Abstract
Background
Agarwal is one of the largest business communities in India. To determine prevalence of cardiovascular risk factors and their distribution according to educational status (ES) in this community we performed a study.
Methods
1781 (men 1039, women 742) of 2500 selected subjects (71.2%) were evaluated and fasting blood sample obtained in 1130.
Results
Age-adjusted prevalence of risk factors was tobacco use 12.2%, sedentary habits 54.2%, overweight/obesity 54.4%, obesity 19.5%, abdominal obesity 61.2%, hypertension 36.0%, diabetes 19.2%, hypercholesterolemia ≥200 mg/dl 25.8%, low HDL cholesterol 29.2%, hypertriglyceridemia 32.8% and metabolic syndrome 22.3%. Low ES subjects had significantly greater prevalence of sedentary habits, low fruit/vegetable intake, hypertension, low HDL cholesterol and diabetes.
Conclusions
Cardiometabolic risk factors are highly prevalent in the Agarwal business community. Prevalence is greater in subjects with low educational status.
Keywords: Cardiovascular epidemiology, Ethnic groups, Diabetes
1. Introduction
Agarwals are the most prominent ethnic group among business communities in India with a population of about 25–30 million.1 It has been serendipitously observed that this community has high incidence of cardiovascular diseases (CVD).2 A study reported that cardio-metabolic risk factors in this community were 2–3 times greater than in tribals of north-east India.3 This community is also genetically homogenous.4 We performed a cardiovascular risk factor epidemiological study among Agarwal community in Jaipur (Rajasthan) to identify prevalence of sociodemographic, lifestyle and biological CVD risk factors. We also evaluated educational status (ES) related differences in various risk factors.
2. Methods
Detailed methodology of the Jaipur Heart Watch cross sectional studies in urban populations has been reported.5 Research Review Board of Department of Home Science, University of Rajasthan, Jaipur, approved the study. Informed consent was obtained from each participant. We used Agarwal community registers as sampling frame and study was performed at 10/72 municipal wards in Jaipur. All the eligible members were invited through distribution of pamphlets and multiple camps organized at local community centres. All the respondents were explained about the study objectives and the expected results. A total of 2500 subjects ≥20 years were invited and 1781 respondents were screened for risk factors. Collection of general information, anthropometric measurements, biophysical parameters and medical history was done on this sample while biochemical estimation was performed on 1130 consenting adults. Details of demographic profile, history of illnesses and risk factors, smoking or tobacco intake, physical activity and dietary history were obtained according to WHO guidelines.6 We inquired details of smoking and smokeless tobacco use, alcohol intake, daily dietary visible fat and fruits and vegetables intake using a 24-h recall and food frequency questionnaire.5–7 Details of physical activity were inquired for exact daily duration (minutes) of work related, commute related and leisure time physical activity.7 Physical examination was performed to assess height, weight, waist and hip circumference and blood pressure (BP). Fasting blood sample was obtained for glucose, total cholesterol (TC), triglycerides (TG), and high density lipoprotein (HDL) cholesterol while low density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula.6
Descriptive statistics are reported. Educational status (ES) was classified according to number of years of formal education into three categories ≤10 years, 10–15 years and >15 years. Low physical activity, high dietary visible fat intake, and low vegetables/fruit intake of were defined as previous studies.7 Overweight and obesity were defined according to body mass index (BMI) cut-offs of ≥25 kg/m2 and ≥30 kg/m2. Abdominal obesity was categorized according to Harmonized Criteria for Asians and defined by waist circumference in men >90 cm and in women >80 cm and waist-hip ratio (WHR) >0.9 in men and >0.8 in women.8 Hypertension was diagnosed when systolic BP was ≥140 mm Hg and/or diastolic BP ≥ 90 mm Hg or a person was a known hypertensive. Dyslipidaemia was defined by the presence of high total cholesterol (≥200 mg/dl), high LDL cholesterol (≥130 mg/dl and ≥100 mg/dl), low HDL cholesterol (men <40 mg/dl, women <50 mg/dl) and high triglycerides (≥150 mg/dl). Diabetes was diagnosed on the basis of either history of known diabetes or fasting blood glucose ≥126 mg/dl. Metabolic syndrome was diagnosed using Asian specific harmonized criteria.8 Age-adjustment of prevalence rates was performed by direct method using standard Jaipur population from 2001 census. Prevalence (%) and 95% confidence intervals (CI) are reported. Intergroup differences were assessed using X2 test and trends examined by Mantel–Haenszel X2 test.
