Abstract
Vitamin B-12 deficiency (<148 pmol/L) is associated with adverse maternal and neonatal outcomes, including developmental anomalies, spontaneous abortions, preeclampsia, and low birth weight (<2500 g). The importance of adequate vitamin B-12 status periconceptionally and during pregnancy cannot be overemphasized, given its fundamental role in neural myelination, brain development, and growth. Infants born to vitamin B-12-deficient women may be at increased risk of neural tube closure defects, and maternal vitamin B-12 insufficiency (<200 pmol/L) can impair infant growth, psychomotor function, and brain development, which may be irreversible. However, the underlying causal mechanisms are unknown. This review was conducted to examine the evidence that links maternal vitamin B-12 status and perinatal outcomes. Despite the high prevalence of vitamin B-12 deficiency and associated risk of pregnancy complications, few prospective studies and, to our knowledge, only 1 randomized trial have examined the effects of vitamin B-12 supplementation during pregnancy. The role of vitamin B-12 in the etiology of adverse perinatal outcomes needs to be elucidated to inform public health interventions.
Keywords: vitamin B-12, cobalamin, one-carbon metabolism, pregnancy, child health
Introduction
Vitamin B-12
Vitamin B-12 is a water-soluble B vitamin required as a cofactor for only 2 enzymes in human physiology: methionine synthase (MTR)6 which generates and uses methylcobalamin and functions in the cytosol to remethylate homocysteine to methionine, and l-methyl-malonyl-coenzyme A mutase, which uses 5′-deoxyadenosylcobalamin as a cofactor and functions in mitochondria to catabolize branched-chain and odd-chain FAs. MTR function is critical in folate-mediated one-carbon metabolism (FOCM), including DNA synthesis and chromatin methylation through the production of S-adenosylmethionine (AdoMet), the universal methyl donor required for methylation reactions throughout the human body. As such, vitamin B-12 is required for erythropoiesis, and the classic presentation of vitamin B-12 deficiency is hematologic: megaloblastic anemia (1). Its classic deficiency syndrome is pernicious anemia, a malabsorption disorder in which autoimmune destruction of gastric parietal cells leads to a lack of intrinsic factor (IF) synthesis and secretion, a vitamin B-12-binding protein required for efficient active transport of vitamin B-12 across the intestinal epithelium. However, vitamin B-12 deficiency also leads to neurologic manifestations, even in the absence of hematologic symptoms, which may be irreversible (2).
Vitamin B-12 is synthesized exclusively by microorganisms and is obtained in the diet through consumption of animal source foods, including meat and dairy. Several studies have noted low vitamin B-12 status in resource-limited settings, particularly in populations with low dietary intake of animal products (3, 4). In these settings, inadequate vitamin B-12 intake may be exacerbated by gastrointestinal infections and subsequent impaired vitamin B-12 absorption. Medications such as proton pump inhibitors and histamine receptor antagonists can also reduce gastric acid production and can decrease vitamin B-12 release from food sources and subsequent absorption (5). Previous studies have noted that use of acid blockers was associated with lower serum vitamin B-12 concentrations (6, 7).
Pathogenesis and mechanisms
This review focuses on vitamin B-12 and its function in FOCM through MTR. Vitamin B-12 is also involved in the catabolism of odd-chain FAs, branched-chain amino acids, and cholesterol through l-methyl-malonyl-coenzyme A mutase (8), which isomerizes methylmalonic-CoA to succinyl-CoA, and leads to elevated plasma methylmalonic acid (MMA) concentrations, an important vitamin B-12 biomarker. However, evidence is limited that this reaction contributes to the pathogenesis of vitamin B-12 deficiency.
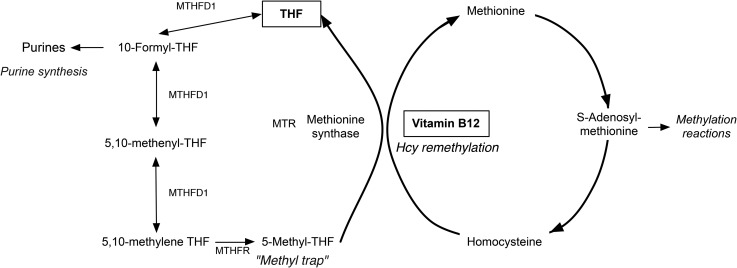
The MTR-catalyzed remethylation of homocysteine to methionine is a 2-step reaction that depends on both folate and vitamin B-12 (9). First, 5-methyltetrahydrofolate (5-methyl-THF) donates the N-5 methyl group to cobalamin, regenerating the unsubstituted tetrahydrofolate (THF) cofactor and methylcobalamin (10). Subsequently, cobalamin donates the methyl group to homocysteine, converting it to methionine (11). Vitamin B-12 deficiency presents direct and indirect consequences for the cell. Vitamin B-12 deficiency decreases MTR activity, directly affecting capacity for methionine synthesis and AdoMet availability for cellular methylation reactions, and accumulation of unmetabolized homocysteine in cells and blood and may be pathogenic (12). AdoMet is a universal methyl donor for nearly 100 cellular reactions and biological processes, including chromatin remodeling, gene transcription, protein localization, and neurotransmitter synthesis (13). Vitamin B-12 deficiency also indirectly disrupts other pathways within FOCM resulting from inability to metabolize 5-methyl-THF; this results in its accumulation in cells at the expense of other folate cofactor forms, a metabolic state known as the methyl-THF trap. Derivatives of folate other than 5-methyl-THF, including 10-formyl-THF and 5,10-methylene-THF are required for synthesis of purines and thymidylate, respectively; thymidylate synthesis is the rate-limiting deoxyribonucleotide in DNA synthesis (9). 5,10-Methylene-THF is reduced to 5-methyl-THF by methylenetetrahdryofolate reductase (MTHFR) in a reaction that is essentially irreversible under physiologic conditions. In the absence of vitamin B-12, cellular folate pools become enriched as 5-methyl-THF, acting as a functional folate deficiency that impairs de novo purine and thymidylate biosynthesis (9, 14).
Vitamin B-12 deficiency and its impact on FOCM result in metabolic impairments: homocysteine accumulation due to failure to remethylate homocysteine to methionine, impaired methionine and AdoMet biosynthesis, and accumulation of 5-methyl-THF, leading to impaired purine and thymidylate synthesis (Figure 1).
FIGURE 1.
Vitamin B-12 in 1-carbon metabolism. Vitamin B-12 deficiency prevents remethylation of homocysteine to methionine; results in an accumulation of 5-methyl-THF, or “folate trap,” decreasing the THF pool for synthesis of 10-formyl-THF; and impairs subsequent purine and thymidylate synthesis. MTHFD1, methylenetetrahydrofolate dehydrogenase 1; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; tHcy, total homocysteine; THF, tetrahydrofolate.
Vitamin B-12 deficiency is associated with biomarkers of genomic instability. Global DNA hypomethylation and decreased purine and pyrimidine synthesis impair genomic stability, causing chromosomal breaks and aberrations (15). A study in Turkey noted that DNA damage was increased in vitamin B-12-deficient (vitamin B-12: <200 pg/mL) children and their mothers; however, intramuscular cobalamin injections significantly decreased DNA damage in leukocytes of vitamin B-12-deficient children 9 d after treatment (P < 0.001) (16). Vitamin B-12 deficiency is associated with retardation of neural myelination in some studies (17–19). Impairment of FOCM by maternal deficiency of vitamin B-12, folate, other one-carbon donors, or genetic polymorphisms may have serious consequences for early neurologic development, although the underlying mechanisms are unknown.
Cobalamin uptake is a multistep process that involves a series of transporters and binding factors that complex with the nutrient to facilitate its absorption and transport in circulation. On ingestion, cobalamin is liberated from protein in food and binds to salivary R binder in the stomach. Vitamin B-12 dissociates from haptocorrin and binds to IF in the duodenum. Parietal cells in the stomach secrete both hydrochloric acid and the vitamin B-12-binding protein IF, and low IF concentrations impair vitamin B-12 absorption. Insufficient IF is caused by pernicious anemia, an autoimmune disease, which typically presents as megaloblastic anemia (20–22). IF-bound vitamin B-12 is absorbed primarily in the ileum by the cubulin receptor. Vitamin B-12 dissociates from IF in endothelial cells and enters portal circulation bound to transcobalamin (TC). Although most vitamin B-12 is bound to haptocorrin, TC-bound vitamin B-12 is delivered to the liver and other tissues where it is taken up by a TC receptor protein (23–25). The physiologic role of haptocorrin in circulation remains unclear, although haptocorrin may act as a scavenger protein to remove inactive vitamin B-12 analogs from circulation (26, 27).
