Abstract
Objectives
Glucagon-like peptide-1 (GLP-1) is an intestinally secreted hormone and it plays an important role in the regulation of glucose homeostasis. However, the possible role of GLP-1 in the differentiation of adipose-derived stem cells (ADSCs) remains unknown. Therefore this study investigated the effect of GLP-1 on the differentiation of ADSCs into osteoblasts and adipocytes.
Methods
ADSCs were isolated from human adipose tissues of the abdomens, cultured and characterized by flow cytometry and multi-lineage potential assay. ADSCs were induced in osteogenic and adipogenic media treated with two different doses (10 and 100 nM) of GLP-1, and then the effect of GLP-1 on differentiation of ADSCs into osteoblast and adipocyte was examined. The signaling pathway involved in these processes was also examined.
Results
Isolated human ADSCs expressed mesenchymal stem cell (MSC) specific markers as well as GLP-1 receptor (GLP-1R) proteins. They also showed multiple-lineage potential of MSC. GLP-1 was upregulated the activity and mRNA expression of osteoblast-specific marker, alkaline phosphatase and the mineralization of calcium. In contrast, GLP-1 significantly suppressed the expression of adipocyte-specific markers, peroxisome proliferator-activated receptor gamma (PPAR-γ), lipoprotein lipase (LPL) and adipocyte protein 2 (AP2). This decreased expression of adipocyte specific markers caused by GLP-1 was significantly reversed by the treatment of extracellular signal-regulated kinase (ERK) inhibitor, PD98059 (P < 0.05).
Conclusion
This result demonstrates that GLP-1 stimulates osteoblast differentiation in ADSCs, whereas it inhibits adipocyte differentiation. The ERK signaling pathway seems to be involved in these differentiation processes mediated by GLP-1.
Keywords: Adipocytes, Adipogenesis, Adipose tissue, Cell differentiation, Glucagon-like peptide 1, Osteogenesis
Introduction
It has been well known that diabetic patients have a greater risk of bone fractures and osteoporosis.1,2 Osteoporosis has been recently suggested as a disorder of lipotoxicity.3 These facts have provided a new dogma that bone loss is accompanied with up-regulation of adipogenesis. Thus, these results mean that if there is a substance that inhibits adipogenesis, whereas it stimulates osteogenesis, it will be more attractive and effective drug in the treatment of type 2 diabetes.
Glucagon-like peptide-1 (GLP-1) is a well known incretin hormone and it is encoded by proglucagon gene in intestinal L-cells and some brain neuron, and degraded rapidly by the enzyme dipeptidyl peptidase-4 (DPP-4).4 It plays a crucial role in blood glucose control and pancreatic islet-cell proliferation.5,6,7 In addition, it has various functions on peripheral tissues and the central nervous system.8,9,10 GLP-1 also stimulates glucose-dependent insulin secretion and has antidiabetogenic effects in the treatment of type 2 diabetes,11 and the GLP-1 agonist is used as a class of drug of the treatment of type 2 diabetes.12,13 In addition, some studies have suggested the potential role of GLP-1 in the differentiation of adipogenesis and osteogenesis of mesenchymal stem cells (MSCs). Sanz et al.14 showed that GLP-1 significantly reduced the expression of peroxisome proliferator-activated receptor (PPAR) in the adipocyte differentiation of human bone marrow (BM)-MSC, meaning that GLP-1 significantly inhibited the adipogenic differentiation. Yamada et al.15 suggested that GLP-1 may have a possible effect on the osteoblast differentiation by showing an essential role of GLP-1 receptor (GLP-1R) in the control of bone resorption. In rat model, GLP-1 reversed hyperlipidemic-related osteopenia.16 In this respect, GLP-1 can be considered to be an excellent candidate for the treatment of type 2 diabetes.
MSCs were initially isolated from BM,17 but they were recently isolated from various adult tissues including the fat, muscle, cartilage, and cord blood.18 Among them, adipose tissue has become an attractive source of MSCs because adipose-derived stem cells (ADSCs) possess higher stem cell population compared to other MSC sources including BM,19,20 and they are easier to obtain, have relatively lower donor site morbidity, a higher yield and rapid ex-vivo expansion. In addition, they are capable of differentiating into at least three lineage (osteogenic, adipogenic and chondrogenic) when cultured under defined in vitro condition. MSCs have a reciprocal relationship between the differentiation of adipogenesis and osteogenesis.21,22,23
Therefore, this study examined the effects of GLP-1 on osteogenic and adipogenic differentiation using ADSCs-derived MSCs.