3. Results
We evaluated 1781/2500 (men 1039, women 742) (71.2%) respondents from Agarwal community. Majority of subjects (68.4%) belonged to age group 30–59 years. Illiteracy and low ES was in 348 (19.5%), middle ES in 843 (47.3%) and high ES in 590 (33.1%). High prevalence of overweight and obesity, abdominal obesity, hypertension, lipid abnormalities and diabetes are observed in the study cohort (Table 1). Age-specific prevalence of various risk factors is shown in Supplementary Table 1 (available online) and shows a high prevalence of cardiometabolic risk factors in age-groups <40 years, both in men and women. Age-adjusted prevalence of various risk factors in different ES groups is shown in Table 2. Prevalence of smoking/tobacco use, high dietary visible fat intake, overweight/obesity, abdominal obesity, high total cholesterol, high LDL cholesterol, high triglycerides and metabolic syndrome is similar across various ES groups. In low vs medium and high ES there is greater prevalence of sedentary lifestyle (82.5 vs 65.4, 62.1%), low fruits/vegetables intake (83.1 vs 64.3, 54.1%), hypertension (33.6 vs 37.6, 33.4%), low HDL cholesterol (38.9 vs 28.4, 26.8%) and diabetes (23.8 vs 20.6, 16.5%) (X2 test, p < 0.05).
Table 1.
Age-adjusted prevalence (percent, 95% confidence intervals) of lifestyle and metabolic cardiovascular risk factors.
| Men (n = 1039) | Women (n = 742) | Total (n = 1781) | |
|---|---|---|---|
| Lifestyle variables | |||
| High dietary fat intake >30 g/day | 33.3 | 34.8 | 34.0 |
| Low fruits/vegetables intake <3 helpings/day | 51.8 | 55.4 | 53.5 |
| Sedentary habits | 47.9 | 61.3 | 54.2 |
| Moderate and intense physical activity | 52.1 | 38.6 | 45.9 |
| Smoking/Tobacco use | 20.4 | 0.8 | 12.2 |
| Overweight and obesity | |||
| BMI ≥ 25 kg/m2 | 50.6 (47.6–53.7) | 59.8 (56.3–63.3) | 54.4 (52.1–56.7) |
| BMI ≥30 kg/m2 | 15.1 (13.1–17.4) | 25.6 (22.6–28.9) | 19.5 (17.7–21.4) |
| Abdominal obesity | |||
| Waist circumference, men >90 cm, women >80 cm | 53.7 (50.6–56.7) | 71.6 (68.3–74.8) | 61.2 (58.9–63.4) |
| Waist:hip ratio, men>0.9, women>0.8 | 65.9 (63.0–68.7) | 74.8 (71.6–77.8) | 69.6 (67.4–71.7) |
| Hypertension | 40.7 (37.7–43.7) | 29.4 (26.2–32.7) | 36.0 (33.8–38.2) |
| Biochemical parameters | n = 725 | n = 405 | n = 1130 |
| Cholesterol ≥200 mg/dl | 25.6 (22.6–28.9) | 26.1 (22.1–30.7) | 25.8 (23.4–28.5) |
| Cholesterol ≥240 mg/dl | 6.2(4.7–8.2) | 4.9(3.2–7.5) | 5.7(4.5–7.3) |
| LDL cholesterol ≥100 mg/dl | 46.2(42.6–49.8) | 43.7(38.9–48.6) | 45.3(42.4–48.2) |
| LDL Cholesterol≥130 mg/dl | 17.3 (14.7–20.2) | 14.8 (11.7–18.6) | 16.4 (14.8–18.9) |
| HDL Cholesterol men <40 mg/dl, women <50 mg/dl | 24.0 (21.2–27.5) | 38.0 (33.4–42.8) | 29.2 (26.6–31.9) |
| Triglycerides ≥150 mg/dl | 40.3 (36.7–43.9) | 20.5 (16.8–24.7) | 32.8 (30.2–35.6) |
| Diabetes (known or fasting glucose ≥126 mg/dl) | 20.5 (17.8–23.6) | 16.8 (13.4–20.7) | 19.2 (17.0–21.6) |
| Metabolic syndrome (harmonized definition) | 26.6 (24.1–29.4) | 16.2 (13.6–19.0) | 22.3 (20.4–24.3) |
BMI body mass index; LDL low density lipoprotein; HDL high density lipoprotein
Table 2.
