Abstract
Plasma transthyretin (TTR) is a plasma protein secreted by the liver that circulates bound to retinol-binding protein 4 (RBP4) and its retinol ligand. TTR is the sole plasma protein that reveals from birth to old age evolutionary patterns that are closely superimposable to those of lean body mass (LBM) and thus works as the best surrogate analyte of LBM. Any alteration in energy-to-protein balance impairs the accretion of LBM reserves and causes early depression of TTR production. In acute inflammatory states, cytokines induce urinary leakage of nitrogenous catabolites, deplete LBM stores, and cause an abrupt decrease in TTR and RBP4 concentrations. As a result, thyroxine and retinol ligands are released in free form, creating a second frontline that strengthens that primarily initiated by cytokines. Malnutrition and inflammation thus keep in check TTR and RBP4 secretion by using distinct and unrelated physiologic pathways, but they operate in concert to downregulate LBM stores. The biomarker complex integrates these opposite mechanisms at any time and thereby constitutes an ideally suited tool to determine residual LBM resources still available for metabolic responses, hence predicting outcomes of the most interwoven disease conditions.
Keywords: transthyretin, lean body mass, malnutrition, inflammation, endocrine implications
Introduction
Transthyretin (TTR)5 is a plasma protein endowed with multiple functional properties (1), which was proposed as an indicator of protein nutritional status in The Lancet in 1972 (2). From the beginning, the use of TTR as a biomarker has received strong support for assessing a broad array of diseases comprising metabolic and septic disorders (3–6) throughout the whole life span from birth (7) to old age (8). Some researchers have nevertheless cast doubt on the clinical reliability of TTR, which explains why the marker soon became a matter of persistent controversy. The most aggressive opposition came from members of the Division of Plasma Proteins of the International Federation of Clinical Chemistry (9). Their criticism has sown confusion in the minds of many clinicians, contributing to prognostic and therapeutic nihilism. The time is ripe to revisit some basic aspects of the physiopathology of TTR and to reconsider its nutritional merits in the light of recently published data showing that fluctuations in plasma TTR reflect the size of and alterations in LBM in humans (10, 11).
Plasma TTR in Healthy Subjects
TTR is a highly conserved protein in animal species, having been secreted by the choroid plexus (CP) and diffused within the cerebrospinal fluid (CSF) of reptiles for 300 million years (12). Liver synthesis of TTR occurred much later, ∼100 million years ago in most classes of vertebrates (birds, Diprotodonta marsupials, eutherian mammals) but restricted to the developmental period in amphibians, reptiles, and Polyprotodonta marsupials (13, 14). Using electrophoretic methods, researchers were able to identify TTR in human CSF (15) and in human blood (16) in 1942. The protein was isolated from human serum and submitted to preliminary chemical analysis in 1956 (17). TTR was rapidly recognized as the third specific binding protein (BP) ensuring the transport of thyroid hormones; the other 2 are serum albumin (ALB) and thyroxine-binding globulin (TBG). The 4 identical subunits [each composed of 127 amino acids (AAs)] coalesce noncovalently to generate a nonglycosylated edifice with a molecular mass (MM) of 55 kDa (18). One of the monomers transports a small companion protein displaying a single binding site for 1 molecule of all-trans retinol (R-OH)—shown as retinol-binding protein (RBP) 4 (holo-RBP, 21-kDa MM) (19). The aggregation of holo-RBP to TTR occurs in the liver and stabilizes TTR before its extracellular export in the form of a retinol circulating complex (RCC) (20, 21), which has an MM of 76 kDa. Despite different biological half-lives (T1/2) 2 d for TTR (22) and 0.5 d for RBP4 (23), both molecules remain attached at a close 1:1:1 stoichiometry (21). RBP4 is a member of the lipocalin superfamily thought to interact with specific receptors to deliver R-OH to target cells (24). In contrast, after having released its retinol ligand, apoRBP exhibits a significantly reduced TTR binding affinity and biological T1/2, undergoing rapid glomerular leakage, tubular disintegration, and subsequent recycling of the released AA residues (23). The data indicate that, within the RCC edifice, TTR protects RBP4 from premature urinary output and serves as a limiting factor for the delivery of retinoid compounds to peripheral tissues (25). The concept is supported by knockout mouse experiments showing rapid kidney clearance of RBP4 from plasma in TTR-deficient mice (26), although intrahepatic sequestration of native RBP molecules in TTR-mutant strains was also advocated (27). Under usual conditions, there exist large excesses of TTR (4.5 mM) over RBP4 (2 mM) in human plasma, which shows that virtually all RBP4 molecules are bound to TTR (21). In contrast, and depending on physiopathologic conditions, variable proportions of TTR may circulate in free and uncomplexed form. It has been shown that RBP4 may be overexpressed in obese and diabetic animals and humans, inducing a stage of insulin resistance (28). The pathway is mediated by a plasma receptor protein known as stimulated by retinoic acid 6 (STRA6), which governs the uptake of R-OH from holo-RBP and triggers a cytosolic cascade of functional abnormalities (29). Interestingly, TTR counteracts these molecular defects by blocking STRA6 receptor activities (29), suggesting that coexisting malnutrition, as assessed by lowered TTR plasma concentrations, would accentuate the STRA6-induced metabolic dysfunction generated by the burden of overweight.
TTR secreted by the liver is detected in the bloodstream as early as 8 wk after conception (30). At birth, plasma TTR concentrations are approximately two-thirds those measured in healthy mothers and thereafter increase linearly without sexual difference during infant growth (31). Human puberty is characterized by major hormonal and metabolic alterations leading to increased height, weight gain, and a substantial redistribution of body tissues (32). Androgens strongly promote the development of muscle mass in male teenagers, whereas estrogens contribute to minimal enlargement of the female musculature and stimulate the accretion of subcutaneous fat depots (32). As a result, a significantly higher S-shaped elevation of TTR is recorded in male adolescents compared with the blunted curve documented in teenaged girls (33, 34) (Figure 1). In healthy adults, the sex-related difference in plasma TTR and RBP4 concentrations is maintained and reaches a plateau during full sexual maturity (33, 34). Normal TTR plasma concentrations are maintained at ∼300–330 mg/L in males and at ∼250–270 mg/L in females (33, 34), whereas RBP4 plasma concentrations manifest a similar sexual difference at ∼63 mg/L and 52 mg/L (34), respectively. Starting at ∼60 y of age, muscle mass undergoes stepwise shrinking leading to sarcopenia, but with a steeper slope in elderly men (35, 36), accounting for the concomitant decline in TTR and RBP4 concentrations (34). Both TTR (15) and RBP (37) are synthesized by the CP and secreted in CSF following regulatory pathways distinct from those of the liver (38), which suggests that the brain might escape the harmful events affecting the overall body economy in the course of nutritional or inflammatory disorders. Moreover, intracerebral TTR and retinoids manifest a multitude of neuroprotective effects that sustain normal brain activities and contain the development of amyloidogenic processes (11). With increasing age, choroidal production rates of TTR (39) and RBP (40) reveal declining trends likely to be genetically programmed, which explains why elderly persons are no longer safeguarded against the risk of neural deterioration (11). The data suggest that the best physiologic way to prevent or delay the onset of neurodegenerative lesions is to promote nutritional rehabilitation and eradicate underlying co-morbidities, thus allowing maintenance of liver-derived RCC concentrations within normal ranges to compensate for CP-born involutive patterns (11).
