cells are found in the adult gastric antrum and, to a lesser extent, in the proximal duodenum in mammals. Intriguingly, most primary gastrinomas are found in the duodenum. The rest are mainly in the pancreas and are only rarely found in the stomach and elsewhere (liver, lung, and ovaries) (2). Gastrinomas develop either sporadically or hereditarily, as seen in patients with multiple endocrine neoplasia type 1 (MEN1; disease identifier OMIM131100). MEN1 patients develop multiple tumors in endocrine organs, primarily affecting parathyroid glands, the pituitary, the pancreas, and the foregut. Gastrinomas are the most common functioning tumors of the gastroenteropancreatic axis in MEN1 patients (3, 4), displaying multiple small lesions in the duodenum and, in rare cases, in the pancreas. Sporadic gastrinomas are often solitary tumors occurring both in the duodenum and in the pancreas, and the etiology is poorly understood. Interestingly, somatic MEN1 mutations and a loss of heterozygosity at the MEN1 locus have been detected in approximately 30% of both sporadic duodenal and pancreatic gastrinomas (5, 6). In addition, we have previously reported gastrin-expressing pancreatic tumors in about 15% of heterozygous Men1 mutant mice (7), confirming that MEN1 inactivation plays a crucial role in the pathogenesis of gastrinomas.
Deciphering the cells of origin of tumors is important not only for improved understanding of tumor biology but also for personalized tumor treatment with targeted therapy (8). Determining the cells of origin of pancreatic gastrinomas is of special interest, since gastrin-expressing cells are not found in the normal adult human or rodent pancreas. Several studies have described gastrin expression in developing and neonatal mammal pancreases, which decreases rapidly after birth (9–13). Analyses of extracts from a neonatal rodent pancreas established that gastrin was fully processed and active in the pancreas at this stage (10). Consistently, using transgenic mice harboring the green fluorescent protein (GFP) gene under the control of the mouse gastrin promoter, Takaishi and colleagues demonstrated that GFP expression could be targeted to pancreatic cells in embryonic and neonatal pancreases until 2 days after birth (14). Passaro et al. postulated that stem cells formed in ventral pancreatic buds could be the cell of origin of pancreatic gastrinomas found in the gastrinoma triangle, based on the clinical features of the tumors (15). Indeed, pancreatic endocrine cells derive from the differentiation of neurogenin 3-expressing (Ngn3+) progenitors, while gastric and duodenal gastrin-expressing cells are also derived from endocrine progenitors expressing Ngn3 in the corresponding tissue (16). This suggests that pancreatic gastrin-expressing cells may also arise from Ngn3+ pancreatic endocrine progenitors. Recently, Suissa et al. (17), using different mouse models and cell lineage tracing, reported the existence of embryonic and perinatal gastrin-expressing cell populations in the pancreas and ascertained that they were a distinct cell lineage derived from pancreatic Ngn3+ progenitors. However, their role in pancreatic gastrinoma development has not been addressed.
It is worth mentioning that several previous attempts to generate mouse gastrinoma models were unsuccessful. Neither the use of a gastrin promoter-driven simian virus 40 (SV40) large T antigen (18) nor Men1 disruption in villin-expressing gastrointestinal cells gave rise to gastrinoma development in the mouse (19). Altogether, the observations described above prompted us to better study gastrin-expressing cells in the pancreas and search for alternative ways to determine the cellular origin of pancreatic gastrinomas.
MATERIALS AND METHODS
Human patient samples.
The study was conducted in accordance with the guidelines in the Declaration of Helsinki, and the use of all patient tissue specimens was carried out according to French laws and regulations.
Animal study approval.
All animal maintenance and experiments were performed in accordance with the animal care guidelines of the European Union and French laws and were validated by the local Animal Ethic Evaluation Committee (protocol CLB 2010/016).
Mouse studies.
Ngn3tTA knock-in mice were generously provided by Jan N. Jensen (20). The tetO-cre mouse line [B6.Cg-Tg(tetO-cre)1Jaw/J MGI:2679524] was purchased from Charles River France. The Men1flox/flox-RIP-Cre+ (βMen1 knockout [KO]) (21), Men1flox/flox-Glu-Cre+ (αMen1 KO) (22), and R26eYFP mouse lines have already been described (23). Ptf1a-Cre knock-in mice (24) were generously provided by Christopher V. E. Wright.
IHC and IF.
Neonate and adult pancreases were fixed in neutral buffered formalin (Lilly's fixative) for 2 h at room temperature and for 18 h at 4°C, respectively, and then embedded in paraffin. Serial sections of 3 μm were prepared, mounted on Superfrost Plus glass slides, and subjected to deparaffinization and rehydration. Immunostainings were performed as previously described (25). Briefly, endogenous peroxidases were quenched in 3% H2O2 solution for 30 min at room temperature. Heat-induced epitope retrieval was performed by immersion in antigen-unmasking solution (catalog no. H-3300; Vector Laboratories) in a microwave oven for 15 min. After blocking with antibody diluent (Dako), sections were incubated overnight with a primary antibody. For immunofluorescence (IF), stainings were revealed with a Cy3 or Cy5 tyramide amplification kit (PerkinElmer), according to the manufacturer's instructions, with prior incubation with the appropriate biotinylated secondary antibody or incubation with appropriate Alexa Fluor 488-, 555-, or 647-coupled secondary antibodies (Life Technologies) for 1 h. For immunohistochemistry (IHC), after incubation with the primary antibody, biotinylated secondary antibody was applied for 1 h and revealed using the avidin-biotin complex (ABC)–3,3′-diaminobenzidine system (Vector Laboratories). Images were captured on either a Leica DRMB epifluorescence microscope (Leica Microsystems), a TCS-SP5 confocal microscope (Leica Microsystems), or a Zeiss 780 confocal microscope. Cell counting was manually performed on multiple-channel pictures with the ImageJ cell counter module (U.S. National Institutes of Health, Bethesda, MD). The data on the graphs and the results of counting are represented as means ± standard errors of the means.
Antibodies.
