Abstract
Oxylipins formed from polyunsaturated fatty acids (PUFAs) are the main mediators of PUFA effects in the body. They are formed via cyclooxygenase, lipoxygenase, and cytochrome P450 pathways, resulting in the formation of prostaglandins, thromboxanes, mono-, di-, and tri-hydroxy fatty acids (FAs), epoxy FAs, lipoxins, eoxins, hepoxilins, resolvins, protectins (also called neuroprotectins in the brain), and maresins. In addition to the well-known eicosanoids derived from arachidonic acid, recent developments in lipidomic methodologies have raised awareness of and interest in the large number of oxylipins formed from other PUFAs, including those from the essential FAs and the longer-chain n–3 (ω-3) PUFAs. Oxylipins have essential roles in normal physiology and function, but can also have detrimental effects. Compared with the oxylipins derived from n–3 PUFAs, oxylipins from n–6 PUFAs generally have greater activity and more inflammatory, vasoconstrictory, and proliferative effects, although there are notable exceptions. Because PUFA composition does not necessarily reflect oxylipin composition, comprehensive analysis of the oxylipin profile is necessary to understand the overall physiologic effects of PUFAs mediated through their oxylipins. These analyses should include oxylipins derived from linoleic and α-linolenic acids, because these largely unexplored bioactive oxylipins constitute more than one-half of oxylipins present in tissues. Because collated information on oxylipins formed from different PUFAs is currently unavailable, this review provides a detailed compilation of the main oxylipins formed from PUFAs and describes their functions. Much remains to be elucidated in this emerging field, including the discovery of more oxylipins, and the understanding of the differing biological potencies, kinetics, and isomer-specific activities of these novel PUFA metabolites.
Keywords: oxylipin, polyunsaturated fatty acid, eicosanoid, lipid mediators, omega-3, omega-6, cyclooxygenase, lipooxygenase, cytochrome P450, lipidomics
Introduction
Oxylipins are PUFA oxidation products formed via one or more mono- or dioxygen-dependent reactions. They are major mediators of PUFA effects in the body, with the most well-known oxylipins being the eicosanoids formed from arachidonic acid (AA)4 (20:4n–6). Oxylipins also can be formed from other PUFAs, with the more common ones being octadecanoids derived from linoleic acid (LA) (18:2n–6) and α-linolenic acid (ALA) (18:3n–3), eicosanoids derived from dihomo-γ-linolenic acid (DGLA) (20:3n–6) and EPA (20:5n–3), and docosanoids derived from adrenic acid (AdA) (22:4n–6) and DHA (22:6n–3). The PUFA precursors to oxylipins can be obtained directly from the diet or from the elongation and desaturation of LA and ALA into longer-chain PUFAs. Hence, a high n–6 PUFA intake is generally associated with a high concentration of n–6 PUFA-derived oxylipins and a high n–3 PUFA intake is generally associated with a high concentration of n–3 PUFA-derived oxylipins.
However, the types of oxylipins produced from tissue PUFAs not only depend on the amount of dietary PUFAs consumed, but also on the amounts of competing PUFAs for incorporation into phospholipids and for elongation and desaturation to longer-chain PUFAs. Further, the oxygenases present for metabolizing these PUFAs into oxylipins in each tissue, as well as enzyme preferences for specific PUFAs, influence oxylipin production. Hence, the tissue oxylipin profile does not necessarily mimic dietary PUFA intake or tissue PUFA profile, necessitating the direct assessment of tissue oxylipins in order to understand the effects of PUFAs that are mediated via oxylipins. The recent advent of lipidomics methodologies has enabled the analyses of oxylipin profiles from all PUFA substrates simultaneously, raising awareness of the vast number of oxylipins in the body. Indeed, these analyses have shown that AA oxylipins comprise less than one-half of all oxylipins. Other studies have shown that oxylipins derived from PUFAs besides AA also have significant biological activity. This necessitates the investigation of the entire oxylipin profile in order to understand the overall effects of dietary PUFAs via their metabolism to oxylipins. Therefore, because there is currently no collated data on oxylipins in mammalian tissue, the purpose of this review is to provide a detailed compilation of the main oxylipins formed from the various PUFAs, and to provide a general overview of their functions.
Oxylipin Formation
Oxylipins are found throughout the body in all tissues, urine, and blood. Classically, they have been described as having a short half-life, acting locally, and not being stored, but being synthesized in situ when needed. However, not all oxylipins are short-lived, as evidenced by the steady-state concentrations of both free and esterified oxylipins in tissues such as the liver, adipose tissue, the kidney, and ileum (1–3). The free forms are presumably the biologically active oxylipins, but the functions of those that are found esterified to phospholipid are not known. It is possible that they may alter membrane properties or act as a storage reservoir.
Oxylipin formation begins with cell activation, which results in precursor PUFAs in the sn-2 position of membrane phospholipids being liberated by cytosolic phospholipase A2 (cPLA2) (4). Evidence for the importance of this enzyme is provided by findings from a patient lacking this enzyme, in whom liberation of free PUFAs and subsequent oxylipin formation is reduced compared with healthy controls (5, 6). However, although only AA oxylipins were examined in these studies, lack of cPLA2 did not completely block oxylipin formation. A recent study showed that inhibition of adipose TG lipase in mast cells also reduced oxylipin formation (7). Because TGs typically contain only small amounts of AA, this raises the question of whether non-AA PUFAs might be released in greater amounts via alternate pathways, such as adipose TG lipase. Further studies examining whether PUFA liberation via this enzyme is a direct source of PUFAs for oxylipin biosynthesis, or whether TG lipase indirectly provides PUFAs for incorporation into phospholipid before liberation via cPLA2 activity, remain to be carried out. Once formed, free oxylipins can mediate their biological effects via interactions with receptors or intracellular effectors, or can be re-esterified into lipids. In addition, small amounts of PUFAs esterified to phospholipid or cholesterol can be converted into oxylipins in situ (8, 9).
PUFA metabolism into oxylipins occurs by 3 main pathways, which are briefly described below. For more details on specific oxylipin generating enzymes, oxylipin receptors, and breakdown products of oxylipins, there are several excellent reviews (10–21).
Cyclooxygenase
The first oxylipin generation pathway involves cyclooxygenase (COX) enzymes, which convert PUFAs into prostanoids, i.e., PGs and thromboxanes (10–12). Prostanoids have one or more double bonds and a characteristic five-carbon ring structure at the 8- to 12-carbon positions of 20-carbon PUFA-derived oxylipins. COX converts DGLA, AA, EPA, and AdA into 1-, 2-, 3- and dihomo-2-series prostanoids, such as prostaglandin D1 (PGD1), PGD2, PGD3, and dihomo-PGD2, respectively (22, 23). After the prostanoids are produced and released, they mediate their effects via binding to G protein–coupled receptors on the surface of cells, or other intracellular effectors, such as PPARγ (10, 12). The number of double bonds and the type of ring structure of a prostanoid determines its receptor specificity. There are 5 classes of prostanoid receptors, including receptors for PGD, PGE, PGI, PGF, and thromboxane A. Each of these receptors can have several isoforms, which may themselves have differing effects. They are characterized by their most potent biological ligand, but there is also some ligand crossreactivity with these receptors (12). In addition to the prostanoids, COX also can produce select hydroxy FAs [e.g., 11-hydroxy-eicosatetraenoic acid (11-HETE) from AA, 13-hydroxy-docosahexaenoic acid (13-HDoHE) from DHA, and 9-hydroxy-octadecadienoic acid (9-HODE) from LA] (24–27).
Lipoxygenase
The second pathway of oxylipin formation involves lipoxygenases (LOXs) that catalyze the formation of hydroxy FAs and their metabolites (including leukotrienes, lipoxins, resolvins, protectins, maresins, hepoxilins, and eoxins). There are multiple LOX enzymes that have traditionally been classified by the position of the hydroperoxy and hydroxy FAs they form from AA [e.g., 5-hydroperoxy-eicosatetraenoic acid (5-HpETE) and 5-HETE are formed from AA by 5-LOX activity]. This nomenclature has limitations because the position is different with PUFAs of differing chain length, some enzymes act at multiple positions, and there can be differences in the positional specificities of the same homolog in different species (11, 15). An alternative nomenclature is to use the gene names to describe the LOX enzymes (15).
Hydroxy FAs (e.g., 5-HETE) produced via LOX are further metabolized to their keto [(e.g., oxo-eicosatetraenoic acid (oxo-ETE)] or dihydroxy derivatives [e.g., 5,15-dihydroxy-eicosatetraenoic acid (5,15-DiHETE)]. 5-LOX activated by 5-lipoxygenase activating protein (FLAP) results in the production of leukotrienes, including leukotriene B4 and those previously known as the slow reacting substance of anaphylaxis, the cysteinyl leukotrienes (19). Combinations of sequential LOX activities (and sometimes including epoxygenase and hydrolase activities) results in the formation of di- and tri-hydroxy FAs, which includes the lipoxins, resolvins, protectins, and maresins (14, 16). Hepoxilins also are formed from 12-HpETE (21) and eoxins from 15-HpETE (28). As with prostanoids, the LOX-derived oxylipins also appear to mediate their effects by binding to G protein–coupled receptors and intracellular effectors, although receptors for all oxylipins have not been identified.
Cytochrome P450
The third pathway of PUFA metabolism to oxylipins involves a diverse array of membrane-bound cytochrome P450 (CYP) enzymes that are so named because of their unique absorbance at 450 nm when reduced and bound by carbon monoxide. Originally known for their roles in xenobiotic metabolism, there are over 50 CYP enzymes expressed in humans, divided into multiple families and subfamilies based on amino acid identity (11). CYP enzymes that form oxylipins can have epoxygenase or ω-hydroxylase activity. For example, they can convert AA, EPA, and DHA into epoxy-eicosatrienoic acid (EpETrE), epoxy-eicosatetraenoic acid (EpETE), and epoxy-docosapentaenoic acid [(EpDPE), sometimes abbreviated EDP] respectively, via epoxygenase, and HETE, hydroxy-eicosapentaenoic acid (HEPE), and HDoHE, respectively, via ω-hydroxylase activity. Epoxygenase products are rapidly metabolized via soluble epoxide hydrolase (sEH) to form dihydroxy FAs such as the AA, EPA, and DHA metabolites dihydroxy-eicosatrienoic acid (DiHETrE), DiHETE, and dihydroxy-docosapentaenoic acid, respectively. Similar to oxylipins formed via the other pathways, these oxylipins also mediate their effects via specific receptors or by crossreacting with other oxylipin receptors (11, 13, 17, 18). In addition, they may also enter cells and mediate effects intracellularly by modulating transcription factors and ion channels (13).
PUFA Substrates for Oxylipin Formation
Oxylipins are formed from a number of n–3 and n–6 PUFA precursors, such as the n–6 PUFAs AA, LA, γ-linolenic acid (GLA), DGLA, and AdA, and the n–3 PUFAs ALA, stearidonic acid, EPA, and DHA. Although studies indicate that cPLA2 exhibits preference for AA and EPA (29, 30), the presence of oxylipins from other PUFAs demonstrates that they can be released in sufficient quantities for oxylipin production. Pathways are shown in the figures and are described by the PUFA precursors below.
N–6 PUFAs
Arachidonic acid.
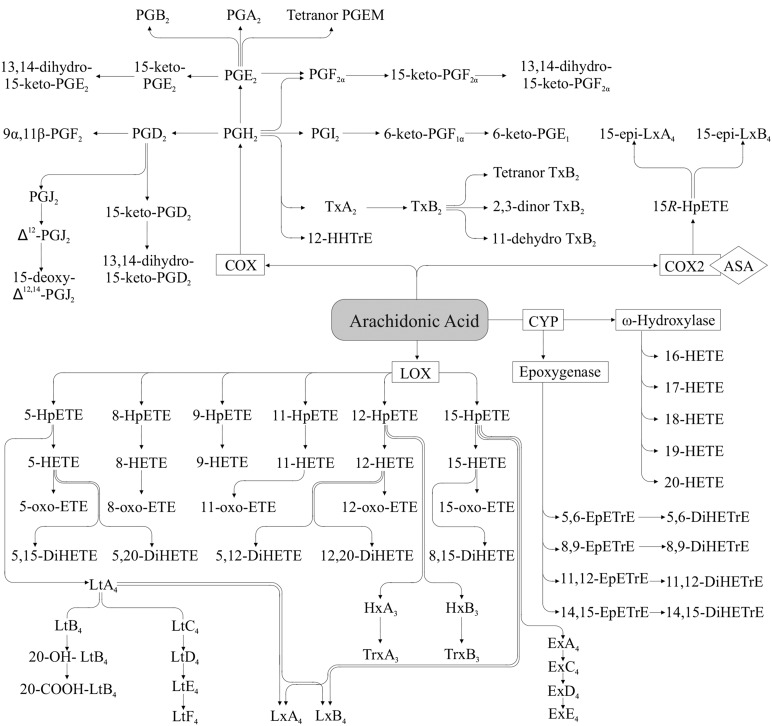
AA produces 2-series oxylipins (Figure 1) via the COX pathway, initially resulting in the formation of PGG2 and subsequently to PGH2, which is then rapidly converted to other PGs (e.g., PGF2α) and thromboxanes (e.g., thromboxane A2) via specific PG and thromboxane synthases (20). As is the case with the other oxylipins, prostanoids are then rapidly degraded to numerous inactive and active metabolites, some of which can be used as markers of the parent compound, whereas others can mediate the same or opposite effects ascribed to the parent compounds (31–33).
FIGURE 1.
Arachidonic acid–derived oxylipins. There is also evidence for thromboxane synthase–independent production of HHTrE (416). 11-HETE and 15-HETE are also produced via the COX pathway (24, 25). ASA, acetylsalicylic acid; COX, cyclooxygenase; CYP, cytochrome P450; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpETrE, epoxy-eicosatrienoic acid; Ex, eoxin; HETE, hydroxy-eicosatetraenoic acid; HHTrE, hydroxy-heptadecatrienoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; Hx, hepoxilin; LOX, lipoxygenase; Lt, Leukotriene; Lx, lipoxin; oxo-ETE, oxo-eicosatetraenoic acid; PGEM, prostaglandin E metabolite; Trx, trioxilin; Tx, thromboxane.
AA also produces oxylipins via the LOX pathway, resulting in HpETEs, (e.g., 12-HpETE), which are further rapidly converted to hydroxy FAs via glutathione peroxidase (34). 5-, 12-, and 15-HETE are the most commonly described HETEs in mammals, although 8-, 9-, and 11-HETE also are produced, and sometimes in greater amounts (35, 36). The 11- or 15-HETE isomers also can be produced via COX activity, as indicated above (24, 25). HETE can be further converted to oxo-ETE via dehydrogenase activity (37, 38), or to DiHETE via further COX (e.g., 5,11-DiHETE), LOX (e.g., 5,15-DiHETE), or CYP ω-hydroxylase (e.g., 5,20-DiHETE) activity (39, 40). In addition, the HpETE formed via LOX can be metabolized via several other routes: 5-HpETE can be further converted to 4-series leukotrienes (e.g., leukotriene C4) via 5-LOX after activation by FLAP; 12-HpETE can be isomerized to hepoxilins (e.g., hepoxilin B3) and subsequently converted to trioxilins (e.g., trioxilin B3) (21, 41); and 15-HpETE can be converted to eoxins (e.g., eoxin C4) (28). Moreover, lipoxins (e.g., lipoxin A4) can be formed from 5- or 15-HpETE via further LOX activity (42–44). Epi-lipoxin (e.g., 15-epi-lipoxin A4) formation can also be initiated by aspirin-acetylated or nitrosylated COX2 and 5-LOX (45–47). AA also can be converted nonenzymatically to HETE (48) and isoprostanes (e.g., iso-PGF2α) (49). The latter are often used as a marker of oxidative stress in vivo; for further discussion of these nonenzymatic oxylipins, see the review by Musiek et al. (49).
AA metabolism via CYP ω-hydroxylase activity results in the formation of HETE with the hydroxy group being at the omega or methyl end of the FA (e.g., 20-HETE), whereas CYP epoxygenase activity yields epoxy FAs (e.g., 14,15-EpETrE), which can be converted to dihydroxy FAs (e.g., 14,15-DiHETE) via sEH activity, as reviewed in several articles (13, 17, 18). Formation of other HETEs (e.g., 13-HETE) may be mediated via CYP bisallylic hydroxylase activity (50–52), but the importance of this pathway is less known.
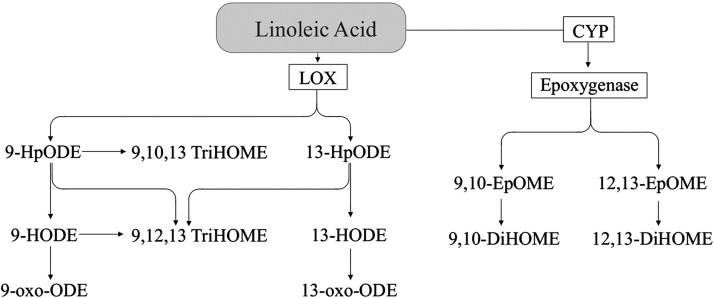
Linoleic acid.
Although the size of the literature for LA oxylipins (Figure 2) is markedly smaller than that for most other oxylipins (especially AA oxylipins), LA oxylipins are usually present in tissues and blood in higher amounts than oxylipins derived from any other PUFA (53–55). LA produces oxylipins through the LOX pathway, resulting in hydroperoxy FAs, which are rapidly converted to hydroxy FAs (e.g., 13-HODE), which can be further metabolized to keto FAs (e.g., 13‑oxo-octadecadienoic acid) (56, 57). LA also can be metabolized via the epoxygenase activity of CYP, resulting in epoxygenated FAs [e.g., 9,10-epoxy-octadecenic acid (9,10-EpOME)], which are metabolized via sEH activity to form dihydroxy FAs (e.g., 9,10-dihydroxy-octadecenoic acid) (58). Further, LA can be converted to trihydroxy FAs (e.g., 9,10,13-trihydroxy-octadecenoic acid) potentially by sequential metabolism of LOX and epoxygenase activity and/or auto-oxidation (59). Several other LA oxylipins also can be produced nonenzymatically (e.g., 9-HODE) (60). There also are reports that the formation of a small amount of the LA oxylipins may be mediated via COX (e.g., 9-HODE) (27, 61) or CYP bisallylic hydroxylation (e.g., 17-HODE) (50–52) activity; the relative importance of these pathways remains to be elucidated.
FIGURE 2.
Linoleic acid–derived oxylipins. 9-HODE and 13-HODE are also produced via the COX pathway (27, 61). COX, cyclooxygenase; CYP, cytochrome P450; DiHOME, dihydroxy-octadecenoic acid; EpOME, epoxy-octadecenoic acid; HODE, hydroxy-octadecadienoic acid; HpODE, hydroperoxy-octadecadienoic acid; LOX, lipoxygenase; oxo-ODE, oxo-octadecadienoic acid; TriHOME, trihydroxy-octadecenoic acid.
γ-Linolenic acid.
GLA can be converted via LOX to 10- and 13-hydroxy-octadecatrienoic acid(γ) [13-HOTrE(γ)] (62) in human platelets and via CYP to γ-6,7-, γ-9,10-, and γ-12,13-epoxy-octadecadienoic acid by human CYP enzymes in vitro (63). Other oxylipins derived from GLA (e.g., 6-HOTrEγ) have been reported to be synthesized in vitro in a patent application (64). Note that oxylipins derived from GLA are distinguished from ALA oxylipins with the use of the γ notation.
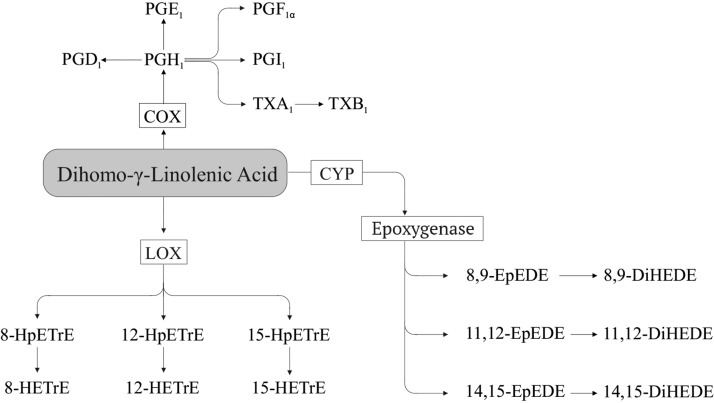
Dihomo-γ-linolenic acid.
DGLA (Figure 3) can be converted via COX to 1-series PGs (e.g., PGI1) and thromboxanes (e.g., thromboxane A1) (22, 65, 66) via LOX to yield hydroperoxy (e.g., 15-hydroperoxy-eicosatrienoic acid) and hydroxy FAs [e.g., 15-hydroxy-eicosatrienoic acid (15-HETrE)] (67–72), and via CYP epoxygenase and sEH to epoxy-eicosadienoic acid (e.g., 8,9-epoxy-eicosadienoic acid) and dihydroxy-eicosadienoic acid (e.g., 8,9- dihydroxy-eicosadienoic acid) (68, 69, 73).
FIGURE 3.
