Abstract
An imbalance between energy intake and energy expenditure is the primary etiology for excess weight gain. Increased energy expenditure via exercise and energy restriction via diet are commonly used approaches to induce weight loss. Such behavioral interventions, however, have generally resulted in a smaller than expected weight loss, which in part has been attributed to compensatory adaptations in other components contributing to energy balance. Current research points to a loose coupling between energy intake and energy expenditure on a daily basis, and evidence for long-term adaptations has been inconsistent. The lack of conclusive evidence on compensatory adaptations in response to alterations in energy balance can be attributed to differences in intervention type and study population. Physical activity (PA) levels may be reduced in response to aerobic exercise but not in response to resistance exercise. Furthermore, athletic and lean adults have been shown to increase their energy intake in response to exercise, whereas no such response was observed in obese adults. There is also evidence that caloric restriction is associated with a decline in PA. Generally, humans seem to be better equipped to defend against weight loss than avoid weight gain, but results also show a large individual variability. Therefore, individual differences rather than group means should be explored to identify specific characteristics of “compensators” and “noncompensators.” This review emphasizes the need for more research with simultaneous measurements of all major components contributing to energy balance to enhance the understanding of the regulation of energy balance, which is crucial to address the current obesity epidemic.
Keywords: physical activity, exercise, nonexercise activity thermogenesis, energy intake, caloric restriction, body composition
Introduction
The high prevalence of overweight and obesity is one of the leading future threats to public health (1). Nearly 69% of US adults are overweight (BMI ≥ 25.0 kg/m2), with almost 36% of adults classified as obese (BMI ≥ 30 kg/m2) (2). Excess body weight increases the risk of numerous chronic diseases, including cardiovascular disease, diabetes, many forms of cancer, and musculoskeletal problems (1), and it has been associated with 300,000 deaths/y in the United States (3). The high prevalence of obesity also puts a substantial burden on the health care system because obesity-related conditions are estimated to account for ~7% of total health care costs or $117 billion/y in direct and indirect costs in the United States (4).
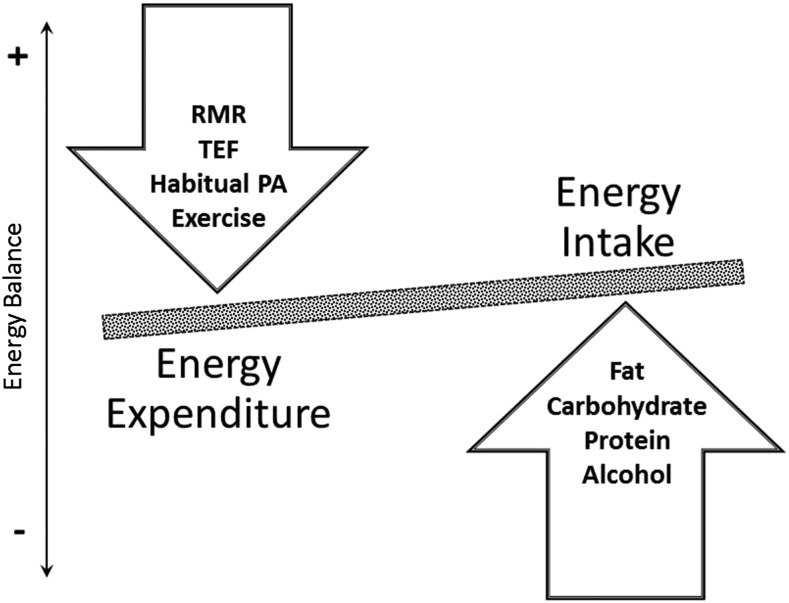
An imbalance between energy intake and energy expenditure is the primary etiology factor related to excess weight gain, with the major components contributing to energy balance displayed in Figure 1. The simple concept of energy balance, however, is highly complex, and the regulation of energy balance is influenced by physiologic and behavioral constraints. Energy intake, e.g., is ultimately determined by eating behavior, which is affected by physiologically driven sensations of hunger and satiety as well as the environment. Similarly, total daily energy expenditure (TDEE)4 is the result of an interaction between physiology and behavior, which are both influenced by the environment. Resting metabolic rate (RMR) and thermic effect of food are predominantly determined by physiologic constraints (5, 6). RMR represents the energy required to maintain essential vital functions and constitutes the majority of TDEE in the general population (5). Physical activity (PA), however, is the most variable component of TDEE because it is mainly determined by a person’s behavior. In the average population PA constitutes between 25% and 35% of TDEE, but it may be up to 75% in extreme situations of sustained heavy exercise (7). To address differences in the nature of PA, a further differentiation between habitual PA or nonexercise activity thermogenesis (NEAT) and exercise is commonly made. Exercise reflects planned, structured, and repetitive movements, which serve a specific objective (8). NEAT reflects the energy expenditure of all PA other than volitional exercise including activities of daily living, small muscle movements, spontaneous muscle contraction, and postural maintenance (9). Although these components of TDEE are predominantly determined by individual behaviors, up to 57% of the variability in spontaneous PA has been attributed to family membership (10).
