Abstract
Restrictive cardiomyopathy is the least common type of primary cardiomyopathies. Electrocardiographic recording is abnormal in 99% of patients with RCM. Biatrial enlargement, obliquely elevated ST segment with notched or biphasic late peaking T waves are considered characteristic ECG finding. Significant ST depression with T inversion mimicking subendocardial ischemia has also been reported in patients with RCM and is even suggested as a predictor of sudden cardiac death. We noted a similar ECG pattern in a 16 yr girl with Idiopathic restrictive cardiomyopathy. Coronaries were normal, stress perfusion imaging did not show any perfusion defect. This diffuse resting ST depression with T inversion in precordial & inferior leads along with ST elevation in aVR was persistent for more than six months.
Keywords: Restrictive cardiomyopathy, Electrocardiogram, Diffuse ST depression, aVR ST elevation
1. Introduction
Restrictive cardiomyopathy (RCM) is the least common type of primary cardiomyopathies Electrocardiographic recording is abnormal in 99% of patients with RCM.1 ST segment, T wave changes are observed in nearly 75% of RCM patients.2 Significant ST depression mimicking ischemia has also been reported especially in idiopathic RCM.3 Classically in a patient with anginal pain, ST elevation in lead aVR with diffuse ST segment depression was considered as a sign of obstructive Left Main Coronary Artery disease. But similar ECG pattern can be seen in other conditions like left ventricular hypertrophy (LVH) as well. Here we report such an ECG pattern mimicking ischemia observed in young girl with RCM.
