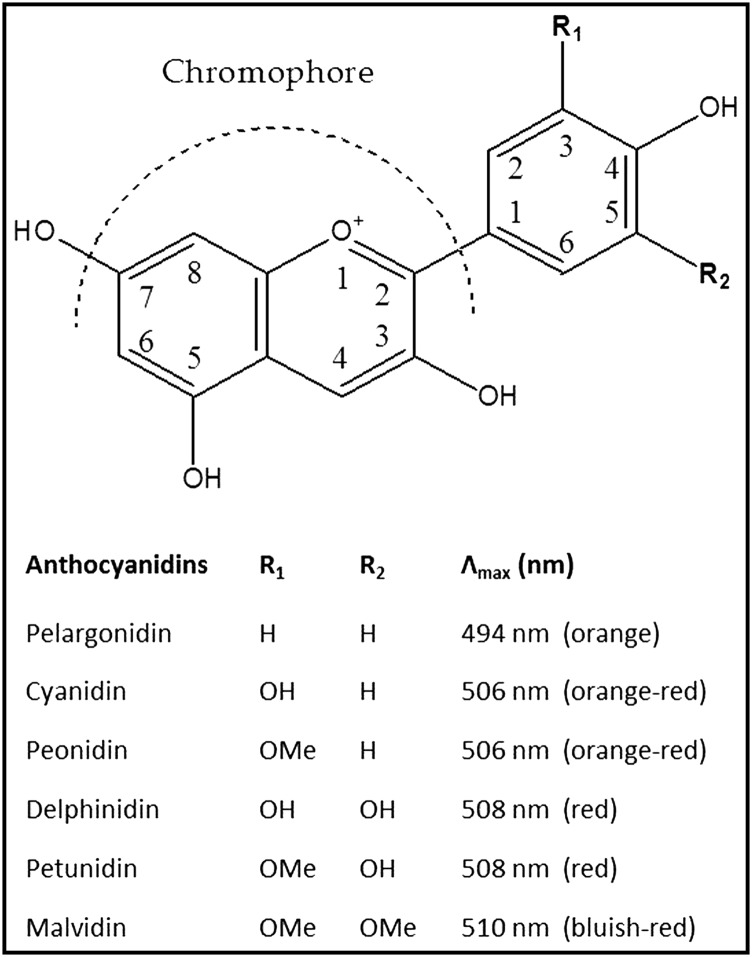
Anthocyanins (Greek anthos = flower and kyáneos = blue) are polyphenolic pigments that belong to the flavonoid group and are responsible for many of the red-orange to blue-violet colors present in plant organs such as fruits, flowers, and leaves. To date, >700 structurally distinct anthocyanin derivatives of 27 aglycons, known as “anthocyanidins,” have been identified in nature. In addition to their multiple phenyl groups, anthocyanins are rarely found as aglycons. The anthocyanidin is typically attached to ≥1 sugar moieties commonly conjugated to the C3 hydroxyl group in the C-ring, making them glycosides. With the exception of the 3-deoxyanthocyanidins, anthocyanins exist almost exclusively in the glycosylated form; their anthocyanidin counterparts are not stable and rarely found in nature (1). Six anthocyanidins are predominant in nature (Figure 1) and represent ∼90% of all anthocyanins identified to date. Adding to their natural diversity in nature, anthocyanins can be acylated, by esterification of a cinnamic or aliphatic acid to ≥1 of the sugar substitutions; ∼50% of anthocyanins found in nature are acylated.
FIGURE 1.
Anthocyanidins more commonly found in nature, their B-ring conjugations (R1 and R2), and maximum absorbance. H, hydrogen group; OH, hydroxy group; OMe, methoxy group.
Anthocyanins have garnered recent interest from researchers because of their potential preventative and/or therapeutic effects on human health. Studies conducted monitoring anthocyanin presence in plasma and urine after dietary intake suggest poor bioavailability, on the order of <1%. However, the relative bioavailability of cyanidin-3-glucoside, the most predominant anthocyanin found in nature, was recently shown to be 12.4% ± 1.38% in a 13C-tracer study (2). The degree of absorption of anthocyanins into the circulation is highly dependent on their structure (3). Chronic consumption of anthocyanins and/or anthocyanin-rich foods has been postulated to exert an array of health benefits, including but not limited to cardiovascular protection, neuroprotection, vision improvement, antidiabetic and antiobesity properties, anti-inflammatory effects, and chemoprevention and cancer protection (4).