Dihomo-γ-linolenic acid–derived oxylipins. COX, cyclooxygenase; CYP, cytochrome P450; DiHEDE, dihydroxy-eicosadienoic acid; EpEDE, epoxy-eicosadienoic acid; HETrE, hydroxy-eicosatrienoic acid; HpETrE, hydroperoxy-eicosatrienoic acid; LOX, lipoxygenase; Tx, thromboxane.
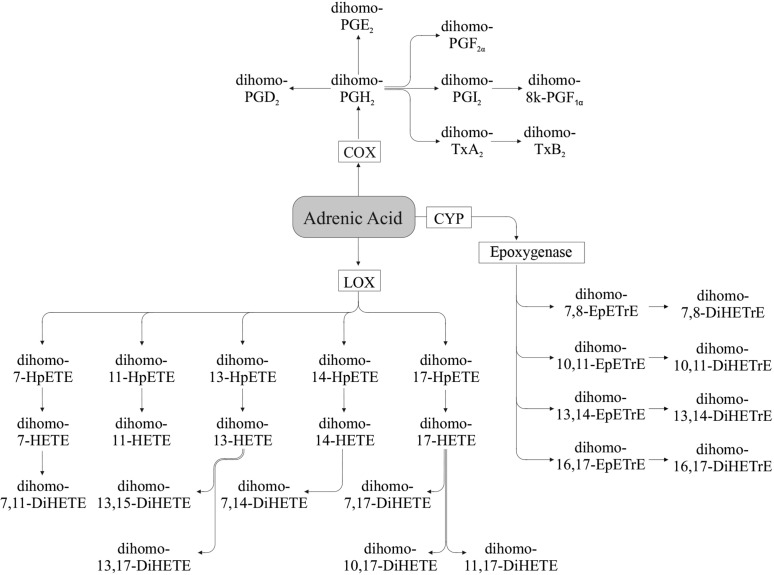
Adrenic acid.
AdA (Figure 4) can be metabolized by COX into dihomo-prostaglandins such as dihomo-PGE2, dihomo-thromboxane B2, and dihomo-PGI2 (74–79). Metabolism via the LOX pathway generates hydroxy-docosatetraenoic acids (also referred to as dihomo-HETE) such as 17-hydroxy-docosatetraenoic acid (dihomo-17-HETE), which can be further converted to dihydroxy compounds (e.g., dihomo-10,17-DiHETE) (76–78), and via the CYP pathway to dihomo-EpETrE (epoxy-docosatrienoic acids) such as dihomo-16,17-EpETrE, which can be further converted to their respective dihydroxy compounds e.g., (dihomo-16,17-DiHETrE) (76).
FIGURE 4.
Adrenic acid–derived oxylipins. Dihomo-7,14-DiHETE and dihomo-7,17-DiHETE also can be formed from dihomo-7-HETE (76). COX, cyclooxygenase; CYP, cytochrome P450; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpETrE, epoxy-eicosatrienoic acid; HETE, hydroxy-eicosatetraenoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; LOX, lipoxygenase; Tx, thromboxane.
n–3 PUFAs
α-Linolenic acid.
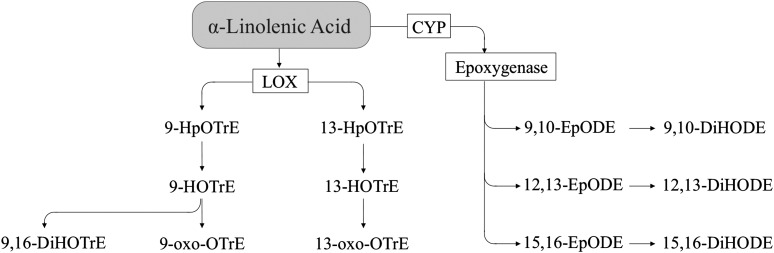
ALA produces oxylipins (Figure 5) via the LOX pathway, resulting in hydroxy FAs (e.g., 9-HOTrE), which can be further metabolized to keto FAs (e.g., 9-oxo-octadecatrienoic acid) (80). As with LA, there are reports that indicate that HOTrE may be formed via COX activity, but the importance of this pathway in vivo remains to be determined (27). ALA also can be metabolized via CYP epoxygenase activity, resulting in epoxygenated FAs, (e.g., 12,13-epoxy-octadecadienoic acid) (63), which can be further converted to dihydroxy FAs (e.g., 12,13-dihydroxy-octadecadienoic acid) via sEH activity (54). Other ALA metabolites that have been reported include 18-HOTrE from ALA via CYP activity (18), 9,16-dihydroxy-octadecatrienoic acid via LOX activity (80), and 12-HOTrE via COX2 activity (27).
FIGURE 5.
α-Linolenic acid–derived oxylipins. CYP, cytochrome P450; DiHODE, dihydroxy-octadecadienoic acid; DiHOTrE, dihydroxy-octadecatrienoic acid; EpODE, epoxy-octadecadienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HpOTrE, hydroperoxy-octadecatrienoic acid; LOX, lipoxygenase; oxo-OTrE, oxo-octadecatrienoic acid.
Stearidonic acid.
Oxylipins derived from stearidonic acid (e.g., 13-hydroxy-octadecatetraenoic acid) have been reported to be produced in vitro in a patent application (64).
Eicosapentaenoic acid.
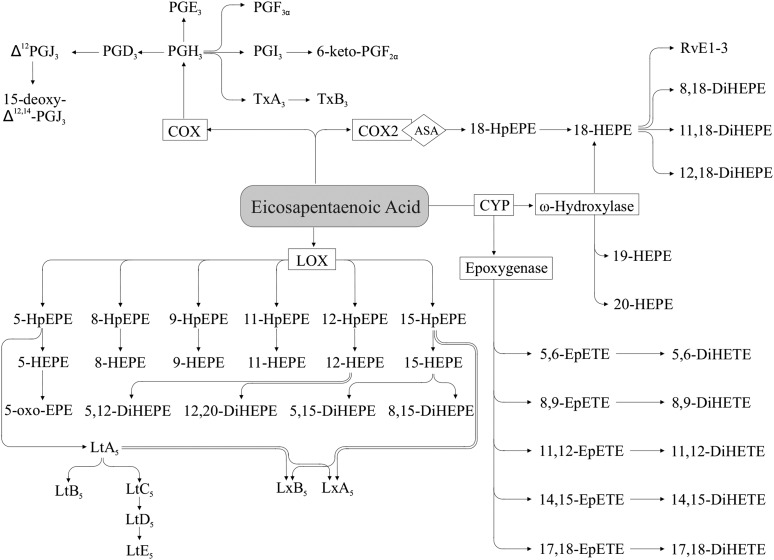
Similarly to AA, EPA produces oxylipins (Figure 6) via the COX pathway, yielding 3-series PGs (e.g., PGE3) and thromboxanes (e.g., thromboxane A3) (23). Compared with AA, EPA is generally a poorer substrate for COX, particularly for the COX1 isoform (81). EPA can produce hydroperoxy FAs (e.g., 5-hydroperoxy-eicosapentaenoic acid), which can be further converted to hydroxy FAs (e.g., 5-HEPE) by LOX activity (23, 82, 83), and 5-series leukotrienes (e.g., leukotriene B5) via combined 5-LOX and FLAP activity (83, 84). HEPE such as 5-HEPE also can be metabolized to dihydroxy-eicosapentaenoic acids such as 5,12-dihydroxy-eicosapentaenoic acid (85) or to keto FAs such as 5-oxo-eicosapentaenoic acid (86). Metabolites of other HEPE isomers are likely to be present, but few have been identified. Hydroxy FAs from EPA with hydroxy groups on the 18–20-carbon positions also are formed via ω-hydroxylase activity of the CYP pathway (e.g., 18-HEPE) (87, 89). The 18-HEPE formed via this pathway (as well as by acetylated COX2) can be further converted to the E-series resolvins (e.g., resolvin E1) via 5-LOX activity (40, 43, 89). EPA can also produce epoxy FAs (e.g., 14,15-EpETE) via CYP epoxygenase activity (90), which can be further converted to dihydroxy FAs (e.g., 14,15-DiHETE) by sEH (91). As with AA and LA, bisallylic hydroxylation of EPA can also yield HEPEs, such as 10-HEPE (92).
FIGURE 6.
EPA-derived oxylipins. ASA, acetylsalicylic acid; COX, cyclooxygenase; CYP, cytochrome P450; DiHEPE, dihydroxy-eicosapentaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; EpETE, epoxy-eicosatetraenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HpEPE, hydroperoxy-eicosapentaenoic acid; LOX, lipoxygenase; Lt, Leukotriene; Lx, lipoxin; oxo-EPE, oxo-eicosapentaenoic acid; Rv, resolvin; Tx, thromboxane.
Docosahexaenoic acid.
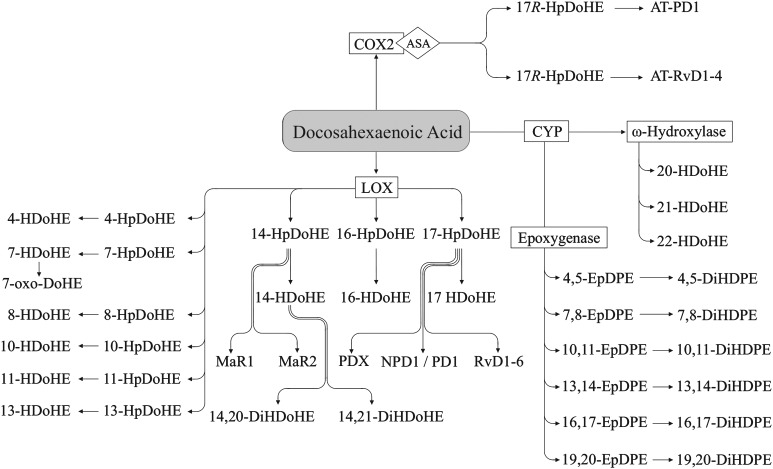
DHA (Figure 7) can be metabolized via the LOX pathway to hydroxy FAs (e.g., 4-HDoHE) with a hydroperoxy intermediate [e.g., 4-hydroperoxy-docosahexaenoic acid (4-HpDoHE) (93)]. 14-HpDoHE can be further metabolized to form maresins (e.g., maresin 1) (94), and 17-HpDoHE can be metabolized to 17-HDoHE, or to resolvins (e.g., resolvin D1) and protectins (e.g., protectin D1) via further LOX and epoxygenation steps. Protectin D1 is produced via LOX, epoxide formation from the hydroperoxide product, and epoxide hydrolase activity (95) while protectin DX is formed via double LOX activity (96). 17-HpDoHE derived from DHA also can be produced via aspirin-acetylated COX2, yielding the aspirin-triggered resolvins (e.g., aspirin-triggered resolvin D1) and aspirin-triggered protectins (e.g., aspirin-triggered protectin D1) (26, 97, 98). DHA also has been shown to yield hydroxy FAs nonenzymatically (e.g., 8-HDoHE) (99, 100), and 13-HDoHE can be formed via COX2 (26). Recent studies provide evidence that HDoHE also can be metabolized to dihydroxy-docosahexaenoic acid (DiHDoHE) (e.g., 14,20-DiHDoHE) (101) and keto FAs (e.g., 7-oxo-docosahexaenoic acid) (102), with more likely to be demonstrated in the future. Oxylipins can be produced from DHA via CYP epoxygenase activity, yielding epoxy FAs (e.g., 16,17-EpDPE) (90, 93), which can be converted to dihydroxy FAs (16,17-dihydroxy-docosapentaenoic acid) via sEH (91). CYP ω-hydroxylase activity produces HDoHE with hydroxy groups near the methyl end of DHA (e.g., 21-HDoHE) (93).
FIGURE 7.
DHA-derived oxylipins. 13-HDoHE also is produced via the COX pathway (26). 14,21-DiHDoHE also may be formed from 21-HDoHE (313, 314). ASA, acetylsalicylic acid; AT, aspirin-triggered; COX, cyclooxygenase; CYP, cytochrome P450; DiHDoHE, dihydroxy-docosahexaenoic acid; DiHDPE, dihydroxy-docosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HpDoHE, hydroperoxy-docosahexaenoic acid; LOX, lipoxygenase; MaR, maresin; oxo-DoHE, oxo-docosahexaenoic acid; PD, protectin; Rv, resolvin.
Oxylipin Functions
Oxylipins have a wide range of functions, many of which are still being elucidated. In addition, oxylipins derived from different pathways, as well as different substrate PUFAs, can have similar or opposing effects, necessitating knowledge of the overall oxylipin profile in order to understand their overall biological effects. Their functions are many, including apoptosis, tissue repair, blood clotting, cell proliferation, blood vessel permeability, pain, inflammation, immune actions, and blood pressure regulation (11, 87). General functions of oxylipins are described below and examples of functions are provided in Tables 1–7.
TABLE 1.
Examples of arachidonic acid–derived oxylipin functions1
| Arachidonic acid–derived oxylipin functions | |
| COX oxylipins | |
| PGA2 | Contributes along with PGE2 to the development of Th1-type immune responses, with PGE2 being more potent in human monocyte–derived dendritic cells (103) |
| Inhibits Ca2+-stimulated ATPase activity of Walker-256 tumor microsomal membranes (104) | |
| Represses insulin-like growth factor I gene expression in C6 rat glioma cells (105) | |
| PGB2 | Mediates mesenteric vascular dose-dependent vasodilatory and vasoconstrictory effects in animal models (106) |
| Elevates blood pressure, tracheal segment pressure, and bronchial resistance in guinea pigs (107) | |
| PGD2 | Inhibits induced apoptosis in human articular chondrocytes (108) |
| Inhibits murine lung inflammation (109) | |
| Promotes sleeping behavior (110) | |
| Regulates body temperature in rodent models (111, 112) | |
| Inhibits tumor cell proliferation in human cells and rodent model (113) | |
| Modulates synaptic transmission via D-type prostanoid receptor (116) | |
| Proinflammatory at nanomolar concentrations and anti-inflammatory at micromolar concentrations [reviewed in (34)] | |
| Inhibits human neutrophil activation in vitro (115, 116) | |
| Causes apoptosis of human eosinophils (117) | |
| Activates human eosinophils (118) | |
| Inhibits human platelet aggregation (119, 120) | |
| PGE2 | Vasodilates cat cerebral arterioles (121) |
| Potentiates human platelet aggregation at lower concentrations and inhibits aggregation at a higher concentrations (122) | |
| Induces human colon cancer cell growth (123) | |
| Stimulates IL-10 production in bone marrow–derived dendritic cells in murine model (124) | |
| Mediates lung inflammation in human cells (125) | |
| 15-keto-PGE2 | Activates PPARγ to enhance adipogenesis of murine 3T3-L1 cells (126) |
| 6-keto-PGF1α | Stable degradation product of PGI2 and useful marker of PGI2 in humans (127, 128) |
| 9α,11β-PGF2 | Activates murine eosinophils (129) |
| PGF2α | Mediates inflammatory tachycardia in the mouse (130) |
| Initiates parturition in the mouse (131) | |
| Vasoconstricts rat brain arterioles (132) | |
| 13,14-dihydro-15-keto-PGF2α | Reflects in vitro PGF2α biosynthesis and is the main inactive degradation product of PGF2α in humans (133) |
| PGI2 | Inhibits ADP-induced hamster platelet aggregation (134) |
| Induces coronary vasodilation in dogs (135) | |
| Inhibits adhesion of human eosinophils to lung endothelial monolayers and transendothelial migration (136) | |
| Inhibits erythrocyte adhesion to bovine aortic endothelial cells (137) | |
| PGJ2 | Causes apoptosis of human eosinophils (117) |
| Induces respiratory burst in human eosinophils (118) | |
| Δ12-PGJ2 | Releases eosinophils from guinea pig bone marrow and induces respiratory burst in human eosinophils (118) |
| Causes apoptosis of human eosinophils and neutrophils (117) | |
| 15-deoxy- Δ12,14-PGJ2 | Inhibits induced apoptosis in human articular chondrocytes (108) |
| Anti-inflammatory via inhibition of NF-κB activation in human and monkey cell culture (138) | |
| Causes apoptosis of human eosinophils and neutrophils (117) | |
| Induces respiratory burst in human eosinophils (118) | |
| Reduces apoptosis in activated human and murine T-lymphocytes (139) | |
| TxA2 | Mediates inflammatory tachycardia in the mouse (130) |
| Causes irreversible platelet aggregation in human platelet-rich plasma (140) | |
| Stimulates mitogenesis of coronary artery smooth muscle cells in guinea pig model (141) | |
| Mediates hypertension in hypertensive rats (142) | |
| Vasoconstricts rabbit aorta (143) | |
| TxB2 | Has a weak bronchoactive effect in guinea pigs and dogs (144) |
| Increases systemic vascular resistance but does not cause platelet aggregation in dogs (145) | |
| Chemotactic in human peripheral PMN (146) | |
| 2,3-dinor-TxB2 | Marker of thromboxane synthesis in urine of rats (147, 148) |
| Possible urinary marker of acute myocardial infarction in humans (149) | |
| Urinary marker for platelet activation (152) | |
| 11-dehydro- TxB2 | Plasma and urinary marker of thromboxane synthesis in human and rabbit models (35, 153, 154) |
| Possible urinary marker of acute myocardial infarction in humans (149) | |
| LOX oxylipins | |
| 5,15-DiHETE | Possesses weak human neutrophil and eosinophil chemotactic activity (153, 154) |
| 8,15-DiHETE | Possesses weak human eosinophil chemotactic activity (153) |
| Exhibits chemotactic activity comparable to that of LtB4 for human PMN (155) | |
| 12,20-DiHETE | Activates cholesterol ester hydrolysis in human vasculature (156) |
| Eoxins | Eoxin C4, D4 and E4 all increase permeability of endothelial cell monolayer from human eosinophils and mast cells in vitro (28) |
| 5-HETE | Inhibits the clonal proliferation of chick embryo fibroblasts and granulocytic progenitors (157) |
| Stimulates human eosinophil chemotaxis and chemokinesis (158) | |
| Stimulates human neutrophil chemokinesis and enhances chemotactic responses (159, 160) | |
| Induces human neutrophil degranulation (161) | |
| Inhibits PGI2 production in porcine coronary artery endothelial cells (162) | |
| Inhibit selenium-induced apoptosis in human prostate cancer cells; 12- and 15-HETE have no effect (163) | |
| Stimulates proliferation of human cancer cells at low concentrations (164) | |
| Promotes bovine neutrophil chemotaxis in vitro more potently than 5-HEPE (165) | |
| 5-HpETE | Inhibits human platelet aggregation similarly to 5-HpEPE, but less potently than 12- or 15-HpETE (166) |
| 5-oxo-ETE | Stimulates human neutrophils and eosinophils (86, 167) |
| Inhibits selenium-induced apoptosis in human prostate cancer cells, with one-half the potency of 5-HETE (163) | |
| Stimulates proliferation of human cancer cells in low concentrations and inhibits proliferation at higher concentrations (164) | |
| Promotes chemotaxis and raises cytosolic calcium concentrations in human neutrophils; more potent than 5-HETE, 15-oxo-ETE, and 5,15-DiHETE (154) | |
| Stimulates human neutrophils more potently than 5-HETE (168) | |
| Does not inhibit LOX enzyme activity (compared to 12- and 15-oxo-ETE) in vitro (169) | |
| 8-HETE | Stimulates human neutrophil chemokinesis and enhances chemotactic responses (159) |
| Promotes wound healing via epithelial cell migration in rat cornea (36) | |
| Induces differentiation of murine 3T3-L1 preadipocytes (170) | |
| 9-HETE | Stimulates human eosinophil chemotaxis and chemokinesis (158) |
| Stimulates human neutrophil chemokinesis and enhances chemotactic responses (159) | |
| 11-HETE | Stimulates human eosinophil chemotaxis and chemokinesis (158) |
| Stimulates human neutrophil chemokinesis and enhances chemotactic responses (159, 160) | |
| Inhibits human vascular smooth muscle cell proliferation (171) | |
| 11-oxo-ETE | Inhibits human colorectal adenocarcinoma epithelial and umbilical vein endothelial cell proliferation in culture (172) |
| 12-HETE | Stimulates human neutrophil chemokinesis and enhances chemotactic responses (159) |
| Induces human neutrophil degranulation (161) | |
| Increases rat heart mitochondrial calcium and nitric oxide, leading to oxidative stress and apoptosis (173) | |
| Increases monocyte adhesion to human endothelial cells leading to aortic fatty streak formation (174, 175) | |
| Enhances tumor cell adhesion to endothelial cells in mice (176) | |
| Enhances thrombin-induced aggregation (177), but suppresses collagen-induced aggregation of bovine platelets (178) | |
| Inhibits U-46619–induced aggregation of human platelets (179, 180) | |
| Reduces ADP-induced aggregation of mouse platelets (181) | |
| Stimulates erythrocyte adhesion to bovine aortic endothelial cells (137) | |
| 12-HpETE | Inhibits human platelet aggregation similarly to 12-HpEPE, and more potently than 5- or 15-HpETE (166, 182) |
| 12-oxo-ETE | Selectively inhibits LOX enzyme activity in vitro (169) |
| Activates human neutrophils (183) | |
| 15-HETE | Exhibits vasodilation or vasoconstriction in isolated arteries from the guinea pig, rabbit, rat, and human, depending on species and conditions (184) |
| Activates PPARγ in human and PPARβ/δ in mouse (185, 186) | |
| Inhibits human PMN migration across cytokine-activated endothelium in vitro (187) | |
| Inhibits degranulation and superoxide production in stimulated human PMN (188) | |
| Mediates hypoxia-induced rabbit pulmonary hypertension (189) | |
| Enhances thrombin-induced human platelet aggregation (190) | |
| Stimulates erythrocyte adhesion to bovine aortic endothelial cells (137) | |
| 15-HpETE | Exhibits vasodilation or vasoconstriction in isolated arteries from the guinea pig, rabbit, rat, and human, depending on species and conditions (184) |
| Stimulates erythrocyte adhesion to bovine aortic endothelial cells (137) | |
| Induces migration of monocyte-like HL-60 cells across a human endothelial cell monolayer (191) | |
| Induces loss of rat cardiomyocyte membrane integrity (192) | |
| Inhibits human platelet aggregation similarly to 15-HpEPE, but less potently than 12-HpETE (166) | |
| 15-oxo-ETE | Selectively inhibits human LOX enzyme activity in vitro (169) |
| Inhibits human vascular vein endothelial cell proliferation (193) | |
| Prevents apoptosis of rat pulmonary arterial smooth muscle cells (194) | |
| HxA3 | Activates human neutrophils (195) |
| Recruits human PMN to the site of inflammation (196) | |
| Promotes murine 3T3-L1 preadipocyte differentiation (197) | |
| HxB3 | Promotes murine 3T3-L1 preadipocyte differentiation (197) |
| LtB4 | Releases human PMN lysosomal enzymes (198) |
| Induces human PMN chemotaxis and aggregation (199, 200) | |
| Stimulates guinea pig lung strip contraction, but less potently than LtC4 (201) | |
| Promotes chemotaxis of bovine neutrophils more potently than LtB5 (165) | |
| 20-OH-LtB4 | Stimulates human neutrophil migration, but less potently than LtB4 (202) |
| Stimulates guinea pig lung strip contraction, but less potently than LtC4 (201) | |
| 20-COOH-LtB4 | Stimulates human neutrophil migration, but less potently than LtB4 (203) |
| Stimulates guinea pig lung strip contraction, but less potently than LtC4 (201) | |
| LtC4 | Causes guinea pig uterine and lung contractions (203) |
| Stimulates guinea pig lung strip contraction more potently than LtB4 (201) | |