2. Case report
SA 16 yr old girl was admitted with history breathlessness class II–III since 3 months, history of atypical anginal type of chest pain since 3 weeks. Her pulse, blood pressure were normal and she had a prominent a wave in JVP with a loud P2.
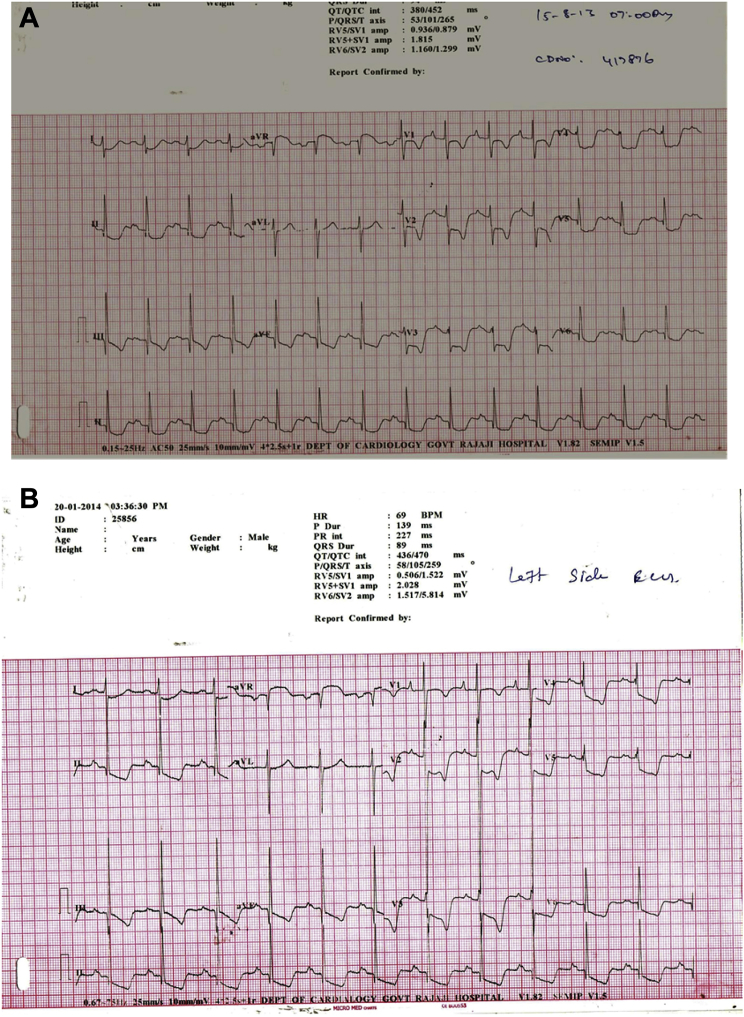
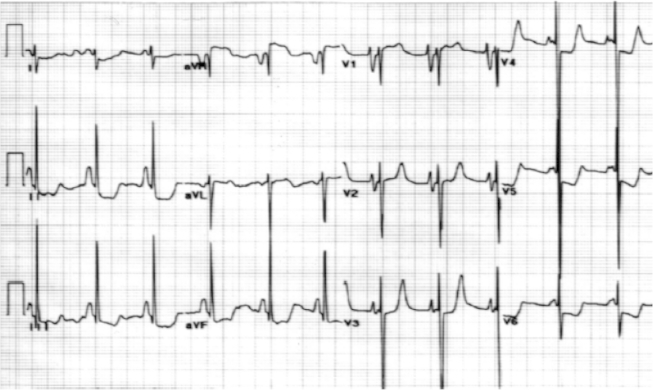
ECG was showing sinus rhythm with QRS axis of 100°, bifid P wave in lead II, 5–7 mm ST horizontal depression with inverted T waves in precordial leads, 3–5 mm ST depression in lead I, II, III aVF, 3 mm ST elevation in lead aVR (ST elevation in aVR with ST depression in >8 leads) (Fig. 1A).
Fig. 1.
A: ECG recording in our patient diffuse ST depression (>7 leads), T inversion with ST elevation in lead aVR (3 mm). B: ECG after 6 months of initial admission – ST depression persisting.
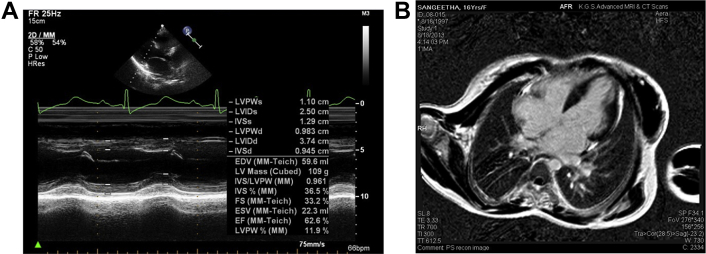
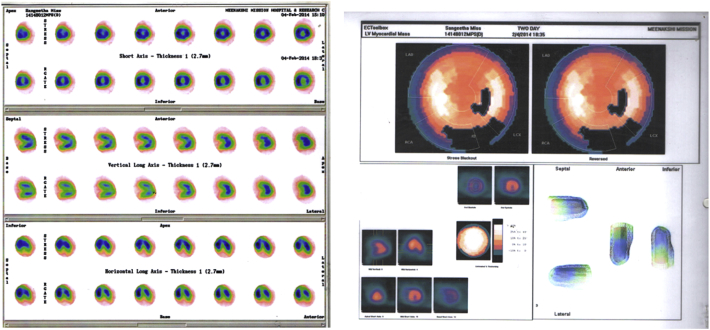
Echocardiography revealed biatrial enlargement normal sized ventricles (Figs 3A, B & 4). Interventricular septal, LV free wall thickness was normal and LV mass was about 110 gm (Fig. 7A). She had no regional wall motion abnormalities and a normal systolic function with EF (68%). Doppler examination was suggestive of restrictive pattern (diastolic dysfunction Grade IV) Figs. 5A, B & 6A, B.
Fig. 3.
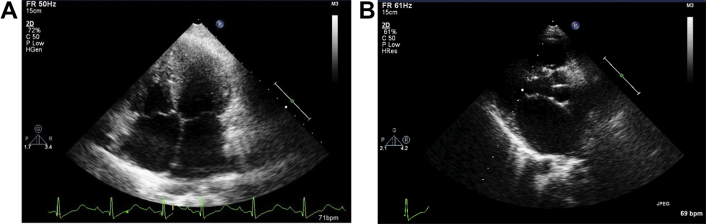
A: Biatrial enlargement with normal ventricles. B: Enlarged left atrium.