Maternal vitamin B-12 concentrations during pregnancy are thought to be closely associated with fetal (28, 29) and early infant (30, 31) vitamin B-12 status. Vitamin B-12 is transported by haptocorrin and TC; >70% of vitamin B-12 transport across the placenta is facilitated by TC, whereas the remainder is bound by haptocorrin (32). Little is known about the pathway of vitamin B-12 transport from the maternal to the fetal circuits, but both vitamin B-12 carrier proteins are produced by the placenta and bind to vitamin B-12 in fetal circulation. Further, the placenta can regulate fetal B-12 uptake by adjusting its rate of TC synthesis (33, 34). Impaired TC synthesis can also lead to vitamin B-12 deficiency in the neonate (33).
Vitamin B-12 biomarkers
There are 4 major biomarkers of cobalamin status: total serum or plasma vitamin B-12, methylmalonic acid, total homocysteine (tHcy), and holotranscobalamin. Assessment of cobalamin status can be classified as 1) circulating biomarkers: vitamin B-12 and holotranscobalamin; 2) functional biomarkers: MMA and tHcy; and 3) other indicators, such as megaloblastic or pernicious anemia (or historically, the Schilling test of vitamin B-12 absorption via radioisotopes, which is no longer used).
Circulating biomarkers.
Total vitamin B-12 is the most widely used indicator of cobalamin status. Holotranscobalamin is the portion of circulating cobalamin (10–30%) that binds to TC for cellular uptake (35). As a result, holotranscobalamin is often referred to as active vitamin B-12. Holotranscobalamin was proposed as an optimal biomarker of early vitamin B-12 deficiency >20 y ago. Data suggest that, although total vitamin B-12 decreases during pregnancy (36, 37), holotranscobalamin concentrations remain relatively constant (36, 37); however, the role of holotranscobalamin in the etiology of perinatal outcomes has not been established. Data are limited regarding the utility of holotranscobalamin for cobalamin assessment compared with other biomarkers (38), and holotranscobalamin is not widely used in population-based assessments of vitamin B-12 status (23).
Functional biomarkers.
MMA and tHcy are functional indicators of vitamin B-12 status. Homocysteine reflects the central role of vitamin B-12 as a cofactor for MTR function, which generates and uses methylcobalamin and remethylates homocysteine to methionine in the cytosol. Homocysteine is commonly used as a biomarker of vitamin B-12 insufficiency, although it is not specific to vitamin B-12 and is influenced by a host of other factors both within (e.g., folate) and outside (e.g., renal disease) FOCM. MMA has been identified as an important functional indicator of cobalamin deficiency, because vitamin B-12 is a required cofactor for isomerization of methylmalonyl-CoA to succinyl-CoA.
A variety of vitamin B-12 biomarkers are used in clinical and population-based studies, including vitamin B-12, MMA, tHcy, and holotranscobalamin. The different biomarkers and cutoffs used to define vitamin B-12 status pose challenges when interpreting findings across studies. The 2011 NHANES roundtable series on vitamin B-12 and folate biomarkers status suggested the inclusion of both circulating and functional biomarkers (vitamin B-12 and MMA) in population-based studies (39–42). Recent data provide support for the potential use of cB12, a combination of all 4 vitamin B-12 biomarkers (vitamin B-12, MMA, homocysteine, and holotranscobalamin), as an indicator of vitamin B-12 status (43), although it has not yet been validated in clinical or field settings.
In this review, the following vitamin B-12 biomarkers were included: total vitamin B-12, MMA, tHcy, and holotranscobalamin. Vitamin B-12 deficiency was defined as total serum or plasma vitamin B-12 concentrations <148 pmol/L and vitamin B-12 insufficiency was defined as vitamin B-12 concentrations <200 pmol/L, unless otherwise noted.
Nutrient–nutrient interactions
Vitamin B-12 deficiency may co-occur with other nutrient deficiencies, including other B vitamins involved in FOCM. These micronutrients, such as folate, riboflavin, and choline, may also influence functional vitamin B-12 biomarkers and risk of adverse perinatal outcomes.
Folate.
Folate and vitamin B-12 are interrelated through the shared MTR pathway in FOCM (9, 44, 45). Folate is also negatively correlated with tHcy, because 5-methyl-THF is the indirect donor of a methyl group to homocysteine (46, 47). A primary function of FOCM is DNA synthesis; vitamin B-12 or folate deficiency leads to impaired DNA synthesis and erythropoiesis and causes megaloblastic anemia (48). Although it represents a small percentage of anemia cases, megaloblastic anemia is a potential consequence of folate deficiency (49, 50). In a study in Canada, 90% of patients with megaloblastic anemia presented with low folate (serum folate: <4.0 ng/mL) and vitamin B-12 (vitamin B-12: <100 pg/mL) status (51). Other adverse perinatal outcomes are also common to vitamin B-12 and folate deficiencies, most notably neural tube closure defects (NTDs).
Choline and B vitamins.
Other nutrients, such as choline, vitamin B-6, and riboflavin, are involved in FOCM. Two pathways methylate homocysteine: vitamin B-12- and betaine-dependent pathways. Betaine methylates homocysteine to methionine via the betaine:homocysteine methyltransferase pathway. Betaine (52) and choline (53) were inversely associated with homocysteine concentrations in previous studies; choline oxidizes irreversibly to betaine, a direct methyl donor for homocysteine. Choline and betaine insufficiency may also affect fetal neurulation. Higher maternal choline intake was associated with a lower odds of NTDs, although findings were not significant (OR: 0.90; 95% CI: 0.78, 1.04; P > 0.05) (54). riboflavin and vitamin B-6 are also required cofactors for folate derivative pathways. Vitamin B-6 is required for conversion of THF to 5,10-methylene-THF, the folate derivative required for thymidylate and purine synthesis (55, 56). Riboflavin is required for conversion of 5,10-methylene-THF to 5-methyl-THF for the MTHFR pathway (55).
The complexity and interrelations of micronutrients in FOCM constrain the ability to ascertain the specific effects of vitamin B-12 on the risk of adverse perinatal outcomes. In addition, the genetic heterogeneity that affects the activity of enzymes in FOCM, such as MTHFR, can modulate FOCM (57). A mathematical model of chemical kinetics of folate pathways in FOCM suggested that folate deficiency has little effect on the rate of these metabolic reactions (58). Compensation between the different pathways of FOCM may help to mitigate the effects of a specific methyl donor deficiency.
Vitamin B-12 and perinatal health
Vitamin B-12 deficiency is a major public health problem worldwide, although representative population-level data are limited (59, 60). Although the prevalence of vitamin B-12 deficiency in the United States is estimated to be relatively low (6%), the burden of vitamin B-12 deficiency is higher in subpopulations across the life cycle (61). The burden of vitamin B-12 deficiency is particularly high in resource-limited settings in Latin America (∼40%) (62), Sub-Saharan Africa (70%) (63, 64), and South Asia (70–80%) (65, 66). For example, in a randomized trial of vitamin B-12 supplementation in Bangalore, India, 51% of pregnant women were vitamin B-12 deficient (vitamin B-12: <150 pmol/L) and 42% had impaired vitamin B-12 status (vitamin B-12: <150 pmol/L; MMA: >0.26 μmol/L) at their first prenatal visit (67).
Maternal vitamin B-12 deficiency is associated with increased risk of common pregnancy complications, including spontaneous abortion, recurrent pregnancy loss, small-for-gestational age (SGA), low birth weight (LBW), intrauterine growth restriction (IUGR), and NTDs (44, 65, 68–77). Infants born to vitamin B-12-deficient women are at increased risk of developmental abnormalities, growth failure, and anemia (18, 78). Vitamin B-12 insufficiency can impair infant growth, psychomotor function, and brain development, which may be irreversible (18, 78).
The high prevalence of vitamin B-12 deficiency and increasing evidence that low periconceptional vitamin B-12 status is a risk factor for adverse perinatal outcomes have stimulated interest in mandatory food fortification with vitamin B-12 (61). However, data are limited on the efficacy of vitamin B-12 interventions on maternal and child health outcomes, and the potential mechanisms need to be elucidated.
The objective of this review was to examine the evidence that links maternal vitamin B-12 status and perinatal outcomes. We examined the evidence from observational studies and intervention trials on the role of vitamin B-12 in the etiology of pregnancy outcomes. We then discuss the potential roles of other micronutrients in FOCM, research gaps, and implications for the development of interventions to improve the health of mothers and young children, with emphasis on resource-limited settings.
Methods
Search strategy and selection process
We conducted a structured literature search with the use of MEDLINE electronic databases. Relevant Medical Subject Heading terms were used to identify published studies from June 24, 1999, to June 25, 2014. The Medical Subject Heading terms used are provided in Table 1, and the search strategy is summarized in Figure 2.