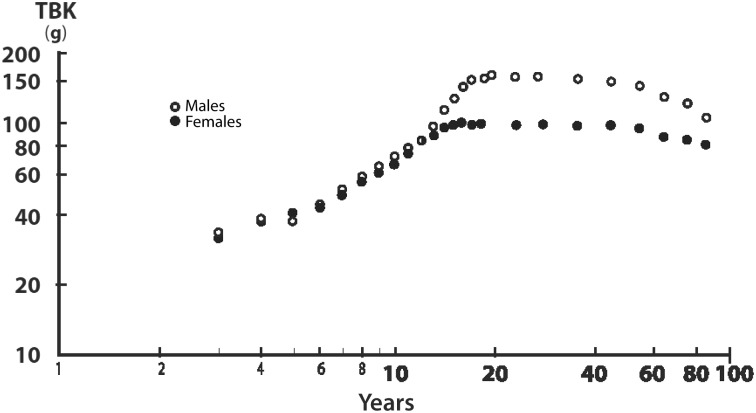
FIGURE 1.
Body accretion of TBK during the life span of healthy subjects. TBK compared with age on double-logarithmic ordinates is shown. TBK was determined by the measurement of 40K with the use of DXA. Averages of normal TBK values were obtained from the compilation of 7 different clinical investigations performed in healthy subjects aged 1 to 85 y. The data reveal that evolutionary patterns of lean body mass disclose linearly increasing, superimposable values from birth until the onset of adolescence and the occurrence of sexual dimorphism between teenaged boys and girls, then stabilization of TBK values until the age of 65 y. Elderly persons are characterized by gradual LBM downsizing in both sexes, with a more pronounced decline in male subjects in relation to more marked involutive trends toward sarcopenia. After the age of 65 y, the loss of TBK per decade is estimated at 5% in men and 3.5% in women. TBK, total body potassium. Adapted from reference 35 (Figure 5.5) with permission.
LBM in Healthy Subjects
The most accurate method for assessing LBM size relies on the use of DXA. Ninety-five percent of body potassium is sequestered within metabolically active tissues; the quantitation of this elemental concentration is achieved by the measurement of gamma rays emitted by the naturally occurring 40K radioisotope (35). DXA is regarded as the gold-standard method, which allows measurement of total body potassium (TBK), known to be tightly correlated with total body nitrogen (TBN), within an average potassium-to-nitrogen ratio of ∼3 mEq K/g N (41). In a healthy reference man weighing 70 kg, LBM constitutes the bulk of TBN (64 mol) and of TBK (3600 mmol) (42). In normal humans, TBK manifests evolutionary patterns closely matching those outlined by TTR plasma concentrations, with minimal concentrations found at birth (35). TBK then exhibits superimposed values and a linear increase during prepubertal growth (35), followed by comparable hormonally induced sex dimorphism at the onset of adolescence. Adulthood is characterized by similar plateaus and senescence by gradual involution of TBK values starting from the age of 65 y (35) (Figure 2). Schematically, LBM may be subdivided into the visceral protein compartment (liver, small intestine, thymo-hemopoietic tissues), which is distinguished by rapid turnover rates, and the structural protein compartment (muscle mass, skin, and connective tissues), which is identified by slower turnover rates (43). According to the pioneering metabolic studies performed by Brožek and Grande (44) in human adults, the fractional synthesis and renewal rates of visceral tissues are ∼10 to 20 times as active as those of the body structural compartment. However, the size of the latter compartment represents almost half the total body weight (BW), reaching in absolute terms an equivalent contribution to the daily turnover of the proteins in the body. The 2 main components of resting energy expenditure, liver (2.6% of BW but oxygen consumption of 44 mL O2/kg) and musculature (37% of BW and 2.3 mL O2/kg), contribute equally to basal metabolic activities, estimated at 26.4% and 25.6%, respectively (44). The data are in close harmony with a more recent clinical investigation showing that these chief organs together generate 50% of resting energy expenditure (45). In terms of specific fractional synthesis, the renewal rate of skeletal muscle mass is calculated at 1.7%/d (46), which is far below that of visceral tissues. Total liver proteins (stationary and exported) have a whole-body daily turnover reaching 25% of daily hepatic protein content (47), whereas the turnover of gut mucosa proteins (48) and lymphocytes (49) is estimated at 10% and 7%, respectively.
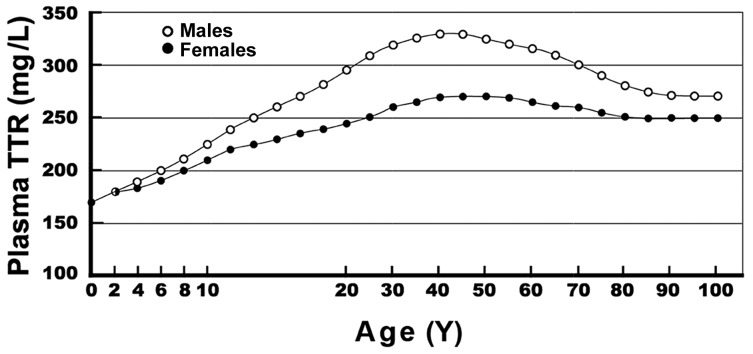
FIGURE 2.
Evolutionary patterns of plasma TTR in healthy subjects from birth to age 100 y. Normal human TTR values measured in both sexes are shown. TTR concentrations were obtained from 68,720 healthy US citizens and measured by immunoturbidimetry with the use of monospecific goat anti-human TTR. Means and SDs stratified by age and sex are provided elsewhere (10). The results show that TTR values are low at birth, manifest a linear increase without sexual difference until the onset of puberty, followed by an increase in TTR values recorded in male teenagers and maintenance reaching a plateau during the period of full sexual maturity. Starting from age 60 y, TTR values show a stepwise decrease, with a steeper slope in elderly men, which reflects a relatively more rapid deterioration of their muscle mass. TTR, transthyretin. Adapted from reference 33 (Figure 4, chapter 9.01) with permission.
Importance of TTR in Protein-Depleted States
Normal growth of neonates from birth to adulthood implies continuing accretion of body protein and is synonymous with positive nitrogen balance leading to LBM expansion, which is regarded as the major outcome measure of protein-related health (50, 51). These growth processes are tightly regulated and require the intake of appropriate energy and AA building blocks (52), in accordance with the concept that “protein synthesis occurs in the flame of sugar”, as expressed by the French physiologist Claude Bernard in 1865 (53). Plasma TTR is obviously situated on the cutting edge of the equilibrium between nutrient classes for which optimal requirements depend on age, physiologic status, and disease conditions. The restriction of dietary AA supply leads to curtailed nitrogen balance and to the concept of unachieved LBM replenishment (54). This is accompanied by depressed hepatic production of TTR mRNA (55), decreased abundance of TTR nuclear transcripts (56), and corresponding reduced exportation of mature TTR molecules in the bloodstream. The exquisitely sensitive response displayed by TTR is attributed to its small pool size, its short T1/2, and its high content of tryptophan (2, 17), which is known to constitute the narrowest of all indispensable AA pools in mammalian tissues (57). TTR has been used as an indicator of energy and protein adequacy in preterm, normal, and sick neonates (7, 58). TTR also proved useful for monitoring the dietetic management of anorexia nervosa (59) and weight-reduction programs (60). Likewise, the use of TTR is recommended for the nutritional follow-up of a variety of genetic and metabolic disorders such as uncontrolled diabetes (61), cystic fibrosis (62), drepanocytosis (63), Reye syndrome (64), inborn errors of AA metabolism (65), and defects of the urea cycle (66).