Antigastrin antibody (Novocastra; Leica Biosystems) recognizing nonsulfated (I) and sulfated (II) gastrin 17 as well as gastrin 34, antigastrin antibody (serum 2717; Jens F. Rehfeld), antigastrin antibody (catalog no. sc-7783; Santa Cruz) recognizing gastrins 17, 34, and 71, antiprogastrin antibody (rabbit polyclonal antibody; a kind gift from Julie Pannequin), and anti-Pax4 antibody (a kind gift from Beatriz Sosa-Pineda [26]) were used in the study (see Table S1 in the supplemental material for a detailed description of all antibodies used in the study). Donkey secondary antibodies coupled with either Alexa Fluor 488, 555, or 647 were used (Life Technologies, Jackson ImmunoResearch). For IHC or immunofluorescence with tyramide signal amplification, biotinylated secondary antibodies from horse or donkey were used (Vector Laboratories). Negative controls were conducted by omitting primary antibody incubation. For the gastrin antibody from Santa Cruz, controls were conducted by incubating the antibody with the corresponding peptide (catalog no. sc-7783 P; Santa Cruz).
Serum gastrin measurement.
The concentration of gastrin in serum from mice was measured with a highly sensitive gastrin radioimmunoassay (RIA) (27). Due to the small serum volume (<50 μl), conventional gastrin RIAs could not be used. The supersensitive RIA (based on antibody no. 921325 from the J. F. Rehfeld laboratory) has a detection limit of 1.0 pmol of gastrin per 15 μl of sample in the incubation mixture, and the cross-reactivity with cholecystokinin (CCK) is minor (<10%), with an intra-assay coefficient variation of 8%. Note that CCK circulates at a 10-fold lower concentration than gastrin.
PCRs.
Mouse islets were purified using the standard collagenase P protocol (Roche). Total RNA was isolated from adult C57BL/6J mouse tissues (Charles River) using an RNeasy minikit (Qiagen). Standard reverse transcription-PCRs were performed (annealing temperature, 60°C; 40 cycles) using the following primers: for gastrin, forward (Fw) primer 5′-AACAGCGCCAGTTCAACAAG-3′ and reverse (Rv) primer 5′-AAGTCCATCCATCCGTAGGC-3′, for cholecystokinin, Fw primer 5′-TGCTAGCGCGATACATCCAG-3′and Rv primer 5′-ATCCATCCAGCCCATGTAGTC-3′, for GIP, Fw primer 5′-TTTCCCTGAGATTGCCCTGC-3′ and Rv primer 5′-CGCAGAGGGGACTTTCATCA-3′, for insulin 2, Fw primer 5′-GCAGCACCTTTGTGGTTCCC-3′ and Rv primer 5′-TGCAGTAGTTCTCCAGCTGG-3′, and for the TATA box binding protein (Tbp) gene, Fw primer 5′-CCCTATCACTCCTGCCACACC-3′ and Rv primer 5′-CGAAGTGCAATGGTCTTTAGGTC-3′.
RESULTS
Pancreatic gastrin+ cells represent transient subpopulations of alpha and beta cells at birth.
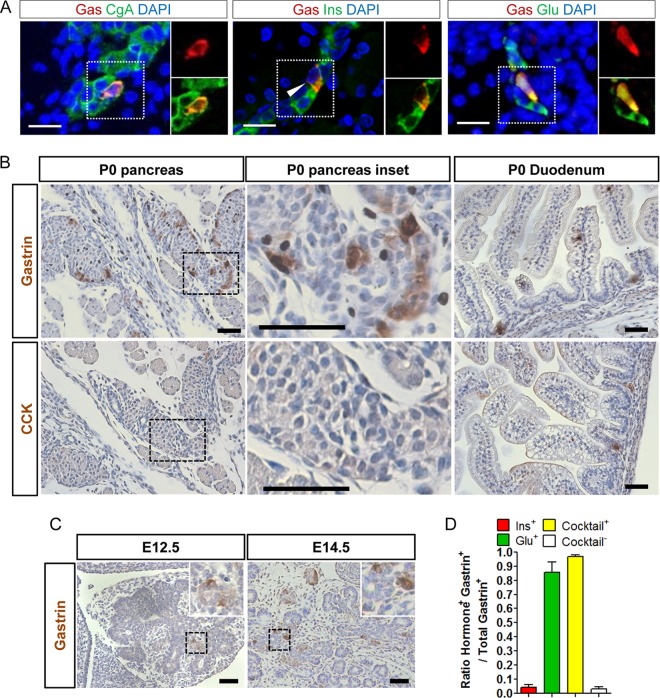
We first tried to better characterize the population of mouse neonatal pancreatic gastrin-expressing (NPG) cells. To achieve that aim, we used different gastrin antibodies that recognize different forms of processed gastrin peptides. The gastrin transcript encodes the progastrin immature peptide that is processed by cleavage and other posttranslational modifications, giving rise to different mature peptides (gastrin 34 and gastrin 17) and immature gastrin peptides (gastrin 71) (28). Thus, the presence of the different gastrin peptides is highly dependent on the expression and activity of the key enzymes necessary for its processing. We used the Novocastra antigastrin antibody widely used by pathologists for the diagnosis of gastrinomas. Both this antibody and the homemade serum 2717 recognize gastrins 17 and 34. The SCBT antibody recognizes gastrins 17 and 34, as well as the gastrin 71 precursor. Immunofluorescence (IF) analyses with Novocastra antigastrin antibody demonstrated that scattered cells with positive cytoplasmic staining could be detected in the pancreas of mouse neonates (Fig. 1A). We quantified the proportion of these NPG cells relative to the total endocrine cell population at birth by performing double IF staining for gastrin and chromogranin A, a marker of endocrine cells (Fig. 1A). The NPG cell population represented 4.3% ± 0.1% of the total endocrine cell number at postnatal day 0 (P0) but became undetectable at P7 (data not shown), consistent with previous results reported in the literature (17, 29). We noticed that in the normal adult mouse pancreas, weak expression of both gastrin and progastrin was detected in alpha cells with one of the antigastrin antibodies used in this study (SCBT) (see Fig. S1A in the supplemental material) and antiprogastrin antibody (from Julie Pannequin) (see Fig. S1B in the supplemental material), with both antibodies giving rise to positively stained cells in the antrum and proximal duodenum (data not shown). Moreover, the gastrin transcript but not the cholecystokinin transcript was also detected in RNA isolated from adult mouse pancreas (see Fig. S1C in the supplemental material). The data support the existence of either residual gastrin expression or a differently processed gastrin peptide, possibly gastrin 71, in adult pancreatic alpha cells on the basis of the specificity of the antibody used. The related peptide hormone CCK was not expressed at P0 in the pancreas but was detectable in the duodenum at birth, as was gastrin (Fig. 1B), confirming that there was no cross-reaction with CCK by the antigastrin antibody used in this study. Next, we checked the existence of gastrin-expressing (gastrin+) cells in the developing mouse pancreas. Gastrin+ cells were readily detected but rarely at embryonic day 12. 5 (E12.5), and the population was markedly increased at E14.5 in the developing pancreas of wild-type mice (Fig. 1C).