TABLE 1.
MeSH Search Terms1
| MeSH Search Terms |
| ((“vitamin b 12"[MeSH Terms] OR “vitamin b 12"[All Fields] OR (“vitamin"[All Fields] AND “B-12"[All Fields]) OR “vitamin B-12"[All Fields]) OR (“vitamin b 12"[MeSH Terms] OR “vitamin b 12"[All Fields] OR “cobalamin"[All Fields]) OR (“vitamin b 12"[MeSH Terms] OR “vitamin b 12"[All Fields])) AND ((“pregnancy"[MeSH Terms] OR “pregnancy"[All Fields]) OR (“infant, newborn"[MeSH Terms] OR (“infant"[All Fields] AND “newborn"[All Fields]) OR “newborn infant"[All Fields] OR “neonate"[All Fields]) OR (“infant"[MeSH Terms] OR “infant"[All Fields]) OR (“birth weight"[MeSH Terms] OR (“birth"[All Fields] AND “weight"[All Fields]) OR “birth weight"[All Fields]) OR (“delivery, obstetric"[MeSH Terms] OR (“delivery"[All Fields] AND “obstetric"[All Fields]) OR “obstetric delivery"[All Fields] OR “delivery"[All Fields]) OR (“parturition"[MeSH Terms] OR “parturition"[All Fields] OR “birth"[All Fields]) OR (“growth and development"[Subheading] OR (“growth"[All Fields] AND “development"[All Fields]) OR “growth and development"[All Fields] OR “growth"[All Fields] OR “growth"[MeSH Terms]) OR (“fetal growth retardation"[All Fields] OR “fetal growth retardation"[MeSH Terms] OR (“fetal"[All Fields] AND “growth"[All Fields] AND “retardation"[All Fields]) OR “fetal growth retardation"[All Fields]) OR (“fetal growth retardation"[MeSH Terms] OR (“fetal"[All Fields] AND “growth"[All Fields] AND “retardation"[All Fields]) OR “fetal growth retardation"[All Fields] OR (“fetal"[All Fields] AND “growth"[All Fields] AND “restriction"[All Fields]) OR “fetal growth restriction"[All Fields]) OR preterm[All Fields] OR intrauterine[All Fields] OR (“neural tube defects"[MeSH Terms] OR (“neural"[All Fields] AND “tube"[All Fields] AND “defects"[All Fields]) OR “neural tube defects"[All Fields] OR (“neural"[All Fields] AND “tube"[All Fields] AND “defect"[All Fields]) OR “neural tube defect"[All Fields])) |
MeSH, Medical Subject Heading.
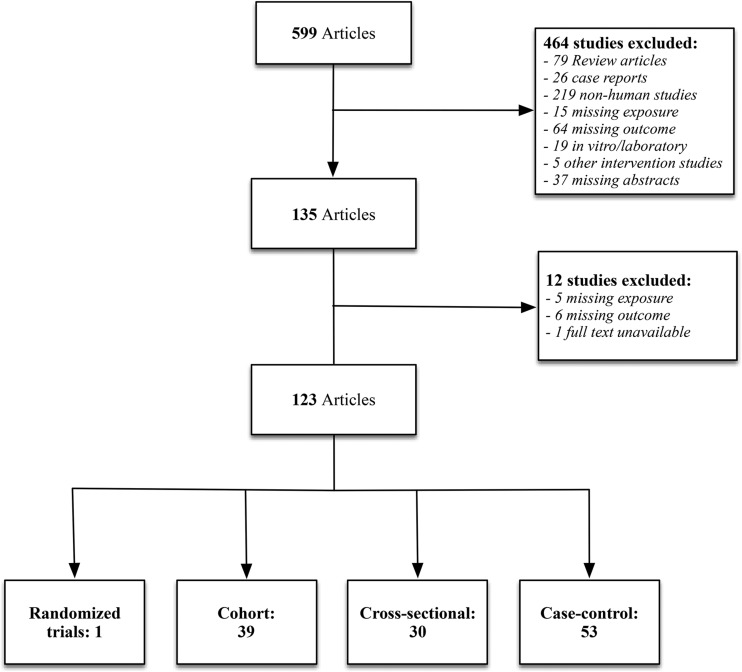
FIGURE 2.
Search strategy. A diagrammatic representation of the retrieval strategy used for identifying and selecting studies for inclusion in the final analysis.
Initial inclusion criteria for this review were the availability of an abstract and inclusion of data on maternal vitamin B-12 status or intake and perinatal outcomes. The following biomarkers of maternal vitamin B-12 status were included in this review: serum and plasma total vitamin B-12, MMA, tHcy, and holotranscobalamin. Abstracts were searched, full-text articles were extracted and reviewed, and the following inclusion criteria were applied: 1) human studies, 2) pregnant women and infants, and 3) availability data on maternal vitamin B-12 status or intake and perinatal outcomes. All observational cross-sectional, case-control, and cohort studies; and randomized trials; interventions; and quasi-randomized and uncontrolled trials that met the above-mentioned selection criteria were included. Sources were retrieved, collected, indexed, and assessed for vitamin B-12 and perinatal outcome data. Additional sources were identified from bibliographies of published studies, manual searches of related articles in references, scientific meeting abstracts, white papers, and expert committee reports. An additional search was conducted to find review articles, which were examined to cross-reference other relevant studies. A standardized data table was used to extract and summarize key information from studies. As part of this protocol, publication date, authors, study design, setting, population, definitions of exposures and outcomes, main findings, and study limitations were recorded.
Results
Literature review
The structured literature search resulted in 599 articles, which were reviewed for potential inclusion in this review. After 464 studies were excluded, 135 studies were extracted for further review. After excluding 12 studies that did not meet inclusion criteria, 123 studies were included in this review. These included 122 observational studies and 1 randomized trial. The structured literature search is summarized in Figure 2, and findings from these studies are summarized in detail in the subsequent tables (Supplemental Tables 1–4).
Vitamin B-12 deficiency and perinatal outcomes
Several studies have reported significant associations between low maternal vitamin B-12 status and increased risk of adverse perinatal outcomes. Maternal vitamin B-12 deficiency was associated with increased risk of gestational diabetes (vitamin B-12: <150 pmol/L) (79), early miscarriage (vitamin B-12: <180 pg/L) (75), and NTDs (vitamin B-12: <133 pg/mL) (44). Although some studies have used a cutoff of serum or plasma vitamin B-12 of <148 or 150 pmol/L, most studies used tertiles, quartiles, or deciles of vitamin B-12 concentrations, based on its distribution in the population. Lower maternal vitamin B-12 status has been associated with increased risk of NTDs (68–72, 80), spontaneous abortions (73), early miscarriage (74), SGA (76), IUGR (77), and LBW (<2500 g) (77).
Maternal anemia.
The classic presentation of vitamin B-12 deficiency is megaloblastic anemia (81). However, evidence is limited for an association between maternal vitamin B-12 status during pregnancy and risk of maternal anemia. In a cross-sectional study in China, serum vitamin B-12 (P < 0.012) and folate (P < 0.010) concentrations were significantly lower in anemic (hemoglobin: <11.0 g/dL) pregnant women than in nonanemic women (82). Similarly, in a cross-sectional study in Brazil, pregnant women in the lowest serum vitamin B-12 quartile (vitamin B-12: <145 pmol/L) had significantly lower hemoglobin (P < 0.05) and serum ferritin (P < 0.05) concentrations than women in the upper 3 vitamin B-12 quartiles (83). However, in a cross-sectional study in Ethiopia, no differences were found in plasma vitamin B-12 concentrations between anemic (hemoglobin: <11.5 g/dL) and nonanemic women (84), and a cross-sectional study in the United Kingdom found that only 5.6% of women with low vitamin B-12 status (vitamin B-12: <211 ng/L) had low hemoglobin (<10.5 g/dL) concentrations (85).
Preeclampsia.
Findings regarding the associations between maternal vitamin B-12 status and risk of preeclampsia have varied among vitamin B-12-related biomarkers. For example, in several studies, women with preeclampsia had significantly higher homocysteine concentrations than normotensive pregnant women; however, no significant differences were found in serum or plasma vitamin B-12 concentrations between groups (86–88). Among patients with severe preeclampsia in Greece (blood pressure: ≥170/110 mm Hg) and Turkey (blood pressure: ≥160/110 mm Hg), only homocysteine concentrations were significantly higher, compared with normotensive pregnant women (P < 0.001 and P < 0.05, respectively) (86, 87). In addition, in a cohort study in Netherlands, pregnant women with the lowest folate quintiles (plasma folate: ≤9.2 nmol/L) and the highest homocysteine quintiles (tHcy: ≥8.3 μmol/L) had a 4-fold greater odds of preeclampsia than women with normal homocysteine (tHcy: <8.3 μmol/L) and folate (folate: >9.2 nmol/L) status [adjusted OR (AOR): 4.27; 95% CI: 1.21, 15.0; P = 0.02] (89).
