Abstract
Double-chambered right ventricle with discrete subaortic stenosis without ventricular septal defect is rare in adults. This report shows incremental value of 3D echocardiography in delineating the pathoanatomy of these lesions.
Keywords: Subaortic stenosis, Double-chambered right ventricle, Real-time live 3D echocardiography
1. Introduction
Co-existent congenital obstruction of the right and the left ventricular outflow tracts without ventricular septal defect was reported for the first time in 1957.1 Very few adults with this combination have been reported ever since. Discrete subaortic stenosis may be an isolated defect or associated with aortic valve stenosis, coarctation of aorta, patent ductus arteriosus, ventricular septal defect and abnormalities of the mitral valve.2 Subvalvular right ventricular outflow obstruction is usually due to anomalous muscle bundles or hypertrophied parieto-septal bands.3 Discrete subpulmonic stenosis due to a fibromuscular diaphragm as an isolated defect is uncommon. Combination of discrete subaortic and subpulmonic obstructions (sequestration of the outlet portion of the ventricle from a circumferential muscular diaphragm) without ventricular septal defect is rare in adults.4 This report illustrates an adult patient with type 2 double-chambered right ventricle without ventricular septal defect, associated subaortic stenosis and aortic regurgitation.
2. Case report
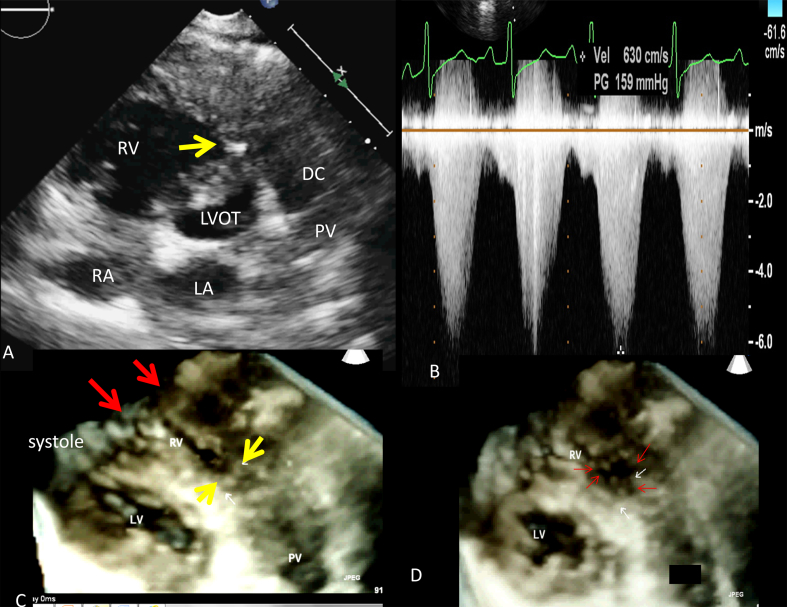
A 22-year young lady presented with history of exertional dyspnoea and fatigue of five years duration. During the physical examination, a prominent grade 5/6 systolic ejection murmur was heard on the left parasternal border. Her blood pressure was 90/72 mmHg, and heart rate was 86/min. In addition to mild cardiomegaly on the chest radiography, the electrocardiogram showed increased amplitude of the R wave on V1 and inverted T waves on V1–V3, suggesting right ventricular overload. The two-dimensional (2-D) transthoracic echocardiogram in the parasternal short axis view showed a marked muscle band protruding from the right ventricular free wall to the interventricular septum dividing the cavity into proximal and distal chambers (Fig. 1). In addition, right atrial enlargement, right ventricular hypertrophy and moderate tricuspid regurgitation were found. A turbulent Doppler color flow jet with a mosaic pattern was seen through the stenotic mid-right ventricle on the parasternal short axis view. Continuous wave Doppler interrogation revealed a systolic flow acceleration of 6.50 m/s, corresponding to a pressure gradient of 159 mmHg calculated using the simplified Bernoulli equation (Δp = 4ν2). A diastolic pressure gradient of 5 mmHg was also observed. In diastole, discrete endocardial thickening of the muscular diaphragm and markedly abnormal aorto-septal angulation due to anterior aortic displacement was observed (Fig. 1). Real-time 3D echocardiography with planar reconstruction was able to reveal two anomalous bundles reaching the site of obstruction. It was also possible to show the muscular diaphragm of RVOT obstruction en face.
Fig. 1.
(A) 2DE parasternal short axis view showing discrete RVOT obstruction: (B) 3D echocardiographic image showing two anomalous bundles (two red arrows), (C) Continuous wave Doppler interrogation of the RVOT showing a peak gradient of 159 mmHg, (D) En face view of the muscular diaphragm in the RVOT denoted by the red arrows.