| Mediates human skin inflammation (204) | |
| Increases permeability of endothelial cell monolayers from human eosinophils and mast cells in vitro (28) | |
| Contracts guinea pig lung parenchymal strips and ileal tissues, with similar potency to LtC5 (205) | |
| LtD4 | Enhances responsiveness to histamine in bovine airway smooth muscle (206) |
| Causes guinea pig uterine and lung contraction (203) | |
| Mediates human skin inflammation (204) | |
| Increases permeability of endothelial cell monolayers from human eosinophils and mast cells in vitro (28) | |
| LtE4 | Causes guinea pig uterine and lung contraction (203) |
| Coronary constrictor in the in situ pig heart (207) | |
| LtF4 | Induces bronchoconstriction in the guinea pig, but less actively than LtD4 (208) |
| LxA4 | Inhibits LtB4-induced human PMN activation (209) |
| Stimulates human monocyte migration and adhesion (210) | |
| Inhibits zymosan A–induced peritonitis in mice (211) | |
| Promotes corneal epithelial cell wound healing in mice (212) | |
| Increases renal plasma flow and glomerular filtration rate in the rat (213) | |
| Stimulates phospholipid remodeling without causing aggregation in human neutrophils (214) | |
| Antagonizes LtD4-induced lowering of glomerular filtration rate in the rat (215) | |
| Induces contraction of isolated guinea pig pulmonary smooth muscle (similar to LxA5 and LxB4 effects), and vasorelaxation of rat or guinea pig aortic rings (similar to LxB4) (216) | |
| Inhibits proliferation of human A549 cells, but less potently than 15-epi LxA4, 15-epi LxB4 or LxB4 (45) | |
| LxB4 | Stimulates human monocyte migration and adhesion (210) |
| Decreases renal plasma flow and glomerular filtration rate in the rat (213) | |
| Inhibits zymosan A-induced peritonitis in mice (211) | |
| Stimulates phospholipid remodeling without causing aggregation in human neutrophils (214) | |
| Induces contraction of isolated guinea pig pulmonary smooth muscle (similar to LxA4 and LxA5 effects), and vasorelaxation of rat or guinea pig aortic rings (similar to LxA4) (216) | |
| Inhibits proliferation of human A549 cells, but less potently than 15-epi LxB5 (45) | |
| 15-epi LxA4 | Inhibits leukocyte-endothelium interactions in mice (217) |
| Blocks reactive oxygen species generation in human endothelial cells (218) | |
| Stimulates human monocyte chemotaxis (219) | |
| Inhibits proliferation of human A549 cells, but less potently than 15-epi LxB5 (45) | |
| 15-epi LxB4 | Inhibits proliferation of human A549 cells more potently than 15-epi LxA4 or LxB4 (45) |
| CYP oxylipins | |
| 5,6-DiHETrE | Vasodilates pre-constricted pressurized mouse arteries more potently than its EpETrE isomer (220) |
| Hyperpolarizes rat vascular smooth muscle from rat small coronary arteries by activating BK channels (221) | |
| 8,9-DiHETrE | Vasodilates pre-constricted pressurized mouse arteries more potently than its EpETrE isomer (220) |
| Vasodilates isolated canine coronary arterioles more potently than EpETrE isomers (222) | |
| Hyperpolarizes rat vascular smooth muscle from rat small coronary arteries by activating BK channels (221) | |
| 11,12-DiHETrE | Vasodilates pre-constricted pressurized mouse arteries more potently than its EpETrE isomer (220) |
| Vasodilates isolated canine coronary arterioles more potently than EpETrE isomers (222) | |
| Hyperpolarizes rat vascular smooth muscle from rat small coronary arteries by activating BK channels (221) | |
| Relaxes porcine coronary artery with similar potency as its EpETrE isomer (223) | |
| 14,15-DiHETrE | Vasodilates preconstricted pressurized mouse arteries more potently than its EpETrE isomer (220) |
| Vasodilates isolated canine coronary arterioles more potently than EpETrE isomers (222) | |
| Hyperpolarizes rat vascular smooth muscle from rat small coronary arteries by activating BK channels (221) | |
| Most potent PPARα activator in a monkey COS-7 cell expression system when compared to other DiHETrE and EpETrE isomers (224) | |
| Stimulates metastasis and escape from tumor dormancy in several murine tumor models (225) | |
| 5,6-EpETrE | Vasodilatory effects in intestinal microcirculation in rat model (226) |
| Promotes angiogenesis by stimulating endothelial cell proliferation in vitro and angiogenesis in vivo in murine model (227) | |
| Vasodilates isolated canine coronary arterioles less potently than DiHETrE isomers (222) | |
| Vasodilates pre-constricted pressurized mouse arteries less potently than its DiHETrE isomer (220) | |
| 8,9-EpETrE | Promotes angiogenesis by stimulating endothelial cell proliferation in vitro and angiogenesis in vivo (227) |
| Dilates coronary microvessels with similar potency to other EpETrE isomers as well as EpETE and EpDPE isomers in canine and porcine models (228) | |
| Attenuates cell apoptosis in rat heart myocytes after hypoxia and reoxygenation (229) | |
| Vasodilates isolated canine coronary arterioles less potently than DiHETrE isomers (222) | |
| Vasodilates preconstricted pressurized mouse arteries less potently than its DiHETrE isomer (220) | |
| 11,12-EpETrE | Vasodilatory effects in intestinal microcirculation (226) |
| Dilates coronary microvessels with similar potency to other EpETrE isomers as well as EpETE and EpDPE isomers in canine and porcine models (228) | |
| Inhibits vascular inflammation distinct from its vasodilatory effects by inhibiting NF-κB and inhibitor of κ B kinase in murine model (230) | |
| Attenuates cell apoptosis in rat heart myocytes after hypoxia and reoxygenation (229) | |
| Vasodilates isolated canine coronary arterioles less potently than DiHETrE isomers (222) | |
| Vasodilates preconstricted pressurized mouse arteries less potently than its DiHETrE isomer (220) | |
| Relaxes porcine coronary artery with similar potency as its DiHETrE isomer (223) | |
| Enhances angiogenesis and tumor progression in murine model (231) | |
| 14,15-EpETrE | Dilates coronary microvessels with similar potency to other EpETrE isomers as well as EpETE and EpDPE isomers in canine and porcine model (228) |
| Attenuates cell apoptosis in rat heart myocytes after hypoxia and reoxygenation (229) | |
| Vasodilates U-46619–preconstricted bovine coronary artery rings more potently than 14,15-DiHETrE (232) | |
| Vasodilates isolated canine coronary arterioles less potently than DiHETrE isomers (222) | |
| Vasodilates preconstricted pressurized mouse arteries less potently than its DiHETrE isomer (220) | |
| Antinociceptive effect in thermally produced tail-flick response in rats, whereas other regioisomers were not effective at same dose (233) | |
| Enhances angiogenesis and tumor progression (231) | |
| 16-HETE | Induces vasodilation in isolated rabbit kidney (234) |
| Inhibits human leukocyte activation (235) | |
| Decreases intracranial pressure in a rabbit model of stroke (235) | |
| 17-HETE | Inhibits rabbit proximal tubule ATPase activity, but has no renal vasodilatory activity (234) |
| 18-HETE | Induces vasodilation in isolated rabbit kidney (234) |
| 19-HETE | Reduces pressure in rabbit-perfused kidneys (236) |
| Induces vasodilation in canine renal arteries (237) | |
| Stimulates rat renal Na+/K+-ATPase (238) | |
| 20-HETE | Reduces pressure in rabbit-perfused kidneys (236) |
| Induces vasoconstriction in canine renal arteries (239) and porcine coronary arteries (239) | |
| Stimulates inflammatory cytokine production in human endothelial cells (240) | |
| Stimulates proliferation of rat vascular smooth muscle cells (241) | |
ADP, adenosine diphosphate; ATPase, adenosine triphosphatase; BK, big potassium; COX, cyclooxygenase; CYP, cytochrome P450; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpDPE, epoxy-docosapentaenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpETrE, epoxy-eicosatrienoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HpEPE, hydroperoxy-eicosapentaenoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; Hx, hepoxilin; LOX, lipoxygenase; Lt, leukotriene; Lx, lipoxin; oxo-ETE, oxo-eicosatetraenoic acid; PMN, polymorphonuclear leukocyte; Th, T-helper; Tx, thromboxane.
TABLE 7.
Examples of DHA-derived oxylipin functions1
| DHA-derived oxylipin functions | |
| LOX oxylipins | |
| 14,20-DiHDoHE | Inhibits PMN infiltration in the mouse peritonitis model (101) |
| 14,21-DiHDoHE | Enhances wound healing in murine models (313, 314) |
| 4-HDoHE | Inhibits endothelial cell proliferation and sprouting angiogenesis in mouse model of oxygen-induced retinopathy (315) |
| 7-HDoHE | Activates PPARγ in transfected monkey kidney COS-7 cells (316) |
| 13-HDoHE | Inhibits TNFα-induced cytokine production in human microglial cells (26) |
| 14-HDoHE | Inhibits human platelet aggregation (180) |
| 17S-HDoHE | Vasodilates bovine coronary arterial smooth muscle cells (317) |
| Reduces genotoxic and oxidative damage in murine hepatocyte cells and TNFα release by murine macrophages (316) | |
| 17R-HDoHE | Inhibits hyperalgesia in a rat model of adjuvant-induced arthritis (318) |
| Has anti-inflammatory effects in a mouse model of dextran sulfate sodium-induced colitis (319) | |
| Inhibits TNFα-induced cytokine production in human microglial cells (26) | |
| 17-HDoHE | Decreases LPS-induced TNFα secretion in a murine macrophage cell line (304) |
| Inhibits 5-LOX in rat basophilic leukemia cells (82) | |
| 17-HpDoHE | Displays cytotoxic potency in human neuroblastoma cells (320) |
| MaR1 | Anti-inflammatory in a murine model of acute respiratory distress syndrome (321) |
| Reduces inflammation- and chemotherapy-induced neuropathic pain in mice (322) | |
| Mitigates inflammatory effects of LPS-induced lung injury in mouse model (323) | |
| PD1 | Reduces genotoxic and oxidative damage in murine hepatocyte cells and TNFα release by murine macrophages (316) |
| Promotes murine phagocyte removal during acute inflammation in vitro and in vivo (318) | |
| Decreases leukocyte accumulation in a mouse model of kidney injury (324) | |
| Protects human retinal pigment epithelial cells from apoptosis due to oxidative stress (325) | |
| Promotes mouse corneal epithelial cell wound healing (212) | |
| PDX | Reduces inflammation in murine peritonitis and inhibits human microglial cell cytokine expression in vitro (91) |
| Inhibits collagen-, AA-, and thromboxane-induced human platelet aggregation (326) | |
| Inhibits PMN infiltration in mouse model of ischemic stroke (327) | |
| Decreases reactive oxygen species production and COX activity in human neutrophils (328) | |
| Improves insulin sensitivity by raising muscle IL-6 without affecting adipose tissue inflammation in a murine model (329) | |
| RvD1 | Reduces reactivity and Ca2+ sensitivity in overactive human pulmonary artery smooth muscle cells (330) |
| Improves bacterial clearance and survival of mice with cecal ligation and puncture-induced sepsis (331) | |
| RvD2 | Has anti-inflammatory effects in a mouse model of dextran sulfate sodium–induced colitis (319) |
| Improves bacterial clearance and survival of mice with cecal ligation and puncture-induced sepsis (332) | |
| Inhibits inflammatory pain in mice (333) | |
| Mitigates neutrophil-mediated damage in mouse burn model (334) | |
| RvD3 | Reduces peritonitis and dermal inflammation in murine model (335) |
| RvD5 | Enhances phagocyte containment of Escherichia coli in a mouse model (336) |
| AT-RvD1 | Inhibits hyperalgesia in a rat model of adjuvant-induced arthritis (318) |
| Has anti-inflammatory effects in a mouse model of dextran sulfate sodium-induced colitis (319) | |
| AT-RvD3 | Reduces murine peritonitis and dermal inflammation with activity similar to RvD3 (335) |
| CYP oxylipins | |
| 7,8-, 10,11-, 13,14-, 16,17-, 19,20-DiHDPE | Inhibit human platelet aggregation with moderately lower potency to EpDPE, and do not affect thromboxane synthesis (298) |
| 13,14-, 16,17- DiHDPE | Reduce pain associated with inflammation more potently than EpETrE and EpEPE (91) |
| 13,14-DiHDPE | Markedly reduces potency to dilate porcine coronary arterioles compared with parent compound (339) |
| 7,8-, 10,11-, 13,14-, 16,17-, 19,10-EpDPE | Dilates porcine coronary arterioles (337) |
| Inhibits human platelet aggregation and thromboxane synthesis, with potency similar to other EpETE and EpDPE isomers, and greater potency than EpETrE isomers (298) | |
| 16,17-, 19,20-EpDPE | Inhibits Met-1 tumor angiogenesis and growth in mice (231) |
| 19,20-EpDPE | Decreases human platelet aggregation (299) |
AA, arachidonic acid; AT, aspirin-triggered; COX, cyclooxygenase; CYP, cytochrome P450; DiHDoHE, dihydroxy-docosahexaenoic acid; DiHDPE, dihydroxy-docosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; EpEPE, epoxy-eicosapentaenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpETrE, epoxy-eicosatrienoic acid; HDoHE, hydroxy-docosahexaenoic acid; HpDoHE, hydroperoxy-docosahexaenoic acid; LOX, lipoxygenase; MaR, maresin; PMN, polymorphonuclear leukocyte; Rv, resolvin.
n–6 PUFA oxylipin functions
COX oxylipins.
The most well known oxylipins are eicosanoids derived from the n–6 PUFA AA (Table 1). COX-derived prostanoids are involved in the regulation of blood pressure, reproduction, diuresis, blood platelet aggregation, modulation of the immune and nervous systems, gastric secretions, cancer, inflammation, and the stimulation of smooth muscle contraction, among other effects, as reviewed in several articles (10, 12, 338–340). Within these COX metabolites there can be similar and differing effects on these functions. For example, PGI2 is an antiaggregatory factor for platelets (341), whereas thromboxane A2 serves as a proaggregatory factor (342). Another example is the vasodilatory effect of PGI2 and PGE2, and the vasoconstrictory effect of PGF2α in some vascular beds (135, 343). PGE2 also can have effects on thrombosis that vary depending on the receptor it interacts with. For example, PGE2 can bind either the EP3 receptor, which makes PGE2 a prothrombotic mediator, or EP4, which makes PGE2 an antithrombotic mediator (344). Similarly, PGD2 and its metabolites can be both proinflammatory and be involved in the resolution of inflammation (32). Compared with COX products formed from AA, those derived from DGLA (Table 3) are usually, but not always, less active or produced less efficiently (345). For example, PGE1 is less stimulatory of aortic smooth muscle cell proliferation than PGE2 (346). The AdA metabolites (Table 4) dihomo-PGE2 and dihomo-PGI2 also are inactive or much less active compared with their AA analogs with respect to their platelet aggregating activity and contractile properties in both vascular and nonvascular smooth muscle (77, 347).
TABLE 3.
Examples of dihomo-γ-linolenic acid–derived oxylipin functions1
| Dihomo-γ-linolenic acid–derived oxylipin functions | |
| COX oxylipins | |
| PGD1 | Activates proinflammatory receptor chemoattractant receptor homologous molecule expressed on T helper type 2 cells/D prostanoid receptor in human kidney cells (compared to PGE1) (262) |
| Inhibits human platelet aggregation, but is 1% as potent as PGD2 or PGD3 (119) | |
| PGE1 | Does not activate proinflammatory receptor CRTH2/DP2 in human kidney cells (compared to PGD1) (262) |
| Reduces healing time of lower limb ulcers in human patients (263) | |
| Alleviates neurologic deteriorations of diabetic rats (264) | |
| Vasodilates rat coronary and systemic circulation (265) | |
| Stimulates peripheral blood flow in humans with peripheral arterial disease (266) | |
| Reduces pulmonary hypertension in patients with pulmonary arterial hypertension (267) | |
| Inhibits human platelet aggregation (120, 268) | |
| 13,14-dihydro-PGE1 | Inhibits human platelet aggregation with similar potency to PGE1 (268) |
| LOX oxylipins | |
| 12-HETrE | Enhances delayed-type hypersensitivity in guinea pig model (269) |
| Inhibits human platelet aggregation (270) | |
| 15-HETrE | Inhibits epidermal hyperproliferation in guinea pig skin (67, 271) |
| Inhibits formation of proinflammatory LtB4 in human neutrophils (70) | |
| Inhibits cellular growth and AA metabolism in human prostatic adenocarcinoma cells (272) | |
AA, arachidonic acid; COX, cyclooxygenase; HETrE, hydroxy-eicosatrienoic acid; LOX, lipoxygenase; Lt, leukotriene.
TABLE 4.
Examples of adrenic acid–derived oxylipin functions1
| Adrenic acid–derived oxylipin functions | |
| COX oxylipins | |
| Dihomo-PGE2 | Stimulates cAMP production in rabbit renal medullary interstitial cells more potently than dihomo-PGI2, but 10 times less potently than PGE2 (77) |
| No contractile activity in vascular and nonvascular smooth muscle tissue at levels at which PGE2 had significant activity (77) | |
| Dihomo-PGI2 | Inhibits thrombin-induced human platelet aggregation, but is 1% as potent as PGI2 (75) |
| Stimulates cAMP production in rabbit renal medullary interstitial cells, but 100 times less potently than PGI2 (77) | |
| Dihomo-TxA2 | No contractile activity in rabbit aorta (79) [compared with constrictory effect of TxA2 (143)] |
| CYP oxylipins | |
| Dihomo-7,8-, Dihomo-10,11-, Dihomo-13,14-, and Dihomo-16,17-EpETrE | Induce vasorelaxation in bovine coronary arterial rings (79) |
| Dilate canine and porcine coronary microvessels with similar potency to other dihomo-EpETE isomers, as well as EpETrE and EpEPE isomers (228) | |
| Dihomo-16,17-EpETrE | Causes concentration-related relaxations in preconstricted bovine adrenal cortical arteries (76) |
COX, cyclooxygenase; CYP, cytochrome P450; EpEPE, epoxy-eicosapentaenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpETrE, epoxy-eicosatrienoic acid; Tx, thromboxane.
LOX oxylipins.