Gestational diabetes.
The data on the associations between maternal vitamin B-12 deficiency and gestational diabetes are limited and conflicting. In a cohort study in India among 785 pregnant women, 43% were vitamin B-12 deficient (vitamin B-12: <150 pmol/L) and only 4% were folate deficient (folate: <7 pmol/L); maternal vitamin B-12 deficiency at 30 wk gestation was associated with significantly increased risk of adiposity and insulin resistance during pregnancy (79). Further, the incidence of gestational diabetes in vitamin B-12-deficient women increased from the lowest to the highest tertiles of plasma folate (79). In a study in Turkey, pregnant women with gestational diabetes (blood glucose: >135 mg/dL; and a positive oral glucose tolerance test with 100 g glucose) had higher homocysteine concentrations than pregnant women without gestational diabetes (90). However, the associations between maternal vitamin B-12 status and gestational diabetes could not be ascertained because vitamin B-12 deficiency was an exclusion criteria for this study (90). A case-control study in Poland found no differences in vitamin B-12, homocysteine, or folate concentrations between women with gestational diabetes (positive oral glucose tolerance test with 75 g glucose) and women without gestational diabetes, although all participants received prenatal multivitamin supplements that contained vitamin B-12 (91).
Fetal outcomes.
Several studies have examined the associations of maternal vitamin B-12 status and risk of adverse fetal outcomes, including spontaneous abortions. In a study in France, maternal vitamin B-12 deficiency (serum vitamin B-12: <180 pg/L) was associated with a 9-fold greater odds of early recurrent abortion (≥2 consecutive abortions with the same partner with <12 wk of amenorrhea), compared with replete vitamin B-12 status (OR: 9.5; 95% CI: 1.2, 75; P < 0.05) (75). In a study among Syrian women, higher maternal vitamin B-12 concentrations were associated with lower odds of recurrent pregnancy loss (OR: 0.99; 95% CI: 0.98, 0.99; P = 0.012) (92). In a study in the United Kingdom, higher maternal homocysteine concentrations were associated with a 1.3 times greater odds of spontaneous abortion (OR: 1.27; 95% CI: 1.01, 1.61; P = 0.041), although no associations were noted with maternal vitamin B-12 status (93).
Preterm birth.
Higher homocysteine concentrations have been associated with increased occurrence of preterm birth or lower gestational age at delivery in several studies, although most studies have not assessed vitamin B-12 concentrations. For example, in a case-control study in China, hyperhomocysteinemia (tHcy: ≥12.4 μM) was associated with 4-fold greater odds of preterm birth (<37 wk gestation; AOR: 3.6; 95% CI: 1.3, 10.0; P < 0.05) (94). In a case-control study in Nepal, higher tHcy concentrations were associated with lower gestational age at delivery (P < 0.05) (95). Additional studies have noted associations between other vitamin B-12 biomarkers and gestational age at delivery, although findings are conflicting. A study in Norway found that higher serum holohaptocorrin concentrations in cord blood were correlated with increased gestational age at delivery (P = 0.026) (96). In contrast, a case-control study in India found that women with preterm delivery had significantly higher plasma vitamin B-12 concentrations at delivery than women with term deliveries (P = 0.003) (96, 97).
LBW.
Lower maternal serum and cord blood vitamin B-12 concentrations have been associated with increased risk of adverse pregnancy outcomes in several studies, including LBW (<2500 g), SGA, and IUGR. For example, lower cord vitamin B-12 concentrations were associated with lower birth weight (P = 0.02) in a study in the United Kingdom (98). In a study in Bangalore, India, lower cord blood vitamin B-12 concentrations were reported in neonates with LBW (<2500 g) than in neonates with normal birth weight (>3000 g) (P = 0.022) (29). Similarly, women with serum vitamin B-12 concentrations in the lowest tertile (median: 157 pg/mL) in the first trimester (10–12 wk gestation) had a 6.2 times greater odds of having an IUGR delivery (OR: 6.22; 95% CI: 1.89, 20.47; P < 0.05) than women in the highest tertile (median: 304 pg/mL) (P = 0.003) (99). Similarly, women with serum vitamin B-12 concentrations in the lowest tertile in the second (OR: 8.35; 95% CI: 2.85, 24.46; P < 0.001) or third (OR: 2.73; 95% CI: 1.04, 7.12; P = 0.048) trimesters had significantly increased odds of IUGR, compared with women in the highest tertile (99). In a prospective analysis in the same cohort in Bangalore (n = 1838), higher intake of prenatal folic acid supplements in combination with low vitamin B-12 intake was associated with increased risk of SGA: women in the lowest tertile of vitamin B-12:folate intake ratio had a 2.7 times greater odds of having a SGA delivery (AOR: 2.73; 95% CI: 1.17, 6.37; P < 0.05), than women in the highest tertile (100). Findings were similar in analyses of serum vitamin B-12 and erythrocyte folate concentrations in a random subset (n = 316) (100). These findings suggest that, in addition to deficiencies of vitamins B-12 and folate alone, unbalanced vitamin B-12 and folate intake or status may interact and increase risk of adverse pregnancy outcomes (100, 101).
Higher tHcy concentrations were associated with significantly increased risk of LBW in a study in Netherlands (89). Similarly, in a study in Japan, maternal tHcy concentrations were associated with significantly lower birth weight: each 0.1-μmol/L increase in maternal tHcy concentrations during the third trimester was associated with a 151-g lower infant birth weight (P < 0.01) (102). In a study in Pakistan, maternal tHcy concentrations were significantly higher in IUGR cases than in control cases (P = 0.02) (103). In the study in Netherlands, maternal tHcy concentrations in the highest quintile (tHcy: ≥8.3 μmol/L) were associated with a 1.7-fold greater odds of SGA (AOR: 1.68; 95% CI: 1.16, 2.43; P = 0.006); maternal tHcy in the highest quintile (homocysteine: ≥8.3 μmol/L) in combination with plasma folate in the lowest quintile (≤9.2 nmol/L) was associated with a 4-fold greater odds of SGA than women with normal tHcy (<8.3 μmol/L) and folate (>9.2 nmol/L) status (AOR: 4.04; 95% CI: 1.97, 8.28; P < 0.001) (89).
NTDs.
Several small case-control studies have reported an association between lower maternal vitamin B-12 concentrations and increased occurrence of NTDs (44, 68–72, 104–106). A case-control study in Egypt reported that low maternal vitamin B-12 status (plasma vitamin B-12: <200 ng/L) was associated with a 2.7 times greater odds of having an NTD-affected pregnancy (OR: 2.67; 95% CI: 1.42, 5.02; P < 0.05) (106). In a similar study in China, low maternal serum vitamin B-12 concentrations (<55 pmol/L) were associated with a 5-fold increase in the odds of NTDs (OR: 4.96; 95% CI: 1.94, 12.7; P < 0.05), after adjusting for maternal age, education, gravidity, and gestational age at delivery (105). In a case-control study in Ontario, Canada, in the folic acid fortification era, women in the lowest quartile of serum holotranscobalamin (≤55.3 pmol/L) had a 2.9 times greater odds of having an NTD-affected pregnancy compared to women in the reference quartile (>121.0 pmol/L; OR: 2.9; 95% CI: 1.2, 6.9; P < 0.05) (71). In case-control studies in the United States among mothers with or without NTD-affected pregnancies, lower maternal serum vitamin B-12 (≤372 pg/mL) and higher tHcy (≥6.7 μmol/L) concentrations were associated with a 2.4 and 2.6 times greater odds of NTDs, respectively; no differences were found in maternal folate concentrations between cases and controls (107). Further, in the case-control study in the United States, the combination of low vitamin B-12 (≤372 pg/mL) and high tHcy (≥6.7 μmol/L) concentrations was associated with a 4.8 times greater odds of NTDs (OR: 4.8; 95% CI: 1.6, 14.8; P < 0.05) (107). Similarly, in a case-control study in Ireland among women with either NTD-affected pregnancies or a history of NTD-affected pregnancies, low maternal vitamin B-12 (<250 ng/L) concentrations predicted a 2.5–3.0 times greater odds of NTDs, independent of folate status (72). Few studies have examined the associations between maternal vitamin B-12 status and specific types of NTDs, such as spina bifida or anencephaly. In a case-control study in Netherlands, women with vitamin B-12 deficiency (<185 pmol/L) had a 3.5 times greater odds of spina bifida than women with replete vitamin B-12 status (OR: 3.5; 95% CI: 1.3, 8.9; P < 0.05) (108). Most studies on maternal vitamin B-12 status and NTD occurrence to date have used a case-control design, in which maternal vitamin B-12 status and NTDs were assessed concurrently at delivery. The lack of assessment of maternal vitamin B-12 status periconceptionally or prospectively during pregnancy constrains the interpretation of findings and establishment of a causal temporal relation.