FIGURE 1.
Major components contributing to energy balance. PA, physical activity; RMR, resting metabolic rate; TEF, thermic effect of food.
Despite the importance of genetics and physiology in the regulation of energy balance, lifestyle changes have been suggested to be the leading contributor to the obesity epidemic (11). Therefore, behavioral interventions such as an increase in energy expenditure, via exercise, and/or a reduction in energy intake are commonly used to induce a negative energy balance, which would initiate weight loss. Such behavioral changes, however, have generally resulted in smaller changes in body weight than what has been expected. This has in part been attributed to reciprocal compensatory adaptations in other components contributing to energy balance—specifically, an increase in dietary intake or a reduction in PA (12). To enhance the efficacy of behavioral interventions for weight loss, a better understanding of compensatory adaptations in response to exercise or dietary restriction is needed. This narrative review describes and evaluates the current evidence on the reciprocal effects of changes in exercise, PA, and dietary intake in adults. Specifically, the effect of exercise on habitual PA and energy intake, as well as the effect of changes in energy intake on PA, will be examined.
Change in Habitual PA in Response to Alterations in Exercise
Habitual PA has been suggested to be an important component in the regulation of energy balance (13, 14), and differences in habitual PA have been shown to account for differences in weight change in response to weight-loss interventions (9, 15). A reduction in NEAT in response to increased exercise could be a possible explanation for the lack of success in exercise-based weight loss (16), but current evidence on compensatory adaptations in NEAT in response to exercise has been equivocal. Inconsistent results may be caused by differences in study designs because duration of the exercise intervention and age potentially affect exercise-induced adaptations in habitual PA (Table 1).
TABLE 1.
Studies examining the effect of exercise on objectively measured habitual PA1
| Reference | Population | Study design | Intervention | Outcome measure | Compensation |
| Alahmadi et al. (17) | 16 Sedentary men (age: 27 ± 3 y; BMI: 30 ± 6) | Crossover | Single aerobic exercise bout (60 min) | Accelerometry | No |
| Cadieux et al. (18) | 16 Sedentary adults (age: 22 ± 3 y; BMI: 23 ± 2) | Crossover | Single aerobic or resistance bout (45 min) | Multisensor device | No |
| Sim et al. (19) | 17 Overweight men (age: 30 ± 8 y; BMI: 28 ± 2) | Crossover | Single moderate or intermittent exercise bout | Accelerometry | No |
| Schutz et al. (20) | 55 Women (age: 34 ± 5 y; BMI: 28 ± 2) | RCT | 4 wk Walking (5 times/wk) | Accelerometry | Yes |
| Colley et al. (21) | 13 Overweight women (age: 34 ± 5 y; BMI: 28 ± 2) | Pre-post | 8 wk Walking (3–4 times/wk) | Accelerometry | Yes |
| Meijer et al. (22) | 22 Adults (age: 58 ± 3 y; BMI: 25 ± 2) | RCT | 12 wk Aerobic + resistance exercise (2 sessions/wk) | Accelerometry | Yes |
| Meijer et al. (23) | 30 Adults (age: 59 ± 4 y; BMI: 31 ± 3) | RCT | 12 wk Aerobic + resistance exercise (2 sessions/wk) | Accelerometry | Yes |
| Di Blasio et al. (24) | 34 Postmenopausal women (age: 56 ± 4 y; BMI: 27 ± 4) | Pre-post | 13 wk Walking (40–50 min; 4 sessions/wk) | Multisensor device | Inconclusive |
| Rosenkilde et al. (25) | 61 Overweight men (age: 29 ± 6 y; BMI: 28 ± 2) | RCT | 13 wk Daily aerobic exercise | Accelerometry | Yes |
| Van Etten et al. (26) | 26 Sedentary men (age: 34 ± 6 y; fat: 23% ± 5%) | RCT | 18 wk Resistance exercise (2 sessions/wk) | Accelerometry | No |
| Turner et al. (27) | 54 Sedentary men (age: 54 ± 5 y; BMI: 28 ± 3) | RCT | 6 mo Aerobic exercise (2–4 times/wk) | Accelerometry | No |
| Hollowell et al. (28) | 50 Overweight adults (age: 53 ± 7 y; BMI: 30 ± 3) | RCT | 8 mo Aerobic exercise 3–5 sessions/wk) | Accelerometry | No |
| Rangan et al. (29) | 82 Overweight adults (age: 50 ± 11 y; BMI: 31 ± 3) | RCT | 8 mo Aerobic/resistance exercise (∼3 d/wk) | Accelerometry | No |
| Willis et al. (30) | 92 Sedentary adults (age: 18–39; BMI: 25–40) | RCT | 10 mo Aerobic exercise (5 sessions/wk) | Accelerometry | No |
| Meijer et al. (31) | 32 Adults (age: 36 ± 4 y; BMI: 23 ± 2) | Pre-post | 10 mo Progressive aerobic exercise | Accelerometry | No |
PA, physical activity; pre-post, comparison of pre-intervention PA to post-intervention PA; RCT, randomized controlled trial.