Materials and Methods
1. Isolation and culture of ADSCs
ADSCs were isolated from subcutaneous adipose tissues of the abdomen of patients undergoing caesarean section in Good Moonhwa Hospital, Busan, Korea. Informed consent was obtained from patients and all procedures were approved from hospital ethic committee and Institutional Review Board (2013-02). Tissues were washed three or four times with phosphate buffered saline (PBS) and digested with same volume of PBS supplemented 0.2% collagenase type I (Gibco BRL, Gaithersburg, MD, USA) for 60 minutes at 37℃ in shaking incubator. And then the tissues were filtered through the 100 µm nylon cell strainer (BD Biosciences Pharmingen, San Diego, CA, USA) and then centrifuged at 1000 rpm for 8 minutes to obtain stromal vascular fraction (SVF). The SVF pellets were resuspended and cultured at 37℃, 5% CO2 in growth media (GM) (Dulbecco's modified Eagle's medium [DMEM] low glucose supplemented with 10% [v/v] fetal bovine serum [FBS; Hyclone, Logan, UT, USA] and 1% [v/v] 100 units/mL of penicillin, and 100 ng/mL streptomycin [Gibco BRL]). This initial culture was referred as passage 0. Fresh media were replaced every third day. When monolayer of adherent cells reached 75% to 90% confluence, the cells were trypsinized (0.25% trypsin-ethylenediaminetetraacetic acid [EDTA]; Gibco BRL) and sub-cultured to passage 4 to 5.
2. Characterization of cells
An analysis of cell surface molecule was executed on passage 4 cultures of human ADSCs using flow cytometry. Briefly, after the media were removed from dish, the cell layer was washed with dPBS and treated with 0.25% trypsin-EDTA to detach cell. ADSCs were harvested by centrifugation and washed with BD PharmigenTM stain buffer (FBS; BD Biosciences). Then, the cells were incubated with fluorescein isothiocyanate (FITC) mouse-anti human CD90, FITC mouse-anti human CD105, FITC mouse anti-human CD73, FITC MSC negative cocktail (FITC mouse-anti human CD31, FITC mouse-anti human CD34, FITC mouse-anti human CD35) and FITC mouse IgG1, Isotype Control (BD Biosciences) for 50 minutes, after which the cells washed with BD PharmagenTM stain buffer (FBS) three times. And then the cells were analyzed on FC500 flow cytometer (Beckman Coulter GmbH, Krefeld, Germany).
3. Cell differentiation
For the differentiation into osteoblasts and adipocytes, ADSCs were cultured with an osteogenic induction media (OIM; GM supplemented with 100 nM dexamethasone, 50 µM ascorbate acid, and 10 mM β-glycerophosphate sodium [Sigma-Aldrich, St. Louis, MO, USA]) and an adipogenic induction media (AIM; GM supplemented with 1 µM dexamethasone, 1 mM 3-isobutyl-1-methylxanthine, 10 ng/mL insulin and 60 µM indomethacin [Sigma]), respectively. In all experiments, media were changed every three days.
4. GLP-1 and protein kinase inhibitor treatment
GLP-1 (PeproTech, Rocky Hill, NJ, USA) was treated to the ADSCs at a concentration of 10 nM and 100 nM. For the study concerning the effects of protein kinase inhibitors, cells were pretreated with 20 µM PD98059 (Gibco BRL) for one hour, followed by treatment with GLP-1 in the presence of the inhibitor.
5. Alizarin red S staining
After ADSCs were grown to confluence in GM, the cells were induced in OIM for 21 days. And then, the cells were estimated using Alizarin red S staining, which indicates the extracellular matrix calcification. Briefly, the media were replaced and then ADSCs were fixed with 70% iced ethanol for one hour at room temperature. After washing in distilled water twice, the cells were stained with 40 mM Alizarin Red S (Sigma, pH 4.1-4.6) for 5 minutes at room temperature. Alizarin Red S solution was removed and the cells were rinced twice with distilled water. Images of cells stained with Alizarin Red S were obtained with a G12 digital camera (Cannon, Tokyo, Japan) or were captured by an optical microscope with a G12 digital camera. Matrix mineralization was quantified by eluting Alizarin Red S staining with 20% methanol, 10% acetic acid and 70% distilled water at room temperature for 10 minutes. The absorbance of the supernatants was measured at 405 nm using a Thermo Multiskan FC Microplate Reader (Scientific).
6. Oil red O staining
After ADSCs were cultured in AIM for two weeks, the cells were fixed with 10% formalin for one hour at room temperature. Fixed cells were washed in 60% isopropanol and stained for 10 minutes with Oil Red O (Sigma) at room temperature. After five consecutive washes in distilled water, the presence of red stained lipid droplets were documented using bright-field microscopy. Images of cells stained with Oil Red O were acquired with a G12 digital camera. In order to elute stained lipid droplet, 100% isopropanol was added to each well followed by 10 minutes at room temperature and quantified by measuring the optical absorbance at 500 nm using a Thermo Multiskan FC Microplate Reader (Scientific).