Other congenital defects.
Several case-control studies in Netherlands have noted that AdoMet, S-adenosyl-l-homocysteine, and tHcy concentrations were significantly higher in women who had neonates with congenital heart defects (CHDs) than newborns without CHDs (109–112). One case-control study in Netherlands also found that women with low serum vitamin B-12 concentrations (≤185 pmol/L) had a 4 times greater odds of having a neonate with nonsyndromic orofacial cleft (OR: 4.4; 95% CI: 1.1, 18.2; P < 0.05) than women with replete vitamin B-12 status (113). The studies that investigated the associations of maternal vitamin B-12 status and CHDs were case-control studies conducted at the same medical facility in Netherlands, which constrains the generalizability of findings.
Infant vitamin B-12 status.
Several cross-sectional studies have examined the associations between maternal and neonatal vitamin B-12 status at delivery and have found strong correlations for serum vitamin B-12 and related biomarkers. For example, in a cross-sectional study in Germany, maternal and cord blood holotranscobalamin concentrations were significantly correlated (r = 0.68, P < 0.001) (28). Several cohort studies have noted associations between maternal serum and neonatal (or cord blood) vitamin B-12 concentrations, and some studies found that neonatal vitamin B-12 concentrations were significantly higher than maternal concentrations. For example, in a cohort study in Bangalore, India, cord blood vitamin B-12 concentrations were 27% higher than maternal vitamin B-12 concentrations (29). Similar results were reported in a study in Pakistan, where cord serum vitamin B-12 concentrations were 2-fold greater than maternal vitamin B-12 concentrations (P < 0.001) (103).
In the first randomized trial of vitamin B-12 supplementation (50 μg/d vitamin B-12 and iron-folic acid, compared to iron-folic acid alone) in 366 pregnant women in Bangalore, India, 51% of women were vitamin B-12 deficient (<150 pmol/L) and 42% had impaired vitamin B-12 status (vitamin B-12: <150 pmol/L; MMA: >0.26 μmol/L) at their first prenatal visit (67). Infants born to vitamin B-12-deficient mothers were 1.6 times more likely to be vitamin B-12 deficient (<150 pmol/L) (P < 0.01) at 6 wk of age compared to infants born to vitamin B-12 replete mothers (31). Daily prenatal vitamin B-12 supplementation with iron-folic acid improved maternal vitamin B-12 status (P < 0.01), reduced the risk of IUGR (P = 0.10), and increased breast milk (P < 0.01) and infant plasma vitamin B-12 concentrations (P < 0.01) at 6 wk of age, compared to iron-folic acid alone. However, the substantial increases in breast milk and infant vitamin B-12 status reverted after cessation of daily vitamin B-12 supplementation.
Infant cognitive development.
Few studies to date have examined the role of vitamin B-12 in cognitive development in infants. In a study in the United States, inadequate vitamin B-12 intake (<2.0 μg/d) was correlated with poorer infant mental development (β = −1.6, P < 0.05) (114). In a study in the United Kingdom, every 2-fold increase in maternal vitamin B-12 intake (log2) was associated with a 2-point increase in infant intelligence quotient (Weschler Intelligence Scale for Children) (P < 0.001), although this was not significant (0.7-u, P = 0.06), after adjusting for potential confounders (115). The association between maternal vitamin B-12 status and cognitive development in children later in life was also noted in several studies.
Other infant outcomes.
Additional infant outcomes assessed in relation to maternal vitamin B-12 status included excessive crying and insulin resistance. Maternal serum vitamin B-12 concentrations in the lowest 4 quintiles (vitamin B-12: ≤425 pg/mL) were associated with a 2.6–3.9 times greater risk of excessive crying in infants, compared to the highest quintile (>425 pg/mL); in women with high depressive and anxiety symptoms, low vitamin B-12 status (<251 pg/mL) was associated with a 12-fold greater odds of having an infant with excessive crying (OR: 12.2; 95% CI: 1.54, 97.0; P < 0.05) than women with high vitamin B-12 status (>425 pg/mL) (116). Low maternal plasma vitamin B-12 concentrations (<150 pmol/L) were also associated with increased risk of infant insulin resistance (P = 0.03) with the use of the homeostatic model assessment of insulin resistance (117). Infant insulin resistance was highest among mothers with the lowest vitamin B-12 (<114 pmol/L) and highest erythrocyte folate (>1144 nmol/L) concentrations, although the nutrient interactions were not significant (P = 0.70). Several studies have also noted an association between low maternal vitamin B-12 status during early pregnancy and insulin resistance in children later in life, including large prospective studies in Nepal (<148 pmol/L) (118) and India (<150 pmol/L) (79).
Discussion
Vitamin B-12 deficiency is common during pregnancy and is associated with adverse outcomes for the mother and infant. Recent observational studies suggest that maternal vitamin B-12 deficiency is associated with increased risk of pregnancy complications, such as NTDs (65, 68–72, 80), spontaneous abortions (73–75), preeclampsia (65), and LBW (76, 77, 99). However, most studies were case-control or cross-sectional in design, which constrains the interpretability of findings and causal inference. Evidence is limited or conflicting to support the role of maternal vitamin B-12 status in other perinatal outcomes. Only 1 randomized trial was conducted to date to examine the effects of vitamin B-12 supplementation during pregnancy, and it was not designed to examine the effects of vitamin B-12 on pregnancy outcomes (67).
The high prevalence of vitamin B-12 deficiency and increasing evidence that low periconceptional vitamin B-12 status may be a risk factor for adverse perinatal outcomes have stimulated interest in mandatory food fortification with vitamin B-12 (61). However, data are lacking on the efficacy of vitamin B-12 interventions on specific maternal and child health outcomes, and the potential mechanisms need to be elucidated.
Findings from observational studies indicate that imbalance in micronutrients involved in FOCM may present deleterious health consequences for the mother and infant. There is also concern regarding the use of folic acid interventions in the context of vitamin B-12 deficiency (i.e., folic acid may have adverse effects in individuals with low vitamin B-12 status) (100, 101). Recent findings suggest that, in addition to vitamin B-12 and folate deficiencies alone, unbalanced vitamin B-12 and folate intake or status may be associated with adverse health outcomes (100).
Research gaps and future directions
Vitamin B-12 deficiency is an important public health issue globally, and poses a severe threat to maternal and child health.
Study design.
The observational studies to date have several limitations, which warrant caution when interpreting findings. Most studies were case-control or cross-sectional in design, which are subject to reverse causation and limit the establishment of causal temporal relations. There is a lack of prospective studies to examine the role of vitamin B-12 in the etiology of perinatal outcomes. Data are limited on maternal vitamin B-12 status periconceptionally when it is critical for risk of birth defects. In addition, few studies have measured maternal vitamin B-12 status prospectively throughout the course of pregnancy. Vitamin B-12 biomarkers need to be examined early in pregnancy and at multiple times during gestation and postpartum periods. In the first randomized trial of prenatal vitamin B-12 supplementation to date in Bangalore, India (67), daily prenatal vitamin B-12 supplementation with iron-folic acid significantly improved maternal and infant vitamin B-12 status, compared to iron-folic acid alone. However the substantial improvements in vitamin B-12 status reverted after cessation of daily vitamin B-12 supplementation, suggesting that longer-term public health approaches are needed.
Vitamin B-12 biomarkers.
The variety of vitamin B-12 biomarkers used in different studies (including serum and plasma vitamin B-12, MMA, tHcy, and holotranscobalamin), laboratory assessment methods, and cutoffs for vitamin B-12 deficiency and insufficiency pose challenges when interpreting findings across different studies. Further research is needed to establish appropriate biomarkers of vitamin B-12 status, particularly in pregnancy and in the context of field and resource-limited settings.
Additional nutrients in FOCM.
One-carbon metabolism is influenced by a variety of nutrients, which interact and can compensate for one another when there is a deficiency of a single nutrient. The role of additional nutrients in FOCM, such as folate and choline, need to be examined in the context of vitamin B-12 deficiency and perinatal outcomes. In particular, maternal folate status needs to be considered in prospective analyses, because it may influence vitamin B-12 status and is an independent risk factor for adverse pregnancy outcomes, such as NTDs. In addition to adjusting for maternal folate status in analyses, the interactions between these nutrients in the etiology of adverse perinatal outcomes need to be elucidated.