A single exercise bout does not appear to induce any changes in habitual PA (17–19). Alahmadi et al. (17) actually reported an increase in PA 2 d after a single exercise bout in previously sedentary participants. Habitual PA, however, was reduced in exercise programs lasting up to 4 mo (20–23, 25). Particularly vigorous exercise has been suggested to induce a reduction in habitual PA because of fatigue and discomfort (32). Accordingly, Schutz et al. (20) showed a greater reduction in PA in response to a higher exercise volume. Nevertheless, evidence of the effect of exercise intensity or exercise volume on habitual PA remains inconsistent (14, 33). There was no change in habitual PA in response to interventions lasting ≥6 mo (27–31). The lack of a compensatory reduction in habitual PA may be because of an increase in fitness in response to prolonged engagement in an exercise protocol, which would increase exercise tolerance and mitigate the negative effects of exercise on habitual PA (34).
Exercise tolerance may also explain differences in the response to exercise in different age groups. Several studies have shown a reduction in habitual PA in response to aerobic exercise in elderly people (22, 23, 35). However, to my knowledge, no study compared younger and older adults, which limits the ability to examine a potential age effect. An additional consideration may be the type of exercise program; Hunter et al. (36) showed an increase in TDEE and habitual PA in response to resistance exercise in elderly people. This has been attributed to an increase in functional capacity (37). This study, however, estimated habitual PA based on TDEE and RMR, rather than objectively measuring it (36). Most exercise interventions relied on aerobic exercise because of the greater energy expenditure associated with this type of exercise compared with resistance training (37). Therefore, more research is needed on potential differences in the effect of various exercise types on correlates of energy balance.
In addition to potential differences by age and type of exercise, there is also a possible difference in the effect of exercise on habitual PA based on its effect on energy balance. Westerterp (7) argues that habitual PA does not change in response to exercise when energy balance is maintained (i.e., increase in energy intake according to increased energy expenditure); however, there is a reduction in habitual PA when exercise induces a negative energy balance. This aspect further underlines the complex interaction of various components contributing to energy balance along with the importance of simultaneous measurements of the various components contributing to energy balance.
Currently, to my knowledge, there is insufficient evidence on hypokinetic effects of exercise. Short-term aerobic exercise interventions potentially induce an incomplete compensatory reduction in habitual PA, particularly in elderly people and less-fit participants. Resistance exercise and a prolonged exercise engagement, on the other hand, were not shown to reduce habitual PA and may actually increase habitual PA as a result of increased fitness and functional capacity.
Change in Energy Intake in Response to Alterations in Exercise
Energy intake has been suggested as the largest source of compensation because it is entirely determined by eating behavior (14). Several reviews have examined the effect of exercise on subsequent energy intake and these should be consulted for more detailed information (38–40). As has been shown for compensatory adaptations in habitual PA, results on adjustments in energy intake in response to exercise have been inconsistent, but some general trends have started to emerge.
The majority of studies have not shown an acute effect of exercise on subsequent energy intake (38, 40). Some studies, however, showed at least a partial compensatory increase in energy intake in the meal after an exercise bout or within a 24-h period (41–49), whereas others reported a decline after a single exercise bout (19, 50-52). The reduction in energy intake after exercise has been attributed to a reduction in appetite, which has been shown, in response to high-intensity aerobic exercise, as a result of a delay in gastric emptying (7, 53, 54). It has also been argued that dietary intake postexercise is driven by the need to restore carbohydrate balance rather than to meet total energy needs (55, 56). This, however, would suggest that higher exercise intensities, which rely predominantly on carbohydrate metabolism, would induce a greater energy intake. Although this hypothesis contradicts the previous statement on an acute reduction in energy intake after exercise bouts of higher intensity, it may affect energy intake over a longer period of time. Alméras et al. (57), e.g., showed that energy intake is higher in subjects who have lower fat oxidation (i.e., greater reliance on carbohydrates) than in those with higher fat oxidation.
The lack of conclusive evidence on acute effects of exercise on energy intake may also be because of a large daily variability in energy intake (SD of 25%) and energy expenditure (SD of 10%) (58). It further supports the commonly observed loose coupling between energy expenditure and energy intake on a daily basis (39, 58). Energy expenditure and energy intake, however, have been shown to correlate well over a period of 2 wk (59), although there is only a partial compensatory increase in energy intake (60). In a recent review, 50% of studies examining changes in energy intake in response to an exercise intervention lasting between 2 and 14 d reported at least partial increases in energy intake in response to an exercise intervention (38). Most studies also showed an increase in carbohydrates, and results for changes in fat and protein intake were less consistent, supporting a close regulation of nutrient availability rather than energy balance. In addition to a physiologic drive to increase energy intake, there may also be a psychological component. Participants in an exercise intervention may feel a greater freedom to eat more and may even reward themselves with a higher energy intake after an exercise bout.