7. RNA extraction and gene expression analysis by reverse transcriptase-polymerase chain reaction (RT-PCR) and quantitative real-time PCR
Total RNA was extracted using a Trisol reagent® (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized from 5 µg of total RNA with Moloney murine-leukaemia virus (MMLV) Reverse Transcriptase (Promega, Madison, WI, USA) using a random hexamer (Bioneer, Daejeon, Korea) at 42℃ for one hour. Template cDNA was subjected to PCR amplification using gene-specific sense and antisense primers (Table 1). RT-PCR conditions were denatured at 95℃ for 5 minutes, followed by 28 to 35 cycles of 95℃ for 30 seconds, annealed at 57℃ for 30 seconds in a thermal cycle. The PCR products were visualized by electrophoresis on 1.5% agarose gel.
Table 1. Sequences of primers used for polymerase chain reaction (PCR) ampification.
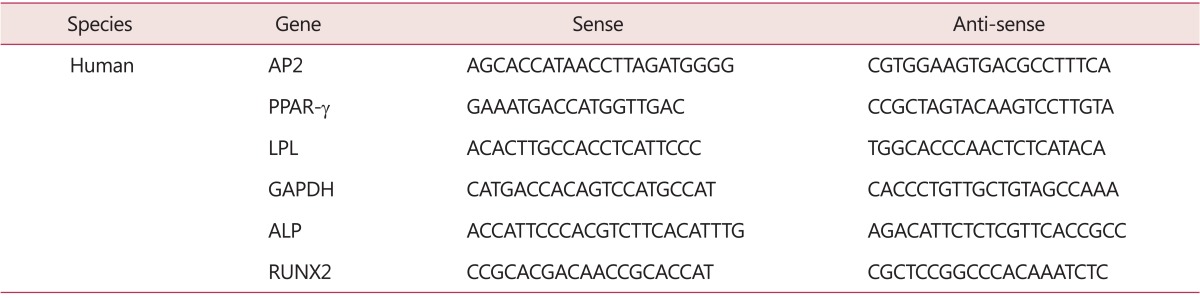
AP2: adipocyte protein 2, PPAR-γ: peroxisome proliferator-activated receptor gamma, LPL: lipoprotein lipase, GAPDH: glyceraldehyde-3-phosphate dehydrogenase, ALP: alkaline phosphatase, RUNX2: runt-related transcription factor 2
Real-time PCR was performed with the SYBR Green I Light cycle system (Roche, Mannheim, Germany). The reaction mixtures were prepared using Light Cycle Fast DNA master mixture for SYBR Green I, 0.5 µM of each primer, 4 mM MgCl2 and 2 µL of cDNA in a final volume of 20 µL. The reaction condition consisted of denaturation at 95℃ for 10 minutes, followed by 40 cycles of 95℃ for 10 seconds and 60℃ for 10 seconds, followed by melting curve analysis. For each sample, PCR were performed in duplicate. The quantitative amount of each gene was normalized against the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
8. Immunofluorescence
For cellular detection of GLP-1R, the cells were fixed with 4% paraformaldehyde in PBS. Rabbit anti-GLP-1R antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used, with diluted 1 : 100 in blocking solution. Fluorescence detection was performed by incubation with FITC conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology) with diluted 1 : 400 in blocking solution for GLP-1R detection. Nuclei staining and mounting were performed with ProLong@Gold antifade reagent with DAPI (Invitrogen, Oregon, USA).
9. Alkaline phosphatase (ALP) staining and activity assay
At day 10 after passage, ALP histochemistry was performed using diagnostic kit 85 (Sigma). ALP activity was determined using a colorimetric end point assay measuring the enzyme conversion of p-nitrophenyl phosphate (pNPP) to the yellowish product, p-nitrophenol (pNP), in the presence of ALP.1 In brief, cells were rinsed with cold PBS, scraped into 1.5 ml Eppendorf tubes in 1ml of lysis buffer (10 mM Tris-HCl, 1 mM magnesium chloride, and 0.05% Triton X-100, pH7.5), and then homogenized on ice for 10 minutes. Afterwards, the resulting mixture was centrifuged at 12,000 rpm for 10 minutes at 4℃. The cell lysates (0.1 mL of the above supernatants) were mixed with 0.5 mL of pNP phosphate solution (Sigma) and 0.5 mL of alkaline buffer solution (Sigma). After incubation at 37℃ for 15 minutes, the above mixture was added to 1 mL of 0.5 N NaOH to stop the reaction and the absorbance was then measured spectrophotometrically at 405 nm. A standard curve of known concentration of pNP was generated concurrently and used to determine sample concentrations.