LOX products such as 5-, 12-, and 15-HETE derived from AA and secreted by epithelial cells and leukocytes are involved in many chronic diseases such as inflammation, obesity, cardiovascular disease, kidney disease, and cancer (348–352) (Table 1). As is the case with COX metabolites, AA-derived LOX products can have effects that are both similar to and differing from each other, as well as from those derived via the COX and CYP pathways. For example, 12-HETE has been shown to have both pro- and antithrombotic effects (179, 353, 354), whereas thromboxane A2 is prothrombotic (342) and PGI2 is antithrombotic (341). LOX-derived HETEs and their oxo-ETE metabolites appear to be primarily proinflammatory; e.g., 5-HETE has chemotactic roles in polymorphonuclear leukocytes (PMNs) and rabbit alveolar macrophages (162, 355, 356) and stimulates specific granule release from human neutrophils (161). Both 5-oxo-ETE and 12-oxo-ETE also can stimulate eosinophils and neutrophils, but appear to have less activity than their corresponding HETEs (154, 357). 5-HETE can also be further converted to 4-series leukotrienes (e.g., leukotriene C4) that play an important role in inflammation, asthma, and allergies (358). Eoxins formed from 15-HpETE also have proinflammatory effects (28), and hepoxilins and their metabolites (trioxilins) are another group of oxylipins derived from 12-HpETE that are involved in neutrophil migration and intracellular calcium release (195, 196).
It is important to note, however, that some AA-derived oxylipins also display anti-inflammatory and anticancer activity. For example, 15-HETE can inhibit degranulation of PMNs, superoxide production, and endothelial PMN interaction (187, 188). In addition, 15-HETE can be metabolized to lipoxins, which can be synthesized by epithelial cells and leukocytes and modulate response to injury by mediating apoptosis and resolution of inflammation, in addition to decreasing pain, angiogenesis, and cell proliferation (14, 42, 359). Aspirin-triggered lipoxins (e.g., 15-epi-lipoxin A4) are formed via aspirin-acetylated COX2 and 5-LOX and have similar properties to the lipoxins (360, 361).
In addition to AA metabolites, LOX also metabolizes other n–6 PUFAs, including LA, GLA, DGLA and AdA (Tables 2–4). As with AA oxylipins, 9-HODE and 13-HODE derived from LA mostly have been related to pathologic conditions such as atherosclerosis, nonalcoholic steatohepatitis, and Alzheimer disease (362–364), but there are also instances in which HODEs and their oxo-octadecadienoic acid metabolites are anti-inflammatory and antiproliferative (176, 271, 365). Although no functions for GLA oxylipins have been reported, DGLA oxylipins also tend to antagonize the analogous LOX-derived AA oxylipins. For example, PGE1 and 15-HETrE from DGLA have antiproliferative effects, inhibit cancer cell growth, and inhibit bleomycin-induced lung fibrosis (366–368), whereas 15-HETrE has anti-inflammatory effects on skin (271). Three-series leukotrienes derived from DGLA may also reduce inflammation and broncho-constriction because of their relatively lower production compared with 4-series leukotrienes from AA and possibly lower bioactivity (369, 370).
TABLE 2.
Examples of linoleic acid–derived oxylipin functions1
| Linoleic acid–derived oxylipin functions | |
| LOX oxylipins | |
| 9-HODE | Induces endoplasmic reticulum stress in human macrophages (242) |
| Inhibits proliferation and induces apoptosis in human U937 cells (243) | |
| Proinflammatory in skin under oxidative conditions in human (244) | |
| Induces maturation, scavenger receptor expression and activates PPARγ-dependent transcription in human monocytes (245) | |
| Does not inhibit tumor cell adhesion to endothelial cells (compared to 13-HODE) in mice (176) | |
| 9-oxo-ODE | Activates PPARγ-dependent transcription in human monocytes (as do 9-HODE and -HpODE) (245) |
| 13-HODE | Prevents platelets from adhering to human vascular endothelium (246) |
| Decreases thrombin-induced platelet adherence to other platelets and to endothelial cells in vitro (247) | |
| Induces maturation and scavenger receptor expression and activates PPARγ-dependent transcription in human monocytes (245) | |
| Inhibits proliferation of hyperproliferative skin in guinea pigs (248) | |
| Inhibits tumor cell adhesion to endothelial cells (176) | |
| Inhibits the secretion and assembly of TG-rich lipoprotein particles in vitro (249) | |
| Inhibits human neutrophil production of LtB4 in vitro (70) | |
| 13-HpODE | Relaxes canine circumflex and splenic arteries, similarly to 13-HODE (250) |
| Relaxes human pulmonary arteries (184) | |
| 13-oxo-ODE | Reduces inflammation in human colonic epithelial cells (251) |
| Does not inhibit tumor cell adhesion to endothelial cells (compared to 13-HODE) in mice (176) | |
| Does not inhibit LOX enzyme activity (compared to 12- and 15-oxo-ETE) in vitro (169) | |
| Activates PPARγ-dependent transcription in human monocytes (as do 13-HODE and -HpODE) (245) | |
| CYP oxylipins | |
| 9,10-DiHOME | Decreases left ventricular–developed pressure recovery and increases coronary resistance after ischemia/reperfusion in the mouse heart (252) |
| Causes mitochondrial dysfunction, leading to cell death in rabbit renal proximal tubular cells, whereas parent epoxy compound is not toxic (253) | |
| 12,13-DiHOME | Causes mitochondrial dysfunction, leading to cell death in rabbit renal proximal tubular cells, whereas parent epoxy compound is not toxic (253) |
| Causes acute respiratory distress syndrome in mice; more toxic than its epoxy parent (254) | |
| Lacks protective effect of 12,13-EpOME in rabbit renal proximal tubular cells exposed to hypoxia/reoxygenation (255) | |
| 9,10-EpOME | Inhibits mitochondrial respiration in perfused rat lung (256) |
| Relaxes rat stomach smooth muscle and uncouples mitochondrial respiration (257) | |
| Induces canine heart failure when injected intravenously (258) | |
| Inhibits growth of normal and transformed human cells in culture (259) | |
| Induces vasoconstriction in isolated perfused cat carotid arteries (260) | |
| 12,13-EpOME | Pretreatment with low concentrations maintains mitochondrial respiration in rabbit renal proximal tubular cells exposed to hypoxia/reoxygenation; 12,13-DiHOME has no effect (255) |
| Induces vasoconstriction in isolated perfused cat carotid arteries (260) | |
| Induces dysfunction in isolated rabbit renal cortical mitochondria, whereas 12,13-DiHOME does not (261) | |
CYP, cytochrome P450; DiHOME, dihydroxy-octadecenoic acid; EpOME, epoxy-octadecenoic acid; HODE, hydroxy-octadecadienoic acid; HpODE, hydroperoxy-octadecadienoic acid; LOX, lipoxygenase; Lt, leukotriene; oxo-ETE, oxo-eicosatetraenoic acid; oxo-ODE, oxo-octadecadienoic acid.
CYP oxylipins.
Oxylipins derived via the CYP pathway from AA include EpETrE and HETE, which have vascular, cardiac and renal functions (13, 371, 372). The effects of these oxylipins also are unique and can be opposing. For example, AA-derived EpETrEs formed via CYP epoxygenase have hypotensive effects, which is opposite to the hypertensive effects of 20-HETE formed via ω-hydroxylase activity (237, 373). In addition, 16-, 18-, and 19-HETE, as well as 20-HETE metabolites (20-COOH-AA and 20-OH-PGE2), also can promote vasodilation (234, 237, 374, 375). In some cases, the DiHETrE metabolites of EpETrE formed via sEH activity have less activity (232), but in other cases the DiHETrE have similar or even greater potency (220, 222). Interestingly, sEH inhibitors are currently being used to treat hypertension pharmacologically by prolonging the effects of the epoxy FAs on vasodilation (376), but polymorphisms in the CYP enzymes that produce EpETrE do not consistently correlate with effects on hypertension, as reviewed in Bellien and Joannides (377). In addition, EpETrEs also play roles in many other biological functions, such as insulin sensitivity (378), hyperalgesia (91), and tumor angiogenesis and metastasis (225, 231).
CYP oxylipins formed from LA appear to have effects similar to those derived from AA. For example, 9,10- and 12,13-EpOME derived from LA are produced by neutrophils and macrophages, mediating inflammatory effects (379, 380) (Table 2). These oxylipins were originally referred to as leukotoxin and isoleukotoxin, respectively, but later studies indicate that their toxic effects may be due to conversion by sEH to their diol metabolites (381). Elevated EpOME also has been related to extensive burns, respiratory syndrome, and systemic organ failure in burned skin of humans and lung (382).
n–3 PUFA oxylipin functions
In general, but not always, oxylipins formed from n–3 PUFAs have lesser biological potency when compared with those derived from n–6 PUFAs, and often compete for the same receptor, further dampening the biological effect (383). In addition, because they also compete with n–6 PUFAs for the same oxylipin biosynthetic enzymes, they may reduce biological activity by reducing the amount of total and n–6 PUFA–derived oxylipins produced and increasing concentrations of less active n–3 PUFA–derived oxylipins (286, 384).
COX oxylipins.
With respect to COX oxylipins, those derived from EPA are similar to DGLA oxylipins, generally being less potent or produced less efficiently (286) than the analogous oxylipins derived from AA (Table 6). Hence, compared with PGE2, PGE3 binds to the EP4 receptor with less affinity and activity in colorectal cancer cells (383) and demonstrates less mitogenetic and inflammatory activity in fibroblasts and monocytes (280, 383, 385). Compared with thromboxane A2, thromboxin A3 is produced less efficiently and was reported to have less vasoconstrictory and aggregatory activity (286), but a later study has attributed this reduced biological effect to the presence of PGD3 in the incubations and found that thromboxane A2 and thromboxane A3 have similar aggregatory activities (81). PGI3 and PGI2 also have similar vasodilatory and antiaggregatory effects on platelets (286) and thromboxane A2 and thromboxane A3 have a similar ability to elevate plasma catecholamines in rats or to activate the thromboxane receptor (81, 283, 286, 384).
TABLE 6.
Examples of EPA-derived oxylipin functions1
| EPA-derived oxylipin functions | |
| COX oxylipins | |
| 15-deoxy-PGJ3 | Increases adiponectin secretion from murine adipocytes (276) |
| PGD3 | Lowers intraocular pressure in rabbit model (277) |
| Decreases peripheral vascular resistance and increases cardiac output and heart rate in dogs (278) | |
| As potent as PGD2 in modulating sympathetic nerve transmission in the eye but less effective in activating vagally mediated bradycardia in cat model (279) | |
| Inhibits human platelet aggregation with similar or greater activity than PGD2 (119, 120) | |
| PGE3 | Lowers intraocular pressure but caused mild conjunctival hyperemia in rabbit model (277) |
| Compared with PGE2, is not mitogenic to and is less efficient in inducing COX2 gene expression in murine NIH 3T3 fibroblasts, and less efficient in inducing IL-6 synthesis in murine RAW 264.7 macrophages (280) | |
| Inhibits proliferation of human A549 cells (281) and mouse melanoma B16 cells (282) | |
| Less effective than PGE2 in elevating plasma noradrenaline when administered intracerebroventricularly in rats (283) | |
| Less potent stimulator of cAMP production than PGE2 in HEK293 human renal cells (81) | |
| PGF3α | Less protective than PGF2α on ethanol induced gastric mucosal injury in rat model (284) |
| PGI3 | Inhibits aggregation in human and rabbit platelets (285, 286) |
| Promotes relaxation of bovine coronary arteries (286) | |
| Δ12-PGJ3 | Inhibits progression of leukemia in a mouse model (287) |
| TxA3 | Synthesized at a much lower rate than TxA2 in human platelets (286) |
| Elevates catecholamines when administered intracerebroventricularly as potently as TxA2 in rats (283) | |
| Activates human platelet aggregation with potency comparable with TxA2 (81) | |
| LOX oxylipins | |
| 5-HEPE | Enhances glucose-dependent insulin secretion in mouse MIN6 insulinoma cells and human NuTu80 intestinal carcinoma cells (288) |
| Promotes bovine neutrophil chemotaxis in vitro, but less potently than 5-HETE (165) | |
| 5-HpETE | Inhibits human platelet aggregation, but less effectively than 12-HpEPE (166) |
| 5-oxo-EPE | Stimulates migration of both human neutrophils and eosinophils at one-tenth the activity of 5-oxo-ETE (86) |
| 8-HEPE | Induces adipogenesis in mouse preadipocytes and glucose uptake in myoblasts via PPAR activation (2) |
| 9-HEPE | Induces adipogenesis in mouse preadipocytes and glucose uptake in myoblasts via PPAR activation (2) |
| 12-HEPE | Inhibits human platelet aggregation similarly to 12-HETE, but less effectively than 12-HpEPE or 12-HpETE (166) |
| 12-HpEPE | Inhibits human platelet aggregation similarly to 12-HpETE, and more potently than 5- or 15-HpEPE (166, 182) |
| 15-HEPE | Inhibits 5-LOX in rat basophilic leukemia cells (84) |
| Inhibits cellular growth and AA metabolism in human prostatic adenocarcinoma cells (272) | |
| 15-HpEPE | Inhibits human platelet aggregation similarly to 15-HpETE, but less potently than 12-HpEPE (166) |
| Inhibits glucosamine synthetase activity in rabbit gastric mucosa (289) | |
| Decreases rabbit renal PG synthesis (290) | |
| Inhibits AA metabolism in rabbit platelets (291) | |
| LtA5 | Inhibits the formation of LtB4 from LtA4 by rat and human neutrophil LtA4 hydrolase (292) |
| LtB5 | Less active than LtB4 in aggregating rat and human neutrophils (83) |
| Promotes chemotaxis of bovine or human neutrophils, but is much less potent than LtB4 (165, 205) | |
| LtC5 | Contracts guinea pig lung parenchymal strips and ileal tissues with potency similar to LtC4 (205) |
| Inhibits the anaphylactic reaction in guinea pig isolated heart, with potency similar to LtC4 (293) | |
| Contracts guinea pig ileum but less potently than LtC4 (294) | |
| LtD5 | Inhibited IL-1β–induced COX2 expression in human pulmonary microvascular endothelial cells (295) |
| Stimulates volume regulation in murine Ehrlich ascites tumor cells (similar potency as LtD4) (296) | |
| LxA5 | Induces contraction of isolated guinea pig pulmonary smooth muscle (similar to LxA4 and LxB4 effects), but does not induce vasorelaxation of rat or guinea pig aortic rings (unlike LxA4 and LxB4) (216) |
| Induces superoxide anion generation from canine neutrophils and contraction of rat tail arteries (297) | |
| LxB5 | Does not induce contraction of isolated guinea pig pulmonary smooth muscle (unlike LxA5, LxA4, and LxB4) or vasorelaxation of rat or guinea pig aortic rings (unlike LxA4 and LxB4) (216) |
| Induces superoxide anion generation from canine neutrophils (with similar activity to 4-series Lx) (297) | |
| CYP oxylipins | |
| 8,9-, 11,12-, 14,15-, 17,18-DiHETE | Inhibit human platelet aggregation, but with much less potency than parent EpETE (298) |
| 8,9-, 11,12-, 14,15-, 17,18-EpETE | Dilate canine and porcine coronary microvessels with similar potency to other EpETE isomers as well as EpETrE and dihomo-EpETrE isomers (228) |
| Inhibit human platelet aggregation and thromboxane synthesis with potency similar to other EpETE and EDPE isomers and potency greater than EpETrE isomers (298) | |
| 17,18-EpETE | Decreases human platelet aggregation (299) |
| Relaxing effect on human bronchi arterial and airway smooth muscles (300) | |
| Anti-inflammatory effect in human lungs (301) | |
| Vasodilator in rat vascular smooth muscle cells (302) | |
| 18-HEPE | Inhibits macrophage-mediated inflammation in cardiac fibroblasts in culture and prevents pressure overload–induced cardiac fibrosis and inflammation in mice (303) |
| Decreases LPS-induced TNFα secretion in the murine macrophage cell line (304) | |
| RvE1 | Reduces dermal inflammation, peritonitis, dendritic cell migration, and IL-12 production in an inflammatory mouse model (305) |
| Reduces total leukocytes and PMN infiltration in murine peritonitis (306) | |
| Reduces hepatic fibrosis in murine model of infection (307) | |
| Promotes phagocyte removal during acute inflammation in vitro and in vivo (308) | |
| RvE2 | Stops zymogen-induced PMN leukocyte infiltration in murine peritonitis (309) |
| Enhances phagocytosis and anti-inflammatory cytokine production in murine peritonitis (310) | |
| Inhibits human neutrophil infiltration and proinflammatory cytokines in an acute peritonitis (311) | |
| RvE3 | Inhibits neutrophil chemotaxis in vitro and reduces neutrophil numbers in zymosan-induced murine peritonitis in vivo (89) |
| Blocks PMN infiltration in a mouse model of peritonitis (312) | |
AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450; diHETE, dihydroxy-eicosatetraenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpETrE, epoxy-eicosatrienoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HpEPE, hydroperoxy-eicosapentaenoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; LOX, lipoxygenase; Lt, leukotriene; Lx, lipoxin; oxo-EPE, oxo-eicosapentaenoic acid; oxo-ETE, oxo-eicosatetraenoic acid; PMN, polymorphonuclear leukocyte; Rv, resolvin; Tx, thromboxane.
LOX oxylipins.
LOX also metabolizes the n–3 PUFAs, ALA to HOTrE, EPA to HEPE and DHA to HDoHE, oxylipins that also tend to have less inflammatory activity or to be anti-inflammatory (Tables 5–7). There is very little information on ALA-derived oxylipins, but recent findings indicate that 9,16-dihydroxy-octadecatrienoic acid has anti-inflammatory and antiaggregatory effects by reducing PG production (80), and that 9- and 13-HOTrE are associated with reduced glomerular hypertrophy in obese rats (55). An earlier paper indicates that 13-HOTrE may have anti-inflammatory effects in chondrocytes (273), and a recent paper showed that 13-oxo-octadecatrienoic acid can stimulate glucose uptake and differentiation in adipocytes (275). EPA oxylipins have been investigated much more and are primarily anti-inflammatory; for example, 5-hydroperoxy-eicosapentaenoic acid can be metabolized to leukotriene B5, which has less activity and also competes with leukotriene B4 and therefore reduces inflammation and broncho-constriction (386–388). 5-oxo-eicosapentaenoic acid derived from 5-HEPE is 10% as potent in stimulating neutrophils than the AA oxylipin (5-oxo-ETE) derived from 5-HETE (86). 15-HEPE derived from EPA also exhibits anticancer effects. For example, in human prostatic adenocarcinoma cells, 15-HEPE can inhibit cancer cell growth and inhibit production of AA oxylipins (272).
TABLE 5.
Examples of α-linolenic acid–derived oxylipin functions1
| α-Linolenic acid–derived oxylipin functions | |
| COX oxylipins | |
| 9-HOTrE | Associated with glomerular hypertrophy in obese rats (55) |
| 9,16-diHOTrE | Inhibits PG synthesis from COX1 and collagen-induced human platelet aggregation (80) |
| 13-HOTrE | Suppresses IL-1β–induced expression of matrix metalloproteinases in human chondrocytes in vitro (273) |
| Associated with glomerular hypertrophy in obese rats (55) | |
| 13-HpOTrE | Causes moderate and reversible depression in action potential markers in rat cardiomyocytes (274) |
| 13-oxo-OTrE | Induces glucose uptake and promotes adipocyte differentiation in murine model (275) |
| CYP oxylipins | |
| 9,10-DiHODE | Lower in blood of hyperlipidemic vs. normolipidemic persons (54) |
| 12,13-DiHODE | Lower in blood of hyperlipidemic vs. normolipidemic persons (54) |
COX, cyclooxygenase; CYP, cytochrome P450; diHODE, dihydroxy-octadecadienoic acid; diHOTrE, dihydroxy-octadecatrienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HpOTrE, hydroperoxy-octadecatrienoic acid; oxo-OTrE, oxo-octadecatrienoic acid.
DHA also is metabolized via LOX, resulting in the production of HDoHE, which also generally exhibits beneficial effects. For example, 4-HDoHE has been reported to inhibit proliferative retinopathy and retinal endothelial cell proliferation (315) and 14-HDoHE can antagonize platelet activation and smooth muscle constriction (180, 389). The functions of 14-HDoHE may be mediated via maresins, given that they have been shown to be involved in resolution of inflammation, tissue regeneration, and analgesia (94, 390), or via other DiHDoHEs, which have similar protective effects, such as the wound healing properties of 14,21-DiHDoHE in mice (313) and the inhibition of PMN infiltration in a mouse peritonitis model by 14,20-DiHDoHE (101). Similarly, 17-HDoHE inhibits 5-LOX in rat leukemia cells (82), reduces inflammation and oxidative damage in murine hepatocyte injury (316), and has antihyperalgesic properties in a rat model of arthritis (318). Some of these actions may be via the D-series resolvins and protectins derived from 17-HpDoHE. Resolvins have been shown to have protective actions in inflammatory diseases (97, 391, 392), whereas the effects of protectins vary by isomer—protectin DX has antiaggregatory effects (326, 393) and can restore insulin sensitivity in obese mice (329), but protectin D1 does not exhibit these activities (329, 394). Both can inhibit influenza virus replication (395, 396), reduce inflammation, and accelerate the resolution of inflammation (392), with the latter study indicating that protectin D1 has greater potency in this regard. Helpful reviews delineating differences in structure and functions of the protectins can be found in 2 articles (18, 97).