Protein malnutrition comprises a large spectrum of deficient states for which the extreme poles are frank kwashiorkor and emaciated marasmus. The former condition is characterized by swollen limbs, heavy liver steatosis (67), and very low plasma TTR concentrations (2). The latter condition is identified by borderline tissue overhydration, lesser hepatic fatty impregnation (68), and moderately depressed TTR concentrations (2). The flattened intestinal mucosa described in children with kwashiorkor (69) and its recovery under dietetic management recalls the malabsorptive events found in celiac patients that may be followed up by the serial measurement of plasma TTR concentrations (70). The same biomarker has been shown to be helpful for the detection and surveillance of elderly persons suffering from thymo-leukocytic disorders (71), likely resulting from a reduced dietary supply of protein resources combined with inadequate zinc intake (72). The data show that LBM stores are distributed into a composite agglomeration of various organs characterized by specific functional properties that are demonstrably interconnected to form an integrated system.
It becomes apparent that the intracellular protein content of each LBM component, taken separately, may undergo nitrogen depletion or recovery processes that can influence the hepatic amount of TTR production. Such coordinated linkages imply centrally mediated regulatory mechanisms governing the balance between protein accretion and protein breakdown as well as interorgan nitrogen fluxes between LBM components. The data provide a unifying concept of body nitrogen compartments for which adaptive alterations are reflected by the liver secretory rate of plasma TTR appearing as the ultimate indicator of LBM reserves. In malnourished children, a plasma TTR concentration of 65 mg/L indicates the upper limit below which the risk of lethality becomes likely (73). Surveys taking into account sex and age differences have shown that the TTR marker is distributed along Gaussian curves, paving the way for epidemiologic approaches seeking to compare protein-related health status in large population groups (74). Note also that, up to now, the synthesis of human TTR was not reportedly altered by ethnic differences or genetic deficiency, as described for TBG (75) and ALB (76). The data provide additional support for the universal reliability of TTR as a nutritional biomarker.
Importance of TTR in Stressful Disorders
Inflammatory disorders of any cause are initiated by activated leukocytes releasing a shower of cytokines working as autocrine, paracrine, and endocrine molecules (77). Proinflammatory cytokines stimulate the oversecretion of counter-regulatory hormones (glucocorticoids, catecholamines, glucagon, and growth hormone) opposing the hypoglycemic and anabolic effects of oversecreted insulin, thus creating a stage of insulin resistance in healthy tissues (78, 79). Despite ambient hyperglycemia, the maintenance of low respiratory quotient values (of ∼0.7) indicates that the energy economy is grounded on the mobilization of fat stores, which allows glucose and AA residues to be spared and preferentially redirected toward injured territories to uphold defense and repair purposes (79). During the course of any inflammatory disorder, cytokines reorganize overall protein metabolism, governing the overproduction of acute-phase reactants (APRs) that contribute in several ways to immune mechanisms using specific kinetic and functional properties (80). The severity and duration of bacterial and viral infections and parasitic infestations (81) or of multiple organ failure (82) lead to increased protein breakdown, which predominates over protein synthesis (82), and negative nitrogen balance; these developments support the concept of excessive LBM losses (54). The urinary leakage of nitrogen catabolites (mainly urea, ammonia, and creatinine) totaling 95% of output together with minor nitrogen compounds such as 3-methylhistidine, hydroxyproline, and AAs reveals that both visceral and structural compartments participate in the depletion of LBM stores of the whole body (43, 79). IL-6 is a key mediator in most chronic and acute inflammatory processes (77, 83), working through the mediation of a nuclear factor (NF) homologous with C/EBP-NF1 and competing for the same DNA-responsive element of the IL-6 gene (83). Stress-induced stimulation of IL-6 thus causes a dramatic elevation in APR values (80) together with reciprocal suppression of TTR synthesis as shown in animal (84) and clinical (85) experiments.
The burden of cytokines on protein metabolism is correlated with the severity and duration of initial impact. Patients afflicted with a combination of 2 distinct diseases or conditions exhibit accelerated LBM downsizing (86) identified by plasma TTR concentrations far worse than those recorded with a single disease (87). The data strongly support the view that the use of TTR enables monitoring of LBM depletion (10) and prediction of outcomes in critically ill patients (88). Clinical teams involved in the treatment of kidney failure (89), cardiac surgery (90), and ovarian cancer (91) have agreed in defining TTR concentrations of 180–200 mg/L as representing the boundary below which the likelihood of serious complications and increased mortality risk may be expected, whereas the threshold of 100–110 mg/L, presumably reflecting the exhaustion of LBM stores, suggests an ominous prognosis (92, 93). The decrease in plasma TTR in inflammatory states has fueled divergent debates. During the past 4 decades, researchers supporting the usefulness of the TTR biomarker had to face observations of its suppressed synthesis by nonnutritional factors (94, 95), which negated, ipso facto, TTR from any clinical relevance (9).
The recent demonstration that protein depletion and stressful disorders operate in concert to deplete LBM reserves along distinct and unrelated physiopathologic pathways helps to reconcile the diversity of opinions. Whatever the causal factors, the synthesis of TTR by the liver integrates opposing influences and yields as a net result a marker of LBM stores that remain available for metabolic processes. As a result, the TTR biomarker benefits from renewed reliability in identifying the most complex clinical situations characterized by the compounding effect of malnutrition and inflammation, as documented in hospitalized patients (4, 5), kidney failure (3), postoperative sepsis (96), cerebral infarction (97), head trauma (98), intensive care management (6, 99), organ transplantation (100), leukemia (101), and cancer (102).
Endocrine Implications
The abrupt decline in plasma TTR and holo-RBP concentrations in stressful disorders has major thyroid and retinoid implications (79, 103), but the importance of those implications is largely unrecognized by the scientific community. The liver serves as a large storage site for thyroxine and is capable of harboring as much as 40% of its total extrathyroidal body pool (104). The liver also secretes the 3 specific BPs ensuring the normal transportation of total thyroxine (TT4) in its intravascular space (5 L). Despite minor disagreements, it is generally held that TBG carries ∼70% of TT4, whereas TTR and ALB equally share the carriage of the remaining 30%. According to the free hormone hypothesis and the law of mass action (105), any endocrine ligand is metabolically inactive as long as it remains attached to the binding sites of specific carrier protein(s). This applies to TT4 because only minute fractions of its ligand are released in free form [free thyroxine (FT4)] to exert hormonal activities (106).