10. Statistical analysis
Statistical analysis was performed with the student t-test using SPSS (version 12.0; SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered to be statistically significant.
Results
1. Characterization of human ADSCs
ADSCs obtained from human adipose tissues exhibited a fibroblast-like cells morphology and adhered to plastic. From one day of ADSCs culture, attachment of spindle-shaped cells was observed. After five days, spindle-shaped cells reached about 80% confluency. Morphology of the cells changed gradually according to passage numbers.
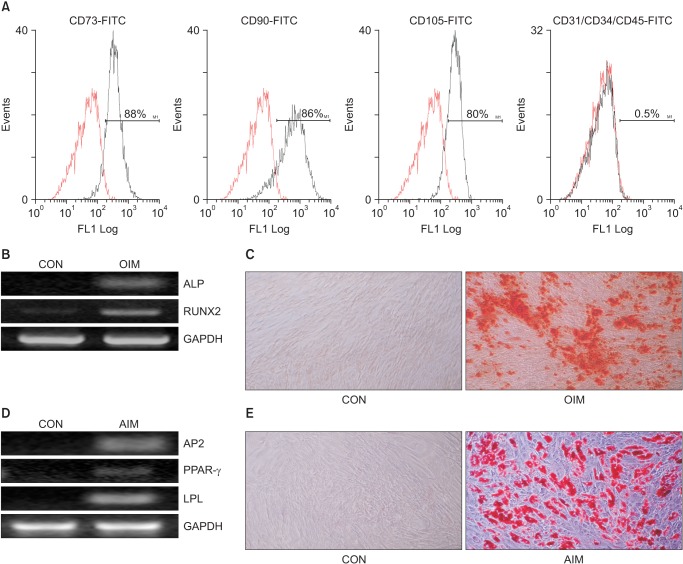
In order to assess whether ADSCs have MSC-specific characteristics, flow cytometry was performed at passage 4. The cells were positively stained for MSC-specific surface markers (CD73, CD90 and CD105), but they were negative for markers of the endothelial cells (CD31) and hematopoietic cells (CD34 and CD45) (Fig. 1A).
Fig. 1. Characterization of adipose-derived stem cells (ADSCs). (A) Flow cytometry analysis of ADSCs. Cells were stained with fluorescein fluorescein isothiocyanate (FITC)-labeled CD73, CD90, CD105, CD31, CD34 and CD45 antibodies. Fluorochrome-conjugated nonspecific mouse IgG1 was used as isotype controls (dotted red lines). ADSCs were stained positively for surface markers characteristic of mesenchymal stem cell, CD73, CD90 and CD105. The cells were negative for markers of the endothelial cells CD31, hematopoietic lineage CD45 and of hematopoietic stem cells CD34. (B, C) Osteogenic differentiation of ADSCs. Seven days of osteogenic induction, mRNA expressions of osteogenic markers were observed by reverse transcriptase-polymerase chain reaction (RT-PCR). Fourteen days of osteogenic induction, the deposition of calcium precipitates was stained by alizarin red S staining. (D, E) Adipogenic differentiation of ADSCs. Seven days of adipogenic induction, mRNA expressions of adipocyte markers were detected by RT-PCR. Fourteen days of adipogenic induction, the lipid droplets were stained by Oil red O staining. Original magnification, × 100. Original magnification, × 100. FITC: fluorescein isothiocyanate, OIM: osteogenic induction media, ALP: alkaline phosphatase, RUNX2: runt-related transcription factor 2, GAPDH: glyceraldehyde-3-phosphate dehydrogenase, AIM: adipogenic induction media, AP2: adipocyte protein 2, LPL: lipoprotein lipase, PPAR-γ: peroxisome proliferator-activated receptor-gamma.
To test the multi-lineage potential of ADSCs, the cells were induced in adipogenic or osteogenic differentiation media. The adipogenic and osteogenic differentiation potential was determined by each differentiation specific gene expression and staining. Seven days after adipogenic or osteogenic induction, specific osteoblast markers, ALP and runt-related transcription factor 2 (RUNX2) were positively expressed (Fig. 1B). The expression of adipogenic-specific genes, PPAR gamma (PPAR-γ), adipocyte protein 2 (AP2) and lipoprotein lipase (LPL) were also observed (Fig. 1D). Fourteen days after adipogenic or osteogenic induction, ADSCs revealed Alizarin Red S positive staining for calcium deposition (Fig. 1C) and Oil Red O positive staining for the lipid droplet (Fig. 1E). These results mean that the isolated ADSCs have the potential of MSCs.