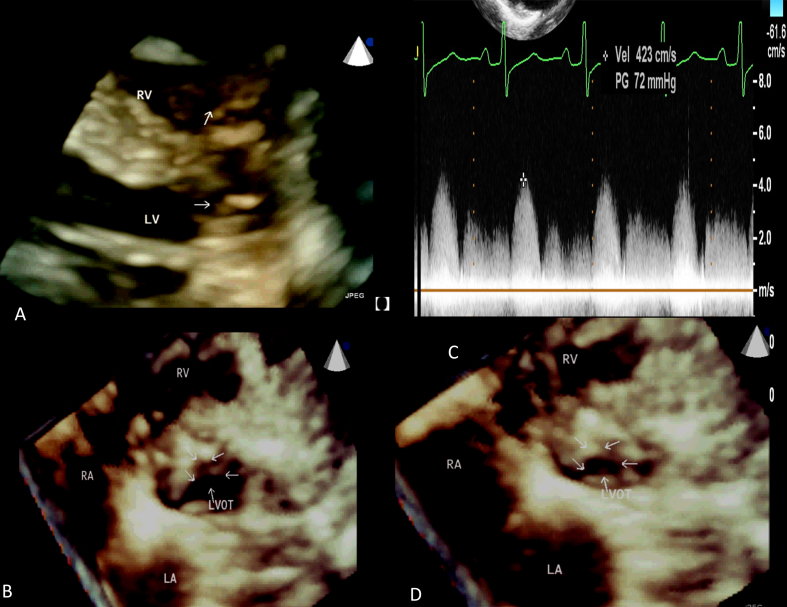
Parasternal long axis view revealed discrete circumferential fibro-muscular diaphragm adjoining the aortic valve with mild thickening of the aortic valve leaflets (Fig. 2). The aortic valve was tricuspid. Continuous wave Doppler showed a peak systolic velocity of 4.2 m/sec (peak gradient 72 mmHg) across the left ventricular outflow tract obtained from parasternal long axis view due to steep aorto-septal angle. Live three-dimensional echocardiography using a fully sampled 3D transducer (Philips IE 33 X-matrix, X5-1) improved the spatial assessment of the subaortic membrane. En-face views of the fibromuscular ring were obtained using real-time 3D-echocardiography.The cross-sectional area of the stenotic site was 0.7 cm2 in mid-systole and 1.7 cm2 in diastole. The end-systolic cross-sectional area of the right ventricular obstruction was 0.5 cm2. The patient awaits surgery.
Fig. 2.
(A) 3D live parasternal long axis view showing subaortic membrane (arrow) along with low infundibular stenosis, (B) 3D echocardiographic reconstructed en face view of the subaortic ring in diastole, (C) Continuous wave Doppler interrogation of the left ventricular outflow tract showing a peak gradient of 72 mmHg in systole and the spectrum of aortic regurgitation. (D) en face view of the subaortic ring in systole.
3. Discussion
A well-known relationship is described among patients with right ventricular outflow tract (RVOT) obstruction, membranous ventricular septal defect (VSD), and subaortic stenosis (SAS) in children. Vogel et al described 36 patients with membranous VSD and double-chambered right ventricle, 88% of whom had echocardiographic evidence of SAS, with evidence of progressive left ventricular outflow tract obstruction.4 It is possible that in adults who have double outflow obstruction without VSD, ventricular septal defect may have closed without leaving a trace and both obstructions may progress over time to become symptomatic later on. It is also possible that both obstructions are acquired. Altered flow patterns due to a steepened aortoseptal angle as in our case, may be a substrate for development of discrete subaortic stenosis as well as RVOT obstruction in adults.5 Evidence suggests that the formation of SAS represents a fibroproliferative reaction of the endocardium occurring in response to alterations in shear stress caused by geometric abnormalities within the left ventricular outflow tract. A similar pathophysiology may be the possible explanation for RVOT obstruction.
Progression of subaortic stenosis may occur before or after VSD closure and/or muscle bundles are resected. Discrete subaortic stenosis is often a circular fibromuscular rim of tissue, with a fibrous inner ring of varying width. The location will vary from just beneath the aortic valve, where occasionally it will be fused with the dependent portion of a cusp as in our case, to discrete structure away from the valve.6
Double-chambered right ventricle is a form of septated right ventricle (RV) caused by the presence of abnormally located or hypertrophied muscular bands. Those muscle bundles run between an area located in the ventricular septum, beneath the level of the septal leaflet of the tricuspid valve, and the anterior wall of the RV. Anomalous muscle bundles divide the RV into a high-pressure proximal chamber and a lower-pressure distal chamber. Evidence suggests that double-chambered right ventricle is an acquired disorder in those patients with appropriate substrate. Obstruction to pulmonary blood flow usually progresses with hypertrophy of the muscle and further obliteration of the RV cavity. The embryologic basis for double-chambered right ventricle is attributed to failure to incorporate bulbus cordis into the RV or an elevated hypertrophied moderator band.3
A contemporary analysis of the origin of the muscle bundles determined the muscular shelf originates from the body of the septomarginal trabeculation. Two positions of muscle bundles are described as high (or horizontal) position and low (or oblique) position.7 Less common, forms of divided RV include those in which a fibromuscular diaphragm partitions the RV.