Energy intake in response to longer exercise interventions (3–72 wk), however, was generally not increased (38). In fact, to my knowledge, only 2 studies showed a substantial increase in energy intake in response to aerobic exercise lasting >8 wk (15, 61). Nevertheless, Cook and Schoeller (62) suggest a better coupling of energy intake and energy expenditure with higher activity levels. In fact, almost 60 y ago Mayer et al. (63) showed that men in occupations with a least light PA matched their energy intake better to energy expenditure than those in sedentary occupations. This led to a differentiation between a “regulated zone” (i.e., energy intake matches energy expenditure) and an “unregulated zone” (i.e., energy intake exceeds energy expenditure), with the unregulated zone being characterized by low levels of PA (64). Accordingly, athletes have been shown to adjust their dietary intake in response to alterations in training regimen (65, 66). Lean participants have also been shown to increase their energy intake in response to an exercise program, and no compensation was observed in obese adults (67–69). This may be because of a higher need of lean individuals to defend their relatively lower body fat stores than individuals with excess fat (70). A reduction in PA, however, was not associated with a compensatory reduction in energy intake in lean men, resulting in significant weight gain (71).
Overall, there seems to be a loose coupling of daily energy expenditure and energy intake because of the ability of humans to rely on the body’s energy stores (16, 72). The majority of studies showed an incomplete adaptation in energy intake in response to exercise, resulting in a negative energy balance. However, Melzer et al. (73) argue that the mismatch between energy intake and energy expenditure does not continue indefinitely and that at some critical point there would be an increase in energy intake in response to increased energy expenditure. The timeline of this adaptation may be influenced by exercise duration, intensity, or type, along with subject characteristics such as activity level, body composition, or race/ethnicity. Subject characteristics may also affect measurement accuracy of energy intake in free-living situations because the majority of studies relied on self-reported dietary intake, which is subject to bias and provides inaccurate estimations of energy intake (74, 75). These limitations in the currently available literature may have contributed to the lack of compelling evidence for an increase in energy intake in response to alterations in energy expenditure.
Change in PA in Response to Alterations in Energy Intake
A reduction in energy intake, rather than an increase in energy expenditure, may be even more popular to induce a negative energy balance compared with starting an exercise program. Research on the effects of dietary changes on correlates of energy balance predominantly focused on TDEE and the effects on thermic effect of food and RMR rather than PA. Only a few studies considered the effect of dietary changes on objectively measured PA. Caloric restriction was generally associated with a decrease in PA (76–80). In addition, a change in substrate metabolism that favors fat deposition and an increase in appetite have been suggested in response to a diet-induced negative energy balance (81). Once energy balance was re-established PA levels returned back to baseline (76) or even increased during weight-loss maintenance (Table 2) (82, 83).
TABLE 2.
Studies examining the effect of caloric restriction on objectively measured habitual PA1
| Reference | Population | Study design | Intervention | Outcome measure | Compensation |
| Bonomi et al. (83) | 66 Overweight/obese adults (age: 51 ± 12 y; BMI: 38 ± 7) | Observation (pre-post) | 12 wk of 67% caloric restriction | DLW, accelerometry | Increased PA after 2 wk of maintenance |
| Camps et al. (76) | 51 Healthy adults (age: 42 ± 8; BMI: 31 ± 3) | Observation (pre-post) | 8 wk very low energy diet (Modifast Nutrition et Sante Benelux; 500 kcal/d) | DLW, accelerometry | Yes (restored after weight loss) |
| Martin et al. (77) and Redman et al. (79) | 48 Overweight adults (age: 37 ± 1 y; BMI: 28 ± 1) | RCT | 6 mo of 25% caloric restriction or 890 kcal/d | DLW, sleep EE-PAL | Yes |
| Martin et al. (78) | 105 Adults (mean age: 41 y; BMI: 28 ± 2) | RCT | Up to 12 mo of 10–30% caloric restriction | DLW, accelerometry | Yes |
| Velthuis-te-Wierik et al. (80) | 24 Healthy men (age: 43 ± 5; BMI: 25 ± 2) | Observation (pre-post) | 10 wk of caloric restriction (80% of TDEE) | DLW, RMR (PAL) | No; trend for decrease (P = 0.066) |
DLW, doubly labeled water; EE, energy expenditure; PA, physical activity; PAL, physical activity level; pre-post, comparison of pre-intervention PA to post-intervention PA; RCT, randomized controlled trial; RMR, resting metabolic rate; TDEE, total daily energy expenditure.
Results on the effects of overfeeding on PA were less consistent. The majority of the studies reported no change in accelerometer determined PA in response to increased caloric intake (9, 84–86), but there is also research that shows an increase (87) or decrease (88) in PA in response to overfeeding. The decline in PA reported by Pasquet et al. (88) may have been caused by the research setting because the goal of this tradition in an African tribe was to put on fat during the overfeeding period. The observed increase in PA may also be explained by the study population, which consisted of nonobese participants who exercised regularly (87). As was shown in the previous section, active adults may be better able to adjust their behavior in order to maintain energy balance (Table 3).
TABLE 3.