Additional outcomes.
Infants are considered a high-risk group for vitamin B-12 deficiency, based on the low vitamin B-12 content in breast milk. However, data are relatively limited on vitamin B-12 breast milk concentrations or related biomarkers and associations with health outcomes in early childhood. In particular, few studies have examined the role of maternal or breast milk vitamin B-12 concentrations in early infant cognition, immune function, and psychomotor development. Future research is needed to determine the role of vitamin B-12 (mechanisms, biomarkers, cutoffs) in the etiology of functional outcomes.
Translational research.
Studies in animal and cell models provide valuable insight into the causal mechanisms and pathways of vitamin B-12 in the etiology of adverse perinatal outcomes. Findings from human and animal studies have identified a potential role of vitamin B-12 deficiency in the development of adverse perinatal outcomes, such as NTDs. However, there is limited prospective and causal data in human populations. The biological mechanisms of vitamin B-12-related disorders need to be determined experimentally in relevant animal and cell models to inform translational research in at-risk human populations.
Conclusions
Maternal vitamin B-12 deficiency is a major public health problem and is associated with increased risk of adverse perinatal outcomes. The importance of adequate vitamin B-12 status, particularly during pregnancy and early childhood, cannot be overemphasized in light of its role in neural myelination, brain development, and fetal and child growth. Further research is urgently needed to elucidate the role of vitamin B-12 in the etiology of perinatal outcomes and to inform public health approaches in at-risk populations.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AdoMet, S-adenosylmethionine; AOR, adjusted odds ratio; CHD, congenital heart defect; FOCM, folate-mediated one-carbon metabolism; IF, intrinsic factor; IUGR, intrauterine growth restriction; LBW, low birth weight; MMA, methylmalonic acid; MTHFR, methylenetetrahdryofolate reductase; MTR, methionine synthase; NTD, neural tube closure defect; SGA, small-for-gestational-age; TC, transcobalamin; tHcy, total homocysteine; THF, tetrahydrofolate; 5-methyl-THF, 5-methyltetrahydrofolate; 5,10-methylene-THF, 5,10-methylenetetrahyhdrofolate.
References
- 1.Aaron S, Kumar S, Vijayan J, Jacob J, Alexander M, Gnanamuthu C. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency-related neurological syndromes. Neurol India 2005;53:55–8. [DOI] [PubMed] [Google Scholar]
- 2.Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, Marcell PD, Stabler SP, Allen RH. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988;318:1720–8. [DOI] [PubMed] [Google Scholar]
- 3.Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull 2008;29(2 Suppl):S20–34; discussion S35–7. [DOI] [PubMed] [Google Scholar]
- 4.Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev 2014;72:68–81. [DOI] [PubMed] [Google Scholar]
- 5.Wolters M, Strohle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prev Med 2004;39:1256–66. [DOI] [PubMed] [Google Scholar]
- 6.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol 2004;57:422–8. [DOI] [PubMed] [Google Scholar]
- 7.Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med 1998;104:422–30. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi-Iñiguez T, Garcia-Hernandez E, Arreguin-Espinosa R, Flores ME. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J Zhejiang Univ Sci B 2012;13:423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shane B, Stokstad EL. Vitamin B12-folate interrelationships. Annu Rev Nutr 1985;5:115–41. [DOI] [PubMed] [Google Scholar]
- 10.Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res 2010;43:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 2004;80:1611–7. [DOI] [PubMed] [Google Scholar]
- 12.Guéant JL, Caillerez-Fofou M, Battaglia-Hsu S, Alberto JM, Freund JN, Dulluc I, Adjalla C, Maury F, Merle C, Nicolas JP, et al. Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase. Biochimie 2013;95:1033–40. [DOI] [PubMed] [Google Scholar]
- 13.Stover PJ. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J Nutrigenet Nutrigenomics 2011;4:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smulders YM, Smith DE, Kok RM, Teerlink T, Swinkels DW, Stehouwer CD, Jakobs C. Cellular folate vitamer distribution during and after correction of vitamin B12 deficiency: a case for the methylfolate trap. Br J Haematol 2006;132:623–9. [DOI] [PubMed] [Google Scholar]
- 15.Fenech M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat Res 2012;733:21–33. [DOI] [PubMed] [Google Scholar]
- 16.Minnet C, Koc A, Aycicek A, Kocyigit A. Vitamin B12 treatment reduces mononuclear DNA damage. Pediatr Int 2011;53:1023–7. [DOI] [PubMed] [Google Scholar]
- 17.Lövblad K, Ramelli G, Remonda L, Nirkko AC, Ozdoba C, Schroth G. Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings. Pediatr Radiol 1997;27:155–8. [DOI] [PubMed] [Google Scholar]
- 18.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008;29(2 Suppl):S126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCaddon A. Vitamin B12 in neurology and ageing; clinical and genetic aspects. Biochimie 2013;95:1066–76. [DOI] [PubMed] [Google Scholar]
- 20.Morel CF, Rosenblatt DS. Inborn errors of folate and cobalamin transport and metabolism. In: Sarafoglou K, Hoffmann G, Roth K, editors. Essential pediatric endocrinology and inborn errors of metabolism. New York: McGraw Hill, 2006. p. 195–210. [Google Scholar]
- 21.O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients 2010;2:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brito A, Hertrampf E, Olivares M, Gaitán D, Sánchez H, Allen LH, Uauy R. [Folate, vitamin B12 and human health] Rev Med Chil 2012;140:1464–75 (in Spanish). [DOI] [PubMed]
- 23.Nexo E, Hoffmann-Lucke E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr 2011;94:359S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bose S, Kalra S, Yammani RR, Ahuja R, Seetharam B. Plasma membrane delivery, endocytosis and turnover of transcobalamin receptor in polarized human intestinal epithelial cells. J Physiol 2007;581:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadros EV, Regec AL, Khan KM, Quadros E, Rothenberg SP. Transcobalamin II synthesized in the intestinal villi facilitates transfer of cobalamin to the portal blood. Am J Physiol 1999;277:G161–6. [DOI] [PubMed] [Google Scholar]
- 26.Furger E, Frei DC, Schibli R, Fischer E, Prota AE. Structural basis for universal corrinoid recognition by the cobalamin transport protein haptocorrin. J Biol Chem 2013;288:25466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stupperich E, Nexø E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur J Biochem 1991;199:299–303. [DOI] [PubMed] [Google Scholar]
- 28.Obeid R, Morkbak AL, Munz W, Nexo E, Herrmann W. The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin Chem 2006;52:263–9. [DOI] [PubMed] [Google Scholar]
- 29.Muthayya S, Dwarkanath P, Mhaskar M, Mhaskar R, Thomas A, Duggan C, Fawzi WW, Bhat S, Vaz M, Kurpad A. The relationship of neonatal serum vitamin B12 status with birth weight. Asia Pac J Clin Nutr 2006;15:538–43. [PubMed] [Google Scholar]
- 30.Deegan KL, Jones KM, Zuleta C, Ramirez-Zea M, Lildballe DL, Nexo E, Allen LH. Breast milk vitamin B-12 concentrations in Guatemalan women are correlated with maternal but not infant vitamin B-12 status at 12 months postpartum. J Nutr 2012;142:112–6. [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein JL, Kurpad AV, Thomas T, Bose B, Samuel T, Srinivasan K, Duggan C. Vitamin B12 status in pregnant women and their children in India (135.6). FASEB J 2014;28(1 Suppl). [Google Scholar]
- 32.Perez-D’Gregorio RE, Miller RK. Transport and endogenous release of vitamin B12 in the dually perfused human placenta. J Pediatr 1998;132:S35–42. [DOI] [PubMed] [Google Scholar]
- 33.Schneider H, Miller RK. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int J Dev Biol 2010;54:367–75. [DOI] [PubMed] [Google Scholar]