The normal plasma concentration of TT4 is 80 μg/L, whereas that of FT4 is 20 ng/L, which indicates a free to bound ratio of 1:4000. In the case of acute inflammatory stress of medium severity, TTR plasma concentrations are usually decreased by 40% within 4 or 5 d, indicating that the 12 μg TT4 transported by TTR should leak ∼5 μg FT4. The freed ligand diffuses uniformly in its normal distribution space (12 L) to reach throughout the critical stress period an estimated FT4 concentration of 400 ng/L (∼20-fold the normal FT4 value). These hormonal alterations thus create a transient hyperthyroid state substantiated by the measurement of significantly higher plasma FT4 concentrations in septic (107) and surgical (108, 109) patients. At least 3 other stress conditions may contribute to release freed ligands in the thyroxine distribution space, as follows: 1) bacterial infections, which cause a 4-fold accelerated peripheral turnover of both thyroid hormones (110); 2) anesthesia and surgery, which mobilize FT4 sequestered in the hepatic parenchymal cells (111); and 3) cytokine-induced inhibition of TBG synthesis, which releases additional amounts of FT4 (112). We conclude that the impact of the hyperthyroid stage associated with acute stress has been underrated as a result of ignoring the effect of increased tissue requirements and immediate cellular overconsumption. FT4 molecules exceeding body tissue needs remain unmetabolized and are excreted by the kidneys (113), consistent with the view that increased amounts of thyroid hormones in the urinary output accurately reflect overall thyroid status (114). This hyperthyroid context has significant functional impact as disclosed by two-dimensional radioautographic experiments indicating that ∼8% of the liver mRNA products (mainly enzymes, proteins, peptides, and components of energy metabolism) are stimulated under thyroid hormone influence (79, 115).
Holo-RBP is, in contrast with TTR, the sole conveyor of retinol, for which the normal concentration in adult humans (60 mg/L) ensures the carriage of ∼500 μg R-OH/L with a free fraction [free retinol (FR-OH)] measured at 1 μg/L, yielding a normal free to bound ratio of 1:500. In the case of acute stress of medium severity, decreasing holo-RBP values by 40%, an estimated proportion of 200 μg retinol is released as FR-OH, which diffuses uniformly in a larger distribution space (18 L) than that of FT4 and causes an augmented FR-OH concentration estimated at 10–12 μg/L or ∼10 times the normal free value. To our knowledge, no study describing the sequential upsurge in plasma FR-OH is available, but there exists clear indirect evidence that this adaptive increase is validated by the recovery of an unexpected retinoluria after surgical stress (113), febrile rotavirus diarrhea (116), shigellosis (117), sepsis, and pneumonia (118). Healthy subjects do not excrete detectable amounts of retinol in the urine, indicating that stress-associated retinoluria, which has been shown to be correlated with the duration and severity of injury, results from an expanded extracellular free pool in which its unmetabolized fractions undergo, like FT4, kidney overflow. The delivery of retinol targets a variety of cell-surface receptors that are unevenly distributed in body tissues, with high concentrations found in the epithelial cells of the CP and in organs belonging to the visceral compartment (liver, intestinal mucosa, bone marrow) (119). Cell surface receptors for holo-RBP operate the transmembrane uptake of the ligand, a process followed by cytosolic internalization (120), although nonspecific intracellular transfer of FR-OH was also documented (121).
Retinoid-induced reactions principally modulate cytokine activities, immune responses, and cellular components implicated in growth and repair processes (79). Taken together, the data show that the transitory hyperthyroid and hyperretinoid conditions created by abrogating liver TTR and RBP4 synthesis last as long as the decrease in both BPs continues. From a strictly mechanistic point of view, the currently used denomination of “negative APRs” applied to the suicidal behavior of TTR/RBP4 molecules is fully justified. From a physiopathologic point of view, this is a misnomer that denies the active participation of these BPs, which preferably deserve the designation of “acute-booster reactants” (57) to highlight the cascade of helpful events generated in the course of stress.
The clinical conditions described above apply to patients undergoing stressful disorders of medium severity. In the case of more grievous injuries (99, 122), the cytokine-induced decrease in TTR/RBP4 plasma concentrations entails longer slopes correlated with the magnitude and duration of the stress impact. The decrease in TTR/RBP4 is an obligatory process lasting some days, which is poorly responsive to dietetic manipulations and associated with culminating FT4 and FR-OH plasma concentrations causing superactivated inflammatory responses. These inflammatory reactions aim at promoting the build-up of a second line of defense processes following that primarily initiated by cytokines (43, 123). Thermally injured patients affected by extensive tissue damage exhibit a decrease in TTR and RBP4 concentrations of 70% of starting concentrations, reaching a nadir on days 6–8 after initial impact (122). Critically ill subjects usually exhibit hyperglycemic status involving the flooding of poorly irrigated tissues via simple diffusion to locally promote anabolic drive and wound healing via anaerobic glycolysis (respiratory quotient ∼1) (79). Serial TTR measurement performed on a daily basis in the most severely affected patients and twice or 3 times per week in illnesses of medium severity allows the identification of LBM stores that remain available to face further inflammatory conditions. Reaching the TTR nadir should initiate the prescription of aggressive nutritional therapy. Two lines of response may develop during the ensuing days. The smallest and most gradual increase in TTR concentrations above nadir values indicate the reversal of nitrogen balance and the progressive restoration of LBM status, which predicts the best possible clinical recovery. It is worth noting that increases in TTR plasma concentrations occur when most other biological and clinical criteria remain silent, which pinpoints the unusual performance of the TTR analyte. In contrast, the maintenance of lowered TTR concentrations during days or weeks (124) indicates that catabolic and anabolic processes neutralize each other. The persistence of lowered TTR values likely indicates inappropriate nutritional management that misses the most effective protein-to-energy ratio and/or harmful effects generated by underlying comorbidities requiring specific therapeutic approaches. When an inflammatory burden is superimposed on pre-existing malnutrition, the secretion of cytokines by activated macrophages is depressed (125), entailing significantly reduced leakage of FT4 and FR-OH ligands whose boosting effects on immune reactions are proportional to the decrement between pre- and poststress RCC values. The data provide a biological explanation for the survival handicap that more severely affects children with kwashiorkor than children with marasmus (126, 127).
In sum, TTR appears to be a unique biomarker of acutely and chronically evolving disorders, hence fulfilling the scoring task claimed by recently published position papers (128, 129). The use of appropriate laboratory equipment for the routine measurement of the TTR indicator (130) allows for early detection and nutritional management of endangered patients, which leads to improved prognoses and alleviation of the financial burdens of hospitalization (131).
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; ALB, serum albumin; APR, acute-phase reactant; BP, binding protein; BW, body weight; C/EBP, transcription factor CCAAT/EBP; CP, choroid plexus; CSF, cerebrospinal fluid; FR-OH, free retinol; FT4, free thyroxine; LBM, lean body mass; MM, molecular mass; NF, nuclear factor; RBP, retinol-binding protein; RCC, retinol circulating complex; R-OH, all-trans retinol; STRA6, stimulated by retinoic acid 6; T1/2, half-life; TBG, thyroxine-binding globulin; TBK, total body potassium; TBN, total body nitrogen; TT4, total thyroxine; TTR, transthyretin.