FIG 1.
Neonatal gastrin-expressing cells represent subsets of alpha and beta cells. (A) IF colocalization of gastrin and chromogranin A, insulin, or glucagon in the pancreases of newborn wild-type mice (3 ≤ n ≤ 6 mice). Magnified views of the area with the dotted outline are shown on the right. Bars = 20 μm. (B) Representative IHC stainings for gastrin and CCK in wild-type mouse pancreas and duodenum at birth. Magnified views of the area with the dashed outline are shown in the middle panel. Bars = 50 μm. (C) Representative IHC staining for gastrin in wild-type mouse pancreas at E12.5 and E14.5 (n = 3 mice of each age). (Insets) Magnified views of the area with the dashed outline. Bars = 50 μm. (D) Percentage of gastrin-expressing cells (3 ≤ n ≤ 7 mice) expressing glucagon, insulin, or any of the pancreatic hormones (Cocktail) (see Results) at birth in wild-type mouse pancreas. DAPI, 4′,6-diamidino-2-phenylindole; Gas, gastrin; Ins, insulin; Glu, glucagon.
Furukawa and colleagues previously observed that some gastrin+ cells were also positive for insulin staining using the restaining method (12). By double IF staining, we could indeed detect rare cells expressing both gastrin (Novocastra antibody) and insulin (Fig. 1A and D), accounting for 4.4% ± 1.8% of total NPG cells, while most NPG cells were negative for insulin. Since we observed that most NPG cells localized to the periphery of developing mouse endocrine islets, where most alpha cells reside, we sought to determine if these NPG cells could express glucagon. Indeed, a very high proportion (85.5% ± 7.3%) of NPG cells was also found to express glucagon (Fig. 1A and D). Thus, it appears that NPG cells represent subpopulations of both developing alpha and beta cells. We performed double staining of gastrin with other pancreatic hormones, and we found that somatostatin-expressing cells did not express gastrin at P0 (see Fig. S2 in the supplemental material). In order to identify cells expressing only gastrin and none of the other pancreatic hormones, we performed double staining with gastrin antibody and a cocktail of pancreatic hormones (insulin, glucagon, somatostatin, and ghrelin). A very small proportion (3.3% ± 1.3%) of gastrin-expressing cells was indeed negative for the hormonal cocktail (Fig. 1D).
Pancreatic gastrin+ cells originate from pancreatic progenitors within the developing pancreas.
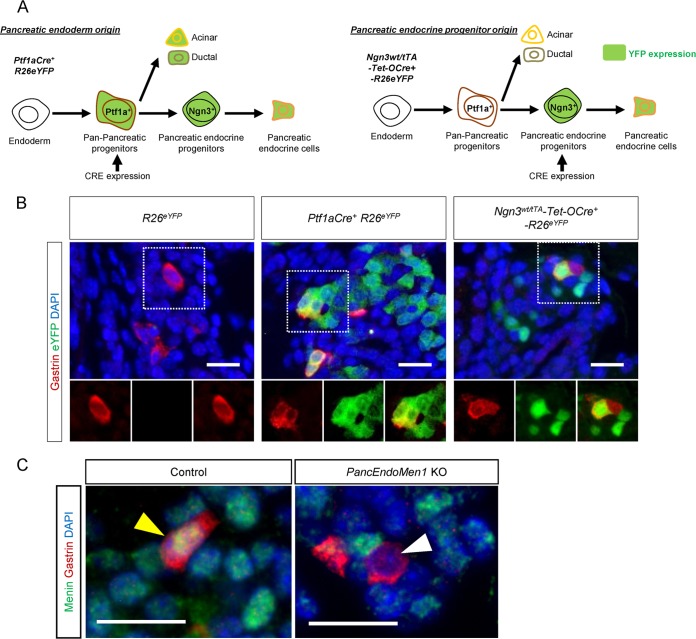
Considering that all alpha and beta cells are derived from Ngn3+ endocrine progenitors, we suspected that NPG cells might also be derived from the differentiation of pancreatic progenitors, as virtually all NPG cells expressed either glucagon or insulin. In order to determine if NPG cells were derived from pancreatic progenitors instead of a result of the migration of gut progenitors or gut differentiated cells, we performed lineage-tracing experiments with Ptf1a-Cre+-R26eYFP mice, since during development Ptf1a-expressing cells give rise only to pancreatic acinar, ductal, and endocrine cells (24) (Fig. 2A). Using double IF staining, 61.3% ± 4.1% of the NPG cells were found to also express the yellow fluorescent protein (YFP) reporter in Ptf1a-Cre+-R26eYFP mice (n = 3 mice) (Fig. 2B), confirming our hypothesis. Similar results were obtained when the proportion of alpha and beta cells expressing the YFP reporter was counted, indicating that the Cre used was not 100% efficient in the targeted cell population (data not shown).
FIG 2.
Pancreatic progenitor tracing and Men1 disruption in pancreatic progenitors define the derivation of NPG cells. (A) Schematic illustration of the two mouse models used for lineage tracing of pancreatic progenitors and pancreatic endocrine progenitors, respectively. (B) Representative IF staining for gastrin and enhanced YFP (eYFP) in the neonatal pancreases of control R26eYFP mice, Ptf1a-Cre+-Rosa26eYFP mice, and Ngn3wt/tTA-tetO-Cre+-R26eYFP mice. Gastrin-expressing cells express the genetic tracer enhanced YFP. (C) IF staining for menin and gastrin in the neonatal pancreases of PancEndoMen1 KO mice. Bars = 20 μm.