Fig. 4.
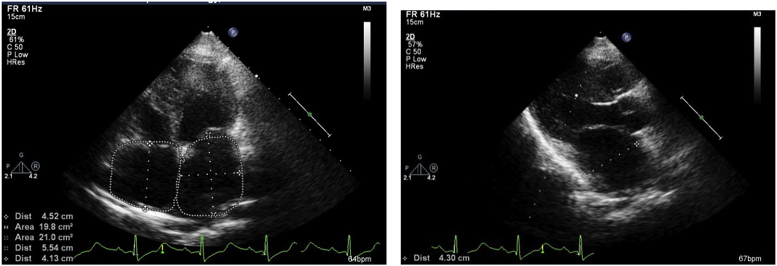
LA volume index = 30.7 ml/m2(Three orthogonal D method), 36.9 ml/m2 (2 areas & length method RA area 19.8 cm2.
Fig. 7.
A: Normal LV systolic function EF −62%, normal sized LV with normal mass. B: Cardiac MR enlarged LA, RA, normal sized ventricles with normal LV mass.
Fig. 5.
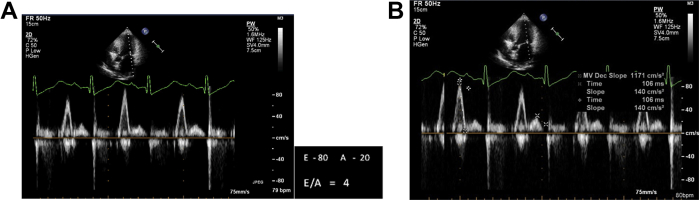
A: E/A >2 Grade IV diastolic dysfunction (Restrictive pattern) B: Deceleration Time 106 ms (<130).
Fig. 6.
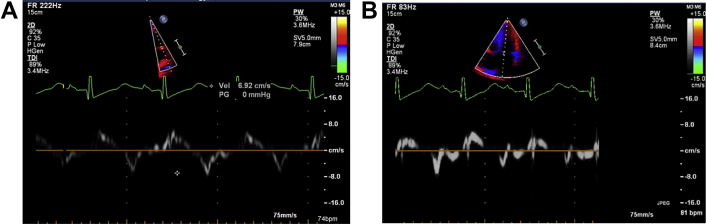
A: Tissue doppler imaging of lateral annulus velocity 6.9 cm/S (<8) B: Medial septal annulus velocity 7 cm/S.
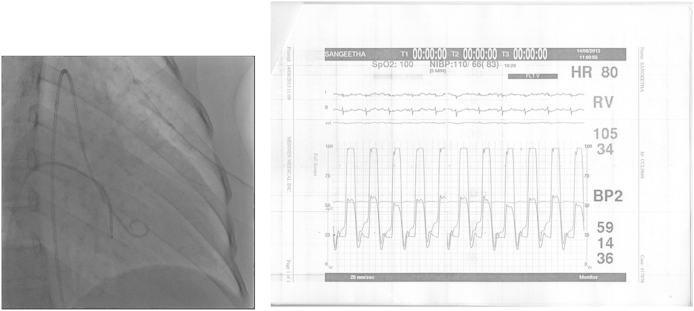
Simultaneous LV and RV pressure tracing on cardiac catheterization showed characteristic dip and plateau pattern (square root sign) with elevated LV and RV end diastolic pressures (Fig. 9) typical of restrictive hemodynamics.
Fig. 9.
Cardiac catheterization: simultaneous RV, LV pressure tracing – characteristic of restrictive physiology – Dip and Plateau pattern elevated RVSP (59 mm Hg), high LV & RVEDP with difference >5 mmHG.
She had normal blood cell count and morphology, serum ferritin and transferrin were within normal range. Liver function test, bone marrow examination and electrophoretic pattern of Immunoglobulins were also normal.