References
- 1.Buxbaum JN, Reixach N. Transthyretin: the servant of many masters. Cell Mol Life Sci 2009;66:3095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet 1972;2:106–9. [DOI] [PubMed] [Google Scholar]
- 3.Cano NJ. Metabolism and clinical interest of serum transthyretin (prealbumin) in dialysis patients. Clin Chem Lab Med 2002;40:1313–9. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MK, Trujillo ER, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr 2003;27:389–95. [DOI] [PubMed] [Google Scholar]
- 5.Devoto G, Gallo F, Marchello C, Racci O, Garbarini R, Bonassi S, Albalustri G, Haupt E. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem 2006;52:2281–5. [DOI] [PubMed] [Google Scholar]
- 6.Devakonda A, George L, Raoof S, Esan A, Saleh A, Bernstein LH. Transthyretin as a marker to predict outcome in critically ill patients. Clin Biochem 2008;41:1126–30. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MR, Massoudi M, Byrne J, Mitchell MA, Eggert LD, Chan GM. Evaluation of transthyretin as a monitor of protein-energy intake in preterm and sick neonatal infants. JPEN J Parenter Enteral Nutr 1988;12:162–6. [DOI] [PubMed] [Google Scholar]
- 8.Mühlethaler R, Stuck AE, Minder CE, Frey BM. The prognostic significance of protein-energy malnutrition in geriatric patients. Age Ageing 1995;24:193–7. [DOI] [PubMed] [Google Scholar]
- 9.Myron Johnson A, Merlini G, Sheldon J, Ichihara K. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. IFCC Scientific Division Committee on Plasma Proteins (C-PP). Clin Chem Lab Med 2007;45:419–26. [DOI] [PubMed] [Google Scholar]
- 10.Ingenbleek Y. Plasma transthyretin reflects the fluctuations of lean body mass in health and disease. In: Richardson SJ, Cody V, editors. Recent advances in transthyretin evolution, structure and biological functions. Berlin: Springer-Verlag; 2009. p. 329–57. [Google Scholar]
- 11.Ingenbleek Y, Bernstein LH. Downsizing of lean body mass is a key determinant of Alzheimer’s disease. J Alzheimers Dis 2015;44:745–54. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber G. The evolution of transthyretin synthesis in the choroid plexus. Clin Chem Lab Med 2002;40:1200–10. [DOI] [PubMed] [Google Scholar]
- 13.Power DM, Elias NP, Richardson SJ, Mendes J, Soares CM, Santos CRA. Evolution of the thyroid-hormone binding protein. Gen Comp Endocrinol 2000;119:241–55. [DOI] [PubMed] [Google Scholar]
- 14.Richardson SJ. Evolutionary changes to transthyretin: evolution of transthyretin biosynthesis. FEBS J 2009;276:5342–56. [DOI] [PubMed] [Google Scholar]
- 15.Kabat EA, Moore D, Landow H. An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to serum proteins. J Clin Invest 1942;21:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebert FB, Nielson JW. Electrophoretic study of the blood protein response in tuberculosis. J Biol Chem 1942;143:29–38. [Google Scholar]
- 17.Schönenberger M, Schultze HE, Schwick G. Uber ein präalbumin des menschlichen serums. About a prälbumin of human serum. Biochem Z 1956;328:267–84 (in German). [PubMed] [Google Scholar]
- 18.Kanda Y, Goodman DS, Canfield RE, Morgan FJ. The amino acid sequence of human plasma prealbumin. J Biol Chem 1974;249:6796–805. [PubMed] [Google Scholar]
- 19.Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest 1968;47:2025–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyung SJ, Deroo S, Robinson CV. Retinol and retinol-binding protein stabilize transthyretin via formation of retinol transport complex. ACS Chem Biol 2010;5:1137–46. [DOI] [PubMed] [Google Scholar]
- 21.Monaco HL. The transthyretin-retinol binding protein complex. In: Richardson SJ, Cody V, editors. Recent advances in transthyretin evolution, structure and biological functions. Berlin: Springer-Verlag; 2009. p. 123–42. [Google Scholar]
- 22.Socolow EL, Woeber KA, Purdy RH, Holloway MT, Ingbar SH. Preparation of I131-labeled human serum prealbumin and its metabolism in normal and sick patients. J Clin Invest 1965;44:1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson PA, Nilsson S, Ostberg L, Rask L, Vahlquist A. Aspects of the metabolism of retinol-binding protein and retinol. Vitam Horm 1974;32:181–214. [DOI] [PubMed] [Google Scholar]
- 24.Ganfornina MD, Guttiérrez G, Bastiani M, Sánchez D. A phylogenetic analysis of the lipocalin protein family. Mol Biol Evol 2000;17:114–26. [DOI] [PubMed] [Google Scholar]
- 25.Ingenbleek Y, Van den Schrieck HG, De Nayer P, De Visscher M. The role of retinol-binding protein in protein-calorie malnutrition. Metabolism 1975;24:633–41. [DOI] [PubMed] [Google Scholar]
- 26.van Bennekum AM, Wei S, Gamble MV, Vogel S, Piantedosi R, Gottesman M, Episkopou W, Blaner WS. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem 2001;276:1107–13. [DOI] [PubMed] [Google Scholar]
- 27.Wei S, Episkopou V, Piantedosi R, Maeda S, Shimada K, Gottesman M, Blaner WS. Studies on the metabolism of retinol and retinol-binding protein in transthyretin-deficient mice produced by homologous recombination. J Biol Chem 1995;270:866–70. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–62. [DOI] [PubMed] [Google Scholar]
- 29.Berry DC, Croniger CM, Ghijselinck NB, Noy A. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol binding protein receptor STRA 6. Mol Cell Biol 2012;32:3851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreoli M, Robbins J. Serum proteins and thyroxine protein interaction in early human fetuses. J Clin Invest 1962;41:1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vahlquist A, Rask L, Peterson PA, Berg T. The concentrations of retinol-binding protein, prealbumin, and transferrin in the sera of newly delivered mothers and children of various ages. Scand J Clin Lab Invest 1975;35:569–75. [PubMed] [Google Scholar]
- 32.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood and puberty. Endocr Rev 2005;26:114–46. [DOI] [PubMed] [Google Scholar]
- 33.Bienvenu J, Jeppson JO, Ingenbleek Y. Transthyretin & retinol-binding protein. In: Ritchie RF, Navolotskaia O, editors. Serum proteins in clinical medicine. Scarborough: Foundation for Blood Research, 1996. p. 9.011–18. [Google Scholar]
- 34.Ingenbleek Y, De Visscher M. Hormonal and nutritional status: critical conditions for endemic goiter epidemiology? Metabolism 1979;28:9–19. [DOI] [PubMed] [Google Scholar]
- 35.Forbes GB. Human body composition: growth, aging, nutrition and activity. Berlin: Springer-Verlag; 1987. [Google Scholar]
- 36.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender and ethnicity. J Appl Physiol 1997;83:229–39. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald PN, Bok D, Ong DE. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat and human. Proc Natl Acad Sci USA 1990;87:4265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickson PW, Aldred AR, Marley PD, Bannister D, Schreiber G. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin): regulation of transthyretin synthesis in choroid plexus is independent from that of liver. J Biol Chem 1986;261:3475–8. [PubMed] [Google Scholar]
- 39.Chen RL, Athauda SB, Kassem NA, Zhang Y, Segal MB, Preston JE. Decrease of transthyretin synthesis at the blood-cerebrospinal fluid barrier of old sheep. J Gerontol A Biol Sci Med Sci 2005;60:852–8. [DOI] [PubMed] [Google Scholar]