Deficiencies
Anthocyanins are not essential nutrients, and no deficiency disorder has been associated with the lack of anthocyanin consumption. The value of anthocyanins and other dietary bioactive compounds may be their potential to promote health maintenance throughout the life span. Regular intake of colorful fruits and vegetables is an important component of a healthy lifestyle that can confer protection against chronic diseases. Low intakes of fruits and vegetables account for an estimated 1.7 million deaths globally, including but not limited to those caused by gastrointestinal cancer (14%), ischemic heart disease (11%), and stroke (9%) (5).
Dietary Recommendations
Dietary reference intakes do not currently exist for anthocyanins and many other dietary bioactive compounds in the United States, Canada, or the European Union. China has currently defined a specific proposed level of 50 mg/d for anthocyanins (6). Although current public health recommendations to increase the consumption of colorful fruits and vegetables in the United States are largely driven by the need to meet essential nutrient intakes from foods, recommendations from policy documents such as the Dietary Guidelines for Americans and groups such as the National Fruit and Vegetable Alliance also take into account the contribution of dietary bioactive compounds such as anthocyanins (7).
A positive association between anthocyanin intake and diet quality suggests that a diet high in anthocyanins is synonymous with greater compliance with national dietary guidance. The dietary intake of anthocyanins, as reported in the 2007–2008 NHANES, has been estimated to be ∼11.6 ± 1.1 mg/d for individuals aged ≥20 y. Women, on average, had a higher daily intake of anthocyanins (12.6 ± 1.5 mg/d) compared with men (10.5 ± 0.8 mg/d). Mean intakes of anthocyanins have also been shown to be significantly different among various racial/ethnic groups, with white individuals having higher mean daily intakes (12.5 ± 1.3 mg/d) than Hispanic (10.1 ± 1.2 mg/d) and non-Hispanic black (8.9 ± 0.9 mg/d) populations in the United States (8).
Clinical Uses
Anthocyanin-rich foods such as chokeberries and bilberries have traditionally been recommended in Europe and Asia for treatment of atherosclerosis, chronic venous insufficiency, and other health effects. In World War II, British fighter pilots were often administered bilberry jam for improved nighttime vision. Despite traditional medicinal uses, anthocyanins are not currently being used for therapeutic uses in Western medicine.
Food Sources
Important sources of anthocyanins in the diet of US adults aged ≥20 y as reported in the USDA’s flavonoid database include berries (20%), wine (16%), grapes (11%), red/purple vegetables (8%), 100% noncitrus juice (6%), yogurt (6%), and other food sources (33%) (6). Berries such as bilberries, blueberries, blackberries, blackcurrants, chokeberries, strawberries, and elderberries are a rich source of anthocyanins.
Anthocyanins are increasing in popularity as colorants for the food industry and may serve as alternatives to the use of synthetic colorants. The replacement of red or blue dyes with anthocyanin sources has potential to substantially increase anthocyanin consumption.
Toxicity
Anthocyanin toxicity, to our knowledge, has not been shown in currently published human intervention studies. The risk of toxicity from the food supply is minute given the low bioavailability of anthocyanins. The Joint FAO/WHO Expert Committee on Food Additives has established an acceptable daily intake of 2.5 mg/kg per day for anthocyanins from grape-skin extracts but not for anthocyanins in general. After a request from the European Commission to the European Food Safety Authority, the Scientific Panel on Food Additives and Nutrient Sources Added to Food was asked to provide a scientific opinion re-evaluating the safety of anthocyanins. The panel concluded that the currently available toxicologic database was inadequate to establish a numerically acceptable daily intake for anthocyanins. The majority of toxicologic data are derived from grape-skin and blackcurrant extracts, which were considered unlikely to be of safety concern by the European Food Safety Authority (9). China, the first country to define a recommended intake for anthocyanins, has not defined a Tolerable Upper Intake Level (6). Animal studies have not identified any toxic effects of anthocyanins (from currants, blueberries, and/or elderberries) at amounts of 20 mg/kg per day in rats, 25 mg/kg per d in mice, >3 g/d for 15 or 90 d in guinea pigs and rats, >2.4% body weight in beagle dogs, and 9 g/kg per day over 3 generations in rats, mice, and rabbits (10).