2. Expression of GLP-1 receptor in ADSCs
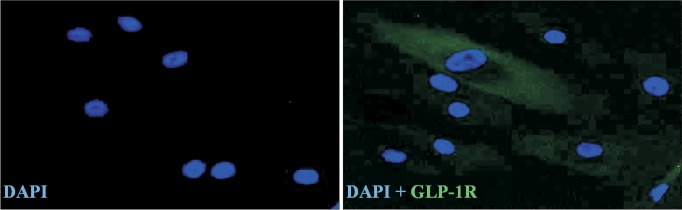
Prior to the examination of the effect of GLP-1 on adipogenic and osteogenic differentiation, whether GLP-1R is expressed in ADSCs was tested by immunofluorescence staining using anti-GLP-1R antibody. Fig. 2 showed GLP-1R positive stained cells.
Fig. 2. Glucagon-like peptide-1 (GLP-1) receptor expression in adipose-derived stem cells (ADSCs) was detected by immunofluorescence staining (green). Nuclei were labeled with DAPI (blue). Original magnification, × 200.
3. Expression of GLP-1 stimulates osteogenic differentiation in ADSCs
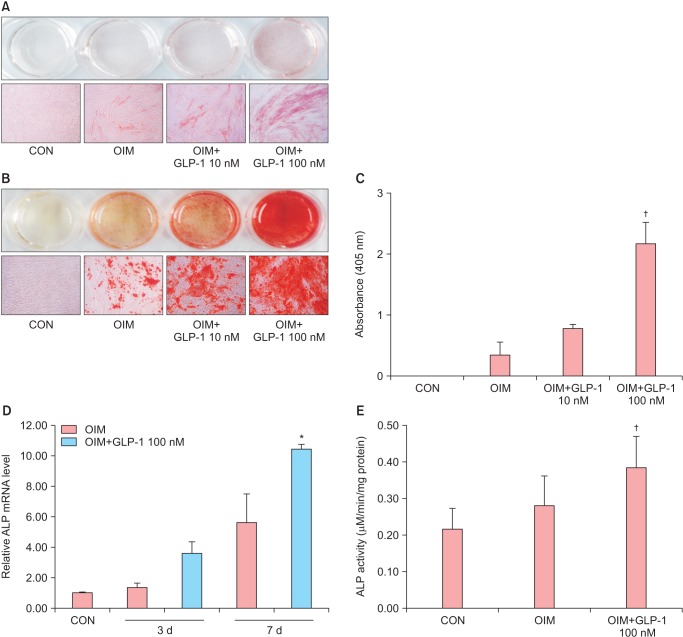
To examine whether GLP-1 has an effect on osteoblast differentiation, two doses of GLP-1 (10 and 100 nM) were treated to ADSCs cultured in OIM, and then Alizarin Red S staining and ALP staining were assessed after 10 days and 21 days of culture, respectively as the first indicator for osteogenic differentiation. The areas of ALP positive staining (Fig. 3A) and calcified nodules by Alizarin Red S staining (Fig. 3B) were increased in a dose-dependent manner in response to GLP-1, and the significant maximal effect was reached at a concentration of 100 nM GLP-1. Quantitative analysis of calcium deposition showed two-fold and five-fold increase in calcium deposition in 10 nM and 100 nM GLP-1, respectively (Fig. 3C).
Fig. 3. Effects of glucagon-like peptide-1 (GLP-1) on the osteogenic differentiation of adipose-derived stem cells (ADSCs). The ADSCs were cultured osteogenic induction medium (OIM) treated with various concentration of GLP-1 (0, 10 nM and 100 nM). The media containing GLP-1 was renewed every day. (A) The mRNA level of alkaline phosphatase (ALP) was determined by real time polymerase chain reaction (PCR) and it was normalized by the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). *P < 0.05 (vs. OIM). (B, C) On day 10, ALP activity was measured by ALP staining and enzyme-linked immunosorbent assay (ELISA). ALP was indicated by the red staining. Original magnification, × 100. ALP activity was determined using a colorimetric end point assay measuring the enzyme p-nitrophenol (pNP) in the presence of ALP. (D) On day 14, cells were stained with alizarin red S and calcium deposition nodules were stained as dark red areas. Original magnification, × 100. (E) Bar graph shows quantitative results of (D). †P < 0.05 (vs. OIM).
Next, to additionally demonstrate the effect of GLP-1 on osteoblast differentiation, 100 nM GLP-1 was treated to ADSCs cultured in OIM, and the expression of ALP mRNA transcript and ALP activity were examined. ALP mRNA expression was increased with culture period and it was significantly increased in GLP-1 treatment compared to the OIM on day 7 of culture (Fig. 3D). After 10 days of induction, the ALP activity also significantly increased in the 100 nM GLP-1-treated cells compared to the OIM group (Fig. 3E). These data indicate that GLP-1 stimulates osteogenic differentiation of ADSCs.