Coronary angiogram revealed normal epicardial coronaries (Figs. 8A, B). Myocardial perfusion study (MPI) using 99 mTc-Sestamibi did not show any evidence of resting or stress induced hypoperfusion or transient ischemic dilatation (TID) (Fig. 10). Cardiac MR revealed biatrial enlargement normal LV mass, pericardium (Fig. 7B).
Fig. 8.
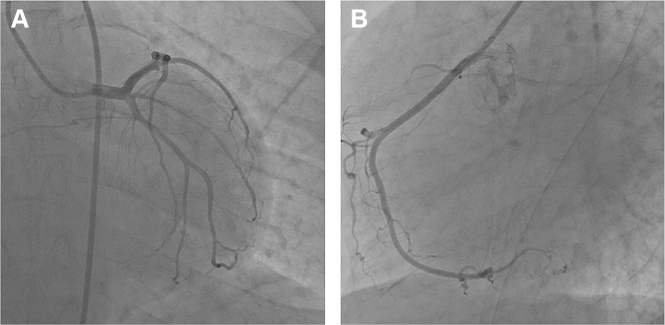
Coronary angiogram. A: AP caudal view of LCA-showing normal left main coronary, left anterior descending and left circumflex artery. B: LAO view of RCA –Normal.
Fig. 10.
Myocardial perfusion imaging (technetium 99 m Sestamibi): No evidence of any perfusion defect during rest or after dobutamine stress. No evidence of TID.
3. Discussion
Restrictive cardiomyopathy has a nonspecific clinical manifestation. It is usually diagnosed by echocardiographic, catheterization findings of restrictive physiology along with normal or near normal sized ventricle and normal systolic LV function. Endomyocardial fibrosis is the commonest cause for RCM worldwide. Others include storage diseases, infiltrative disorders, hypereosinophilia and idiopathic RCM (IRCM). IRCM is considered as a spectrum of hypertrophic cardiomyopathy and associated with mutation in the sarcomeric proteins. Restrictive hemodynamic pattern without evidence for secondary causes suggested idiopathic RCM as the possible diagnosis in our patient. The crux of discussion is unique ECG pattern with normal coronaries without any perfusion defect.
In the setting of coronary artery disease widespread ST depression in >7 leads with ST segment elevation in aVR is an indicator of left main coronary obstruction, multivessel disease or proximal Left anterior descending artery occlusion.4 However, similar ECG pattern may be seen in common conditions like LVH characterized by ST depression, T wave inversion in left sided leads (I, aVL, V5, V6) and ST elevation in right sided leads mainly in V2 V3, rarely in aVR also. The absence of LVH by QRS voltage criteria does not exclude this possibility.5 Cases of RCM showing such ECG pattern mimicking severe subendocardial ischemia have been rarely reported3 (Fig. 2).
Fig. 2.
ECG reported by Rivenes et al, demonstrating biatrial enlargement ST depression in inferior lateral, lateral precordial leads, also ST elevation in aVR but not mentioned.
ECG is a useful screening tool in RCM patients and is abnormal in nearly 90–99% of patients especially in idiopathic RCM. Biatrial enlargement evidenced by biphasic P waves in precordial leads was reported in 91%. 74% of patients demonstrated atrial fibrillation. ST, T changes observed in nearly 80% of individuals was considered nonspecific by Naser M. Ammash et al.,2 But Rivenes et al demonstrated 3–12 mm ST depression suggestive of ischemia preceding sudden cardiac death (SCD) and Torsade's de pontes in patients with RCM. Pathological changes of acute ischemia but without coronary obstruction were seen in autopsied heart. Hence they co-related ST depression (a sign of ischemia) to SCD in RCM.
Hayashi et al, suggested obliquely elevated ST segment with notched or biphasic late peaking T waves (67%), and ST depression with T inversion (25%) were characteristic of idiopathic RCM. By demonstrating normal epicardial coronaries in angiogram and absence of any perfusion defect on MPI, they concluded ECG changes were a reflection of repolarization abnormalities, and SCD was due to arrhythmias rather than ischemia.6
We hypothesize that ST depression observed in precordial, inferior and lateral leads may be due to high end diastolic pressure (EDP). High EDP can produce repolarization abnormalities by impairing perfusion in the subendocardial region inducing ischemic repolarization abnormality, or by stretching the myocardium and activating stretch sensitive channels. In our patients resting ST depression lasted for more than 6 months (Fig. 1 B).