CYP oxylipins.
n–3 PUFA oxylipins derived via the CYP pathway also have some similar and some differing effects compared with their n–6 PUFA–derived counterparts (Tables 5–7). EpETEs derived from EPA have vasodilatory and anti-inflammatory effects (339, 399, 400), which is similar to EpETrE derived from AA, with the vasodilatory effects of EpETE possibly exceeding those of EpETrE in some vascular beds (337, 398). In addition, several CYP isoforms preferentially metabolize n–3 over n–6 PUFAs, as reviewed in 2 articles (87, 399). EpETE can also inhibit Ca2+ and isoproterenol-induced contractility of neonatal cardiomyocytes, suggesting that they have antiarrhythmic effects (400). EpDPE derived from DHA has anti-inflammatory, vasodilatory, and anticancer effects, similar to EpETE (231, 299, 337). EpDPE also can inhibit angiogenesis and metastasis (231), unlike the AA derived EpETrE, which promote these functions (225). 18-HEPE derived from EPA via ω-hydroxylase also appears to have an anticancer role by downregulating proinflammatory and pro-proliferative factors (304), possibly via conversion to E-series resolvins. These resolvins have effects similar to the D-series resolvins, markedly reducing PMN infiltration, decreasing proinflammatory cytokines, and enhancing the resolution of inflammation (359, 401, 402).
In summary, oxylipins have important biological effects that mediate normal physiology and function. However, compared with oxylipins derived from n–3 PUFAs, those derived from n–6 PUFAs have more inflammatory, vasoconstrictory, and proliferative effects, with the exception of several examples, such as some prostanoids and/or their metabolites, lipoxins, some oxylipins from DGLA and LA, EpETrE, and some CYP-derived HETEs. But most oxylipins derived from n–3 PUFAs tend to have less activity or be anti-inflammatory, proresolving, vasodilatory, and antiproliferative. In addition, some of the anti-inflammatory and vasodilatory CYP oxylipins derived from EPA and DHA have even greater potency than their AA counterparts.
Future Developments in Nutrition and Oxylipin Research
Given the vastly differing and often opposing functions, it is critical that comprehensive analyses of the oxylipin profile be performed in order to gain an overall understanding of the biological effects. To date, few studies have examined the whole range of PUFA-derived oxylipins, but the recent development of MS-based methods is enabling this possibility (403). The number of oxylipins being measured by these methods continues to grow (e.g., novel protectin- and maresin-like products from both the n–3 and n–6 docosapentaenoic acid isomers) (18, 97). Recently, several reports have described the oxylipin profile in human blood (53, 404) and a small number of studies have examined the serum oxylipin profile in response to fish oil supplementation in healthy individuals (405–408), as well as in those who have asthma (409). These analyses and other studies that have increased dietary LA or ALA have revealed that the type of dietary fat significantly alters oxylipin profiles (55, 410–412). Furthermore, these studies have demonstrated that the oxylipins derived from LA and ALA make up more than one-half of the total oxylipin content measured. Despite this, much less is known about these oxylipins, and future studies characterizing concentrations, as well as determining their biological activities, will greatly increase our understanding of the effects of nutritional interventions in health and disease.
In this regard, there are some studies that have examined oxylipin activities side-by-side, such as for those derived from EPA or DHA compared with those derived from AA (see Tables 6 and 7), which generally, but not always, exhibit less activity in the former than the latter. However, comparisons of the biopotencies of most of the LA and ALA oxylipins are unknown, either to each other, or to their elongation counterparts. These comparisons and other studies that examine the relative biological activities of oxylipins are needed in order to further our understanding of the physiologic effects of the entire oxylipin profile. In addition, although some studies have compared the effects of oxylipin stereoisomers, much more knowledge in this area also is required. Differentiation between enzyme-mediated and autooxidation products and their potential effects in biology will also be facilitated by these studies.
It is important to note that tissue PUFA composition cannot be used to reliably predict the oxylipin content of tissues, despite the fact that this has routinely been done in the past literature. This was illustrated in a recent targeted lipidomic analysis of renal oxylipins in obese rats, which demonstrated that although the PUFA content generally reflected oxylipin content, there were notable discrepancies. For example, with 9-fold differences in the amounts of LA in the diets of these rats, the AA content of the renal phospholipid was the same, but the concentrations of several AA-derived oxylipins were different (55). This has important implications for the current debate surrounding the dietary recommendations for LA (413). Furthermore, this study indicated that PUFA conversion to oxylipins varies by as much as 10-fold between PUFAs, with ALA being metabolized to oxylipins at a greater rate than LA, AA, or EPA. This may be due to differences in incorporation and release of phospholipid FAs, as well as differences in conversion to metabolites, which may be less, more, or equally active. ALA also increased the concentration of oxylipins derived from EPA and DHA, although no EPA or DHA was present in the diets, demonstrating that PUFAs also may mediate some of their effects via oxylipins derived from PUFAs formed via elongation and desaturation of the shorter PUFAs (55). Hence, there is also a need for kinetic analysis of oxylipin formation and turnover [also referred to as fluxolipidomics (414, 415)], which also will improve our understanding of the physiologic effects of oxylipins in vivo. Comprehensive analyses that include the LA and ALA oxylipins in differing tissues in response to dietary interventions promises to yield significant novel information on the large numbers of these bioactive compounds.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; AdA, adrenic acid; ALA, α-linolenic acid; COX, cyclooxygenase; cPLA2, cytosolic phospholipase A2; CYP, cytochrome P450; DGLA, dihomo-γ-linolenic acid; DiHDoHE, dihydroxy-docosahexaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; EpDPE, epoxy-docosapentaenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpETrE, epoxy-eicosatrienoic acid; EpOME, epoxy-octadecenoic acid; FLAP, 5-lipoxygenase activating protein; GLA, γ-linolenic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HETrE, hydroxy-eicosatrienoic acid; HODE, hydroxy-octadecadienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HpDoHE, hydroperoxy-docosahexaenoic acid; HpETE, hydroperoxy-eicosatetraenoic acid; LA, linoleic acid; LOX, lipoxygenase; oxo-ETE, oxo-eicosatetraenoic acid; PMN, polymorphonuclear leukocyte; sEH, soluble epoxide hydrolase.
References
- 1.Balvers MG, Verhoeckx KC, Bijlsma S, Rubingh CM, Meijerink J, Wortelboer HM, Witkamp RF. Fish oil and inflammatory status alter the n–3 to n–6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics. 2012;8:1130–47. [DOI] [PMC free article] [PubMed]
- 2.Yamada H, Oshiro E, Kikuchi S, Hakozaki M, Takahashi H, Kimura K. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J Lipid Res 2014;55:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat 2014;113–115:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 2011;111:6130–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed KA, Tucker DE, Aloulou A, Adler D, Ghomashchi F, Gelb MH, Leslie CC, Oates JA, Boutaud O. Functional characterization of mutations in inherited human cPLA(2) deficiency. Biochemistry 2011;50:1731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler DH, Cogan JD, Phillips JA 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest 2008;118:2121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lipid Res 2014;55:2471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schewe T, Halangk W, Hiebsch C, Rapoport SM. A lipoxygenase in rabbit reticulocytes which attacks phospholipids and intact mitochondria. FEBS Lett 1975;60:149–52. [DOI] [PubMed] [Google Scholar]
- 9.Belkner J, Wiesner R, Kuhn H, Lankin VZ. The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett 1991;279:110–4. [DOI] [PubMed] [Google Scholar]
- 10.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001;294:1871–5. [DOI] [PubMed] [Google Scholar]
- 11.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res 2009;50:1015–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 2004;36:1187–205. [DOI] [PubMed] [Google Scholar]
- 13.Spector AA, Kim HY. Cytochrome P epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta 2015;1851:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014;40:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 2015;1851:308–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 2015;1851:397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahabi P, Siest G, Meyer UA, Visvikis-Siest S. Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders. Pharmacol Ther 2014;144:134–61. [DOI] [PubMed] [Google Scholar]
- 18.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta 2011;1814:210–22. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 1987;237:1171–6. [DOI] [PubMed] [Google Scholar]
- 20.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 2011;111:5821–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace-Asciak CR. Pathophysiology of the hepoxilins. Biochim Biophys Acta 2015;1851:383–96. [DOI] [PubMed] [Google Scholar]
- 22.Fan YY, Chapkin RS. Mouse peritoneal macrophage prostaglandin E1 synthesis is altered by dietary gamma-linolenic acid. J Nutr 1992;122:1600–6. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni PS, Srinivasan BD. Eicosapentaenoic acid metabolism in human and rabbit anterior uvea. Prostaglandins 1986;31:1159–64. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill GP, Mancini JA, Kargman S, Yergey J, Kwan MY, Falgueyret JP, Abramovitz M, Kennedy BP, Ouellet M, Cromlish W, et al. Overexpression of human prostaglandin G/H synthase-1 and -2 by recombinant vaccinia virus: inhibition by nonsteroidal anti-inflammatory drugs and biosynthesis of 15-hydroxyeicosatetraenoic acid. Mol Pharmacol 1994;45:245–54. [PubMed] [Google Scholar]
- 25.Thuresson ED, Lakkides KM, Smith WL. Different catalytically competent arrangements of arachidonic acid within the cyclooxygenase active site of prostaglandin endoperoxide H synthase-1 lead to the formation of different oxygenated products. J Biol Chem 2000;275:8501–7. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laneuville O, Breuer DK, Xu N, Huang ZH, Gage DA, Watson JT, Lagarde M, DeWitt DL, Smith WL. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)- octadecatrienoic acids from alpha-linolenic acid. J Biol Chem 1995;270:19330–6. [DOI] [PubMed] [Google Scholar]
- 28.Feltenmark S, Gautam N, Brunnstrom A, Griffiths W, Backman L, Edenius C, Lindbom L, Bjorkholm M, Claesson HE. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci USA 2008;105:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underwood KW, Song C, Kriz RW, Chang XJ, Knopf JL, Lin LL. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J Biol Chem 1998;273:21926–32. [DOI] [PubMed] [Google Scholar]
- 30.Grandits M, Oostenbrink C. Selectivity of cytosolic phospholipase A2 type IV toward arachidonyl phospholipids. J Mol Recognit 2015 Feb 23 (Epub ahead of print; DOI: 10.1002/jmr.2462.) [DOI] [PubMed] [Google Scholar]
- 31.Buczynski MW, Dumlao DS, Dennis EA. An integrated omics analysis of eicosanoid biology. J Lipid Res 2009;50:1015–38. [DOI] [PMC free article] [PubMed]
- 32.Sandig H, Pease JE, Sabroe I. Contrary prostaglandins: the opposing roles of PGD(2) and its metabolites in leukocyte function. J Leukoc Biol 2007;81:372–82. [DOI] [PubMed] [Google Scholar]
- 33.Catella F, Healy D, Lawson JA, Fitzgerald GA. 11-Dehydrothromboxane-B2: a quantitative index of thromboxane-A2 formation in the human circulation. Proc Natl Acad Sci USA 1986;83:5861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland M, Shankaranarayanan P, Schewe T, Nigam S. Evidence for the presence of phospholipid hydroperoxide glutathione peroxidase in human platelets: implications for its involvement in the regulatory network of the 12-lipoxygenase pathway of arachidonic acid metabolism. Biochem J 2001;353:91–100. [PMC free article] [PubMed] [Google Scholar]
- 35.Goetzl EJ, Sun FF. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med 1979;150:406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada M, Proia AD. 8(S)-hydroxyeicosatetraenoic acid is the lipoxygenase metabolite of arachidonic acid that regulates epithelial cell migration in the rat cornea. Cornea 2000; 19(3, Suppl)S13–20. [DOI] [PubMed] [Google Scholar]
- 37.Fruteau de Laclos B, Maclouf J, Poubelle P, Borgeat P. Conversion of arachidonic acid into 12-oxo derivatives in human platelets. A pathway possibly involving the heme-catalysed transformation of 12-hydroperoxy-eicosatetraenoic acid. Prostaglandins 1987;33:315–37. [DOI] [PubMed] [Google Scholar]
- 38.Erlemann KR, Cossette C, Gravel S, Lesimple A, Lee GJ, Saha G, Rokach J, Powell WS. Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress. Free Radic Biol Med 2007;42:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Flaherty JT, Wykle RL, Redman J, Samuel M, Thomas M. Metabolism of 5-hydroxyicosatetraenoate by human neutrophils: production of a novel omega-oxidized derivative. J Immunol 1986;137:3277–83. [PubMed] [Google Scholar]
- 40.Tejera N, Boeglin WE, Suzuki T, Schneider C. COX-2-dependent and -independent biosynthesis of dihydroxy-arachidonic acids in activated human leukocytes. J Lipid Res 2012;53:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryant RW, Bailey JM. Altered lipoxygenase metabolism and decreased glutathione peroxidase activity in platelets from selenium-deficient rats. Biochem Biophys Res Commun 1980;92:268–76. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA 1984;81:5335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta 2010;1801:1260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romano M, Chen XS, Takahashi Y, Yamamoto S, Funk CD, Serhan CN. Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem J 1993;296:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med 1996;2:583–96. [PMC free article] [PubMed] [Google Scholar]
- 46.Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Claria J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A(4). Am J Physiol 1999;277:C870–7. [DOI] [PubMed] [Google Scholar]
- 47.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Huang MH, Perez-Polo JR, Uretsky BF. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostaglandins Other Lipid Mediat 2007;83:89–98. [DOI] [PubMed] [Google Scholar]
- 48.Guido DM, McKenna R, Mathews WR. Quantitation of hydroperoxy-eicosatetraenoic acids and hydroxy-eicosatetraenoic acids as indicators of lipid peroxidation using gas chromatography-mass spectrometry. Anal Biochem 1993;209:123–9. [DOI] [PubMed] [Google Scholar]
- 49.Musiek ES, Yin H, Milne GL, Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids 2005;40:987–94. [DOI] [PubMed] [Google Scholar]
- 50.Oliw EH, Bylund J, Herman C. Bisallylic hydroxylation and epoxidation of polyunsaturated fatty acids by cytochrome P450. Lipids 1996;31:1003–21. [DOI] [PubMed] [Google Scholar]
- 51.Bylund J, Kunz T, Valmsen K, Oliw EH. Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J Pharmacol Exp Ther 1998;284:51–60. [PubMed] [Google Scholar]
- 52.Bylund J, Ericsson J, Oliw EH. Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Anal Biochem 1998;265:55–68. [DOI] [PubMed] [Google Scholar]
- 53.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, et al. The human serum metabolome. PLoS ONE 2011;6:e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuchardt JP, Schmidt S, Kressel G, Dong H, Willenberg I, Hammock BD, Hahn A, Schebb NH. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot Essent Fatty Acids 2013;89:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caligiuri SP, Love K, Winter T, Gauthier J, Taylor CG, Blydt-Hansen T, Zahradka P, Aukema HM. Dietary linoleic acid and alpha-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J Nutr 2013;143:1421–31. [DOI] [PubMed] [Google Scholar]
- 56.Reinaud O, Delaforge M, Boucher JL, Rocchiccioli F, Mansuy D. Oxidative metabolism of linoleic acid by human leukocytes. Biochem Biophys Res Commun 1989;161:883–91. [DOI] [PubMed] [Google Scholar]
- 57.Bull AW, Earles SM, Bronstein JC. Metabolism of oxidized linoleic acid: distribution of activity for the enzymatic oxidation of 13-hydroxyoctadecadienoic acid to 13-oxooctadecadienoic acid in rat tissues. Prostaglandins 1991;41:43–50. [DOI] [PubMed] [Google Scholar]
- 58.Askari AA, Thomson S, Edin ML, Lih FB, Zeldin DC, Bishop-Bailey D. Basal and inducible anti-inflammatory epoxygenase activity in endothelial cells. Biochem Biophys Res Commun 2014;446:633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsson N, Lundstrom SL, Pinto R, Rankin G, Karimpour M, Blomberg A, Sandstrom T, Pourazar J, Trygg J, Behndig AF, et al. Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans. Eur Respir J 2014;43:453–63. [DOI] [PubMed] [Google Scholar]
- 60.Niki E, Yoshida Y. Biomarkers for oxidative stress: measurement, validation, and application. The journal of medical investigation. J Med Invest 2005;52: Suppl:228–30. [DOI] [PubMed] [Google Scholar]
- 61.Funk CD, Powell WS. Metabolism of linoleic acid by prostaglandin endoperoxide synthase from adult and fetal blood vessels. Biochim Biophys Acta 1983;754:57–71. [DOI] [PubMed] [Google Scholar]
- 62.Hamberg M. Omega 6-oxygenation of 6, 9, 12-octadecatrienoic acid in human platelets. Biochem Biophys Res Commun 1983;117:593–600. [DOI] [PubMed] [Google Scholar]
- 63.Laethem RM, Balazy M, Koop DR. Epoxidation of C18 unsaturated fatty acids by cytochromes P4502C2 and P4502CAA. Drug Metab Dispos 1996;24:664–8. [PubMed] [Google Scholar]
- 64.Directory Patent [Internet]. [cited 2015 Jan 13]. Available from: http://www.directorypatent.com/U2S/20070248586-a1.html.