- 34.Miller RK, Faber W, Asai M, D’Gregorio RP, Ng WW, Shah Y, Neth-Jessee L. The role of the human placenta in embryonic nutrition. Impact of environmental and social factors. Ann N Y Acad Sci 1993;678:92–107. [DOI] [PubMed] [Google Scholar]
- 35.Nexø E, Andersen J. Unsaturated and cobalamin saturated transcobalamin I and II in normal human plasma. Scand J Clin Lab Invest 1977;37:723–8. [DOI] [PubMed] [Google Scholar]
- 36.Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, Hertz J. Iron prophylaxis during pregnancy–how much iron is needed? A randomized dose- response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstet Gynecol Scand 2005;84:238–47. [DOI] [PubMed] [Google Scholar]
- 37.Morkbak AL, Hvas AM, Milman N, Nexo E. Holotranscobalamin remains unchanged during pregnancy. Longitudinal changes of cobalamins and their binding proteins during pregnancy and postpartum. Haematologica 2007;92:1711–2. [DOI] [PubMed] [Google Scholar]
- 38.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94:348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr 2011;94:297S–302S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 2011;94:322S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:303S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedosov SN, Brito A, Miller JW, Green R, Allen LH. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med. 2015. Jul 1;53(8):1215–25. [DOI] [PubMed] [Google Scholar]
- 44.Gu Q, Li Y, Cui ZL, Luo XP. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Paediatr 2012;101:e486–90. [DOI] [PubMed]
- 45.Jones KM, Ramirez-Zea M, Zuleta C, Allen LH. Prevalent vitamin B-12 deficiency in twelve-month-old Guatemalan infants is predicted by maternal B-12 deficiency and infant diet. J Nutr 2007;137:1307–13. [DOI] [PubMed] [Google Scholar]
- 46.Vanderjagt DJ, Ujah IA, Patel A, Kellywood J, Crossey MJ, Allen RH, Stabler SP, Obande OS, Glew RH. Subclinical vitamin B12 deficiency in pregnant women attending an antenatal clinic in Nigeria. J Obstet Gynaecol 2009;29:288–95. [DOI] [PubMed] [Google Scholar]
- 47.Couto FD, Moreira LM, Dos Santos DB, Reis MG, Goncalves MS. Folate, vitamin B12 and total homocysteine levels in neonates from Brazil. Eur J Clin Nutr 2007;61:382–6. [DOI] [PubMed] [Google Scholar]
- 48.Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician 2009;79:203–8. [PubMed] [Google Scholar]
- 49.Panigrahi A, Sahoo PB. Nutritional anemia and its epidemiological correlates among women of reproductive age in an urban slum of Bhubaneswar, Orissa. Indian J Public Health 2011;55:317–20. [DOI] [PubMed] [Google Scholar]
- 50.Cardoso MA, Scopel KK, Muniz PT, Villamor E, Ferreira MU. Underlying factors associated with anemia in Amazonian children: a population-based, cross-sectional study. PLoS One 2012;7:e36341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowenstein L, Brunton L, Hsieh YS. Nutritional anemia and megaloblastosis in pregnancy. Can Med Assoc J 1966;94:636–45. [PMC free article] [PubMed] [Google Scholar]
- 52.Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J Jr, Mar MH, Zeisel SH, Castro C, Garrow T, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J 2003;17:512–4. [DOI] [PubMed] [Google Scholar]
- 53.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Ann Rev Nutr 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9. [DOI] [PubMed] [Google Scholar]
- 55.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 2012;23:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood) 2004;229:988–95. [DOI] [PubMed] [Google Scholar]
- 57.McNulty H, Dowey le RC, Strain JJ, Dunne A, Ward M, Molloy AM, McAnena LB, Hughes JP, Hannon-Fletcher M, Scott JM. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C->T polymorphism. Circulation 2006;113:74–80. [DOI] [PubMed] [Google Scholar]
- 58.Nijhout HF, Reed MC, Budu P, Ulrich CM. A mathematical model of the folate cycle: new insights into folate homeostasis. J Biol Chem 2004;279:55008–16. [DOI] [PubMed] [Google Scholar]
- 59.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89:693S–6S. [DOI] [PubMed] [Google Scholar]
- 60.McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull 2008;29(2 Suppl):S38–51. [DOI] [PubMed] [Google Scholar]
- 61.Allen LH, Rosenberg IH, Oakley GP, Omenn GS. Considering the case for vitamin B12 fortification of flour. Food Nutr Bull 2010;31(1 Suppl):S36–46. [DOI] [PubMed] [Google Scholar]
- 62.Allen LH. Folate and vitamin B12 status in the Americas. Nutr Rev 2004;62(6 Pt 2):S29–33; discussion S34. [DOI] [PubMed] [Google Scholar]
- 63.Siekmann JH, Allen LH, Bwibo NO, Demment MW, Murphy SP, Neumann CG. Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B-12 is the only detectable micronutrient response to meat or milk supplementation. J Nutr 2003;133(11 Suppl 2):3972S–80S. [DOI] [PubMed] [Google Scholar]
- 64.McLean ED, Allen LH, Neumann CG, Peerson JM, Siekmann JH, Murphy SP, Bwibo NO, Demment MW. Low plasma vitamin B-12 in Kenyan school children is highly prevalent and improved by supplemental animal source foods. J Nutr 2007;137:676–82. [DOI] [PubMed] [Google Scholar]
- 65.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Guttormsen AB, Joglekar A, Sayyad MG, Ulvik A, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr 2001;74:233–41. [DOI] [PubMed] [Google Scholar]
- 66.Taneja S, Bhandari N, Strand TA, Sommerfelt H, Refsum H, Ueland PM, Schneede J, Bahl R, Bhan MK. Cobalamin and folate status in infants and young children in a low-to-middle income community in India. Am J Clin Nutr 2007;86:1302–9. [DOI] [PubMed] [Google Scholar]
- 67.Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, Finkelstein JL, Lukose A, Fawzi W, Allen LH, et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr 2014;144:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ratan SK, Rattan KN, Pandey RM, Singhal S, Kharab S, Bala M, Singh V, Jhanwar A. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr Surg Int 2008;24:803–8. [DOI] [PubMed] [Google Scholar]
- 69.Adams MJ Jr, Khoury MJ, Scanlon KS, Stevenson RE, Knight GJ, Haddow JE, Sylvester GC, Cheek JE, Henry JP, Stabler SP, et al. Elevated midtrimester serum methylmalonic acid levels as a risk factor for neural tube defects. Teratology 1995;51:311–7. [DOI] [PubMed] [Google Scholar]
- 70.Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, Yang H, Gravel RA, Rozen R. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab 1999;67:317–23. [DOI] [PubMed] [Google Scholar]
- 71.Ray JG, Wyatt PR, Thompson MD, Vermeulen MJ, Meier C, Wong PY, Farrell SA, Cole DE. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology 2007;18:362–6. [DOI] [PubMed] [Google Scholar]
- 72.Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics 2009;123:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowland AS, Baird DD, Shore DL, Weinberg CR, Savitz DA, Wilcox AJ. Nitrous oxide and spontaneous abortion in female dental assistants. Am J Epidemiol 1995;141:531–8. [DOI] [PubMed] [Google Scholar]
- 74.Hübner U, Alwan A, Jouma M, Tabbaa M, Schorr H, Herrmann W. Low serum vitamin B12 is associated with recurrent pregnancy loss in Syrian women. Clin Chem Lab Med 2008;46:1265–9. [DOI] [PubMed] [Google Scholar]
- 75.Reznikoff-Etiévant MF, Zittoun J, Vaylet C, Pernet P, Milliez J. Low vitamin B(12) level as a risk factor for very early recurrent abortion. Eur J Obstet Gynecol Reprod Biol 2002;104:156–9. [DOI] [PubMed] [Google Scholar]
- 76.Hogeveen M, Blom HJ, den Heijer M. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr 2012;95:130–6. [DOI] [PubMed] [Google Scholar]
- 77.Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr 2006;60:791–801. [DOI] [PubMed] [Google Scholar]
- 78.Pepper MR, Black MM. B12 in fetal development. Semin Cell Dev Biol 2011;22:619–23. [DOI] [PubMed] [Google Scholar]
- 79.Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CL, Yajnik CS, Fall CH. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009;52:2350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 81.de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 2008;29(2 Suppl):S238–44. [DOI] [PubMed] [Google Scholar]
- 82.Ma AG, Chen XC, Wang Y, Xu RX, Zheng MC, Li JS. The multiple vitamin status of Chinese pregnant women with anemia and nonanemia in the last trimester. J Nutr Sci Vitaminol (Tokyo) 2004;50:87–92. [DOI] [PubMed] [Google Scholar]