Type 1 DCRV is characterized by an anomalous muscle bundle crossing the right ventricular cavity, recognized as the cause of the intraventricular obstruction. In type 2 DCRV however, no anomalous muscle bundle is found. The obstruction in this case is caused by marked parietal and septal muscle hypertrophy. The intraventricular pressure gradient of type 1 DCRV is greater than that of type 2 DCRV, whereas VSD is found to be highly associated with type 2 DCRV.
The patient described in this report has Type 2 DCRV, SAS, steep aorto-septal angle with anteriorly displaced aortic root and no VSD. Association of DCRV with SAS without VSD is rare. Hoffman and colleagues reported two such cases out of the 32 studied.8 In a large series of 48 cases of DCRV reported from India, no patient was detected to have SAS, although 69% of the patients had VSD.9 Similarly, two large series of 73 and 52 cases respectively with DCRV did not report a single case of associated SAS.10,11
Using real-time live 3D echocardiography and multiplanar reconstruction, not only it was possible to visualize the subaortic ring en face and measure its area in systole and diastole but also its relationship with the aortic valve leaflets.2D-echocardiography did not reveal the exact cause of RV obstruction and it appeared discrete. Using real-time 3D-echo, it was possible to delineate two anomalous bundles which ran parallel in the cavity to be attached to the parietal and the septal margins of the obstruction very much proximal the pulmonary valve. It was possible to show the muscular diaphragm en face. The incremental value of 3D echocardiography was obvious.
Conflicts of interest
The authors have none to declare.
References
- 1.Beard E.F., Cooley D.A., Latson J.R. Combined congenital subaortic stenosis and infundibular subpulmonic stenosis. Report of a case with successful surgical treatment. AMA Arch Intern Med. 1957;100:647–650. doi: 10.1001/archinte.1957.00260100131016. [DOI] [PubMed] [Google Scholar]
- 2.van der Linde D., Takkenberg J.J., Rizopoulos D. Natural history of discrete subaortic stenosisin adults: a multicentre study. Eur Heart J. 2013;34:1548–1556. doi: 10.1093/eurheartj/ehs421. [DOI] [PubMed] [Google Scholar]
- 3.Restivo A., Cameron A.H., Anderson R.H., Allwork S.P. Divided right ventricle: a review of its anatomical varieties. Pediatr Cardiol. 1984;5:197–204. doi: 10.1007/BF02427045. [DOI] [PubMed] [Google Scholar]
- 4.Vogel M., Smallhorn J.F., Freedom R.M. An echocardiographic study of the association of ventricular septal defect and right ventricular muscle bundles with a fixed subaortic abnormality. Am J Cardiol. 1988;61 doi: 10.1016/0002-9149(88)91079-x. 857–604. [DOI] [PubMed] [Google Scholar]
- 5.Yap S.C., Roos-Hesselink J.W., Bogers A.J., Meijboom F.J. Steepened aortoseptal angle may be a risk factor for discrete subaortic stenosis in adults. Int J Cardiol. 2008;126:138–139. doi: 10.1016/j.ijcard.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Maréchaux S., Juthier F., Banfi C., Vincentelli A., Prat A., Ennezat P.V. Illustration of the echocardiographic diagnosis of subaortic membrane stenosis in adults: surgical and live three-dimensional transoesophageal findings. Eur J Echocardiogr. 2011;12:E2. doi: 10.1093/ejechocard/jeq096. [DOI] [PubMed] [Google Scholar]
- 7.Folger G.M. Right ventricular outflow pouch associated with double chambered right ventricle. Am Heart J. 1985;109:1044–1049. doi: 10.1016/0002-8703(85)90248-0. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman P., Wojcik A.W., Rozanski J. The role of echocardiography in diagnosing double chambered right ventricle in adults. Heart. Jul 2004;90:789–793. doi: 10.1136/hrt.2003.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh M., Agarwala M.K., Grover A. Clinical, echocardiographic and angiographic profile of patients with double chambered right ventricle: experience with 48 cases. Angiology. 1999;50:223–231. doi: 10.1177/000331979905000307. [DOI] [PubMed] [Google Scholar]
- 10.Galal O., Al-Halees Z., Solymar L. Double-chambered right ventricle in 73 patients: spectrum of the disease and surgical results of transartial repair. Can J Cardiol. 2000;16:167–174. [PubMed] [Google Scholar]
- 11.Cil E., Saraclar M., Özkutlu S. Double-chambered right ventricle: experience with 52 cases. Int J Cardiol. 1995;50:19–29. doi: 10.1016/0167-5273(95)02343-u. [DOI] [PubMed] [Google Scholar]