4. GLP-1 inhibits adipogenic differentiation in ADSCs
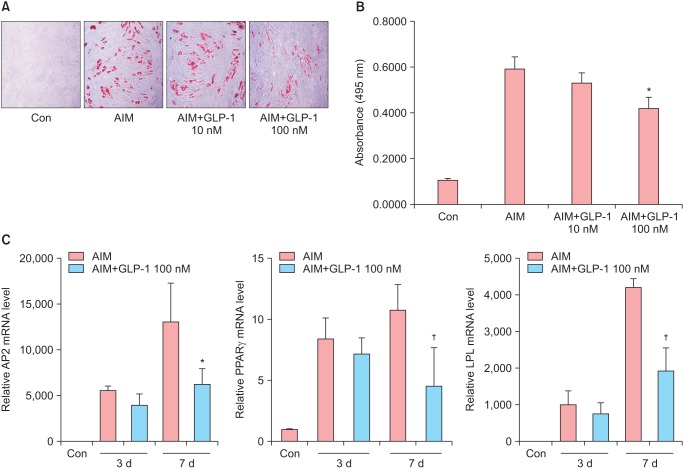
To examine the effect of GLP-1 on adipogenic differentiation in ADSCs, ADSCs cultured in AIM were treated with two doses of GLP-1 (10 nM and 100 nM). And then Oil-Red O staining and adipogenic specific gene expression were determined as an indicator for adipogenic differentiation. After 14 days of culture, the lipid accumulation by Oil Red O staining and quantitative analysis of lipid-droplet formation was decreased in a dose-dependent manner in response to GLP-1 treatment. Especially, a concentration of 100 nM GLP-1 significantly decreased the lipid accumulation compared to AIM without GLP-1 (Fig. 4A, 4B). Next, when the ADSCs were treated with 100 nM GLP-1 on day 0, 3 and 7 of culture, the expressions of AP2, PPAR-γ and LPL were significantly down-regulated at day 7 compared to AIM group (P < 0.05) (Fig. 4C). These data indicate that adipogenic differentiation of ADSCs may be inhibited by GLP-1.
Fig. 4. Effects of glucagon-like peptide-1 (GLP-1) on the adipogenic differentiation of adipose-derived stem cells (ADSCs). The ADSCs were induced to with adipogenic induction medium (AIM) with or without GLP-1 (0, 10 nM and 100 nM). (A, B) On day 14, cells were stained with oil red O to visualize lipid droplets. Original magnification, × 100. *P < 0.05 (vs. AIM). (C) On day 3 and 7, the mRNA levels of aP2, peroxisome proliferator-activated receptor (PPAR) and lipoprotein lipase (LPL) were determined by real time polymerase chain reaction (PCR). †P < 0.05 (vs. AIM).
5. Regulation of GLP-1 in adipogenesis of ADSCs via ERK activation
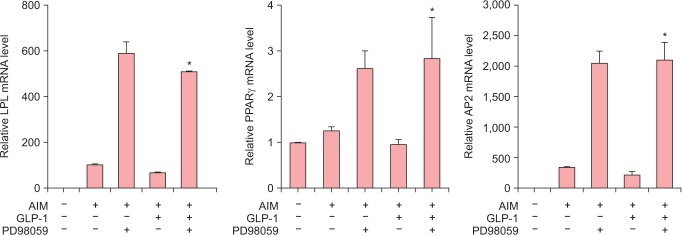
To understand the signaling pathway involved in the inhibitory effect of GLP-1 on the adipocyte differentiation of ADSCs, ADSCs cultured in AIM in the presence or absence of GLP-1 were treated with ERK inhibitor, PD98059. GLP-1 treatment decreased expression of PPAR-γ, LPL and AP2 mRNA levels and this decreased effect caused by GLP-1 was significantly reversed by the treatment of ERK inhibitor (P < 0.05) (Fig. 5).
Fig. 5. The treatment of extracellular signal-regulated kinase (ERK) inhibitor in adipogenic differentiation of adipose-derived stem cells (ADSCs) by glucagon-like peptide-1 (GLP-1). ADSCs were induced with adipogenic induction medium (AIM) for 7 days in the presence or absence of GLP-1 and then they were treated with ERK inhibitor PD98059. Real time polymerase chain reaction (PCR) for indicated genes were performed. *P < 0.05 (vs. GLP-1(+) / PD98059(-) group).