Studies examining the effect of overfeeding on objectively measured habitual PA1
| Reference | Population | Study design | Intervention | Outcome measure | Compensation |
| Apolzan et al. (87) | 25 Healthy adults (age: 18–35 y; BMI: 25 ± 1) | RCT | 56 d (140% of energy needs; average intake 954 kcal/d above energy needs) | 24-h Calorimetry, accelerometry, DLW | Yes |
| Diaz et al. (84) | 10 Men (age: 30 ± 8 y; BMI: 23 ± 3) | Observation (pre-post) | 42 d (150% of energy needs) | DLW, direct calorimetry | No |
| Joosen et al. (85) | 14 Healthy women (age: 25 ± 4 y; BMI 2 ± 2) | Observation (pre-post) | 14 d (150% of energy needs) | DLW, accelerometry | No |
| Levine et al. (9) | 16 Nonobese adults (age: 25–36y; BMI: N/A) | Observation (pre-post) | 8 wk (1000 kcal/d above energy needs) | DLW, BMR, accelerometry | No |
| Pasquet et al. (88) | 9 Lean men (age: 29 ± 5 y; BMI: 20 ± 2) | Observation (pre-post) | 2 mo Traditional overfeeding (3600 kcal/d) | DLW, RMR, PA diary, accelerometry | Reduction in PA |
| Roberts et al. (86) | 7 Healthy men (age: 24 ± 1 y; BMI: 24 ± 1) | Observation (pre-post) | 21 d (1000 kcal/d above energy needs) | DLW, accelerometry | No |
BMR, basal metabolic rate; DLW, doubly labeled water; PA, physical activity; pre-post, comparison of pre-intervention PA to post-intervention PA; RCT, randomized controlled trial; RMR, resting metabolic rate.
Taken together, these findings suggest that a reduction in energy intake is associated with a decline in energy expenditure, which, at least partially, offsets the dietary-induced energy deficit. Overfeeding, on the other hand, was generally not associated with compensatory adaptations in PA, resulting in subsequent weight gain.
Conclusions
The limited success of behavioral interventions to induce weight loss on a population level suggests some underlying compensatory mechanisms that offset a negative energy balance induced by exercise or caloric restriction. There appears to be a stronger mechanism to defend against weight loss, and compensatory adaptations seem to be less pronounced to prevent weight gain (72). This phenomenon has been attributed to a genetic drift, which predisposes certain individuals to an increased risk of excess weight gain (89). This hypothesis further accounts for the large variability in individual response rate, which may at least partially explain the inconsistent findings regarding compensatory behavioral adaptations in response to a disruption in energy balance.
Overall, data presented in this review provide limited evidence for behavioral adjustments in response to exercise-based or dietary interventions. The lack of conclusive evidence can be partially attributed to large individual variability in the response to alterations in energy balance and the focus on average values for specific groups, which may not accurately represent the response of an individual (40). Therefore, a stronger focus on individual differences in response to alterations in PA or dietary intake is warranted (14). Subject characteristics such as age, sex, body weight, or fitness potentially affect compensatory adaptations. It also remains to be determined whether behavioral changes are intentional (i.e., I can eat more because I exercised; I already exercised, so I don’t need to move the rest of the day) or not (14). Furthermore, differences in the characteristics of the exercise (e.g., duration, intensity, frequency, and type of exercise) or dietary interventions (e.g., macronutrient content, meal frequency, and timing) could have different effects on energy balance.
Compensatory adaptations are also driven by physiologic responses to alterations in energy balance, but the effect of physiologic changes on specific behaviors may vary considerably among individuals. A negative energy balance has generally been associated with an increased orexigenic drive (90, 91), which would induce an increase in energy intake. Exercise, however, has been associated with increased satiety signaling, which could offset the orexigenic effect of a negative energy balance (91). These 2 independent processes are regulated by multiple hormones, which potentially lead to individual differences in the response to alterations in energy balance (62). King et al. (91), e.g., showed a greater increase in energy intake in participants who did not lose weight in response to a 12-wk exercise program, suggesting the existence of specific phenotypes that are more or less likely to compensate for changes in energy balance. On the other hand, Manthou et al. (15) argue that a difference in habitual PA is the major contributor to individual variability in weight loss in response to exercise interventions.
To enhance the understanding of the regulation of energy balance, simultaneous objective measurements of all major contributors (i.e., body composition, various components of energy expenditure, and energy intake) are needed (14). Further physiologic and environmental constraints need to be considered when examining the effects of diet and exercise on energy balance. This may help with the identification of specific characteristics that contribute to compensations via dietary intake or PA. A better understanding of specific characteristics and settings that contribute to a compensatory response appears to be crucial for the development of successful weight-loss and weight-management interventions. It would also allow for identification of individuals who may require assistance beyond a behavioral intervention to achieve their goals. Perhaps there is no single best approach to target the current obesity epidemic, but a better understanding of the contribution of physiologic, behavioral, and environmental constraints to the regulation of energy balance will help in the development of successful strategies to address this problem.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: NEAT, nonexercise activity thermogenesis; PA, physical activity; RMR, resting metabolic rate; TDEE, total daily energy expenditure.