We next genetically traced the progeny of Ngn3+ pancreatic endocrine progenitors using Ngn3wt/tTA-tetO-Cre+-R26eYFP mice (Fig. 2A). Consistent with the findings of Suissa et al. (17), YFP expression could be detected in 10.87% ± 5.46% of NPG cells from these mice (Fig. 2B), with a similar YFP expression rate being detected in alpha cells from the same mice. We noticed that in wild-type neonatal mouse pancreases, all the gastrin+ cells expressed menin protein at the same level observed in other islet cells (Fig. 2C). We then analyzed Men1flox/flox-Ngn3wt/tTA-tetO-Cre+-R26eYFP mice, where the Men1 gene can be disrupted in Ngn3-expressing cells and which are referred to as PancEndoMen1 KO mice. Menin expression was lost in about 20% (specifically, 20.2% ± 4.7%) of NPG cells from PancEndoMen1 KO mice (Fig. 2C), further confirming the above-presented cell-tracing data obtained using YFP. Our results indicate that NPG cells are derived from the pan-pancreatic (Ptf1a+) and pancreatic endocrine (Ngn3+) progenitors. Taking these results together, we define, for the first time, NPG cells to be temporal subpopulations of either glucagon-expressing or insulin-expressing cells, with pancreatic progenitors being their common cell of origin.
Perinatal pancreatic gastrin-expressing cells display a distinct molecular signature.
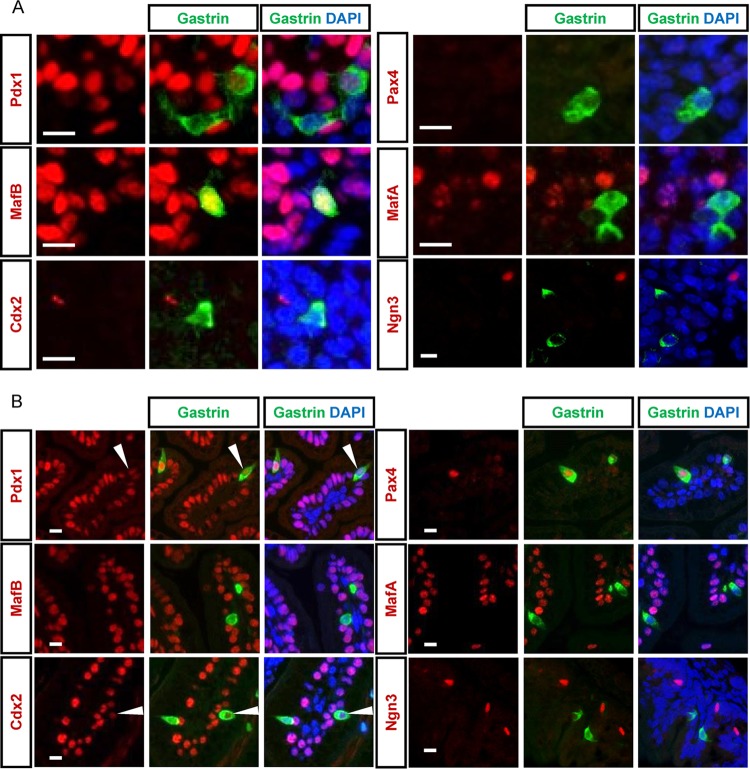
To study the relation between NPG cells and duodenal G cells, we compared the protein expression profiles of several factors known to be expressed in these cells. Analyses of the transcription factors expressed by NPG cells showed that they displayed high levels of MafB expression, and some also expressed low levels of Pdx1 at P0, whereas they did not express MafA, Pax4, Cdx2, or Ngn3 (Fig. 3A). Interestingly, at P0 duodenal G cells displayed an MafB−, Pdx1high/low, MafA−, Pax4+, Cdx2−/low expression profile (Fig. 3B), showing that they are a cell population distinct from NPG cells.
FIG 3.
Differential molecular expression profiles between perinatal pancreatic gastrin+ and perinatal duodenal gastrin+ cells. (A and B) Double IF immunostaining with antibodies against gastrin and the indicated transcription factors in the pancreases (A) and duodenums (B) of newborn wild-type mice. Bars = 10 μm.
Mouse pancreatic gastrin-expressing tumors develop upon Men1 disruption in both Ngn3+ pancreatic progenitors and insulin- or glucagon-expressing cells.
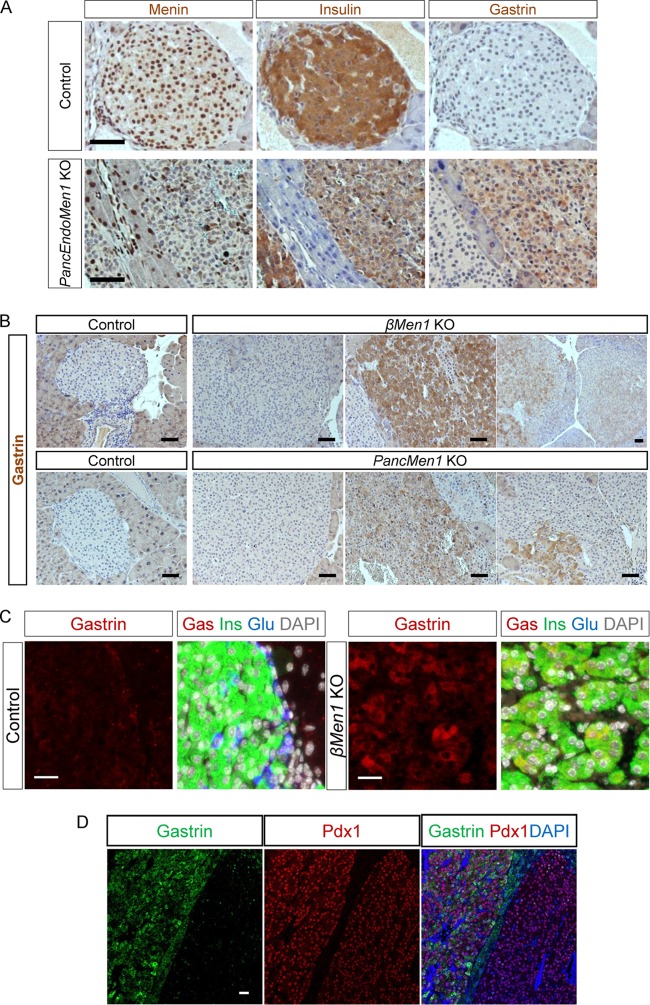
Having identified that NPG cells were derived from pancreatic progenitors, we hypothesized that the pancreatic progenitors and their derivatives, including NPG cells, could be among the cells of origin of pancreatic gastrinomas. To test this hypothesis, we examined aged mice with Men1 disruption in Ngn3+ progenitors. Histological analyses of pancreases dissected from aged PancEndoMen1 KO mice showed that 12-month-old mutant mice displayed a large amount of endocrine lesions. Tumor lesions exhibited a complete loss of menin expression, as expected (Fig. 4A), due to Ngn3-Cre-mediated gene disruption in pancreatic endocrine progenitors, with most of them being positive for insulin staining by IHC (the detailed data concerning the analysis of other islet tumors will be published elsewhere). Interestingly, by using gastrin antibody, several tumors were positively stained (Fig. 4A), whereas other adjacent islet tumors were not, and CCK was not detected with two different antibodies in these gastrin-immunoreactive tumor cells (data not shown). The frequency of gastrin+ lesions in the PancEndoMen1 KO mouse model is reported in Table 1.