- 83.de Azevedo Paiva A, Rondó PH, Guerra-Shinohara EM, Silva CS. The influence of iron, vitamin B(12), and folate levels on soluble transferrin receptor concentration in pregnant women. Clin Chim Acta 2003;334–:197–203. [DOI] [PubMed] [Google Scholar]
- 84.Gibson RS, Abebe Y, Stabler S, Allen RH, Westcott JE, Stoecker BJ, Krebs NF, Hambidge KM. Zinc, gravida, infection, and iron, but not vitamin B-12 or folate status, predict hemoglobin during pregnancy in Southern Ethiopia. J Nutr 2008;138:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shields RC, Caric V, Hair M, Jones O, Wark L, McColl MD, Ramsay JE. Pregnancy-specific reference ranges for haematological variables in a Scottish population. J Obstet Gynaecol 2011;31:286–9. [DOI] [PubMed] [Google Scholar]
- 86.Makedos G, Papanicolaou A, Hitoglou A, Kalogiannidis I, Makedos A, Vrazioti V, Goutzioulis M. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch Gynecol Obstet 2007;275:121–4. [DOI] [PubMed] [Google Scholar]
- 87.Acilmis YG, Dikensoy E, Kutlar AI, Balat O, Cebesoy FB, Ozturk E, Cicek H, Pence S. Homocysteine, folic acid and vitamin B12 levels in maternal and umbilical cord plasma and homocysteine levels in placenta in pregnant women with pre-eclampsia. J Obstet Gynaecol Res 2011;37:45–50. [DOI] [PubMed] [Google Scholar]
- 88.Patrick TE, Powers RW, Daftary AR, Ness RB, Roberts JM. Homocysteine and folic acid are inversely related in black women with preeclampsia. Hypertension 2004;43:1279–82. [DOI] [PubMed] [Google Scholar]
- 89.Bergen NE, Jaddoe VW, Timmermans S, Hofman A, Lindemans J, Russcher H, Raat H, Steegers-Theunissen RP, Steegers EA. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG 2012;119:739–51. [DOI] [PubMed] [Google Scholar]
- 90.Tarim E, Bagis T, Kilicdag E, Erkanli S, Aslan E, Sezgin N, Kuscu E. Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand 2004;83:543–7. [DOI] [PubMed] [Google Scholar]
- 91.Idzior-Waluś B, Cyganek K, Sztefko K, Seghieri G, Breschi MC, Waluś-Miarka M, Kawalec E, Seretny M, Sieradzki J. Total plasma homocysteine correlates in women with gestational diabetes. Arch Gynecol Obstet 2008;278:309–13. [DOI] [PubMed] [Google Scholar]
- 92.Hübner U, Alwan A, Jouma M, Tabbaa M, Schorr H, Herrmann W. Low serum vitamin B12 is associated with recurrent pregnancy loss in Syrian women. Clin Chem Lab Med 2008;46:1265–9. [DOI] [PubMed] [Google Scholar]
- 93.Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, Templeton A, Haites N, Campbell D, Bhattacharya S. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet 2006;367:1513–9. [DOI] [PubMed] [Google Scholar]
- 94.Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J, Xu X. Preconception homocysteine and B vitamin status and birth outcomes in Chinese women. Am J Clin Nutr 2002;76:1385–91. [DOI] [PubMed] [Google Scholar]
- 95.Bondevik GT, Schneede J, Refsum H, Lie RT, Ulstein M, Kvale G. Homocysteine and methylmalonic acid levels in pregnant Nepali women. Should cobalamin supplementation be considered? Eur J Clin Nutr 2001;55:856–64. [DOI] [PubMed] [Google Scholar]
- 96.Hay G, Clausen T, Whitelaw A, Trygg K, Johnston C, Henriksen T, Refsum H. Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. J Nutr 2010;140:557–64. [DOI] [PubMed] [Google Scholar]
- 97.Dhobale M, Chavan P, Kulkarni A, Mehendale S, Pisal H, Joshi S. Reduced folate, increased vitamin B(12) and homocysteine concentrations in women delivering preterm. Ann Nutr Metab 2012;61:7–14. [DOI] [PubMed] [Google Scholar]
- 98.Relton CL, Pearce MS, Parker L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br J Nutr 2005;93:593–9. [DOI] [PubMed] [Google Scholar]
- 99.Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr 2006;60:791–801. [DOI] [PubMed] [Google Scholar]
- 100.Dwarkanath P, Barzilay JR, Thomas T, Thomas A, Bhat S, Kurpad AV. High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am J Clin Nutr 2013;98:1450–8. [DOI] [PubMed] [Google Scholar]
- 101.Gadgil M, Joshi K, Pandit A, Otiv S, Joshi R, Brenna JT, Patwardhan B. Imbalance of folic acid and vitamin B12 is associated with birth outcome: an Indian pregnant women study. Eur J Clin Nutr 2014;68:726–9. [DOI] [PubMed] [Google Scholar]
- 102.Takimoto H, Mito N, Umegaki K, Ishiwaki A, Kusama K, Abe S, Yamawaki M, Fukuoka H, Ohta C, Yoshiike N. Relationship between dietary folate intakes, maternal plasma total homocysteine and B-vitamins during pregnancy and fetal growth in Japan. Eur J Nutr 2007;46:300–6. [DOI] [PubMed] [Google Scholar]
- 103.Lindblad B, Zaman S, Malik A, Martin H, Ekstrom AM, Amu S, Holmgren A, Norman M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet Gynecol Scand 2005;84:1055–61. [DOI] [PubMed] [Google Scholar]
- 104.Zhang HY, Luo GA, Liang QL, Wang Y, Yang HH, Wang YM, Zheng XY, Song XM, Chen G, Zhang T, et al. Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp Neurol 2008;212:515–21. [DOI] [PubMed] [Google Scholar]
- 105.Zhang T, Xin R, Gu X, Wang F, Pei L, Lin L, Chen G, Wu J, Zheng X. Maternal serum vitamin B12, folate and homocysteine and the risk of neural tube defects in the offspring in a high-risk area of China. Public Health Nutr 2009;12:680–6. [DOI] [PubMed] [Google Scholar]
- 106.Gaber KR, Farag MK, Soliman SE, El-Bassyouni HT, El-Kamah G. Maternal vitamin B12 and the risk of fetal neural tube defects in Egyptian patients. Clin Lab 2007;53:69–75. [PubMed] [Google Scholar]
- 107.Felkner M, Suarez L, Canfield MA, Brender JD, Sun Q. Maternal serum homocysteine and risk for neural tube defects in a Texas-Mexico border population. Birth Defects Res A Clin Mol Teratol 2009;85:574–81. [DOI] [PubMed] [Google Scholar]
- 108.Groenen PM, van Rooij IA, Peer PG, Gooskens RH, Zielhuis GA, Steegers-Theunissen RP. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am J Obstet Gynecol 2004;191:11–7. [DOI] [PubMed] [Google Scholar]
- 109.Obermann-Borst SA, van Driel LM, Helbing WA, de Jonge R, Wildhagen MF, Steegers EA, Steegers-Theunissen RP. Congenital heart defects and biomarkers of methylation in children: a case-control study. Eur J Clin Invest 2011;41:143–50. [DOI] [PubMed] [Google Scholar]
- 110.Verkleij-Hagoort AC, van Driel LM, Lindemans J, Isaacs A, Steegers EA, Helbing WA, Uitterlinden AG, Steegers-Theunissen RP. Genetic and lifestyle factors related to the periconception vitamin B12 status and congenital heart defects: a Dutch case-control study. Mol Genet Metab 2008;94:112–9. [DOI] [PubMed] [Google Scholar]
- 111.Verkleij-Hagoort AC, de Vries JH, Ursem NT, de Jonge R, Hop WC, Steegers-Theunissen RP. Dietary intake of B-vitamins in mothers born a child with a congenital heart defect. Eur J Nutr 2006;45:478–86. [DOI] [PubMed] [Google Scholar]
- 112.Verkleij-Hagoort AC, Verlinde M, Ursem NT, Lindemans J, Helbing WA, Ottenkamp J, Siebel FM, Gittenberger-de Groot AC, de Jonge R, Bartelings MM, et al. Maternal hyperhomocysteinaemia is a risk factor for congenital heart disease. BJOG 2006;113:1412–8. [DOI] [PubMed] [Google Scholar]
- 113.van Rooij IA, Swinkels DW, Blom HJ, Merkus HM, Steegers-Theunissen RP. Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts. Am J Obstet Gynecol 2003;189:1155–60. [DOI] [PubMed] [Google Scholar]
- 114.del Río Garcia C, Torres-Sánchez L, Chen J, Schnaas L, Hernández C, Osorio E, Portillo MG, López-Carrillo L. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci 2009;12:13–20. [DOI] [PubMed] [Google Scholar]
- 115.Bonilla C, Lawlor DA, Taylor AE, Gunnell DJ, Ben-Shlomo Y, Ness AR, Timpson NJ, St Pourcain B, Ring SM, Emmett PM, et al. Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS One 2012;7:e51084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goedhart G, van der Wal MF, van Eijsden M, Bonsel GJ. Maternal vitamin B-12 and folate status during pregnancy and excessive infant crying. Early Hum Dev 2011;87:309–14. [DOI] [PubMed] [Google Scholar]
- 117.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 2008;51:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, West KP Jr. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr 2011;141:1912–7. [DOI] [PubMed] [Google Scholar]