Age adjusted prevalence (95% confidence intervals) of various lifestyle and cardiometabolic risk factors according to educational status.
| Low (n = 348) | Middle (n = 843) | High (n = 590) | X2 for trend (p value) | |
|---|---|---|---|---|
| Smoking/Tobacco use | 12.6 (9.5–16.6) | 12.6 (10.5–15.0) | 10.8 (8.6–13.6) | 0.652 |
| Dietary visible fat intake <30 g/day | 22.4 (18.3–27.1) | 35.7 (32.5–39.0) | 34.9 (31.2–38.5) | 0.156 |
| Fruits & vegetables ≥3 servings/day | 16.9 (13.3–21.3) | 35.7 (32.5–39.0) | 45.9 (41.9–50.0) | <0.001 |
| Physical activity ≥ moderate | 17.5 (13.9–21.8) | 34.6 (31.5–37.9) | 37.9 (34.1–41.9) | <0.001 |
| Body mass index ≥25 kg/m2 | 49.1 (43.9–54.4) | 55.4 (52.0–58.7) | 53.5 (49.5–57.5) | 0.165 |
| Waist circumference: men >90, women >80 cm | 57.5 (52.2–62.5) | 63.3 (60.0–66.5) | 59.8 (55.8–63.7) | 0.504 |
| Hypertension | 33.6 (28.8–38.7) | 37.6 (34.4–40.9) | 33.4 (29.7–37.3) | <0.001 |
| Total cholesterol ≥200 mg/dl | 23.8 (18.7–29.7) | 23.3 (19.9–27.2) | 27.8 (23.1–32.0) | 0.697 |
| LDL cholesterol ≥100 mg/dl | 40.2 (34.1–46.7) | 46.1 (41.9–50.4) | 44.6 (39.7–49.6) | 0.528 |
| HDL cholesterol: men <40, women <50 mg/dl | 38.9 (32.9–45.4) | 28.4 (24.7–32.4) | 26.8 (22.6–31.4) | 0.036 |
| Triglycerides ≥150 mg/dl | 27.3 (21.9–33.3) | 32.8 (28.9–37.0) | 35.2 (30.5–40.1) | 0.213 |
| Diabetes | 23.8 (18.0–28.8) | 20.6 (17.4–24.3) | 16.5 (13.1–20.6) | 0.023 |
| Metabolic syndrome | 22.9 (18.9–27.7) | 22.4 (19.7–25.3) | 21.3 (18.2–24.8) | 0.277 |
4. Discussion
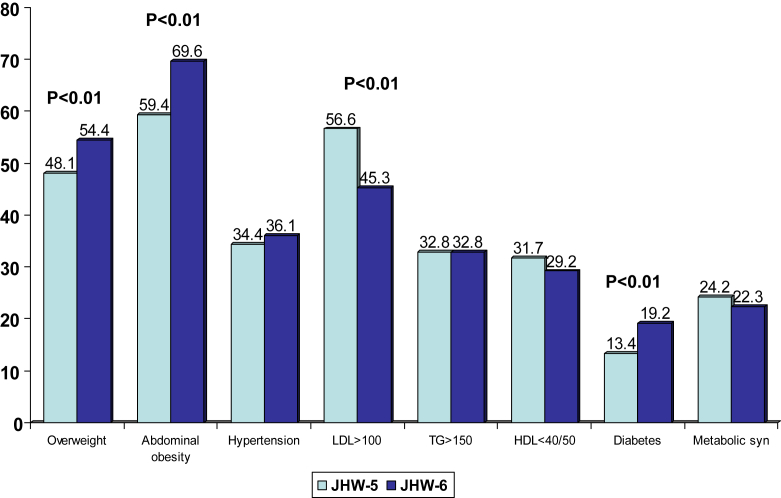
This study shows that low educational status Agarwal community subjects in India have greater prevalence of sedentary lifestyles, poor diet, hypertension, low HDL cholesterol and diabetes. Overall, a high prevalence of cardiometabolic risk factors is noted in this community. This is greater than contemporary population based studies in India9 including contemporary middle-class studies, Jaipur Heart Watch-5 and India Heart Watch (Fig. 1).5,7 Recent nationwide studies to assess prevalence of cardiovascular risk factors include the Industrial Population Surveillance Study,10 India Migration Study,11 India Women's Health Study,12 India Heart Watch7 and INDIAB Study.13 These studies reported that while prevalence of risk factors is low in rural subjects, among industrial workers and urban participants high prevalence of abdominal obesity (40–60%), hypertension (30–40%), diabetes (10–15%) and metabolic syndrome (20–40%) is present. These rates are similar to the present study. Prevalence of smoking and other tobacco use is low in the present study and reflects greater ES similar to previous studies from India.7 Prevalence of hypertension and hypercholesterolemia is similar to previous studies.9 Diabetes prevalence is especially high and is similar to studies in South India,14 and greater than population-based studies in other parts of the country.9 This study also shows that Agarwal men have high prevalence of risk factors at a younger age (Supplementary Table 1, online only).