FIG 4.
PancEndoMen1 KO mice, PancMen1 KO mice, and βMen1 KO mice develop tumors expressing gastrin. (A) IHC stainings for the indicated factors in 12-month-old control and PancEndoMen1 KO mice. Note that the tumor located on the right expressed gastrin, whereas the tumor on the left did not. Bars = 50 μm. (B) IHC detection of gastrin in 12-month-old control and βMen1 KO or PancMen1 KO mice. Gastrin is not expressed in normal islet cells of control mice. Most lesions in mutant mice do not express gastrin (left), whereas subsets of tumors express gastrin at high levels (middle) or at low levels or focally (right). Bars = 50 μm. (C) Triple IF stainings for insulin, glucagon, and gastrin in 12-month-old control and βMen1 KO mice. Gastrin-expressing cells in tumors from βMen1 KO mice also expressed insulin. Bars = 20 μm. (D) Representative IF staining for Pdx1, gastrin, and DAPI (4′,6-diamidino-2-phenylindole). The lesion on the right did not express gastrin but expressed Pdx1, whereas the lesion on the left expressed both gastrin and Pdx1. Bar = 25 μm.
TABLE 1.
Summary of gastrin-expressing lesions in different models of Men1 disruption
| Model | No. of mice with gastrin lesions/total no. of mice | No. of gastrin+ lesions/total no. of lesions (%) |
|---|---|---|
| PancMen1 KO | 8/9 | 14/346 (4.0) |
| PancEndoMen1 KO | 2/4 | 2/70 (2.9) |
| βMen1 KO | 3/4 | 5/106 (4.7) |
| αMen1 KO | 1/6 | 1/34 (2.9) |
Unexpectedly, we found that gastrin+ tumors also expressed insulin (insulin+) with a much lower intensity than other insulin+ tumors negative for gastrin and other hormones (Fig. 4A; see also Fig. S3A in the supplemental material). Similar results were obtained when we studied the occurrence of gastrin+ lesions in PancMen1 KO (Men1flox/flox-Ptf1a-Cre+) mice, but a higher proportion of analyzed mice (8/9) displayed pancreatic gastrin+ tumors (Fig. 4B and Table 1). The higher percentage of gastrin+ lesions in the latter mice than in the PancEndoMen1 KO mice most likely resulted from the better Cre efficiency in PancMen1 KO mice, since we observed 3-fold more pancreatic endocrine cells expressing the reporter at birth in Ptf1a-Cre+-R26eYFP mice than Ngn3wt/tTA-tetO-Cre+-R26eYFP mice (data not shown). Since NPG cells coexpress insulin or glucagon at birth, we next sought to determine whether perinatal Men1 disruption specifically in insulin+ or glucagon+ cells led to gastrin+ tumor development. Careful analysis of menin-deficient lesions from Men1flox/flox-Rip-Cre (βMen1 KO) mice at 12 months of age revealed the occurrence of gastrin+ lesions (Fig. 4B). The cells expressing gastrin in tumors also expressed insulin but not glucagon, as demonstrated by triple IF staining (Fig. 4C; see also Fig. S3A in the supplemental material). Three out of four analyzed βMen1 KO mice developed at least one gastrin+ tumor, with a total of 5 lesions out of 106 lesions analyzed being gastrin+ (Table 1). Molecular characterization of gastrin+ tumors from PancEndoMen1 and βMen1 KO mice demonstrated that gastrin-expressing tumors were Pdx1+, consistent with a gastrin+ beta cell origin of these tumors (Fig. 4D). Nevertheless, Ki67 analyses did not reveal any difference in proliferation from that of other insulin-expressing tumors (see Fig. S3B in the supplemental material). In parallel, the same analyses were also carried out in the mutant mice where the Men1 gene was specifically disrupted in glucagon+ cells (αMen1). All the glucagon-expressing lesions in 12- to 18-month-old αMen1 KO mice were positive for gastrin detection with the antigastrin antibody (SCBT) but negative for progastrin detection with a progastrin antibody (homemade). When using other gastrin antibodies, we observed an occurrence of gastrin+ lesions similar to that found in βMen1 KO mice (Table 1). To homogenize the data, only those lesions revealed to be positive by the gastrin antibody used in the clinic (Novocastra) were considered gastrin+ and are included in Table 1. We additionally measured the concentration of gastrin in the serum of 12-month-old PancEndoMen1 and βMen1 KO mice. We did not detect significantly elevated serum gastrin levels in mice presenting with pancreatic gastrin+ tumors, possibly reflecting the nonfunctionality of these lesions (see Fig. S3C and D in the supplemental material).