- 40.Chen CP, Chen RL, Preston JE. The influence of ageing in the cerebrospinal fluid concentrations of proteins that are derived from the choroid plexus, brain and plasma. Exp Gerontol 2012;47:323–8. [DOI] [PubMed] [Google Scholar]
- 41.Cohn SH, Vartsky D, Yasumura S, Vaswani AN, Elis KJ. Indexes of body cell mass: nitrogen versus potassium. Am J Physiol 1983;244:E305–10. [DOI] [PubMed] [Google Scholar]
- 42.Forbes GB. Body composition. In: Brown ML, editor. Present knowledge in nutrition. 6th ed. Washington (DC): ILSI Nutrition Foundation; 1990. p. 7–12. [Google Scholar]
- 43.Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med 2002;40:1281–91. [DOI] [PubMed] [Google Scholar]
- 44.Brožek J, Grande F. Body composition and basal metabolism in man: correlation analysis versus physiological approach. Hum Biol 1955;27:22–31. [PubMed] [Google Scholar]
- 45.Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Müller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab 2000;278:E308–15. [DOI] [PubMed] [Google Scholar]
- 46.McNurlan MA, Essén P, Heys SD, Buchan V, Garlick PJ, Wernerman J. Measurement of protein synthesis in human skeletal muscle: further investigation of the flooding technique. Clin Sci (Lond) 1991;81:557–64. [DOI] [PubMed] [Google Scholar]
- 47.Barle H, Nyberg B, Essén P, Andersson K, McNurlan MA, Wernerman J, Garlick PJ. The synthesis rates of total liver protein and plasma albumin determined simultaneously in vivo in humans. Hepatology 1997;25:154–8. [DOI] [PubMed] [Google Scholar]
- 48.Nakshabendi IM, McKee R, Downie S, Russell RI, Rennie MJ. Rates of small intestinal mucosal protein synthesis in human jejunum and ileum. Am J Physiol 1999;277:E1028–31. [DOI] [PubMed] [Google Scholar]
- 49.McNurlan MA, Sandgren A, Hunter K, Essén P, Garlick PJ, Wernerman J. Protein synthesis rates of skeletal muscle, lymphocytes, and albumin with stress hormone infusion in healthy man. Metabolism 1996;45:1388–94. [DOI] [PubMed] [Google Scholar]
- 50.Golden M, Waterlow JC, Picou D. The relationship between dietary intake, weight change, nitrogen balance, and protein turnover in man. Am J Clin Nutr 1977;30:1345–8. [DOI] [PubMed] [Google Scholar]
- 51.Millward DJ, Jackson AA. Protein/energy ratio of current diets in developed and developing countries with a safe protein/energy ratio: implications for recommended protein and amino acid intakes. Public Health Nutr 2004;7:387–405. [DOI] [PubMed] [Google Scholar]
- 52.Young VR, Yu YM, Fukagawa NK. Energy and protein turnover. In: Kinney JM, Tucker HN, editors. Energy, metabolism, tissue determinants and cellular corollaries. New York: Raven Press; 1992. p. 439–66. [Google Scholar]
- 53.Bernard C. Introduction à l’étude de la médecine expérimentale. [Introduction to experimental medicine investigation.] Paris: JB Baillière et Fils; 1865 (in French). [Google Scholar]
- 54.Ingenbleek Y, Kimura H. Nutritional essentiality of sulfur in health and disease. Nutr Rev 2013;71:413–32. [DOI] [PubMed] [Google Scholar]
- 55.de Jong FA, Schreiber G. Messenger RNA levels of plasma proteins in rat liver during protein depletion and refeeding. J Nutr 1987;117:1795–800. [DOI] [PubMed] [Google Scholar]
- 56.Straus DS, Marten NW, Hayden JM, Burke EJ. Protein restriction specifically decreases the abundance of serum albumin and transthyretin nuclear transcripts in rat liver. J Nutr 1994;124:1041–51. [DOI] [PubMed] [Google Scholar]
- 57.Ingenbleek Y, Young VR. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr 1994;14:495–533. [DOI] [PubMed] [Google Scholar]
- 58.Moskowitz SR, Pereira G, Spitzer A, Heaf L, Amsel J, Watkins JB. Prealbumin as a biochemical marker of nutritional adequacy in premature infants. J Pediatr 1983;102:749–53. [DOI] [PubMed] [Google Scholar]
- 59.Gaudiani JL, Sabel AL, Mehler PS. Low prealbumin is a significant predictor of medical complications in severe anorexia nervosa. Int J Eat Disord 2014;47:148–56. [DOI] [PubMed] [Google Scholar]
- 60.Göfferje H, Kozlik V. Blood proteins during short-term fasting and during supply of essential amino acids. Infusionsther Klin Ernahr 1977;4:320–4. [PubMed] [Google Scholar]
- 61.Kobbah AM, Hellsing K, Tuvemo T. Early changes of some serum proteins and metals in diabetic children. Acta Paediatr Scand 1988;77:734–40. [DOI] [PubMed] [Google Scholar]
- 62.Adde FV, Dolce P, Tanikawa CE, Uehara DY, Cardoso AL, Rozov T. Nutritional supplementation in patients with cystic fibrosis. J Pediatr (Rio J) 1997;73:317–23. [DOI] [PubMed] [Google Scholar]
- 63.Jain SK, Ross JD, Duett J, Herbst JJ. Low plasma prealbumin and carotenoid levels in sickle cell disease patients. Am J Med Sci 1990;299:13–5. [DOI] [PubMed] [Google Scholar]
- 64.Bosin E, Glasgow AM, Monji N. Retinol binding protein and prealbumin in Reye’s syndrome. Clin Biochem 1986;19:189–91. [DOI] [PubMed] [Google Scholar]
- 65.Rocha JC, Almeida MF, Carmona C, Cardoso ML, Borges N, Soares I, Salcedo G, Lima MR, Azevedo I, van Spronsen FJ. The use of prealbumin concentration as biomarker of nutritional status in treated phenylketonuric patients. Ann Nutr Metab 2010;56:207–11. [DOI] [PubMed] [Google Scholar]
- 66.Acosta PB, Yannicelli S, Ryan AS, Arnold G, Marriage BJ, Plewinska M, Bernstein L, Fox J, Lewis V, Miller MP, et al. Nutritional therapy improves growth and protein status of children with an urea cycle enzyme defect. Mol Genet Metab 2005;86:448–55. [DOI] [PubMed] [Google Scholar]
- 67.Waterlow JC. Amount and rate of disappearance of liver fat in malnourished infants in Jamaica. Am J Clin Nutr 1975;28:1330–6. [DOI] [PubMed] [Google Scholar]
- 68.Doherty JF, Adam EJ, Griffin GE, Golden MH. Ultrasonographic assessment of the extent of hepatic steatosis in severe malnutrition. Arch Dis Child 1992;67:1348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbezat GO, Bowie MD, Kashula ROC, Hansen JDL. Studies of the small intestinal mucosa of children with protein-calorie malnutrition. S Afr Med J 1967;41:1031–6. [PubMed] [Google Scholar]
- 70.McMillan SA, Dickey W, Douglas JP, Hughes DF. Transthyretin values correlate with mucosal recovery in patients with coeliac disease taking a gluten-free diet. J Clin Pathol 2001;54:783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moulias R, Deville-Chabrolle A, Congy F, Wang A, Marescot MR, Lesourd B. Low prealbumin: a correlate of immunodeficiency in elderly patients. In: Chandra RK, editor. Nutrition, immunity and illness. New York: Pergamon Press; 1985. p. 165–72. [Google Scholar]
- 72.Bates J, McClain CJ. The effect of severe zinc deficiency on serum levels of albumin, transferrin and prealbumin in man. Am J Clin Nutr 1981;34:1655–60. [DOI] [PubMed] [Google Scholar]
- 73.Dramaix M, Brasseur D, Donnen P, Bawhere P, Porignon D, Tonglet R, Hennart P. Prognostic indices for mortality of hospitalized children in central Africa. Am J Epidemiol 1996;143:1235–43. [DOI] [PubMed] [Google Scholar]
- 74.Ingenbleek Y. La malnutrition protéino-calorique chez l’enfant en bas âge. Répercussions sur la fonction thyroïdienne et les protéines vectrices du serum [thesis]. [Protein-calorie malnutrition in the young child. Consequences on thyroid function and serum carrier-proteins.] Acco Press, Leuven (Belgium): Catholic University of Louvain: 1977 (in French).