Discussions
The present study shows that GLP-1 stimulates osteoblast differentiation in ADSCs, whereas it inhibits adipocyte differentiation. Although several studies have suggested a potential role of GLP-1 in bone,16,24 the exact physiological effect of GLP-1 on bone is known incompletely. Even in the previous study investigating the role of GLP-1 in the regulation of bone metabolism using GLP-1R knockout mice, the authors suggested that endogeneous GLP-1R signaling may have an essential role in the control of bone resorption, but GLP-1 had no direct effect on osteoclast and osteoblast. In this respect, the finding of pro-osteogenic function of GLP-1 in ADSCs in the present study may be very notable finding and this is the first report according to the literature.
It has been well known that GLP-1 acts through GLP-1R.25 However, reports on expression of GLP-1R in osteoblastic cells are inconsistent. Some studies reported the presence of GLP-1R in various osteoblastic cell lines.26,27,28 Another study failed to show GLP-1R in osteoblasts.15 Sanz et al.14 showed the expression of GLP-1R protein in human BM-derived MSCs. Our present study showed that isolated human ADSCs had MSC characteristics and expressed GLP-1R. This result means that the effect of GLP-1 on differentiation of ADSC-derived MSCs into osteoblast and adipocyte may be mediated through GLP-1R.
In the present study, the fact that GLP-1 stimulates osteoblast differentiation of ADSCs was demonstrated by the two methods. One is Alizarin Red S staining and the other is ALP staining, ALP activity, and ALP gene expression. As we all know, Alizarin Red S staining has been widely used in the characterization of osteoblast differentiation. ALP is produced by liver as well as osteoblast or bone and osteocalcin is well known to be mature osteoblast marker. Nevertheless, the present study used ALP as an osteoblast marker due to the following reason: ALP has been solidified as a specific osteogenic marker to be detected in the early stage of osteogenic differentiation from MSCs and it is a specific marker for mineralization.29,30,31 This study showed that GLP-1 increased not only ALP activity and mRNA expression, but also the area of ALP and Alizarin Red S staining.
In contrast to the osteogenic effect, the present study found that GLP-1 not only reduced lipid accumulation, but also down-regulated the expressions of adipocyte specific markers AP2 and LPL as well as PPAR-γ, especially on day 7 of culture. This result means that GLP-1 inhibited the adipogenic differentiation of ADSCs. Consistent with this data, Sanz et al.14 have reported that GLP-1 significantly prevents the adipocyte differentiation of human BM-derived MSC. However, our result is inconsistent with other two studies, showing that GLP-1 increases the differentiation of pre-adipocyte into mature adipocyte. One study used the 3T3-L1 fibroblast and primary pre-adipocyte derived from SVF of mouse adipose tissues.29 The other study used 3T3-L1.32 Given that human MSCs have a little different characteristics in differentiation potential with those of mouse MSCs and pre-adipocyte cell line, this discrepancy might be due to difference in cell types used as experimental subjects. Further study needs to elucidate clearly this issue.
In this respect, one of interesting finding in the present study is that GLP reciprocally regulates both differentiation of adipogenesis and osteogenesis of ADSCs. To our acknowledgement, this is the first study to report that GLP-1 plays an additional role in the regulation of osteoblast and adipocyte differentiation in ADSCs. Numerous studies have demonstrated that there is a reciprocal relationship between adipogenesis and osteogenesis of human MSCs.21,22 In vivo, a decrease of bone formation accompanied by increasing adipogenesis may lead to osteoporosis.33,34 Especially in the postmenopausal women, age-related osteoporosis is accompanied by an increase in MSC adipogenesis. To maintain the balance between osteoblasts and adipocytes is a key goal of pharmacological approach in the treatment of osteoporosis. Some studies have attempted to find factors that increase fat accumulation lead to enhanced bone loss,35,36 but the mechanism that regulates these two differential differentiations is not clearly understood yet. In this respect, this result suggests that GLP-1 may act as an effective regulator for a reciprocal relationship between adipogenesis and osteoporosis and it can be used as a potent drug for osteoporosis treatment. In addition, this result provides a strong information that GLP-1 based therapy can be used as a more effective and powerful agent for the treatment of type II diabetes due to the following reasons: One is that type II diabetes is closely related to bone fractures and osteoporosis,1,2 which is accompanied with up-regulation of adipogenesis.3 Second is that thiazolidinediones (TZD) is a drug to bieng widely used in the treatment of type II diabetes by activating PPARs, but long term usage of TZD for diabetic patients cause a greater risk of bone fractures and osteoporosis.1,2 This could be attributed to the fact that TZD drugs promote adipocyte differentiation of preadipocyte, which eventually lead to the imbalance in bone remodeling process and bone loss. Finally, the GLP-1 agonist has been being used as a class of drug of the treatment of type II diabetes12,13 because GLP-1 stimulates glucose-dependent insulin secretion and has antidiabetogenic effects.6,11
Extracellular signal-regulated kinas (ERK) pathway plays a key role in signaling cascade regulating proliferation and the earliest phase of differentiation.37,38 GLP-1 activates ERK 1/2 in pancreatic β-cell through GLP-1R.24 Therefore we next investigated whether ERK signaling pathway involves in GLP-1-mediated pre-adipocyte differentiation by the treatment of ERK inhibitor, PD98059. It was found that the inhibitor reversed the decreased expression of adipocyte specific markers induced by GLP-1. This result suggests the possibility that ERK signaling pathway may be involved in the inhibition process of adipocyte differentiation by GLP-1, but to further study is needed to clearly conclude it.