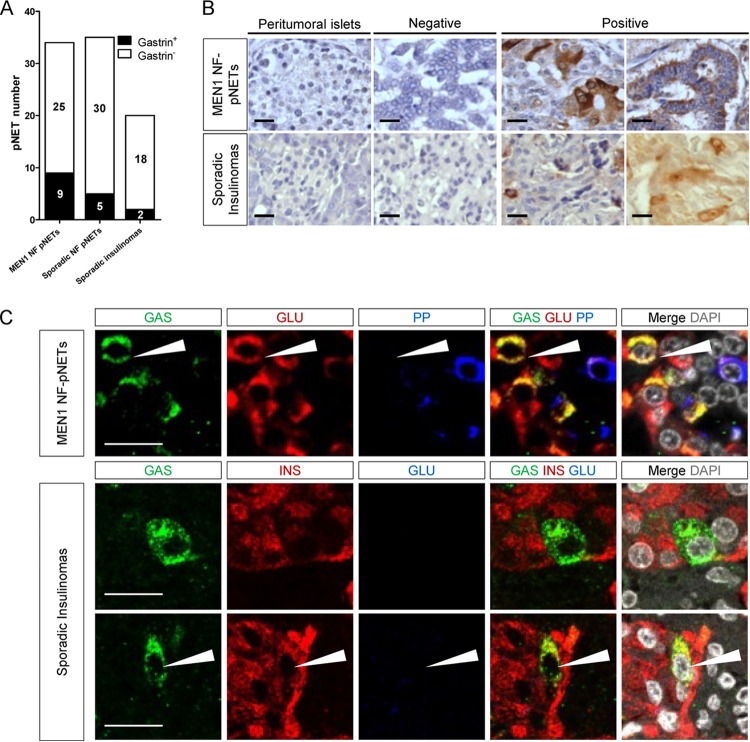
Gastrin-expressing cells can be seen in a substantial number of human islet tumors that were not diagnosed as gastrinomas.
The observations presented above prompted us to examine how frequently gastrin coexpression was found in human islet tumor lesions that were not previously diagnosed as gastrinomas. To this end, we performed gastrin detection by IHC using gastrin antibody (Novocastra) in a panel of 34 pancreatic endocrine tumors (pNETs) from 14 MEN1 patients, 35 sporadic nonfunctioning pNETs and another panel of 20 sporadic insulinomas. As shown in Fig. 5A and B, gastrin expression was detected in 9/34 MEN1 pNETs tested (from 6/14 MEN1 patients), in 5/35 sporadic nonfunctioning pNETs, and in 2/20 cases of sporadic insulinomas. Among the five sporadic nonfunctioning pNETs expressing gastrin, two displayed additional insulin and glucagon expression. Among 34 MEN1 pNETs, only 2 tumors were positive for insulin, and both were negative for gastrin. The remaining MEN1 pNETs were glucagon and/or pancreatic polypeptide (PP) positive. Double staining further demonstrated that gastrin+ cells in the MEN1 pNETs tested also coexpressed glucagon or PP (Fig. 5C), while in the two gastrin-positive sporadic insulinomas, gastrin+ cells were negative for glucagon but frequently coexpressed insulin (Fig. 5C).
FIG 5.
Detection of gastrin-expressing cells in human nongastrinoma pancreatic endocrine tumors. (A) Number of tumors with gastrin expression among the three cohorts with pNETs analyzed. (B) Representative IHC stainings from two cohorts of human pancreatic endocrine tumors (MEN1 or sporadic). (C) IF stainings for the indicated factors in pancreatic endocrine tumors containing gastrin-immunoreactive cells. Arrows, gastrin-expressing cells coexpressing glucagon (top) or insulin (bottom). NF, nonfunctioning. Bars = 20 μm.
DISCUSSION
By better defining the pancreatic gastrin-expressing cells, the current work revealed some new features of perinatal pancreatic gastrin+ cells. Our data extend the origin of NPG cells further to pancreatic Ptf1a+ progenitors. We report that about 60% of NPG cells expressed the YFP reporter, as determined by lineage tracing. The 40% of gastrin+ cells negative for the reporter could be the result of low Cre-mediated recombination efficiency. Importantly, the presence of only a few gastrin+ cells not coexpressing other islet hormones at the perinatal stage, the period, unfortunately, omitted in the work of Suissa et al. (17), adds important information for the definition of NGP cells. The low number of monohormonal gastrin+ cells at birth detected in our study suggests that, at birth, the monohormonal population initially described by Suissa et al. (17) either undergoes cell death or further differentiates into double-positive cells: glucagon-expressing gastrin+ cells or insulin-expressing gastrin+ cells. Assays with a future generation of inducible gastrin+ cell-specific Cre driver mouse lines combined with lineage tracing will certainly provide information on which of these possibilities occurs. Our data also provide evidence suggesting that adult alpha cells may still express some residual level of gastrin, since adult alpha cells are weakly, yet consistently, positive for both gastrin antibodies (SCBT) and progastrin antibodies, and that the gastrin transcript can be detected in the adult pancreas. However, the fact that only one of the three gastrin antibodies used in this study detected gastrin suggests that the gastrin detected in adult alpha cells may represent an immature processed peptide form of gastrin (gastrin 71). Considering the existence of the numerous gastrin peptides reported in the literature (28) and the incomplete information about gastrin antibodies, more specific tools and approaches will be needed to completely clarify the issue. This is of great interest, as several groups have recently reported on the biological activities of both progastrin and gastrin intermediates (30). Taking our results together, we defined the NPG cells to be temporal subpopulations of islet cells, with pancreatic progenitors being their common cell origin.