References
- 1.WHO. World health report 2002: reducing risk—promoting healthy life. Geneva (Switzerland): WHO Press; 2002. [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services (USDHHS). The Surgeon General’s call to action to prevent and decrease overweight and obesity. Rockville (MD): USDHHS, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- 4.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab 2004;89:2522–5. [DOI] [PubMed] [Google Scholar]
- 5.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care 2004;7:599–605. [DOI] [PubMed] [Google Scholar]
- 6.Vermorel M, Lazzer S, Bitar A, Ribreye J, Montaurier C, Fellmann N, Coudert J, Meyer M, Broirie Y. Contributing factors and variability of energy expenditure in non-obese, obese, and post-obese adolescents. Reprod Nutr Dev 2005;45:129–42. [DOI] [PubMed] [Google Scholar]
- 7.Westerterp KR. Alterations in energy balance with exercise. Am J Clin Nutr 1998;68:970S–4S. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999;283:212–4. [DOI] [PubMed] [Google Scholar]
- 10.Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol 1992;263:E296–300. [DOI] [PubMed] [Google Scholar]
- 11.Frühbeck G. Does a NEAT difference in energy expenditure lead to obesity? Lancet 2005;366:615–6. [DOI] [PubMed] [Google Scholar]
- 12.Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes (Lond) 2014 Oct 17 (Epub ahead of print; DOI: 10.1038/ijo.2014.184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes Rev 2012;13(Suppl 2):105–21. [DOI] [PubMed] [Google Scholar]
- 14.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc 2013;45:1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manthou E, Gill JM, Wright A, Malkova D. Behavioural compensatory adjustments to exercise training in overweight women. Med Sci Sports Exerc 2010;42:1121–8. [DOI] [PubMed] [Google Scholar]
- 16.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, Stubbs JR, Blundell JE. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring) 2007;15:1373–83. [DOI] [PubMed] [Google Scholar]
- 17.Alahmadi MA, Hills AP, King NA, Byrne NM. Exercise intensity influences nonexercise activity thermogenesis in overweight and obese adults. Med Sci Sports Exerc 2011;43:624–31. [DOI] [PubMed] [Google Scholar]
- 18.Cadieux S, McNeil J, Lapierre MP, Riou M, Doucet É. Resistance and aerobic exercises do not affect post-exercise energy compensation in normal weight men and women. Physiol Behav 2014;130:113–9. [DOI] [PubMed] [Google Scholar]
- 19.Sim AY, Wallman KE, Fairchild TJ, Guelfi KJ. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond) 2014;38:417–22. [DOI] [PubMed] [Google Scholar]
- 20.Schutz Y, Nguyen DM, Byrne NM, Hills AP. Effectiveness of three different walking prescription durations on total physical activity in normal- and overweight women. Obes Facts 2014;7:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colley RC, Hills AP, King NA, Byrne NM. Exercise-induced energy expenditure: implications for exercise prescription and obesity. Patient Educ Couns 2010;79:327–32. [DOI] [PubMed] [Google Scholar]
- 22.Meijer EP, Westerterp KR, Verstappen FT. Effect of exercise training on total daily physical activity in elderly humans. Eur J Appl Physiol Occup Physiol 1999;80:16–21. [DOI] [PubMed] [Google Scholar]
- 23.Meijer EP, Westerterp KR, Verstappen FT. Effect of exercise training on physical activity and substrate utilization in the elderly. Int J Sports Med 2000;21:499–504. [DOI] [PubMed] [Google Scholar]
- 24.Di Blasio A, Ripari P, Bucci I, Di Donato F, Izzicup P, D’Angelo E, De Nenno B, Taglieri M, Napolitano G. Walking training in postmenopause: effects on both spontaneous physical activity and training-induced body adaptations. Menopause 2012;19:23–32. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkilde M, Auerbach P, Reichkendler MH, Ploug T, Stallknecht BM, Sjödin A. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol 2012;303:R571–9. [DOI] [PubMed] [Google Scholar]
- 26.Van Etten LM, Westerterp KR, Verstappen FT, Boon BJ, Saris WH. Effect of an 18-week weight-training program on energy expenditure and physical activity. J Appl Physiol 1997;82:298–304. [DOI] [PubMed] [Google Scholar]
- 27.Turner JE, Markovitch D, Betts JA, Thompson D. Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. Am J Clin Nutr 2010;92:1009–16. [DOI] [PubMed] [Google Scholar]
- 28.Hollowell RP, Willis LH, Slentz CA, Topping JD, Bhakpar M, Kraus WE. Effects of exercise training amount on physical activity energy expenditure. Med Sci Sports Exerc 2009;41:1640–4. [DOI] [PubMed] [Google Scholar]
- 29.Rangan VV, Willis LH, Slentz CA, Bateman LA, Shields AT, Houmard JA, Kraus WE. Effects of an 8-month exercise training program on off-exercise physical activity. Med Sci Sports Exerc 2011;43:1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis EA, Herrmann SD, Honas JJ, Lee J, Donnelly JE, Washburn RA. Nonexercise energy expenditure and physical activity in the midwest exercise trial 2. Med Sci Sports Exerc 2014;46:2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer GA, Janssen GM, Westerterp KR, Verhoeven F, Saris WH, ten Hoor F. The effect of a 5-month endurance-training programme on physical activity: evidence for a sex-difference in the metabolic response to exercise. Eur J Appl Physiol Occup Physiol 1991;62:11–7. [DOI] [PubMed] [Google Scholar]