- 65.Amagai Y, Oida K, Matsuda A, Jung K, Kakutani S, Tanaka T, Matsuda K, Jang H, Ahn G, Xia Y, et al. Dihomo-gamma-linolenic acid prevents the development of atopic dermatitis through prostaglandin D1 production in NC/Tnd mice. J Dermatol Sci 2015;79:30–7. [DOI] [PubMed] [Google Scholar]
- 66.Manku MS, Oka M, Horrobin DF. Differential regulation of the formation of prostaglandins and related substances from arachidonic acid and from dihomogammalinolenic acid. II. Effects of vitamin C. Prostaglandins Med 1979;3:129–37. [DOI] [PubMed] [Google Scholar]
- 67.Xi S, Pham H, Ziboh WA. 15-hydroxyeicosatrienoic acid (15-HETrE) suppresses epidermal hyperproliferation via the modulation of nuclear transcription factor (AP-1) and apoptosis. Arch Dermatol Res 2000;292:397–403. [DOI] [PubMed] [Google Scholar]
- 68.Miller CC, Ziboh VA. Gammalinolenic acid-enriched diet alters cutaneous eicosanoids. Biochem Biophys Res Commun 1988;154:967–74. [DOI] [PubMed] [Google Scholar]
- 69.Miller CC, McCreedy CA, Jones AD, Ziboh VA. Oxidative metabolism of dihomogammalinolenic acid by guinea pig epidermis: evidence of generation of anti-inflammatory products. Prostaglandins 1988;35:917–38. [DOI] [PubMed] [Google Scholar]
- 70.Iversen L, Fogh K, Bojesen G, Kragballe K. Linoleic acid and dihomogammalinolenic acid inhibit leukotriene B4 formation and stimulate the formation of their 15-lipoxygenase products by human neutrophils in vitro. Evidence of formation of antiinflammatory compounds. Agents Actions 1991;33:286–91. [DOI] [PubMed] [Google Scholar]
- 71.Heitmann J, Iversen L, Kragballe K, Ziboh VA. Incorporation of 15-hydroxyeicosatrienoic acid in specific phospholipids of cultured human keratinocytes and psoriatic plaques. Exp Dermatol 1995;4:74–8. [DOI] [PubMed] [Google Scholar]
- 72.Chapkin RS, Miller CC, Somers SD, Erickson KL. Ability of 15-hydroxyeicosatrienoic acid (15-OH-20:3) to modulate macrophage arachidonic acid metabolism. Biochem Biophys Res Commun 1988;153:799–804. [DOI] [PubMed] [Google Scholar]
- 73.Yamane M, Abe A, Yamane S. High-performance liquid chromatography-thermospray mass spectrometry of epoxy polyunsaturated fatty acids and epoxyhydroxy polyunsaturated fatty acids from an incubation mixture of rat tissue homogenate. J Chromatogr 1994;652:123–36. [DOI] [PubMed] [Google Scholar]
- 74.Cagen LM, Zusman RM, Pisano JJ. Formation of 1a, 1b dihomoprostaglandin E2 by rabbit renal intersititial cell cultures. Prostaglandins 1979;18:617–21. [DOI] [PubMed] [Google Scholar]
- 75.Campbell WB, Falck JR, Okita JR, Johnson AR, Callahan KS. Synthesis of dihomoprostaglandins from adrenic acid (7,10,13,16-docosatetraenoic acid) by human endothelial cells. Biochim Biophys Acta 1985;837:67–76. [DOI] [PubMed] [Google Scholar]
- 76.Kopf PG, Zhang DX, Gauthier KM, Nithipatikom K, Yi XY, Falck JR, Campbell WB. Adrenic acid metabolites as endogenous endothelium-derived and zona glomerulosa-derived hyperpolarizing factors. Hypertension 2010;55:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sprecher H, VanRollins M, Sun F, Wyche A, Needleman P. Dihomo-prostaglandins and -thromboxane. A prostaglandin family from adrenic acid that may be preferentially synthesized in the kidney. J Biol Chem 1982;257:3912–8. [PubMed] [Google Scholar]
- 78.VanRollins M, Horrocks L, Sprecher H. Metabolism of 7,10,13,16-docosatetraenoic acid to dihomo-thromboxane, 14-hydroxy-7,10,12-nonadecatrienoic acid and hydroxy fatty acids by human platelets. Biochim Biophys Acta 1985;833:272–80. [DOI] [PubMed] [Google Scholar]
- 79.Yi XY, Gauthier KM, Cui L, Nithipatikom K, Falck JR, Campbell WB. Metabolism of adrenic acid to vasodilatory 1alpha,1beta-dihomo-epoxyeicosatrienoic acids by bovine coronary arteries. Am J Physiol Heart Circ Physiol 2007;292:H2265–74. [DOI] [PubMed] [Google Scholar]
- 80.Liu M, Chen P, Vericel E, Lelli M, Beguin L, Lagarde M, Guichardant M. Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. J Lipid Res 2013;54:2083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem 2007;282:22254–66. [DOI] [PubMed] [Google Scholar]
- 82.Miller C, Yamaguchi RY, Ziboh VA. Guinea pig epidermis generates putative anti-inflammatory metabolites from fish oil polyunsaturated fatty acids. Lipids 1989;24:998–1003. [DOI] [PubMed] [Google Scholar]
- 83.Terano T, Salmon JA, Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins 1984;27:217–32. [DOI] [PubMed] [Google Scholar]
- 84.Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clinical chemistry and laboratory medicine: CCLM/FESCC 2010;48:1063–73. [DOI] [PubMed]
- 85.von Schacky C, Marcus AJ, Safier LB, Ullman HL, Islam N, Broekman MJ, Fischer S. Platelet-neutrophil interactions. 12S,20- and 5S,12S-dihydroxyeicosapentaenoic acids: two novel neutrophil metabolites from platelet-derived 12S-hydroxyeicosapentaenoic acid. J Lipid Res 1990;31:801–10. [PubMed] [Google Scholar]
- 86.Powell WS, Gravel S, Gravelle F. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res 1995;36:2590–8. [PubMed] [Google Scholar]
- 87.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep 2010;62:536–47. [DOI] [PubMed]
- 88.Westphal C, Konkel A, Schunck WH. CYP-eicosanoids–a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat 2011;96:99–108. [DOI] [PubMed] [Google Scholar]
- 89.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem 2012;287:10525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, Amet Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys 2008;471:116–25. [DOI] [PubMed] [Google Scholar]
- 91.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res 2010;51:3481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hörnsten L, Bylund J, Oliw EH. Dexamethasone induces bisallylic hydroxylation of polyunsaturated fatty acids by rat liver microsomes. Arch Biochem Biophys 1996;332:261–8. [DOI] [PubMed] [Google Scholar]
- 93.VanRollins M, Baker RC, Sprecher HW, Murphy RC. Oxidation of docosahexaenoic acid by rat liver microsomes. J Biol Chem 1984;259:5776–83. [PubMed] [Google Scholar]
- 94.Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014;9:e102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balas L, Guichardant M, Durand T, Lagarde M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie 2014;99:1–7. [DOI] [PubMed] [Google Scholar]
- 96.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278:14677–87. [DOI] [PubMed] [Google Scholar]
- 98.Shinohara M, Mirakaj V, Serhan CN. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front Immunol 2012;3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.VanRollins M, Murphy RC. Autooxidation of docosahexaenoic acid: analysis of ten isomers of hydroxydocosahexaenoate. J Lipid Res 1984;25:507–17. [PubMed] [Google Scholar]
- 100.Reynaud D, Thickitt CP, Pace-Asciak CR. Facile preparation and structural determination of monohydroxy derivatives of docosahexaenoic acid (HDoHE) by alpha-tocopherol-directed autoxidation. Anal Biochem 1993;214:165–70. [DOI] [PubMed] [Google Scholar]
- 101.Yokokura Y, Isobe Y, Matsueda S, Iwamoto R, Goto T, Yoshioka T, Urabe D, Inoue M, Arai H, Arita M. Identification of 14,20-dihydroxy-docosahexaenoic acid as a novel anti-inflammatory metabolite. J Biochem 2014;156:315–21. [DOI] [PubMed] [Google Scholar]
- 102.Cipollina C, Salvatore SR, Muldoon MF, Freeman BA, Schopfer FJ. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS ONE 2014;9:e94836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thurnher M, Putz T, Gander H, Rahm A, Bartsch G, Ramoner R. The cyclopentenone prostaglandin PGA2 costimulates the maturation of human dendritic cells. Exp Hematol 2005;33:144–50. [DOI] [PubMed] [Google Scholar]
- 104.Deliconstantinos G, Kopeikina L, Ramantanis G. PGE2 and PGA2 affect the allosteric properties and the activities of calmodulin-dependent guanylate cyclase and Ca2+-stimulated ATPase of Walker-256 tumour microsomal membranes. Anticancer Res 1989;9:fluorescence polarization. [PubMed]
- 105.Bui T, Kuo C, Rotwein P, Straus DS. Prostaglandin A2 specifically represses insulin-like growth factor-I gene expression in C6 rat glioma cells. Endocrinology 1997;138:985–93. [DOI] [PubMed] [Google Scholar]
- 106.Fara JW, Barth KH, White RI Jr, Bynum TE. Mesenteric vascular effects of prostaglandins F2 alpha and B2. Possible advantages over vasopressin in control of gastrointestinal bleeding. Radiology 1979;133:317–20. [DOI] [PubMed] [Google Scholar]
- 107.Hall DW, Jaitly KD. Structure-activity relationships in a series of 11-deoxy prostaglandins. Prostaglandins 1976;11:573–87. [DOI] [PubMed] [Google Scholar]
- 108.Relic B, Benoit V, Franchimont N, Ribbens C, Kaiser MJ, Gillet P, Merville MP, Bours V, Malaise MG. 15-deoxy-delta12,14-prostaglandin J2 inhibits Bay 11–7085-induced sustained extracellular signal-regulated kinase phosphorylation and apoptosis in human articular chondrocytes and synovial fibroblasts. J Biol Chem 2004;279:22399–403. [DOI] [PubMed] [Google Scholar]
- 109.Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol 2003;171:3936–40. [DOI] [PubMed] [Google Scholar]
- 110.Urade Y, Hayaishi O. Prostaglandin D2 and sleep regulation. Biochim Biophys Acta 1999;1436:606–15. [DOI] [PubMed] [Google Scholar]
- 111.F örstermann U, Heldt R, Hertting G. Effects of intracerebroventricular administration of prostaglandin D2 on behaviour, blood pressure and body temperature as compared to prostaglandins E2 and F2 alpha. Psychopharmacology (Berl) 1983;80:365–70. [DOI] [PubMed] [Google Scholar]
- 112.Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc Natl Acad Sci USA 1982;79:6093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kikuchi Y, Miyauchi M, Oomori K, Kita T, Kizawa I, Kato K. Inhibition of human ovarian cancer cell growth in vitro and in nude mice by prostaglandin D2. Cancer Res 1986;46:3364–6. [PubMed] [Google Scholar]
- 114.Tachikawa M, Hosoya K, Terasaki T. Pharmacological significance of prostaglandin E2 and D2 transport at the brain Barriers. In: Davis TP, editor, Advances in pharmacology, Academic Press; 2014;71:337–60. [DOI] [PubMed] [Google Scholar]
- 115.Darius H, Michael-Hepp J, Thierauch KH, Fisch A. Inhibition of human platelets and polymorphonuclear neutrophils by the potent and metabolically stable prostaglandin D2 analog ZK 118.182. Eur J Pharmacol 1994;258:207–13. [DOI] [PubMed] [Google Scholar]
- 116.Ney P, Schror K. PGD2 and its mimetic ZK 110.841 are potent inhibitors of receptor-mediated activation of human neutrophils. Eicosanoids 1991;4:21–8. [PubMed] [Google Scholar]
- 117.Ward C, Dransfield I, Murray J, Farrow SN, Haslett C, Rossi AG. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of I kappa B alpha degradation using a peroxisome proliferator-activated receptor-gamma-independent mechanism. J Immunol 2002;168:6232–43. [DOI] [PubMed] [Google Scholar]
- 118.Heinemann A, Schuligoi R, Sabroe I, Hartnell A, Peskar BA. Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J Immunol 2003;170:4752–8. [DOI] [PubMed] [Google Scholar]
- 119.Bundy GL, Morton DR, Peterson DC, Nishizawa EE, Miller WL. Synthesis and platelet aggregation inhibiting activity of prostaglandin D analogues. J Med Chem 1983;26:790–9. [DOI] [PubMed] [Google Scholar]
- 120.Whitaker MO, Wyche A, Fitzpatrick F, Sprecher H, Needleman P. Triene prostaglandins: prostaglandin D3 and icosapentaenoic acid as potential antithrombotic substances. Proc Natl Acad Sci USA 1979;76:5919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ellis EF, Wei EP, Kontos HA. Vasodilation of cat cerebral arterioles by prostaglandins D2, E2, G2, and I2. Am J Physiol 1979;237:H381–5. [DOI] [PubMed] [Google Scholar]
- 122.Gray SJ, Heptinstall S. Interactions between prostaglandin E2 and inhibitors of platelet aggregation which act through cyclic AMP. Eur J Pharmacol 1991;194:63–70. [DOI] [PubMed] [Google Scholar]
- 123.Dufour M, Faes S, Dormond-Meuwly A, Demartines N, Dormond O. PGE2-induced colon cancer growth is mediated by mTORC1. Biochem Biophys Res Commun 2014;451:587–91. [DOI] [PubMed] [Google Scholar]
- 124.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 2002;168:2255–63. [DOI] [PubMed] [Google Scholar]
- 125.Lee IT, Lin CC, Lin WN, Wu WL, Hsiao LD, Yang CM. Lung inflammation caused by adenosine-5′-triphosphate is mediated via Ca2+/PKCs-dependent COX-2/PGE2 induction. Int J Biochem Cell Biol 2013;45:1657–68. [DOI] [PubMed] [Google Scholar]
- 126.Chou WL, Chuang LM, Chou CC, Wang AH, Lawson JA, FitzGerald GA, Chang ZF. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. J Biol Chem 2007;282:18162–72. [DOI] [PubMed] [Google Scholar]
- 127.Kelton JG, Blajchman MA. Prostaglandin I2 (prostacyclin). Can Med Assoc J 1980;122:175–9. [PMC free article] [PubMed] [Google Scholar]
- 128.Brash AR, Jackson EK, Saggese CA, Lawson JA, Oates JA, FitzGerald GA. Metabolic disposition of prostacyclin in humans. J Pharmacol Exp Ther 1983;226:78–87. [PubMed] [Google Scholar]
- 129.Sandig H, Andrew D, Barnes AA, Sabroe I, Pease J. 9alpha,11beta-PGF2 and its stereoisomer PGF2alpha are novel agonists of the chemoattractant receptor, CRTH2. FEBS Lett 2006;580:373–9. [DOI] [PubMed] [Google Scholar]
- 130.Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, et al. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med 2005;11:562–6. [DOI] [PubMed] [Google Scholar]
- 131.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science 1997;277:681–3. [DOI] [PubMed] [Google Scholar]
- 132.Morrow JD, Minton TA, Roberts LJ 2nd. The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins 1992;44:155–63. [DOI] [PubMed] [Google Scholar]
- 133.Basu S. Novel cyclooxygenase-catalyzed bioactive prostaglandin F2alpha from physiology to new principles in inflammation. Med Res Rev 2007;27:435–68. [DOI] [PubMed] [Google Scholar]
- 134.Higgs EA, Higgs GA, Moncada S, Vane JR. Prostacyclin (PGI2) inhibits the formation of platelet thrombi in arterioles and venules of the hamster cheek pouch. 1977. Br J Pharmacol 1997; 120(4, Suppl)439–43, discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dusting GJ, Chapple DJ, Hughes R, Moncada S, Vane JR. Prostacyclin (PGI2) induces coronary vasodilatation in anaesthetised dogs. Cardiovasc Res 1978;12:720–30. [PubMed] [Google Scholar]
- 136.Konya V, Sturm EM, Schratl P, Beubler E, Marsche G, Schuligoi R, Lippe IT, Peskar BA, Heinemann A. Endothelium-derived prostaglandin I(2) controls the migration of eosinophils. J Allergy Clin Immunol 2010;125:1105–13. [DOI] [PubMed] [Google Scholar]
- 137.Setty BN, Dampier CD, Stuart MJ. Arachidonic acid metabolites are involved in mediating red blood cell adherence to endothelium. J Lab Clin Med 1995;125:608–17. [PubMed] [Google Scholar]
- 138.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000;403:103–8. [DOI] [PubMed] [Google Scholar]
- 139.Cippitelli M, Fionda C, Di Bona D, Lupo A, Piccoli M, Frati L, Santoni A. The cyclopentenone-type prostaglandin 15-deoxy-delta 12,14-prostaglandin J2 inhibits CD95 ligand gene expression in T lymphocytes: interference with promoter activation via peroxisome proliferator-activated receptor-gamma-independent mechanisms. J Immunol 2003;170:4578–92. [DOI] [PubMed] [Google Scholar]
- 140.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 1975;72:2994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morinelli TA, Zhang LM, Newman WH, Meier KE. Thromboxane A2/prostaglandin H2-stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J Biol Chem 1994;269:5693–8. [PubMed] [Google Scholar]
- 142.Geoffroy J, Benzoni D, Sassard J. Antihypertensive effect of thromboxane A2 receptor blockade in genetically hypertensive rats of the Lyon strain. J Hypertens 1989;7:S272–3. [DOI] [PubMed]
- 143.Uchida M, Iida H, Iida M, Dohi S. Changes in cerebral microcirculation during and after abdominal aortic cross-clamping in rabbits: the role of thromboxane A2 receptor. Anesth Analg 2003;96:651–6. [DOI] [PubMed] [Google Scholar]
- 144.Wasserman MA, Griffin RL. Thromboxane B2–comparative bronchoactivity in experimental systems. Eur J Pharmacol 1977;46:303–13. [DOI] [PubMed] [Google Scholar]
- 145.Friedman LS, Fitzpatrick TM, Bloom MF, Ramwell PW, Rose JC, Kot PA. Cardiovascular and pulmonary effects of thromboxane B2 in the dog. Circ Res 1979;44:748–51. [DOI] [PubMed] [Google Scholar]
- 146.Kitchen EA, Boot JR, Dawson W. Chemotactic activity of thromboxane B2, prostaglandins and their metabolites for polymorphonuclear leucocytes. Prostaglandins 1978;16:239–44. [DOI] [PubMed] [Google Scholar]
- 147.Benigni A, Chiabrando C, Perico N, Fanelli R, Patrono C, FitzGerald GA, Remuzzi G. Renal metabolism and urinary excretion of thromboxane B2 in the rat. Am J Physiol 1989;257:F77–85. [DOI] [PubMed] [Google Scholar]
- 148.Chiabrando C, Corada M, Bachi A, Fanelli R. Urinary excretion of 2,3-dinor-thromboxane B1, a major metabolite of thromboxane B2 in the rat. Prostaglandins 1994;47:409–22. [DOI] [PubMed] [Google Scholar]
- 149.Foegh ML, Zhao Y, Madren L, Rolnick M, Stair TO, Huang KS, Ramwell PW. Urinary thromboxane A2 metabolites in patients presenting in the emergency room with acute chest pain. J Intern Med 1994;235:153–61. [DOI] [PubMed] [Google Scholar]
- 150.Catella F, Healy D, Lawson JA, FitzGerald GA. 11-Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA 1986;83:5861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lopez LR, Guyer KE, Torre IG, Pitts KR, Matsuura E, Ames PR. Platelet thromboxane (11-dehydro-Thromboxane B2) and aspirin response in patients with diabetes and coronary artery disease. World J Diabetes 2014;5:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Westlund P, Kumlin M, Nordenstrom A, Granstrom E. Circulating and urinary thromboxane B2 metabolites in the rabbit: 11-dehydro-thromboxane B2 as parameter of thromboxane production. Prostaglandins 1986;31:413–43. [DOI] [PubMed] [Google Scholar]
- 153.Morita E, Schroder JM, Christophers E. Identification of a novel and highly potent eosinophil chemotactic lipid in human eosinophils treated with arachidonic acid. J Immunol 1990;144:1893–900. [PubMed] [Google Scholar]
- 154.Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem 1993;268:9280–6. [PubMed] [Google Scholar]
- 155.Shak S, Perez HD, Goldstein IM. A novel dioxygenation product of arachidonic acid possesses potent chemotactic activity for human polymorphonuclear leukocytes. J Biol Chem 1983;258:14948–53. [PubMed] [Google Scholar]
- 156.Hajjar DP, Marcus AJ, Etingin OR. Platelet-neutrophil-smooth muscle cell interactions: lipoxygenase-derived mono- and dihydroxy acids activate cholesteryl ester hydrolysis by the cyclic AMP dependent protein kinase cascade. Biochemistry 1989;28:8885–91. [DOI] [PubMed] [Google Scholar]
- 157.Dodge W, Thomas M. The effect of 5-hydroxyeicosatetraenoic acid on the proliferation of granulocyte progenitors and embryonic fibroblasts of the chick. Biochem Biophys Res Commun 1985;131:731–5. [DOI] [PubMed] [Google Scholar]
- 158.Goetzl EJ, Weller PF, Sun FF. The regulation of human eosinophil function by endogenous mono-hydroxy-eicosatetraenoic acids (HETEs). J Immunol 1980;124:926–33. [PubMed] [Google Scholar]
- 159.Goetzl EJ, Brash AR, Tauber AI, Oates JA, Hubbard WC. Modulation of human neutrophil function by monohydroxy-eicosatetraenoic acids. Immunology 1980;39:491–501. [PMC free article] [PubMed] [Google Scholar]
- 160.Valone FH, Franklin M, Sun FF, Goetzl EJ. Alveolar macrophage lipoxygenase products of arachidonic acid: isolation and recognition as the predominant constituents of the neutrophil chemotactic activity elaborated by alveolar macrophages. Cell Immunol 1980;54:390–401. [DOI] [PubMed] [Google Scholar]
- 161.Stenson WF, Parker CW. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol 1980;124:2100–4. [PubMed] [Google Scholar]
- 162.Gordon EE, Gordon JA, Spector AA. HETEs and coronary artery endothelial cells: metabolic and functional interactions. Am J Physiol 1991;261:C623–33. [DOI] [PubMed] [Google Scholar]
- 163.Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun 2004;315:624–35. [DOI] [PubMed] [Google Scholar]
- 164.O'Flaherty JT, Rogers LC, Paumi CM, Hantgan RR, Thomas LR, Clay CE, High K, Chen YQ, Willingham MC, Smitherman PK, et al. 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochim Biophys Acta 2005;1736:228–36. [DOI] [PubMed] [Google Scholar]
- 165.Heidel JR, Taylor SM, Laegreid WW, Silflow RM, Liggitt HD, Leid RW. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. Am J Pathol 1989;134:671–6. [PMC free article] [PubMed] [Google Scholar]
- 166.Takenaga M, Hirai A, Terano T, Tamura Y, Kitagawa H, Yoshida S. Comparison of the in vitro effect of eicosapentaenoic acid (EPA)-derived lipoxygenase metabolites on human platelet function with those of arachidonic acid. Thromb Res 1986;41:373–84. [DOI] [PubMed] [Google Scholar]
- 167.Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol 1995;154:4123–32. [PubMed] [Google Scholar]
- 168.O'Flaherty JT, Cordes J, Redman J, Thomas MJ. 5-Oxo-eicosatetraenoate, a potent human neutrophil stimulus. Biochem Biophys Res Commun 1993;192:129–34. [DOI] [PubMed] [Google Scholar]
- 169.Armstrong MM, Diaz G, Kenyon V, Holman TR. Inhibitory and mechanistic investigations of oxo-lipids with human lipoxygenase isozymes. Bioorg Med Chem 2014;22:4293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 1995;270:23975–83. [DOI] [PubMed] [Google Scholar]
- 171.Brinkman HJ, van Buul-Wortelboer MF, van Mourik JA. Involvement of cyclooxygenase- and lipoxygenase-mediated conversion of arachidonic acid in controlling human vascular smooth muscle cell proliferation. Thromb Haemost 1990;63:291–7. [PubMed] [Google Scholar]