To our knowledge, the attempt to generate a mouse gastrinoma model has so far been unsuccessful, hampering the ability to perform detailed experimental analyses. Interestingly, targeting of distinct islet cell lineages, including, in particular, Ptf1a+ and Ngn3+ pancreatic progenitors and alpha and beta cells, triggered the development of pancreatic gastrin-expressing tumors in all the different mouse Men1 models. Importantly, our data show that these mouse gastrin-expressing tumors do not exactly mimic human pancreatic gastrinomas, giving rise to hormonal symptoms due to hypergastrinemia, which is rarely seen in MEN1 patients, according to detailed analyses provided by Pipeleers-Marichal et al. (31). Keeping the clinical definition of human pancreatic gastrinoma in mind, we prefer to describe the lesions found in our mouse models as pancreatic gastrin-expressing tumors. Interestingly, we detected gastrin-expressing lesions in a substantial proportion of patients from three cohorts with human islet tumors not previously diagnosed as gastrinoma: MEN1 islet tumors, sporadic nonfunctioning islet tumors, and insulinomas. Our data provide evidence showing not only that human gastrin-expressing lesions similar to those found in our murine models exist but also that they may be more frequent than may be expected. These gastrin+ cells could have been missed at diagnosis because of the lack of clinical symptom and of specific but sensitive antibodies for gastrin detection. Importantly, the gastrin+ tumors in our mouse models and in human patients that we have described here may represent prelesions of pancreatic gastrinoma and could clinically awaken, giving rise to gastrinoma. Taken together, the observations made in Men1-deficient mouse models, revealing no gastrinoma associated with hypergastrinemia but pancreatic gastrin-expressing tumors, are consistent with the data obtained by analyses of three patient cohorts and are also reminiscent of what has been described by many clinical studies. Also, in the future it will be interesting and useful to study the similarity and the difference between gastrinomas and pancreatic gastrin+ tumors in patients without clinical gastrinoma symptoms.
By direct cell-specific Men1 ablation, the current work affords, for the first time, strong evidence showing that islet cells themselves, either progenitors or differentiated cells, serve as the cells of origin of pancreatic gastrin-expressing tumors. Our data may also suggest that the above-mentioned NPG cells could also be among the cells of origin of these tumors, considering their ontogeny described in the current study. Remarkably, the frequency of gastrin+ tumors found in each of the above-mentioned models resembles the proportion of gastrin-positive cells found during the late embryonic and neonatal period. Interestingly, a similar situation may occur in humans, since Anlauf and colleagues reported that in two tumor banks comprising a total of 300 nonfunctioning pNETs, 3% of pNETs (9/300) stained positive for gastrin (32). However, we consider the possibility that NPG cells are by no means the unique cell of origin of pancreatic gastrinomas. Other possibilities may also be involved in pancreatic gastrinoma development, such as the ectopic expression or reexpression of gastrin by islet tumor cells that may dedifferentiate to the stage of the perinatal transient population coexpressing gastrin-glucagon and gastrin-insulin. The latter possibility can be tested in the future by disrupting the Men1 gene uniquely in adult islet cells using an inducible Cre-loxP system.
Although pancreatic gastrinomas are described in general to be monohormonal tumors, clinical observations often report that they coexpress other islet hormones (33, 34). The data from our analysis of human islet tumors are consistent with those from these previous works. Intriguingly, we noticed that the gastrin+ islet lesions observed in our PancEndoMen1 mutant mice coexpress insulin. This finding may suggest that the gastrin+ glucagon-expressing subpopulation could be less sensitive to Men1 disruption and other tumorigenic stimuli than the gastrin+ insulin-expressing subpopulation. The fact that the transgenic mice carrying the SV40 large T antigen under the control of the gastrin promoter gave rise only to insulinomas and not glucagonomas may support such a hypothesis (18). However, this may not be the case in humans, since many gastrin+ MEN1 islet tumors expressed glucagon in our study. Due to the rarity of MEN1 islet tumors, the significance of this will need to be further studied in a larger cohort.
In conclusion, the current study provides meaningful insights into both the ontogeny of islet NPG cells and the histogenesis of pancreatic gastrinomas. Our data pave the way for further studies of the molecular mechanisms controlling the development of pancreatic gastrinomas. The mouse gastrin-expressing tumor models generated in the current study, the first of their kind, will also be of help for the conception of new strategies for diagnosis and treatment of the disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the members of the animal facilities ALECS-Co, ALECS-SPF, and ANICAN. We are also grateful to the staff from the CECIL imaging facility and especially Christophe Vanbelle. We are especially grateful to Jan N. Jensen for providing the Ngn3wt/tTA mouse line, Christopher V. E. Wright for providing Ptf1a-Cre mice, J. Chayvialle and Colette Roche for anti-CCK antibodies, Beatriz Sosa-Pineda for anti-Pax4 antibodies, and Julie Pannequin for antiprogastrin antibodies. We thank Chloe Tessereau, Philippe Ruszniewski, Gérard Gradwohl, and Xianxin Hua for stimulating discussions.
This study was supported by the Ligue contre le Cancer du Rhône (to C.X.Z.) and de la Loire (to P.B.), the Agence National de Recherche (grant SVSE2 ANR 10 BLAN 1240 04), and the CMIRA program of the Region Rhône-Alpes (12004959-01); R.B. was the recipient of a 4th year doctoral fellowship from the Fondation ARC (DOC20120605135).
R.B. conducted the experiments, analyzed and interpreted the data, and prepared the figures and manuscript; R.T., R.J., and D.R. provided technical and material support and participated in interpretation of the data; E.L., J.-Y.S., F.L., V.H., Y.-J.C., F.P., and M.-C.V. supervised the development of the tissue banks, performed pathological analyses, provided material support, and participated in interpretation of the data; J.F.R. participated in development of the study concept and provided critical revision of the manuscript; P.B. participated in development of the study concept, design, and supervision and provided critical revision of the manuscript; and C.X.Z. conceived of the study, supervised the study and manuscript preparation, and obtained funding.
We have nothing to disclose.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00302-15.