- 32.Westerterp KR. Pattern and intensity of physical activity. Nature 2001;410:539. [DOI] [PubMed] [Google Scholar]
- 33.Li J, O’Connor LE, Zhou J, Campbell WW. Exercise patterns, ingestive behaviors, and energy balance. Physiol Behav 2014;134:70–5. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev 2005;33:169–74. [DOI] [PubMed] [Google Scholar]
- 35.Morio B, Montaurier C, Pickering G, Ritz P, Fellmann N, Coudert J, Beaufrère B, Vermorel M. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br J Nutr 1998;80:511–9. [DOI] [PubMed] [Google Scholar]
- 36.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol (1985) 2000;89:977–84. [DOI] [PubMed] [Google Scholar]
- 37.Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes 2011 2011;pii:482564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One 2014;9:e83498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, Finlayson G. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech 2012;5:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopkins M, King NA, Blundell JE. Acute and long-term effects of exercise on appetite control: is there any benefit for weight control? Curr Opin Clin Nutr Metab Care 2010;13:635–40. [DOI] [PubMed] [Google Scholar]
- 41.Erdman J, Tahbaz R, Lippl F, Wagenpfeil S, Schusdziarra V. Plasma ghrelin levels during exercise—effects of intensity and duration. Regul Pept 2007;143:127–35. [DOI] [PubMed] [Google Scholar]
- 42.Laan DJ, Leidy HJ, Lim E, Campbell WW. Effects and reproducibility of aerobic and resistance exercise on appetite and energy intake in young, physically active adults. Appl Physiol Nutr Metab 2010;35:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol 2007;193:251–8. [DOI] [PubMed] [Google Scholar]
- 44.Pomerleau M, Imbeault P, Parker T, Doucet E. Effects of exercise intensity on food intake and appetite in women. Am J Clin Nutr 2004;80:1230–6. [DOI] [PubMed] [Google Scholar]
- 45.Shorten AL, Wallman KE, Guelfi KJ. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am J Clin Nutr 2009;90:1215–21. [DOI] [PubMed] [Google Scholar]
- 46.Verger P, Lanteaume MT, Louis-Sylvestre J. Free food choice after acute exercise in men. Appetite 1994;22:159–64. [DOI] [PubMed] [Google Scholar]
- 47.Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM. Influence of running and walking on hormonal regulators of appetite in women. J Obes 2012; 2012:730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha J, Paxman J, Dalton C, Winter E, Broom D. Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive men. Appetite 2013;71:369–78. [DOI] [PubMed] [Google Scholar]
- 49.Charlot K, Chapelot D. Energy compensation after an aerobic exercise session in high-fat/low-fit and low-fat/high-fit young male subjects. Br J Nutr 2013;110:1133–42. [DOI] [PubMed] [Google Scholar]
- 50.Jokisch E, Coletta A, Raynor HA. Acute energy compensation and macronutrient intake following exercise in active and inactive males who are normal weight. Appetite 2012;58:722–9. [DOI] [PubMed] [Google Scholar]
- 51.Kissileff HR, Pi-Sunyer FX, Segal K, Meltzer S, Foelsch PA. Acute effects of exercise on food intake in obese and nonobese women. Am J Clin Nutr 1990;52:240–5. [DOI] [PubMed] [Google Scholar]
- 52.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Fujimoto S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J Endocrinol 2009;203:357–64. [DOI] [PubMed] [Google Scholar]
- 53.Cammack J, Read NW, Cann PA, Greenwood B, Holgate AM. Effect of prolonged exercise on the passage of a solid meal through the stomach and small intestine. Gut 1982;23:957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neufer PD, Young AJ, Sawka MN. Gastric emptying during walking and running: effects of varied exercise intensity. Eur J Appl Physiol Occup Physiol 1989;58:440–5. [DOI] [PubMed] [Google Scholar]
- 55.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007;86:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snitker S, Larson DE, Tataranni PA, Ravussin E. Ad libitum food intake in humans after manipulation of glycogen stores. Am J Clin Nutr 1997;65:941–6. [DOI] [PubMed] [Google Scholar]
- 57.Alméras N, Lavallée N, Després JP, Bouchard C, Tremblay A. Exercise and energy intake: effect of substrate oxidation. Physiol Behav 1995;57:995–1000. [DOI] [PubMed] [Google Scholar]
- 58.Schoeller DA, Thomas D. Energy balance and body composition. World Rev Nutr Diet 2015;111:13–8. [DOI] [PubMed] [Google Scholar]
- 59.Edholm OG, Fletcher JG, Widdowson EM, McCance RA. The energy expenditure and food intake of individual men. Br J Nutr 1955;9:286–300. [DOI] [PubMed] [Google Scholar]