Elevated EDP can induce diastolic stretching of the myocardial fibers, and this stretch may increase calcium leak at the sarcoplasmic reticulum level (Iribe et al, 2009).7 Calcium leak activates BKca (excessive in endocardium), or ClCswell channels and may alter the repolarization and induce ST T changes and arrhythmias.8 But Restrictive cardiomyopathy (RCM) myocardium too stiff to be stretched, hence stretch sensitive channel activation is unlikely to be the culprit for the observed repolarization abnormality in our restrictive cardiomyopathy patients.
High EDP will reduce the coronary perfusion pressure and induce subendocardial hypoperfusion in the ventricle globally. Diffusely impaired subendocardial blood flow in all the 3 vascular territories may not be diagnosed by SPECT Technetium 99 m MPI (False Negative).9 Hence normal coronaries and negative myocardial perfusion study by SPECT cannot exclude diffuse subendocardial hypoperfusion. ECG is a sensitive tool to diagnose subendocardial ischemia and perfusion MRI10 might be the ideal investigation for quantitative estimation of blood flow but could not be done in our patient due to non-availability.
We speculate that in our patient ischemia induced repolarization abnormality might be the most likely mechanism for ST depression, ClCswell channel activation may also be (albeit less likely) contributor for this exaggerated ST depression in our case.
4. Conclusion
Diffuse ST depression in the precordial, lateral and inferior leads with ST elevation in aVR may be seen in RCM. Severe subendocardial ischemia involving whole of the ventricle due to high EDP is a well-known phenomenon and may be most likely mechanism of ST T changes observed in RCM. ClCswell channel activation could be a less likely contributor for this exaggerated ST depression.
Conflicts of interest
The authors have none to declare.
References
- 1.Jean-Charles Pierre-Yves, Li Yue-Jin, Nan Chang-Long, Huang Xu-Pei. Insights into restrictive cardiomyopathy from clinical and animal studies. J Geriatr Cardiol. 2011;8:168–183. doi: 10.3724/SP.J.1263.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammash N.M., Seward J.B., Bailey K.R. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490–2496. doi: 10.1161/01.cir.101.21.2490. [DOI] [PubMed] [Google Scholar]
- 3.Rivenes S.M., Kearney D.L., Smith E.O. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000;102:876–882. doi: 10.1161/01.cir.102.8.876. [DOI] [PubMed] [Google Scholar]
- 4.Kosuge M., Ebina T., Hibi K. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011;107:495–500. doi: 10.1016/j.amjcard.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Zema Michael J. MD ST-segment elevation in Lead aVR on the presenting electrocardiogram. Arch Intern Med. 2012;172:1190–1191. doi: 10.1001/archinternmed.2012.2079. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki Hayashi M.D., Etsuko Tsuda M.D., Kenichi Kurosaki M.D. Electrocardiographic and clinical characteristics of idiopathic restrictive cardiomyopathy in children. Circ J. 2007;71:1534–1539. doi: 10.1253/circj.71.1534. [DOI] [PubMed] [Google Scholar]
- 7.Iribe G., Ward C.W., Camelliti P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circulation Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Da-yu, Liu Luis Lh, Nathan Bozeat. Funtional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005 (Mar);26:265–278. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 9.Denfield Susan W., Moss, Adams . 8th ed. Lippincott Wiliams&Wilkins; 2013. Heart Disease in Infant, Children, Adolascents Including the Foetus and Young Adult; pp. p1267–1276. [Chapter 57 Resrictive cardiomyopathy] [Google Scholar]
- 10.Salerno M., Beller G.A. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2:412–424. doi: 10.1161/CIRCIMAGING.109.854893. [DOI] [PubMed] [Google Scholar]