Most studies concerning the effect of GLP-1 on osteoblast and adipocyte differentiation have used 10 nM and 100 nM as the treatment concentration of GLP-1.14,28,32 Due to these background data, we firstly treated with these concentrations of GLP-1. After we confirmed the maximal effect at a concentration of 100 nM GLP-1, we choose the concentration of GLP-1 to perform the next experiment.
In summary, it is suggested that GLP-1 effects on ADSCs differentiation by enhancing osteogenic differentiation and concomitantly inhibiting adipogenic differentiation. Therefore, the enhancement of osteogenesis with preventing adipogenesis could provide a therapeutic target to osteoporosis. Considering that GLP-1 could be function as anti-osteoporotic agents, this study provides an important clue that GLP-1 could be applied as an effective drug in the treatment of osteoporosis of postmenopausal women, obesity and diabetes mellitus patients.
Acknowledgements
This study was supported by a grant of the Dong-A University research fund.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Schwartz AV. TZDs and Bone: A Review of the Recent Clinical Evidence. PPAR Res. 2008;2008:297893. doi: 10.1155/2008/297893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–434. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 4.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60:1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 5.Creutzfeldt W. The entero-insular axis in type 2 diabetes--incretins as therapeutic agents. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S288–S303. doi: 10.1055/s-2001-18589. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ma X, Hui H, Liu Z, He G, Hu J, Meng J, et al. Poly-GLP-1, a novel long-lasting glucagon-like peptide-1 polymer, ameliorates hyperglycaemia by improving insulin sensitivity and increasing pancreatic beta-cell proliferation. Diabetes Obes Metab. 2009;11:953–965. doi: 10.1111/j.1463-1326.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–927. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 11.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 12.Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3853–3860. doi: 10.1210/jcem.86.8.7743. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534–539. doi: 10.1001/jamainternmed.2013.2720. [DOI] [PubMed] [Google Scholar]
- 14.Sanz C, Vazquez P, Blazquez C, Barrio PA, Alvarez Mdel M, Blazquez E. Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab. 2010;298:E634–E643. doi: 10.1152/ajpendo.00460.2009. [DOI] [PubMed] [Google Scholar]
- 15.Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- 16.Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P, et al. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011;209:203–210. doi: 10.1530/JOE-11-0015. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 19.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 20.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006;5:176–179. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 23.Lee BI. Hormone replacement therapy following stem cell transplant. J Korean Soc Menopause. 2007;13:8–13. [Google Scholar]
- 24.Nuche-Berenguer B, Moreno P, Esbrit P, Dapia S, Caeiro JR, Cancelas J, et al. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009;84:453–461. doi: 10.1007/s00223-009-9220-3. [DOI] [PubMed] [Google Scholar]
- 25.Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010;225:585–592. doi: 10.1002/jcp.22243. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. doi: 10.1186/1472-6793-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012;287:6421–6430. doi: 10.1074/jbc.M111.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 30.Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–448. [Google Scholar]
- 31.Ahn KH, Jung SE, Yi KW, Park HT, Shin JH, Kim YT, et al. Barium stimulates the expression of osteogenic genes in human mesenchymal stem cells into osteoblasts in vitro. J Korean Soc Menopause. 2011;17:81–87. [Google Scholar]
- 32.Yang J, Ren J, Song J, Liu F, Wu C, Wang X, et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int J Mol Med. 2013;31:1429–1435. doi: 10.3892/ijmm.2013.1350. [DOI] [PubMed] [Google Scholar]
- 33.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 37.Choi SS, Cha BY, Iida K, Sato M, Lee YS, Teruya T, et al. Honokiol enhances adipocyte differentiation by potentiating insulin signaling in 3T3-L1 preadipocytes. J Nat Med. 2011;65:424–430. doi: 10.1007/s11418-011-0512-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Ikeda K, Xu JW, Yamori Y, Gao XM, Zhang BL. Genistein suppresses adipogenesis of 3T3-L1 cells via multiple signal pathways. Phytother Res. 2009;23:713–718. doi: 10.1002/ptr.2724. [DOI] [PubMed] [Google Scholar]