- 172.Liu X, Zhang S, Arora JS, Snyder NW, Shah SJ, Blair IA. 11-Oxoeicosatetraenoic acid is a cyclooxygenase-2/15-hydroxyprostaglandin dehydrogenase-derived antiproliferative eicosanoid. Chem Res Toxicol 2011;24:2227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, Sen CK, Ghafourifar P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys 2007;468:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol 1999;19:2615–22. [DOI] [PubMed] [Google Scholar]
- 175.Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, et al. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem 2004;279:9440–50. [DOI] [PubMed] [Google Scholar]
- 176.Honn KV, Nelson KK, Renaud C, Bazaz R, Diglio CA, Timar J. Fatty acid modulation of tumor cell adhesion to microvessel endothelium and experimental metastasis. Prostaglandins 1992;44:413–29. [DOI] [PubMed] [Google Scholar]
- 177.Sekiya F, Takagi J, Usui T, Kawajiri K, Kobayashi Y, Sato F, Saito Y. 12S-hydroxyeicosatetraenoic acid plays a central role in the regulation of platelet activation. Biochem Biophys Res Commun 1991;179:345–51. [DOI] [PubMed] [Google Scholar]
- 178.Sekiya F, Takagi J, Sasaki K, Kawajiri K, Kobayashi Y, Sato F, Saito Y. Feedback regulation of platelet function by 12S-hydroxyeicosatetraenoic acid: inhibition of arachidonic acid liberation from phospholipids. Biochim Biophys Acta 1990;1044:165–8. [DOI] [PubMed] [Google Scholar]
- 179.Fonlupt P, Croset M, Lagarde M. 12-HETE inhibits the binding of PGH2/TXA2 receptor ligands in human platelets. Thromb Res 1991;63:239–48. [DOI] [PubMed] [Google Scholar]
- 180.Croset M, Sala A, Folco G, Lagarde M. Inhibition by lipoxygenase products of TXA2-like responses of platelets and vascular smooth muscle. 14-Hydroxy from 22:6n-3 is more potent than 12-HETE. Biochem Pharmacol 1988;37:1275–80. [DOI] [PubMed] [Google Scholar]
- 181.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci USA 1998;95:3100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tamura Y, Hirai A, Terano T, Takenaga M, Saitoh H, Tahara K, Yoshida S. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res 1986;25:461–6. [DOI] [PubMed] [Google Scholar]
- 183.Naccache PH, Leblanc Y, Rokach J, Patrignani P, Fruteau de Laclos B, Borgeat P. Calcium mobilization and right-angle light scatter responses to 12-oxo-derivatives of arachidonic acid in neutrophils: evidence for the involvement of the leukotriene B4 receptor. Biochim Biophys Acta 1991;1133:102–6. [DOI] [PubMed] [Google Scholar]
- 184.Matsuda H, Miyatake K, Dahlen SE. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J Pharmacol Exp Ther 1995;273:1182–9. [PubMed] [Google Scholar]
- 185.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 1999;400:378–82. [DOI] [PubMed] [Google Scholar]
- 186.Naruhn S, Meissner W, Adhikary T, Kaddatz K, Klein T, Watzer B, Muller-Brusselbach S, Muller R. 15-hydroxyeicosatetraenoic acid is a preferential peroxisome proliferator-activated receptor beta/delta agonist. Mol Pharmacol 2010;77:171–84. [DOI] [PubMed] [Google Scholar]
- 187.Takata S, Papayianni A, Matsubara M, Jimenez W, Pronovost PH, Brady HR. 15-Hydroxyeicosatetraenoic acid inhibits neutrophil migration across cytokine-activated endothelium. Am J Pathol 1994;145:541–9. [PMC free article] [PubMed] [Google Scholar]
- 188.Smith RJ, Justen JM, Nidy EG, Sam LM, Bleasdale JE. Transmembrane signaling in human polymorphonuclear neutrophils: 15(S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid modulates receptor agonist-triggered cell activation. Proc Natl Acad Sci USA 1993;90:7270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res 2003;92:992–1000. [DOI] [PubMed] [Google Scholar]
- 190.Setty BN, Werner MH, Hannun YA, Stuart MJ. 15-Hydroxyeicosatetraenoic acid-mediated potentiation of thrombin-induced platelet functions occurs via enhanced production of phosphoinositide-derived second messengers–sn-1,2-diacylglycerol and inositol-1,4,5-trisphosphate. Blood 1992;80:2765–73. [PubMed] [Google Scholar]
- 191.Sultana C, Shen Y, Rattan V, Kalra VK. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J Cell Physiol 1996;167:477–87. [DOI] [PubMed] [Google Scholar]
- 192.Thollon C, Iliou JP, Cambarrat C, Robin F, Vilaine JP. Nature of the cardiomyocyte injury induced by lipid hydroperoxides. Cardiovasc Res 1995;30:648–55. [PubMed] [Google Scholar]
- 193.Wei C, Zhu P, Shah SJ, Blair IA. 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol 2009;76:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sugumaran PK, Wang S, Song S, Nie X, Zhang L, Feng Y, Ma W, Zhu D. 15-oxo-Eicosatetraenoic acid prevents serum deprivation-induced apoptosis of pulmonary arterial smooth muscle cells by activating pro-survival pathway. Prostaglandins Leukot Essent Fatty Acids 2014;90:89–98. [DOI] [PubMed] [Google Scholar]
- 195.Dho S, Grinstein S, Corey EJ, Su WG, Pace-Asciak CR. Hepoxilin A3 induces changes in cytosolic calcium, intracellular pH and membrane potential in human neutrophils. Biochem J 1990;266:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA 2004;101:7421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hallenborg P, Jorgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, Langer T, Furstenberger G, Krieg P, Koppen A, et al. Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Mol Cell Biol 2010;30:4077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hafstrom I, Palmblad J, Malmsten CL, Radmark O, Samuelsson B. Leukotriene B4–a stereospecific stimulator for release of lysosomal enzymes from neutrophils. FEBS Lett 1981;130:146–8. [DOI] [PubMed] [Google Scholar]
- 199.Ringertz B, Palmblad J, Radmark O, Malmsten C. Leukotriene-induced neutrophil aggregation in vitro. FEBS Lett 1982;147:180–2. [DOI] [PubMed] [Google Scholar]
- 200.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980;286:264–5. [DOI] [PubMed] [Google Scholar]
- 201.Hansson G, Lindgren JA, Dahlen SE, Hedqvist P, Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett 1981;130:107–12. [DOI] [PubMed] [Google Scholar]
- 202.Palmblad J, Uden AM, Lindgren JA, Radmark O, Hansson G, Malmsten CL. Effects of novel leukotrienes on neutrophil migration. FEBS Lett 1982;144:81–4. [DOI] [PubMed] [Google Scholar]
- 203.Cheng JB, Lang D, Bewtra A, Townley RG. Tissue distribution and functional correlation of [3H]leukotriene C4 and [3H]leukotriene D4 binding sites in guinea-pig uterus and lung preparations. J Pharmacol Exp Ther 1985;232:80–7. [PubMed] [Google Scholar]
- 204.Camp RD, Coutts AA, Greaves MW, Kay AB, Walport MJ. Responses of human skin to intradermal injection of leukotrienes C4, D4 and B4. Br J Pharmacol 1983;80:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Leitch AG, Lee TH, Ringel EW, Prickett JD, Robinson DR, Pyne SG, Corey EJ, Drazen JM, Austen KF, Lewis RA. Immunologically induced generation of tetraene and pentaene leukotrienes in the peritoneal cavities of menhaden-fed rats. J Immunol 1984;132:2559–65. [PubMed] [Google Scholar]
- 206.Carbajal V, Vargas MH, Flores-Soto E, Martinez-Cordero E, Bazan-Perkins B, Montano LM. LTD4 induces hyperresponsiveness to histamine in bovine airway smooth muscle: role of SR-ATPase Ca2+ pump and tyrosine kinase. Am J Physiol Lung Cell Mol Physiol 2005;288:L84–92. [DOI] [PubMed] [Google Scholar]
- 207.Ezra D, Boyd LM, Feuerstein G, Goldstein RE. Coronary constriction by leukotriene C4, D4, and E4 in the intact pig heart. Am J Cardiol 1983;51:1451–4. [DOI] [PubMed] [Google Scholar]
- 208.Denis D, Charleson S, Rackham A, Jones TR, Ford-Hutchinson AW, Lord A, Cirino M, Girard Y, Larue M, Rokach J. Synthesis and biological activities of leukotriene F4 and leukotriene F4 sulfone. Prostaglandins 1982;24:801–14. [DOI] [PubMed] [Google Scholar]
- 209.Patcha V, Wigren J, Winberg ME, Rasmusson B, Li J, Sarndahl E. Differential inside-out activation of beta2-integrins by leukotriene B4 and fMLP in human neutrophils. Exp Cell Res 2004;300:308–19. [DOI] [PubMed] [Google Scholar]
- 210.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996;183:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol 2004;143:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem 2005;280:15267–78. [DOI] [PubMed] [Google Scholar]
- 213.Katoh T, Takahashi K, DeBoer DK, Serhan CN, Badr KF. Renal hemodynamic actions of lipoxins in rats: a comparative physiological study. Am J Physiol 1992;263:F436–42. [DOI] [PubMed] [Google Scholar]
- 214.Nigam S, Fiore S, Luscinskas FW, Serhan CN. Lipoxin A4 and lipoxin B4 stimulate the release but not the oxygenation of arachidonic acid in human neutrophils: dissociation between lipid remodeling and adhesion. J Cell Physiol 1990;143:512–23. [DOI] [PubMed] [Google Scholar]
- 215.Badr KF, DeBoer DK, Schwartzberg M, Serhan CN. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc Natl Acad Sci USA 1989;86:3438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Stahl GL, Tsao P, Lefer AM, Ramphal JY, Nicolaou KC. Pharmacologic profile of lipoxins A5 and B5: new biologically active eicosanoids. Eur J Pharmacol 1989;163:55–60. [DOI] [PubMed] [Google Scholar]
- 217.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med 2004;200:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost 2007;97:88–98. [PubMed] [Google Scholar]
- 219.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 1997;272:6972–8. [DOI] [PubMed] [Google Scholar]
- 220.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves A, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 2009;29:54–60. [DOI] [PubMed] [Google Scholar]
- 221.Lu T, Katakam PV, VanRollins M, Weintraub NL, Spector AA, Lee HC. Dihydroxyeicosatrienoic acids are potent activators of Ca(2+)-activated K(+) channels in isolated rat coronary arterial myocytes. J Physiol 2001;534:651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res 1998;83:932–9. [DOI] [PubMed] [Google Scholar]
- 223.Fang X, Kaduce TL, Weintraub NL, VanRollins M, Spector AA. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res 1996;79:784–93. [DOI] [PubMed] [Google Scholar]
- 224.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, Liu Y, Sangras B, Falck JR, Weintraub NL, et al. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol 2006;290:H55–63. [DOI] [PubMed] [Google Scholar]
- 225.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnes CM, Mammoto A, Mammoto T, Luria A, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest 2012;122:178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Proctor KG, Falck JR, Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res 1987;60:50–9. [DOI] [PubMed] [Google Scholar]
- 227.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem 2005;280:27138–46. [DOI] [PubMed] [Google Scholar]
- 228.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol 2001;280:H2430–40. [DOI] [PubMed] [Google Scholar]
- 229.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 2008;294:H724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999;285:1276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA 2013;110:6530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li PL. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am J Physiol Heart Circ Physiol 2002;282:H1656–64. [DOI] [PubMed] [Google Scholar]
- 233.Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther 2008;326:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol 1996;271:R863–9. [DOI] [PubMed] [Google Scholar]
- 235.Bednar MM, Gross CE, Russell SR, Fuller SP, Ahern TP, Howard DB, Falck JR, Reddy KM, Balazy M. 16(R)-hydroxyeicosatetraenoic acid, a novel cytochrome P450 product of arachidonic acid, suppresses activation of human polymorphonuclear leukocyte and reduces intracranial pressure in a rabbit model of thromboembolic stroke. Neurosurgery 2000;47:1410–8, discussion 8–9. [PubMed] [Google Scholar]
- 236.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther 1992;260:104–9. [PubMed] [Google Scholar]
- 237.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 1993;72:126–36. [DOI] [PubMed] [Google Scholar]
- 238.Escalante B, Falck JR, Yadagiri P, Sun LM, Laniado-Schwartzman M. 19(S)-hydroxyeicosatetraenoic acid is a potent stimulator of renal Na+-K+-ATPase. Biochem Biophys Res Commun 1988;152:1269–74. [DOI] [PubMed] [Google Scholar]
- 239.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension 2003;41:801–6. [DOI] [PubMed] [Google Scholar]
- 240.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther 2008;324:103–10. [DOI] [PubMed] [Google Scholar]
- 241.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 1998;31:242–7. [DOI] [PubMed] [Google Scholar]
- 242.Niculescu LS, Sanda GM, Sima AV. HDL inhibits endoplasmic reticulum stress by stimulating apoE and CETP secretion from lipid-loaded macrophages. Biochem Biophys Res Commun 2013;434:173–8. [DOI] [PubMed] [Google Scholar]
- 243.Hampel JK, Brownrigg LM, Vignarajah D, Croft KD, Dharmarajan AM, Bentel JM, Puddey IB, Yeap BB. Differential modulation of cell cycle, apoptosis and PPARgamma2 gene expression by PPARgamma agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot Essent Fatty Acids 2006;74:283–93. [DOI] [PubMed] [Google Scholar]
- 244.Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, Izumi T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol 2008;128:1123–33. [DOI] [PubMed] [Google Scholar]
- 245.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998;93:229–40. [DOI] [PubMed] [Google Scholar]
- 246.Buchanan MR, Haas TA, Lagarde M, Guichardant M. 13-Hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J Biol Chem 1985;260:16056–9. [PubMed] [Google Scholar]
- 247.Tloti MA, Moon DG, Weston LK, Kaplan JE. Effect of 13-hydroxyoctadeca-9,11-dienoic acid (13-HODE) on thrombin induced platelet adherence to endothelial cells in vitro. Thromb Res 1991;62:305–17. [DOI] [PubMed] [Google Scholar]
- 248.Miller CC, Ziboh VA. Induction of epidermal hyperproliferation by topical n-3 polyunsaturated fatty acids on guinea pig skin linked to decreased levels of 13-hydroxyoctadecadienoic acid (13-hode). J Invest Dermatol 1990;94:353–8. [DOI] [PubMed] [Google Scholar]
- 249.Murthy S, Born E, Mathur S, Field FJ. 13-hydroxy octadecadienoic acid (13-HODE) inhibits triacylglycerol-rich lipoprotein secretion by CaCo-2 cells. J Lipid Res 1998;39:1254–62. [PubMed] [Google Scholar]
- 250.De Meyer GR, Bult H, Verbeuren TJ, Herman AG. The role of endothelial cells in the relaxations induced by 13-hydroxy- and 13-hydroperoxylinoleic acid in canine arteries. Br J Pharmacol 1992;107:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, et al. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol 2007;74:612–22. [DOI] [PubMed] [Google Scholar]
- 252.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J 2011;25:3436–47. [DOI] [PMC free article] [PubMed]
- 253.Moran JH, Weise R, Schnellmann RG, Freeman JP, Grant DF. Cytotoxicity of linoleic acid diols to renal proximal tubular cells. Toxicol Appl Pharmacol 1997;146:53–9. [DOI] [PubMed] [Google Scholar]
- 254.Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 2001;25:434–8. [DOI] [PubMed] [Google Scholar]
- 255.Nowak G, Grant DF, Moran JH. Linoleic acid epoxide promotes the maintenance of mitochondrial function and active Na+ transport following hypoxia. Toxicol Lett 2004;147:161–75. [DOI] [PubMed] [Google Scholar]
- 256.Sakai T, Ishizaki T, Ohnishi T, Sasaki F, Ameshima S, Nakai T, Miyabo S, Matsukawa S, Hayakawa M, Ozawa T. Leukotoxin, 9,10-epoxy-12-octadecenoate inhibits mitochondrial respiration of isolated perfused rat lung. Am J Physiol 1995;269:L326–31. [DOI] [PubMed] [Google Scholar]
- 257.Ozawa T, Hayakawa M, Takamura T, Sugiyama S, Suzuki K, Iwata M, Taki F, Tomita T. Biosynthesis of leukotoxin, 9,10-epoxy-12 octadecenoate, by leukocytes in lung lavages of rat after exposure to hyperoxia. Biochem Biophys Res Commun 1986;134:1071–8. [DOI] [PubMed] [Google Scholar]
- 258.Sugiyama S, Hayakawa M, Nagai S, Ajioka M, Ozawa T. Leukotoxin, 9, 10-epoxy-12-octadecenoate, causes cardiac failure in dogs. Life Sci 1987;40:225–31. [DOI] [PubMed] [Google Scholar]
- 259.Ozawa T, Nishikimi M, Sugiyama S, Taki F, Hayakawa M, Shionoya H. Cytotoxic activity of leukotoxin, a neutrophil-derived fatty acid epoxide, on cultured human cells. Biochem Int 1988;16:369–73. [PubMed] [Google Scholar]
- 260.Siegfried MR, Aoki N, Lefer AM, Elisseou EM, Zipkin RE. Direct cardiovascular actions of two metabolites of linoleic acid. Life Sci 1990;46:427–33. [DOI] [PubMed] [Google Scholar]
- 261.Moran JH, Nowak G, Grant DF. Analysis of the toxic effects of linoleic acid, 12,13-cis-epoxyoctadecenoic acid, and 12,13-dihydroxyoctadecenoic acid in rabbit renal cortical mitochondria. Toxicol Appl Pharmacol 2001;172:150–61. [DOI] [PubMed] [Google Scholar]
- 262.Schröder R, Xue L, Konya V, Martini L, Kampitsch N, Whistler JL, Ulven T, Heinemann A, Pettipher R, Kostenis E. PGH1, the precursor for the anti-inflammatory prostaglandins of the 1-series, is a potent activator of the pro-inflammatory receptor CRTH2/DP2. PLoS ONE 2012;7:e33329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.De Caridi G, Massara M, Stilo F, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Effectiveness of prostaglandin E1 in patients with mixed arterial and venous ulcers of the lower limbs. Int Wound J 2014 Aug 5 (Epub ahead of print; DOI: 10.1111/iwj.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Natsume T, Iwatsuki K, Nishizuka T, Arai T, Yamamoto M, Hirata H. Prostaglandin E1 alleviates neuropathic pain and neural dysfunction from entrapment neuropathy associated with diabetes mellitus. Microsurgery 2014;34:568–75. [DOI] [PubMed] [Google Scholar]
- 265.Ney P, Feelisch M. Vasodilator effects of PGE1 in the coronary and systemic circulation of the rat are mediated by ATP-sensitive potassium (K+) channels. Agents Actions Suppl 1995;45:71–6. [DOI] [PubMed] [Google Scholar]
- 266.Makino H, Aoki M, Hashiya N, Yamasaki K, Hiraoka K, Shimizu H, Azuma J, Kurinami H, Ogihara T, Morishita R. Increase in peripheral blood flow by intravenous administration of prostaglandin E1 in patients with peripheral arterial disease, accompanied by up-regulation of hepatocyte growth factor. Hypertens Res 2004;27:85–91. [DOI] [PubMed]
- 267.Zhang CY, Ma ZS, Ma LL, Wang LX. Effect of prostaglandin E1 inhalation on pulmonary hypertension following corrective surgery for congenital heart disease. Exp Clin Cardiol 2013;18:13–6. [PMC free article] [PubMed]
- 268.Westwick J. The effect of pulmonary metabolites of prostaglandins E1, E2 and F2alpha on ADP-induced aggregation of human and rabbit platelets. [proceedings] Br J Pharmacol 1976;58:297P–8P. [PMC free article] [PubMed] [Google Scholar]
- 269.Conners MS, Schwartzman ML, Quan X, Heilman E, Chauhan K, Falck JR, Godfrey HP. Enhancement of delayed hypersensitivity inflammatory reactions in guinea pig skin by 12(R)-hydroxy-5,8,14-eicosatrienoic acid. J Invest Dermatol 1995;104:47–51. [DOI] [PubMed] [Google Scholar]
- 270.Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res 2012;53:2546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ziboh VA, Miller CC, Cho Y. Significance of lipoxygenase-derived monohydroxy fatty acids in cutaneous biology. Prostaglandins Other Lipid Mediat 2000;63:3–13. [DOI] [PubMed] [Google Scholar]
- 272.Vang K, Ziboh VA. 15-lipoxygenase metabolites of gamma-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: possible implications of dietary fatty acids. Prostaglandins Leukot Essent Fatty Acids 2005;72:363–72. [DOI] [PubMed] [Google Scholar]
- 273.Schulze-Tanzil G. de SP, Behnke B, Klingelhoefer S, Scheid A, Shakibaei M. Effects of the antirheumatic remedy hox alpha–a new stinging nettle leaf extract–on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol 2002;17:477–85. [DOI] [PubMed] [Google Scholar]
- 274.Durot I, Devillard L, Tissier C, Vandroux D, Voisin S, Jaquir S, Rochette L, Athias P. Dependence on the phospholipid polyunsaturated fatty acids of the oxidative injury of isolated cardiomyocytes. Free Radic Res 2006;40:251–61. [DOI] [PubMed] [Google Scholar]
- 275.Takahashi H, Hara H, Goto T, Kamakari K, Wataru N, Mohri S, Takahashi N, Suzuki H, Shibata D, Kawada T. 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic acid activates peroxisome proliferator-activated receptor gamma in adipocytes. Lipids 2015;50:3–12. [DOI] [PubMed] [Google Scholar]
- 276.Lefils-Lacourtablaise J, Socorro M, Geloen A, Daira P, Debard C, Loizon E, Guichardant M, Dominguez Z, Vidal H, Lagarde M, et al. The eicosapentaenoic acid metabolite 15-deoxy-delta(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARgamma-dependent mechanism. PLoS ONE 2013;8:e63997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kulkarni PS, Srinivasan BD. Prostaglandins E3 and D3 lower intraocular pressure. Invest Ophthalmol Vis Sci 1985;26:1178–82. [PubMed] [Google Scholar]
- 278.Wendling MG, DuCharme DW. Cardiovascular effects of prostaglandin D3 and D2 in anesthetized dogs. Prostaglandins 1981;22:235–43. [DOI] [PubMed] [Google Scholar]
- 279.Hemker DP, Aiken JW. Effects of prostaglandin D3 on nerve transmission in nictitating membrane of cats. Eur J Pharmacol 1980;67:155–8. [DOI] [PubMed] [Google Scholar]
- 280.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 2003;100:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yang P, Chan D, Felix E, Cartwright C, Menter DG, Madden T, Klein RD, Fischer SM, Newman RA. Formation and antiproliferative effect of prostaglandin E(3) from eicosapentaenoic acid in human lung cancer cells. J Lipid Res 2004;45:1030–9. [DOI] [PubMed] [Google Scholar]