REFERENCES
- 1.Zollinger RM. 1987. Gastrinoma: the Zollinger-Ellison syndrome. Semin Oncol 14:247–252. [PubMed] [Google Scholar]
- 2.Tartaglia A, Vezzadini C, Bianchini S, Vezzadini P. 2005. Gastrinoma of the stomach: a case report. Int J Gastrointest Cancer 35:211–216. doi: 10.1385/IJGC:35:3:211. [DOI] [PubMed] [Google Scholar]
- 3.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. 2008. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 15:409–427. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakker RV. 2014. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol 386:2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debelenko LV, Zhuang Z, Emmert-Buck MR, Chandrasekharappa SC, Manickam P, Guru SC, Marx SJ, Skarulis MC, Spiegel AM, Collins FS, Jensen RT, Liotta LA, Lubensky IA. 1997. Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1-associated and sporadic gastrinomas and pancreatic endocrine tumors. Cancer Res 57:2238–2243. [PubMed] [Google Scholar]
- 6.Zhuang Z, Vortmeyer AO, Pack S, Huang S, Pham TA, Wang C, Park WS, Agarwal SK, Debelenko LV, Kester M, Guru SC, Manickam P, Olufemi SE, Yu F, Heppner C, Crabtree JS, Skarulis MC, Venzon DJ, Emmert-Buck MR, Spiegel AM, Chandrasekharappa SC, Collins FS, Burns AL, Marx SJ, Jensen RT, Liotta LA, Lubensky IA. 1997. Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas. Cancer Res 57:4682–4686. [PubMed] [Google Scholar]
- 7.Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. 2003. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol 17:1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 8.Blanpain C. 2013. Tracing the cellular origin of cancer. Nat Cell Biol 15:126–134. doi: 10.1038/ncb2657. [DOI] [PubMed] [Google Scholar]
- 9.Larsson LI, Rehfeld JF, Sundler F, Hakanson R. 1976. Pancreatic gastrin in foetal and neonatal rats. Nature 262:609–610. doi: 10.1038/262609a0. [DOI] [PubMed] [Google Scholar]
- 10.Bardram L, Hilsted L, Rehfeld JF. 1990. Progastrin expression in mammalian pancreas. Proc Natl Acad Sci U S A 87:298–302. doi: 10.1073/pnas.87.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand SJ, Fuller PJ. 1988. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem 263:5341–5347. [PubMed] [Google Scholar]
- 12.Furukawa M, Magami Y, Azuma T, Inokuchi H, Nakayama D, Moriyasu F, Kawai K, Hattori T. 2001. Proliferation and functional changes of pancreatic gastrin cells in neonatal rat. Pancreas 23:421–426. doi: 10.1097/00006676-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Gittes GK, Rutter WJ, Debas HT. 1993. Initiation of gastrin expression during the development of the mouse pancreas. Am J Surg 165:23–25. doi: 10.1016/S0002-9610(05)80399-X. [DOI] [PubMed] [Google Scholar]
- 14.Takaishi S, Shibata W, Tomita H, Jin G, Yang X, Ericksen R, Dubeykovskaya Z, Asfaha S, Quante M, Betz KS, Shulkes A, Wang TC. 2011. In vivo analysis of mouse gastrin gene regulation in enhanced GFP-BAC transgenic mice. Am J Physiol Gastrointest Liver Physiol 300:G334–G344. doi: 10.1152/ajpgi.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passaro E Jr, Howard TJ, Sawicki MP, Watt PC, Stabile BE. 1998. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg 133:13–16. [DOI] [PubMed] [Google Scholar]
- 16.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suissa Y, Magenheim J, Stolovich-Rain M, Hija A, Collombat P, Mansouri A, Sussel L, Sosa-Pineda B, McCracken K, Wells JM, Heller RS, Dor Y, Glaser B. 2013. Gastrin: a distinct fate of neurogenin3 positive progenitor cells in the embryonic pancreas. PLoS One 8:e70397. doi: 10.1371/journal.pone.0070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montag AG, Oka T, Baek KH, Choi CS, Jay G, Agarwal K. 1993. Tumors in hepatobiliary tract and pancreatic islet tissues of transgenic mice harboring gastrin simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A 90:6696–6700. doi: 10.1073/pnas.90.14.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veniaminova NA, Hayes MM, Varney JM, Merchant JL. 2012. Conditional deletion of menin results in antral G cell hyperplasia and hypergastrinemia. Am J Physiol Gastrointest Liver Physiol 303:G752–G764. doi: 10.1152/ajpgi.00109.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. 2009. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A 106:9715–9720. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, Wang ZQ. 2003. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res 63:4836–4841. [PubMed] [Google Scholar]
- 22.Lu J, Herrera PL, Carreira C, Bonnavion R, Seigne C, Calender A, Bertolino P, Zhang CX. 2010. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology 138:1954–1965. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. 2002. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 25.Bonnavion R, Jaafar R, Kerr-Conte J, Assade F, van Stralen E, Leteurtre E, Pouponnot C, Gargani S, Pattou F, Bertolino P, Cordier-Bussat M, Lu J, Zhang CX. 2013. Both PAX4 and MAFA are expressed in a substantial proportion of normal human pancreatic alpha cells and deregulated in patients with type 2 diabetes. PLoS One 8:e72194. doi: 10.1371/journal.pone.0072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J, Sosa-Pineda B. 2008. Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn 237:51–61. doi: 10.1002/dvdy.21379. [DOI] [PubMed] [Google Scholar]
- 27.Rehfeld JF, Ericsson P. 2012. Supersensitive gastrin assay using antibodies raised against a cholecystokinin homolog. Scand J Clin Lab Invest 72:175–179. doi: 10.3109/00365513.2011.637130. [DOI] [PubMed] [Google Scholar]
- 28.Rehfeld JF. 2006. The endoproteolytic maturation of progastrin and procholecystokinin. J Mol Med 84:544–550. doi: 10.1007/s00109-006-0055-3. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu K, Shiratori K, Sakayori N, Kobayashi M, Hayashi N. 1999. Expression of cholecystokinin in the pancreas during development. Pancreas 19:98–104. doi: 10.1097/00006676-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Dimaline R, Varro A. 2014. Novel roles of gastrin. J Physiol 592:2951–2958. doi: 10.1113/jphysiol.2014.272435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, Polak JM, Hacki WH, Stamm B, Heitz PU, Klöppel G. 1990. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med 322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 32.Anlauf M, Garbrecht N, Henopp T, Schmitt A, Schlenger R, Raffel A, Krausch M, Gimm O, Eisenberger CF, Knoefel WT, Dralle H, Komminoth P, Heitz PU, Perren A, Kloppel G. 2006. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features. World J Gastroenterol 12:5440–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapran Y, Bauersfeld J, Anlauf M, Sipos B, Kloppel G. 2006. Multihormonality and entrapment of islets in pancreatic endocrine tumors. Virchows Arch 448:394–398. doi: 10.1007/s00428-005-0147-4. [DOI] [PubMed] [Google Scholar]
- 34.Larsson LI, Grimelius L, Hakanson R, Rehfeld JF, Stadil F, Holst J, Angervall L, Sundler F. 1975. Mixed endocrine pancreatic tumors producing several peptide hormones. Am J Pathol 79:271–284. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.