Fig. 1.
Comparison of the prevalence of cardiometabolic risk factors in Jaipur Heart Watch (JHW-5) and the present JHW-6 study. In JHW-6 there is significantly greater prevalence of overweight/obesity, abdominal obesity and diabetes.
We also determined association of ES, as marker of socioeconomic status, with prevalence of cardiometabolic risk factors (Table 2). Greater prevalence of hypertension and diabetes in low ES participants is indicative of advanced epidemiological transition in this community. It is well known that in the initial phases of socioeconomic development cardiovascular risk factors are greater in higher vs. lower social groups but with continuing development there is a transition and risk factors are more in lower ES groups.15 It has been argued that there are multiple levels of risk factor transitions including demographic transition, epidemiological transition and health transitions.16 In the global health context, a multilevel eco-epidemiological life course framework for the health, disease and mortality cross-continuum has been suggested for deepening understandings of the demographic and epidemiological transitions.16 Such a framework is designed to help construct a precise understanding of mechanisms of change and to develop suitable interventions and appropriate health policies.
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ihj.2015.03.011.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Timberg T.A. Allen Lane; New Delhi: 2014. The Marwaris: From Jagat Seths to the Birlas; pp. 1–23. [Google Scholar]
- 2.Gupta R., Agrawal M. High cardiovascular risks in a North Indian Agarwal community: a case series. Cases J. 2009;2:e007870. doi: 10.1186/1757-1626-2-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg P.R., Kabita S., Singh H.S. Differences in conventional cardiovascular risk factors in two ethnic groups in India. Ethn Dis. 2013;22:372–376. [PubMed] [Google Scholar]
- 4.Gupta V., Khadgawat R., Ng H.K., Kumar S., Rao V.R., Sachdeva M.P. Population structure of Aggarwals of North India as revealed by molecular markers. Genet Test Mol Biomarkers. 2010;14:781–785. doi: 10.1089/gtmb.2010.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta R., Sharma K.K., Gupta A. Persistent high prevalence of cardiovascular risk factors in the urban middle-class in India: Jaipur Heart Watch-5. J Assoc Physicians India. 2012;60:11–16. [PubMed] [Google Scholar]
- 6.Luepkar R.V., Evans A., McKeigue P.M., Reddy K.S. 3rd ed. World Health Organization; Geneva: 2002. Cardiovascular Survey Methods. [Google Scholar]
- 7.Gupta R., Deedwania P.C., Sharma K.K. Association of education, occupation and socioeconomic status with cardiovascular risk factors in Asian Indians: a cross-sectional study. PLoS One. 2012;7:e044098. doi: 10.1371/journal.pone.0044098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti K.G., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation; National Heart, Lung and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R., Guptha S., Sharma K.K., Gupta A., Deedwania P.C. Regional variations in cardiovascular risk factors in India: India Heart Watch. World J Cardiol. 2012;4:112–120. doi: 10.4330/wjc.v4.i4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy K.S., Prabhakaran D., Chaturvedi V. Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bull WHO. 2006;84:461–469. doi: 10.2471/blt.05.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinra S., Bowen L.J., Lyngdoh T. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010;341:c4974. doi: 10.1136/bmj.c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey R.M., Gupta R., Misra A. Determinants of urban-rural differences in cardiovascular risk factors in middle-aged women in India: a cross-sectional study. Int J Cardiol. 2013;163:157–162. doi: 10.1016/j.ijcard.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Bhansali A., Dhandhania V.K., Deepa M. Prevalence of and risk factors for hypertension in urban and rural India: the ICMR-INDIAB study. J Hum Hypertens. 2015;29:204–209. doi: 10.1038/jhh.2014.57. [DOI] [PubMed] [Google Scholar]
- 14.Mohan V., Deepa M., Deepa R. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban south India – the Chennai Urban Rural Epidemiology Study. Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan G.A., Keil J.E. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 16.Defo B.K. Beyond the transition framework: the cross continuum of health, disease and mortality framework. Glob Health Action. 2014;7:e24804. doi: 10.3402/gha.v7.24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.