- 60.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, Blundell J. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol 2004;286:R350–8. [DOI] [PubMed] [Google Scholar]
- 61.Brandon LJ, Elliott-Lloyd MB. Walking, body composition, and blood pressure dose-response in African American and white women. Ethn Dis 2006;16:675–81. [PubMed] [Google Scholar]
- 62.Cook CM, Schoeller DA. Physical activity and weight control: conflicting findings. Curr Opin Clin Nutr Metab Care 2011;14:419–24. [DOI] [PubMed] [Google Scholar]
- 63.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr 1956;4:169–75. [DOI] [PubMed] [Google Scholar]
- 64.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012;126:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin MK, Martin DT, Collier GR, Burke LM. Voluntary food intake by elite female cyclists during training and racing: influence of daily energy expenditure and body composition. Int J Sport Nutr Exerc Metab 2002;12:249–67. [DOI] [PubMed] [Google Scholar]
- 66.Barr SI, Costill DL. Effect of increased training volume on nutrient intake of male collegiate swimmers. Int J Sports Med 1992;13:47–51. [DOI] [PubMed] [Google Scholar]
- 67.Woo R, Garrow JS, Pi-Sunyer FX. Voluntary food intake during prolonged exercise in obese women. Am J Clin Nutr 1982;36:478–84. [DOI] [PubMed] [Google Scholar]
- 68.Woo R, Pi-Sunyer FX. Effect of increased physical activity on voluntary intake in lean women. Metabolism 1985;34:836–41. [DOI] [PubMed] [Google Scholar]
- 69.Durrant ML, Royston JP, Wloch RT. Effect of exercise on energy intake and eating patterns in lean and obese humans. Physiol Behav 1982;29:449–54. [DOI] [PubMed] [Google Scholar]
- 70.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, Caudwell P, Finlayson G, Gibbons C, Hopkins M, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br J Sports Med 2012;46:315–22. [DOI] [PubMed] [Google Scholar]
- 71.Stubbs RJ, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell JE. A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. Am J Clin Nutr 2004;79:62–9. [DOI] [PubMed] [Google Scholar]
- 72.Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev 2010;68:148–54. [DOI] [PubMed] [Google Scholar]
- 73.Melzer K, Kayser B, Saris WH, Pichard C. Effects of physical activity on food intake. Clin Nutr 2005;24:885–95. [DOI] [PubMed] [Google Scholar]
- 74.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill RJ, Davies PSW. The validity of self-reported energy intake as determined using the doubly labeled water technique. Br J Nutr 2001;85:415–30. [DOI] [PubMed] [Google Scholar]
- 76.Camps SG, Verhoef SP, Westerterp KR. Weight loss-induced reduction in physical activity recovers during weight maintenance. Am J Clin Nutr 2013;98:917–23. [DOI] [PubMed] [Google Scholar]
- 77.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15:2964–73. [DOI] [PubMed] [Google Scholar]
- 78.Martin CK, Das SK, Lindblad L, Racette SB, McCrory MA, Weiss EP, Delany JP, Kraus WE; CALERIE Study Team. Effect of calorie restriction on the free-living physical activity levels of nonobese humans: results of three randomized trials. J Appl Physiol (1985) 2011;110:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 2009;4:e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int J Obes Relat Metab Disord 1995;19:318–24. [PubMed] [Google Scholar]
- 81.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci (Lond) 2013;124:231–41. [DOI] [PubMed] [Google Scholar]
- 82.Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, Newcomer BR, Goran MI. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 2000;71:1138–46. [DOI] [PubMed] [Google Scholar]
- 83.Bonomi AG, Soenen S, Goris AH, Westerterp KR. Weight-loss induced changes in physical activity and activity energy expenditure in overweight and obese subjects before and after energy restriction. PLoS One 2013;8:e59641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 1992;56:641–55. [DOI] [PubMed] [Google Scholar]
- 85.Joosen AM, Bakker AH, Westerterp KR. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav 2005;85:593–7. [DOI] [PubMed] [Google Scholar]
- 86.Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol 1990;259:R461–9. [DOI] [PubMed] [Google Scholar]
- 87.Apolzan JW, Bray GA, Smith SR, de Jonge L, Rood J, Han H, Redman LM, Martin CK. Effects of weight gain induced by controlled overfeeding on physical activity. Am J Physiol Endocrinol Metab 2014;307:E1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, de Garine I, Apfelbaum M. Massive overfeeding and energy balance in men: the Guru Walla model. Am J Clin Nutr 1992;56:483–90. [DOI] [PubMed] [Google Scholar]
- 89.Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the ‘drifty gene’ hypothesis. Int J Obes (Lond) 2008;32:1611–7. [DOI] [PubMed] [Google Scholar]
- 90.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr 1998;68:794–801. [DOI] [PubMed] [Google Scholar]
- 91.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr 2009;90:921–7. [DOI] [PubMed] [Google Scholar]