- 282.Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, Kang JX. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci USA 2006;103:12499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shimizu T, Yokotani K. Effects of centrally administered prostaglandin E(3) and thromboxane A(3) on plasma noradrenaline and adrenaline in rats: comparison with prostaglandin E(2) and thromboxane A(2). Eur J Pharmacol 2009;611:30–4. [DOI] [PubMed] [Google Scholar]
- 284.Faust TW, Lee E, Redfern JS, Feldman M. Effect of prostaglandin F3 alpha on gastric mucosal injury by ethanol in rats: comparison with prostaglandin F2 alpha. Prostaglandins 1989;37:493–504. [DOI] [PubMed] [Google Scholar]
- 285.Kobzar G, Mardla V, Jarving I, Samel N. Comparison of anti-aggregatory effects of PGI2, PGI3 and iloprost on human and rabbit platelets. Cell Physiol 2001;11:279–84. [DOI] [PubMed]
- 286.Needleman P, Raz A, Minkes MS, Ferrendelli JA, Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci USA 1979;76:944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hegde S, Kaushal N, Ravindra KC, Chiaro C, Hafer KT, Gandhi UH, Thompson JT, van den Heuvel JP, Kennett MJ, Hankey P, et al. Delta12-prostaglandin J3, an omega-3 fatty acid-derived metabolite, selectively ablates leukemia stem cells in mice. Blood 2011;118:6909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kogure R, Toyama K, Hiyamuta S, Kojima I, Takeda S. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochem Biophys Res Commun 2011;416:58–63. [DOI] [PubMed] [Google Scholar]
- 289.Fujita T, Sakuma S, Yamamoto N, Fujimoto Y. Effects of eicosapentaenoic acid and its 15-hydroperoxy and 15-hydroxy derivatives on glucosamine synthetase activity in rabbit gastric mucosa. Biochem Mol Biol Int 1998;46:157–63. [DOI] [PubMed] [Google Scholar]
- 290.Sakuma S, Usa K, Fujimoto Y. 15-Hydroperoxyeicosapentaenoic acid, but not eicosapentaenoic acid, shifts arachidonic acid away from cyclooxygenase pathway into acyl-CoA synthetase pathway in rabbit kidney medulla microsomes. Prostaglandins Leukot Essent Fatty Acids 2006;75:69–74. [DOI] [PubMed] [Google Scholar]
- 291.Tsunomori M, Fujimoto Y, Muta E, Nishida H, Sakuma S, Fujita T. 15-Hydroperoxyeicosapentaenoic acid inhibits arachidonic acid metabolism in rabbit platelets more potently than eicosapentaenoic acid. Biochim Biophys Acta 1996;1300:171–6. [DOI] [PubMed] [Google Scholar]
- 292.Nathaniel DJ, Evans JF, Leblanc Y, Leveille C, Fitzsimmons BJ, Ford-Hutchinson AW. Leukotriene A5 is a substrate and an inhibitor of rat and human neutrophil LTA4 hydrolase. Biochem Biophys Res Commun 1985;131:827–35. [DOI] [PubMed] [Google Scholar]
- 293.Juan H, Peskar BA, Simmet T. Effect of exogenous 5,8,11,14,17-eicosapentaenoic acid on cardiac anaphylaxis. Br J Pharmacol 1987;90:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hammarström S. Leukotriene C5: a slow reacting substance derived from eicosapentaenoic acid. J Biol Chem 1980;255:7093–4. [PubMed] [Google Scholar]
- 295.Ait-Said F, Elalamy I, Werts C, Gomard MT, Jacquemin C, Couetil JP, Hatmi M. Inhibition by eicosapentaenoic acid of IL-1beta-induced PGHS-2 expression in human microvascular endothelial cells: involvement of lipoxygenase-derived metabolites and p38 MAPK pathway. Biochim Biophys Acta 2003;1631:77–84. [DOI] [PubMed] [Google Scholar]
- 296.Lauritzen L, Hoffmann EK, Hansen HS, Jensen B. Dietary n-3 and n-6 fatty acids are equipotent in stimulating volume regulation in Ehrlich ascites tumor cells. Am J Physiol 1993;264:C109–17. [DOI] [PubMed] [Google Scholar]
- 297.Lam BK, Wong PY. Biosynthesis and biological activities of lipoxin A5 and B5 from eicosapentaenoic acid. Adv Exp Med Biol 1988;229:51–9. [DOI] [PubMed] [Google Scholar]
- 298.VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Ther 1995;274:798–804. [PubMed] [Google Scholar]
- 299.Jung F, Schulz C, Blaschke F, Muller DN, Mrowietz C, Franke RP, Lendlein A, Schunck WH. Effect of cytochrome P450-dependent epoxyeicosanoids on Ristocetin-induced thrombocyte aggregation. Clin Hemorheol Microcirc 2012;52:403–16. [DOI] [PubMed] [Google Scholar]
- 300.Morin C, Sirois M, Echave V, Rizcallah E, Rousseau E. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am J Physiol Lung Cell Mol Physiol 2009;296:L130–9. [DOI] [PubMed] [Google Scholar]
- 301.Morin C, Sirois M, Echave V, Albadine R, Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol 2010;43:564–75. [DOI] [PubMed] [Google Scholar]
- 302.Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, Ruth P, Schunck WH, Luft FC, Gollasch M. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp Physiol 2007;92:1067–76. [DOI] [PubMed] [Google Scholar]
- 303.Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 2014;211:1673–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, Waechter SF, Fischer A, Rothe M, Kang JX. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis 2011;32:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005;201:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol 2005;174:4345–55. [DOI] [PubMed] [Google Scholar]
- 307.Qiu W, Guo K, Yi L, Gong Y, Huang L, Zhong W. Resolvin E1 reduces hepatic fibrosis in mice with infection. Exp Ther Med 2014;7:1481–5. [DOI] [PMC free article] [PubMed]
- 308.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol 2006;13:1193–202. [DOI] [PubMed] [Google Scholar]
- 310.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol 2012;188:4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ogawa S, Urabe D, Yokokura Y, Arai H, Arita M, Inoue M. Total synthesis and bioactivity of resolvin E2. Org Lett 2009;11:3602–5. [DOI] [PubMed] [Google Scholar]
- 312.Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K, Inoue M, Arai H. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J Biochem 2013;153:355–60. [DOI] [PubMed] [Google Scholar]
- 313.Lu Y, Tian H, Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res 2010;51:923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tian H, Lu Y, Shah SP, Hong S. Novel 14S,21-dihydroxy-docosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure. J Cell Biochem 2010;111:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med 2011;3:69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, Horrillo R, Ferre N, Deulofeu R, Arroyo V, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J 2006;20:2537–9. [DOI] [PubMed]
- 317.Li X, Hong S, Li PL, Zhang Y. Docosahexanoic acid-induced coronary arterial dilation: actions of 17S-hydroxy docosahexanoic acid on K+ channel activity. J Pharmacol Exp Ther 2011;336:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol 2011;164:278–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 2011;187:1957–69. [DOI] [PubMed] [Google Scholar]
- 320.Gleissman H, Yang R, Martinod K, Lindskog M, Serhan CN, Johnsen JI, Kogner P. Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates. FASEB J 2010;24:906–15. [DOI] [PMC free article] [PubMed]
- 321.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci USA 2014;111:16526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 2012;26:1755–65. [DOI] [PMC free article] [PubMed]
- 323.Gong J, Wu ZY, Qi H, Chen L, Li HB, Li B, Yao CY, Wang YX, Wu J, Yuan SY, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol 2014;171:3539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol 2006;177:5902–11. [DOI] [PubMed] [Google Scholar]
- 325.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA 2004;101:8491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chen P, Vericel E, Lagarde M, Guichardant M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J 2011;25:382–8. [DOI] [PubMed]
- 327.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem 2003;278:43807–17. [DOI] [PubMed] [Google Scholar]
- 328.Liu M, Boussetta T, Makni-Maalej K, Fay M, Driss F, El-Benna J, Lagarde M, Guichardant M. Protectin DX, a double lipoxygenase product of DHA, inhibits both ROS production in human neutrophils and cyclooxygenase activities. Lipids 2014;49:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.White PJ, St-Pierre P, Charbonneau A, Mitchell PL, St-Amand E, Marcotte B, Marette A. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med 2014;20:664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hiram R, Rizcallah E, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin D1 reverses reactivity and Ca2+ sensitivity induced by ET-1, TNF-alpha, and IL-6 in the human pulmonary artery. Am J Physiol Heart Circ Physiol 2014;307:H1547–58. [DOI] [PubMed] [Google Scholar]
- 331.Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, Deng XM. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis 2014;33:457–64. [DOI] [PubMed]
- 332.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009;461:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 2011;31:18433–8. [DOI] [PMC free article] [PubMed]
- 334.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen 2013;21:35–43. [DOI] [PMC free article] [PubMed]
- 335.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol 2013;20:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012;484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther 2002;303:768–76. [DOI] [PubMed] [Google Scholar]
- 338.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev 2011;30:277–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Dogné JM, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends Pharmacol Sci 2005;26:639–44. [DOI] [PubMed] [Google Scholar]
- 341.Tateson JE, Moncada S, Vane JR. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins 1977;13:389–97. [DOI] [PubMed] [Google Scholar]
- 342.Svensson J, Hamberg M, Samuelsson B. On the formation and effects of thromboxane A2 in human platelets. Acta Physiol Scand 1976;98:285–94. [DOI] [PubMed] [Google Scholar]
- 343.Eklund B, Carlson LA. Central and peripheral circulatory effects and metabolic effects of different prostaglandins given I.V. to man. Prostaglandins 1980;20:333–47. [DOI] [PubMed] [Google Scholar]
- 344.Iyú D, Juttner M, Glenn JR, White AE, Johnson AJ, Fox SC, Heptinstall S. PGE1 and PGE2 modify platelet function through different prostanoid receptors. Prostaglandins Other Lipid Mediat 2011;94:9–16. [DOI] [PubMed] [Google Scholar]
- 345.Needleman P, Minkes M, Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science 1976;193:163–5. [DOI] [PubMed] [Google Scholar]
- 346.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation 1999;100:48–54. [DOI] [PubMed] [Google Scholar]
- 347.Marcus AJ, Weksler BB, Jaffe EA. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem 1978;253:7138–41. [PubMed] [Google Scholar]
- 348.Uderhardt S, Kronke G. 12/15-lipoxygenase during the regulation of inflammation, immunity, and self-tolerance. J Mol Med 2012;90:1247–56. [DOI] [PubMed] [Google Scholar]
- 349.Martínez-Clemente M, Claria J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care 2011;14:347–53. [DOI] [PubMed] [Google Scholar]
- 350.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res 2010;86:243–53. [DOI] [PubMed] [Google Scholar]
- 351.Ardaillou R, Baud L, Sraer J. Leukotrienes and other lipoxygenase products of arachidonic acid synthesized in the kidney. Am J Med 1986;81: 2B:12–22. [DOI] [PubMed] [Google Scholar]
- 352.Menna C, Olivieri F, Catalano A, Procopio A. Lipoxygenase inhibitors for cancer prevention: promises and risks. Curr Pharm Des 2010;16:725–33. [DOI] [PubMed] [Google Scholar]
- 353.Aharony D, Smith JB, Silver MJ. Regulation of arachidonate-induced platelet aggregation by the lipoxygenase product, 12-hydroperoxyeicosatetraenoic acid. Biochim Biophys Acta 1982;718:193–200. [DOI] [PubMed] [Google Scholar]
- 354.Katoh A, Ikeda H, Murohara T, Haramaki N, Ito H, Imaizumi T. Platelet-derived 12-hydroxyeicosatetraenoic acid plays an important role in mediating canine coronary thrombosis by regulating platelet glycoprotein IIb/IIIa activation. Circulation 1998;98:2891–8. [DOI] [PubMed] [Google Scholar]
- 355.O'Flaherty JT, Taylor JS, Thomas MJ. Receptors for the 5-oxo class of eicosanoids in neutrophils. J Biol Chem 1998;273:32535–41. [DOI] [PubMed] [Google Scholar]
- 356.Goetzl EJ, Woods JM, Gorman RR. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest 1977;59:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Powell WS, Hashefi M, Falck JR, Chauhan K, Rokach J, Wang SS, Mills E, MacLeod RJ. Effects of oxo and dihydro metabolites of 12-hydroxy-5,8,10,14-eicosatetraenoic acid on chemotaxis and cytosolic calcium levels in human neutrophils. J Leukoc Biol 1995;57:257–63. [DOI] [PubMed] [Google Scholar]
- 358.Samuelsson B. Leukotrienes: mediators of allergic reactions and inflammation. Int Arch Allergy Appl Immunol 1981;66: Suppl 1:98–106. [DOI] [PubMed] [Google Scholar]
- 359.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA 1995;92:9475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Serhan CN, Maddox JF, Petasis NA, Akritopoulouzanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin a(4) stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995;34:14609–15. [DOI] [PubMed] [Google Scholar]
- 362.Ku G, Thomas CE, Akeson AL, Jackson RL. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. J Biol Chem 1992;267:14183–8. [PubMed] [Google Scholar]
- 363.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res 2010;51:3046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yoshida Y, Yoshikawa A, Kinumi T, Ogawa Y, Saito Y, Ohara K, Yamamoto H, Imai Y, Niki E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer's disease patients and their potential as biomarkers. Neurobiol Aging 2009;30:174–85. [DOI] [PubMed] [Google Scholar]
- 365.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AA, Lawrence TS, et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis 1999;20:1985–95. [DOI] [PubMed] [Google Scholar]
- 366.Tabolacci C, Lentini A, Provenzano B, Gismondi A, Rossi S, Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16–F10 murine melanoma cells. Melanoma Res 2010;20:273–9. [DOI] [PubMed] [Google Scholar]
- 367.Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ziboh VA, Yun M, Hyde DM, Giri SN. gamma-Linolenic acid-containing diet attenuates bleomycin-induced lung fibrosis in hamsters. Lipids 1997;32:759–67. [DOI] [PubMed] [Google Scholar]
- 369.Evans JF, Nathaniel DJ, Zamboni RJ, Ford-Hutchinson AW. Leukotriene A3. A poor substrate but a potent inhibitor of rat and human neutrophil leukotriene A4 hydrolase. J Biol Chem 1985;260:10966–70. [PubMed] [Google Scholar]
- 370.Evans J, Zamboni R, Nathaniel D, Leveille C, Ford-Hutchinson AW. Characterization of biological properties of synthetic and biological leukotriene B3. Prostaglandins 1985;30:981–8. [DOI] [PubMed] [Google Scholar]
- 371.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Archiv 2010;459:881–95. [DOI] [PMC free article] [PubMed]
- 372.McGiff JC, Quilley J. 20-HETE and the kidney: resolution of old problems and new beginnings. Am J Physiol 1999;277:R607–23. [DOI] [PubMed] [Google Scholar]
- 373.Salmon ED, Goode D, Maugel TK, Bonar DB. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J Cell Biol 1976;69:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kaduce TL, Fang X, Harmon SD, Oltman CL, Dellsperger KC, Teesch LM, Gopal VR, Falck JR, Campbell WB, Weintraub NL, et al. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem 2004;279:2648–56. [DOI] [PubMed] [Google Scholar]
- 375.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol 2006;291:H2301–7. [DOI] [PubMed] [Google Scholar]
- 376.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 2002;39:690–4. [DOI] [PubMed] [Google Scholar]
- 377.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol 2013;61:188–96. [DOI] [PubMed] [Google Scholar]
- 378.Ramirez CE, Shuey MM, Milne GL, Gilbert K, Hui N, Yu C, Luther JM, Brown NJ. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins Other Lipid Mediat 2014;113–115:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ozawa T, Sugiyama S, Hayakawa M, Satake T, Taki F, Iwata M, Taki K. Existence of leukotoxin 9,10-epoxy-12-octadecenoate in lung lavages from rats breathing pure oxygen and from patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1988;137:535–40. [DOI] [PubMed] [Google Scholar]
- 380.Zhang W, Nagao M, Takatori T, Iwadate K, Itakura Y, Yamada Y, Iwase H, Oono T. Immunohistochemical dynamics of leukotoxin (9,10-epoxy-12-octadecenoic acid) in lungs of rats. Int J Legal Med 1995;107:174–8. [DOI] [PubMed] [Google Scholar]
- 381.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 1997;3:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ozawa T, Sugiyama S, Hayakawa M, Taki F, Hanaki Y. Neutrophil microsomes biosynthesize linoleate epoxide (9,10-epoxy-12-octadecenoate), a biological active substance. Biochem Biophys Res Commun 1988;152:1310–8. [DOI] [PubMed] [Google Scholar]
- 383.Hawcroft G, Loadman PM, Belluzzi A, Hull MA. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signaling in human colorectal cancer cells. Neoplasia 2010;12:618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Krämer HJ, Stevens J, Grimminger F, Seeger W. Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol 1996;52:1211–7. [DOI] [PubMed] [Google Scholar]
- 385.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. omega-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat 2014;113–115:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Miller AM, van Bekkum DW, Kobb SM, McCrohan MB, Knaan-Shanzer S. Dietary fish oil supplementation alters LTB4:LTB5 ratios but does not affect the expression of acute graft versus host disease in mice. Prostaglandins Leukot Essent Fatty Acids 1993;49:561–8. [DOI] [PubMed] [Google Scholar]
- 387.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest 2006;129:39–49. [DOI] [PubMed] [Google Scholar]
- 388.Lee TH, Menica-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem 1984;259:2383–9. [PubMed] [Google Scholar]
- 389.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA 1999;96:12010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 2009;206:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA 2010;107:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol 2006;176:1848–59. [DOI] [PubMed] [Google Scholar]
- 393.Chen P, Fenet B, Michaud S, Tomczyk N, Vericel E, Lagarde M, Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett 2009;583:3478–84. [DOI] [PubMed] [Google Scholar]
- 394.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008;112:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013;153:112–25. [DOI] [PubMed] [Google Scholar]
- 396.Imai Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim Biophys Acta 2015;1851:496–502. [DOI] [PubMed] [Google Scholar]
- 397.Morin C, Sirois M, Échavé V, Albadine R, Rousseau E. 17,18-Epoxyeicosatetraenoic acid targets pparγ and p38 mitogen–activated protein kinase to mediate its anti-inflammatory effects in the lung. Am J Respir Cell Mol Biol 2010;43:564–75. [DOI] [PubMed] [Google Scholar]
- 398.Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kargel E, Theuer J, Schwartzman ML, Haller H, Luft FC, Gollasch M, et al. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension 2002;39:609–13. [DOI] [PubMed] [Google Scholar]
- 399.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, et al. Dietary Omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-epoxygenase Pathway. J Lipid Res 2014;55:1150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem 2010;285:32720–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J 2006;20:401–3. [DOI] [PubMed]
- 402.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care 2005;8:115–21. [DOI] [PubMed] [Google Scholar]
- 403.Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J Chromatogr A 2014;1359:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med 2011;365:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, Hogg RJ, Trygg J, Hammock BD, Zivkovic AM. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 fatty acids. PLoS ONE 2013;8:e76575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Schuchardt JP, Schneider I, Willenberg I, Yang J, Hammock BD, Hahn A, Schebb NH. Increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acid levels in human plasma after a single dose of long-chain omega-3 PUFA. Prostaglandins Other Lipid Mediat 2014;109–111:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res 2010;51:2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res 2012;53:1662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Lundström SL, Yang J, Brannan JD, Haeggstrom JZ, Hammock BD, Nair P, O'Byrne P, Dahlen SE, Wheelock CE. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res 2013;57:1378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostag –Leukot Essent Fatty Acids 2012;87:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an alpha-linolenic acid-induced inhibition of soluble epoxide hydrolase. Hypertension 2014;64:53–9. [DOI] [PubMed] [Google Scholar]
- 412.Caligiuri SP, Aukema HM, Ravandi A, Pierce GN. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp Gerontol 2014;59:51–7. [DOI] [PubMed] [Google Scholar]
- 413.Calder PC, Deckelbaum RJ. Harmful, harmless or helpful? The n-6 fatty acid debate goes on. Curr Opin Clin Nutr Metab Care 2011;14:113–4. [DOI] [PubMed] [Google Scholar]
- 414.Lagarde M, Bernoud-Hubac N, Guichardant M. Expanding the horizons of lipidomics. Towards fluxolipidomics. Mol Membr Biol 2012;29:222–8. [DOI] [PubMed] [Google Scholar]
- 415.Lagarde M, Bernoud-Hubac N, Calzada C, Vericel E, Guichardant M. Lipidomics of essential fatty acids and oxygenated metabolites. Mol Nutr Food Res 2013;57:1347–58. [DOI] [PubMed] [Google Scholar]
- 416.Matsunobu T, Okuno T, Yokoyama C, Yokomizo T. Thromboxane A synthase-independent production of 12-hydroxyheptadecatrienoic acid, a BLT2 ligand. J Lipid Res 2013;54:2979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]