- 75.Watson F, Dick M. Distribution and inheritance of low thyroxine-binding globulin levels in Australian aborigenes: a new genetic variation. Med J Aust 1980;2:385–7. [DOI] [PubMed] [Google Scholar]
- 76. Minchiotti L, Galliano M, Caridi G, Kragh-Hansen U, Peters T Jr. Congenital analbuminaemia: molecular defects and biochemical and clinical aspects. Biochim Biophys Acta 2013;1830:5494–502. [DOI] [PubMed]
- 77.Bienvenu J, Monneret G, Fabien N, Revillard JP. The clinical usefulness of the measurement of cytokines. Clin Chem Lab Med 2000;38:267–85. [DOI] [PubMed] [Google Scholar]
- 78.Gelfand RA, Matthews DE, Bier DM, Sherwin RS. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest 1984;74:2238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ingenbleek Y, Bernstein LH. The stressful condition as a nutritionally dependent adaptive dichotomy. Nutrition 1999;15:305–20. [DOI] [PubMed] [Google Scholar]
- 80.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 81.Beisel WR. Metabolic response to infection. Annu Rev Med 1975;26:9–20. [DOI] [PubMed] [Google Scholar]
- 82.Arnold J, Campbell IT, Samuels TA, Devlin JC, Green CJ, Hipkin LJ, MacDonald IA, Scrimgeour CM, Smith K, Rennie MJ. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci 1993;84:655–61. [DOI] [PubMed] [Google Scholar]
- 83.Isshiki H, Akira S, Sugita T, Nishio Y, Hashimoto S, Pawlowski T, Suematsu S, Kishimoto T. Reciprocal expression of NF-IL-6 and in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol 1991;3:63–70. [PubMed] [Google Scholar]
- 84.Murakami T, Ohnishi S, Nishiguchi S, Maeda S, Araki S, Shimada K. Acute-phase response of mRNAs for serum amyloid P component, C-reactive protein and prealbumin (transthyretin) in mouse liver. Biochem Biophys Res Commun 1988;155:554–60. [DOI] [PubMed] [Google Scholar]
- 85.Banks RE, Forbes MA, Storr M, Higginson J, Thompson D, Raynes J, Illingworth JM, Perren TJ, Selby PJ, Whicher JT. The acute phase protein response in patients receiving subcutaneous IL-6. Clin Exp Immunol 1995;102:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pupim LB, Heimbürger O, Qureshi AR, Ikizler TA, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int 2005;68:2368–74. [DOI] [PubMed] [Google Scholar]
- 87.Niyongabo T, Henzel D, Idi M, Nimubona S, Gikoro E, Melchior JC, Matheron S, Kamanfu G, Samb B, Messing B, et al. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition 1999;15:289–93. [DOI] [PubMed] [Google Scholar]
- 88. Huang JW, Lien YC, Wu HY, Yen CJ, Pan CC, Hung TW, Su CT, Chiang CK, Cheng HT, Hung KY. Lean body mass predicts long-term survival in Chinese patients on peritoneal dialysis. PLoS One 2013;8.1:e54976. [DOI] [PMC free article] [PubMed]
- 89.Rambod M, Koverdy CP, Bross R, Kopple JD, Kalantar-Zadeh K. Association of serum prealbumin and its changes over time with clinical outcome and survival in patients receiving hemodialysis. Am J Clin Nutr 2008;88:1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu PJ, Cassiere HA, Dellis SL, Manetta F, Kohn N, Hartman AR. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J Parenter Enter Nutr 2014;Jun4pii:0148607114536735. [DOI] [PubMed]
- 91.Geisler JP, Linnemeirer GC, Thomas AJ, Manahan KJ. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol 2007;106:128–31. [DOI] [PubMed] [Google Scholar]
- 92.Perez Valdivieso JR, Bes-Rastrollo M, Monedoro P, de Irala J, Lavilla FJ. Impact of prealbumin levels on mortality in patients with acute kidney injury: an observational cohort study. J Ren Nutr 2008;18:262–8. [DOI] [PubMed] [Google Scholar]
- 93.Ho SY, Guo HR, Chen HH, Peng CJ. Nutritional predictors of survival in terminally ill cancer patients. J Formos Med Assoc 2003;102:544–50. [PubMed] [Google Scholar]
- 94.Johnson AM. Low levels of plasma proteins: malnutrition or inflammation? Clin Chem Lab Med 1999;37:91–6. [DOI] [PubMed] [Google Scholar]
- 95.Shenkin A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin Chem 2006;52:2177–9. [DOI] [PubMed] [Google Scholar]
- 96.Bae HJ, Lee HJ, Han DS, Suh YS, Lee YH, Lee HS, Cho JJ, Kong SH, Yang HK. Prealbumin levels as a useful marker for predicting infectious complications after gastric surgery. J Gastrointest Surg 2011;15:2136–44. [DOI] [PubMed] [Google Scholar]
- 97.Gao C, Zhang B, Zhang W, Pu S, Yin J, Gao Q. Serum prealbumin (transthyretin) predicts good outcome in young patients with cerebral infarction. Clin Exp Med 2011;11:49–54. [DOI] [PubMed] [Google Scholar]
- 98.Nataloni S, Gentili P, Marini B, Guidi A, Marconi P, Busco F, Pelaia P. Nutritional assessment in head injured patients through the study of rapid turnover visceral proteins. Clin Nutr 1999;18:247–51. [DOI] [PubMed] [Google Scholar]
- 99.Raguso CA, Dupertuis YM, Pichard C. The role of visceral proteins in the nutritional assessment of intensive care unit patients. Curr Opin Clin Nutr Metab Care 2003;6:211–6. [DOI] [PubMed] [Google Scholar]
- 100.González-Castro A, Llorca J, Suberviola B, Diaz-Regañón G, Ordóñez J, Miñambres E. Influence of nutritional status in lung transplant recipients. Transplant Proc 2006;38:2539–40. [DOI] [PubMed] [Google Scholar]
- 101.Yu LC, Kuvibidila S, Ducos R, Warrier RP. Nutritional status of children with leukemia. Med Pediatr Oncol 1994;22:73–7. [DOI] [PubMed] [Google Scholar]
- 102.Elhasid R, Laor A, Lischinsky S, Postovsky S, Weyl Ben Arush M. Nutritional status of children with solid tumors. Cancer 1999;86:119–25. [DOI] [PubMed] [Google Scholar]
- 103.Ingenbleek Y. Thyroid function in nonthyroid illnesses. In: De Visscher M, editor. The thyroid gland. New York: Raven Press; 1980. p. 499–527. [Google Scholar]