Recent Research
Human studies have indicated that anthocyanins are rapidly absorbed, appearing in the bloodstream a few minutes after consumption. Anthocyanin absorption can start as early as in the oral cavity, where they can be taken up by tissues lining the oral cavity. Degradation begins in the oral cavity and was recently shown to be structure-dependent and mediated by oral microbiota (11). Anthocyanins are absorbed throughout the gastrointestinal tract, from the stomach and the intestines. Maximum blood concentrations are achieved at ∼1 h. In both humans and animals, many ingested anthocyanins can be absorbed intact, circulating in the plasma and passing into urine without undergoing metabolic changes. Until recently, only a few methylated and sulfoconjugated metabolites had been identified; however, new 13C-tracer research has helped to identify many degradation products that enter the circulation and are excreted via the urine and breath (2). The ability of anthocyanins to target certain tissues appears limited; however, they have been shown to cross the blood-brain barrier and to be taken up into human vascular endothelial cells.
The capacity of anthocyanins to affect mammalian metabolism was recently demonstrated in an investigation of metabolomic changes in adult rats after intravenous administration of cyanidin-3-glucoside. It was shown that cyanidin-3-glucoside alters important cellular metabolites such as bile acids, glutathione, oxidized glutathione, and lipids in the blood, kidneys, and liver of rats (12). Randomized controlled trials suggest that purified anthocyanins and/or anthocyanin-rich extracts exert a beneficial significant effect on LDL cholesterol among individuals with hyperlipidemia. Less consistent (many nonsignificant) beneficial trends for total cholesterol and HDL cholesterol among those with hyperlipidemia have been reported in the peer-reviewed literature (13).
For future work, a standardized set of analytical methodologies that provide more homogeneous results would promote more rapid and productive comparisons between studies. Current analytical methodologies have limitations, such as underestimating the concentration of anthocyanins, as well as metabolites and degradation products, in the plasma, urine, and feces. Although research on anthocyanins has rapidly expanded, more human studies are needed with regard to their pharmacokinetics and to identify the mechanisms involved in their biological activities.
Acknowledgment
Both authors read and approved the final manuscript.
References
- 1.Andersen OM, Jordheim M. Basic anthocyanin chemistry and dietary sources. In: Wallace TC, Giusti MM, editors. Anthocyanins in health and disease. Boca Raton (FL): CRC Press; 2013. p. 13–90. [Google Scholar]
- 2.Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr 2013;97:995–1003. [DOI] [PubMed] [Google Scholar]
- 3.Novotny JA, Clevidence BA, Kurilich AC. Anthocyanin kinetics are dependent on anthocyanin structure. Br J Nutr 2012;107:504–9. [DOI] [PubMed] [Google Scholar]
- 4.Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Safety 2013;12:483–508. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global strategy on diet, physical activity, and health: promoting fruit and vegetable consumption around the world. 2004 [cited 2015 Jun 11]. Available from: http://www.who.int/dietphysicalactivity/fruit/en/index2.html.
- 6.Chinese Nutrition Society. Chinese DRIs handbook. Beijing (China): Standards Press of China; 2013. [Google Scholar]
- 7.Wallace TC, Blumberg JB, Johnson EJ, Shao A. Dietary bioactives: establishing a framework for recommended intakes. Adv Nutr 2015;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastian RS, Enns CW, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, Moshfegh AJ. New database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among U.S. adults. J Nutr 2015;145:1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Food Safety Authority. Scientific opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J 2013;11:1–51. [Google Scholar]
- 10.Pourrat H, Bastide P, Dorier P, Tronche P. Préparation et activité thérapeutique de quelques glycosides d’anthocyanes. [Preparation and therapeutic activity of some anthocyanin glycosides.] Chim Ther 1967;2:33–8 (in French). [Google Scholar]
- 11.Kamonpatana K, Giusti MM, Chitchumroonchokchai C, Moreno Cruz M, Riedl KM, Kumar P, Failla ML. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem 2012;135:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanzo A, Scholz M, Gasperotti M, Tramer F, Passamonti S, Vrhovsek U, Mattivi F. Metabonomic investigation of rat tissues following intravenous administration of cyanidin 3-glucoside at a physiologically relevant dose. Metabolomics 2013;9:88–100. [Google Scholar]
- 13.Wallace TC. Anthocyanins in cardiovascular disease prevention. In: Wallace TC and Giusti MM, editors. Anthocyanins in health and disease. New York: CRC Press; 2014. p. 165–97. [Google Scholar]