- 104.Cavalieri RR, Searle GL. The kinetics of distribution between plasma and liver of 131I-labeled L-thyroxine in man: observations of subjects with normal and decreased serum thyroxine-binding globulin. J Clin Invest 1966;45:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 1989;10:232–74. [DOI] [PubMed] [Google Scholar]
- 106.Mendel CM, Cavalieri RR, Weisiger RA. Uptake of thyroxine by the perfused rat liver: implications for the free hormone hypothesis. Am J Physiol 1988;255:E110–9. [DOI] [PubMed] [Google Scholar]
- 107.Talwar KK, Sawhney RC, Rastogi GK. Serum levels of thyrotropin, thyroid hormones and their responses to thyrotropin releasing hormone in infective febrile illnesses. J Clin Endocrinol Metab 1977;44:398–403. [DOI] [PubMed] [Google Scholar]
- 108.Prescott RW, Yeo PP, Watson MJ, Johnston ID, Ratcliffe JG, Evered DC. Total and free thyroid hormone concentrations after elective surgery. J Clin Pathol 1979;32:321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saatvedt K, Lindberg H, Geiran OR, Fiane A, Seem E, Michelsen S, Pedersen T, Hagve TA. Thyroid function during and after cardiopulmonary bypass in children. Acta Anaesthesiol Scand 1998;42:1100–3. [DOI] [PubMed] [Google Scholar]
- 110.Gregerman RI, Solomon N. Acceleration of thyroxine and triiodothyronine turnover during bacterial pulmonary infections and fever: implications for the functional state of the thyroid during stress and in senescence. J Clin Endocrinol Metab 1967;27:93–105. [DOI] [PubMed] [Google Scholar]
- 111.Harland WA, Horton PW, Strang R, Fitzgerald B, Richards JR, Holloway KB. Release of thyroxine from the liver during anaesthesia and surgery. Br J Anaesth 1974;46:818–20. [DOI] [PubMed] [Google Scholar]
- 112.Kobayashi M, Horiuchi R, Hachisu T, Takikawa H. Dualistic effects of thyroid hormone on a human hepatoma cell line: inhibition of thyroxine-binding globulin synthesis and stimulation of α1-acid glycoprotein synthesis. Endocrinology 1988;123:631–40. [DOI] [PubMed] [Google Scholar]
- 113.Ramsden DB, Princé HP, Burr WA, Bradwell AR, Black EG, Evans AE, Hoffenberg R. The inter-relationship of thyroid hormones, vitamin A and their binding proteins following acute stress. Clin Endocrinol (Oxf) 1978;8:109–22. [DOI] [PubMed] [Google Scholar]
- 114.Rogowski P, Siersbaek-Nielsen K, Hansen JM. Urinary excretion of thyroxine and triiodothyronine in different thyroid function states in man. Acta Endocrinol 1978;87:525–34. [DOI] [PubMed] [Google Scholar]
- 115.Seelig S, Liaw C, Towle HC, Oppenheimer JH. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci USA 1981;78:4733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvarez JO, Salazar-Lindo E, Kohatsu J, Miranda P, Stephensen CB. Urinary excretion of retinol in children with acute diarrhea. Am J Clin Nutr 1995;61:1273–6. [DOI] [PubMed] [Google Scholar]
- 117.Mitra AK, Alvarez JO, Guay-Woodford L, Fuchs GJ, Wahed MA, Stephensen CB. Urinary retinol excretion and kidney function in children with shigellosis. Am J Clin Nutr 1998;68:1095–103. [DOI] [PubMed] [Google Scholar]
- 118.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI Jr, Gammon RB Jr. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 119.Smeland S, Bjerknes T, Malaba L, Eskild W, Norum KR, Blomhoff R. Tissue distribution of the receptor for plasma retinol-binding protein. Biochem J 1995;305:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Senoo H, Stang E, Nilsson A, Kindberg GM, Berg T, Roos N, Norum KR, Blomhoff R. Internalization of retinol-binding protein in parenchymal and stellate cells of rat liver. J Lipid Res 1990;31:1229–39. [PubMed] [Google Scholar]
- 121.Hodam JR, Creek KE. Comparison of the metabolism of retinol delivered to human keratinocytes either bound to serum retinol-binding protein or added directly to the culture medium. Exp Cell Res 1998;238:257–64. [DOI] [PubMed] [Google Scholar]
- 122.Cynober L, Prugnaud O, Lioret N, Duchemin C, Saizy R, Giboudeau J. Serum transthyretin levels in patients with burn injury. Surgery 1991;109:640–4. [PubMed] [Google Scholar]
- 123.Bernstein LH. Transthyretin and the systemic inflammatory response. Curr Nutr Food Sci 2009;5:71–4. [Google Scholar]
- 124.Dalrymple LS, Johansen KL, Chertow GM, Grimes B, Anand S, McCulloch CE, Kaysen CA. Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the comprehensive dialysis study. J Ren Nutr 2013;23:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aslan Y, Erduran E, Gedik Y, Mocan H, Okten A, Orem A. Serum interleukin-1 and granulocyte-macrophage colony-stimulating factor levels in protein malnourished patients during acute infection. Cent Afr J Med 1996;42:179–84. [PubMed] [Google Scholar]
- 126.Doherty JF, Golden MH, Raynes JG, Griffin GE, McAdam KP. Acute-phase response is impaired in severely malnourished children. Clin Sci 1993;84:169–75. [DOI] [PubMed] [Google Scholar]
- 127.Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. The acute-phase protein response to infection in edematous and non-edematous protein-energy malnutrition. Am J Clin Nutr 2002;76:1409–15. [DOI] [PubMed] [Google Scholar]
- 128.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med 2014;370:1227–36. [DOI] [PubMed] [Google Scholar]
- 129.Jensen GL. Malnutrition and inflammation—“ burning down the house “: inflammation as an adaptive physiological response versus self-destruction? JPEN J Parenter Enteral Nutr 2015;39:52–62. [DOI] [PubMed] [Google Scholar]
- 130.David G, Bernstein LH, Coifman RR. The automated malnutrition assessment. Nutrition 2013;29:113–21. [DOI] [PubMed] [Google Scholar]
- 131.Bernstein LH, Ingenbleek Y. Transthyretin: its response to malnutrition and stress injury—clinical usefulness and economic implications. Clin Chem Lab Med 2002;40:1344–8. [DOI] [PubMed] [Google Scholar]