Abstract
Purpose
Curative therapy for childhood glioma presents challenges when complete resection is not possible. Patients with recurrent low-grade tumors or anaplastic astrocytoma may receive radiation treatment, however, the long-term sequellae from radiation treatment can be severe. As many childhood gliomas are associated with activation of BRAF, we have explored the combination of ionizing radiation with MEK inhibition in a model of BRAF-mutant anaplastic astrocytoma.
Experimental Design
The regulation of TORC1 signaling by BRAF was examined in BT-40 (BRAF mutant) and BT-35 (BRAF wild type) xenografts, in a cell line derived from the BT-40 xenograft and two adult BRAF mutant glioblastoma cell lines. The effect of MEK inhibition (selumetinib), XRT (total dose10 Gy as 2 Gy daily fractions), or the combination of selumetinib and XRT was evaluated in subcutaneous BT-40 xenografts.
Results
Inhibition of MEK signaling by selumetinib, suppressed TORC1 signaling only in the context of the BRAF-mutant both in vitro and in vivo. Inhibition of MEK signaling in BT-40 cells or in xenografts lead to a complete suppression of FANCD2 and conferred hypersensitivity to XRT in BT-40 xenografts without increasing local skin toxicity.
Conclusions
Selumetinib suppressed TORC1 signaling in the context of BRAF mutation. Selumetinib caused a rapid downregulation of FANCD2 and markedly potentiated the effect of XRT. These data suggest the possibility of potentiating the effect of XRT selectively in tumor cells by MEK inhibition in the context of mutant BRAF or maintaining tumor control at lower doses of XRT that would decrease long-term sequelae.
Keywords: childhood astrocytoma, BRAF mutation, TORC1 signaling
Introduction
Low-grade gliomas, are the most common tumors of the central nervous system in children, [1]. Classified as grade I and II by the World Health Organization (WHO), they are a histologically diverse group of tumors which include pilocytic astrocytomas (most common), fibrillary astrocytomas and less commonly, pilomyxoid astrocytoma, gangliogliomas, pleomorphic xanthroastrocytomas, and oligodendrogliomas, among others [2]. Although outcome for children with low-grade gliomas is, in general, excellent, with a reported 5-year overall survival of approximately 90%, extent of resection, histology, tumor location and age are important prognostic factors [2,3]. reference In children where tumor is accessible, surgical resection is the standard therapy, with a gross total resection considered to be curative [4]; however, a significant percentage of tumors are not resectable due to involvement of midline structures such as the diencephalon, optic pathway and brainstem. In such cases,adjuvant chemotherapy is warranted in younger patients. Five-year EFS with the most common upfront chemotherapy regimen (carboplatin and vincristine) is 39±4% demonstrating the chronic and intermittently progressive course of these tumors [5]. In older patients and those who have failed chemotherapy regimens, radiotherapy is considered standard [6]. As a result, many patients with LGGs experience significant treatment and tumor-related morbidity, such as neuroendocrine-cognitive deficits, visual deficits, vasculopathy and secondary tumors [7,8]. Moreover, a small subgroup of patients experience metastatic disease and malignant transformation to a high-grade astrocytoma [9,10].
Considerable advances have been made in defining subsets of pediatric tumors through use of genotyping and expression profiling [11–13]. Low-grade gliomas are primarily associated with activation of BRAF through a tandem duplication that results in the KIAA1549-BRAF fusion [14] leading to constitutively activated BRAF or through an activating point mutation of BRAF (predominantly V600E), leading to activation of the MAP kinase pathway. The KIAA1549-BRAF fusion is largely restricted to juvenile pilocytic astrocytoma (65- 80%) [14–16] whereas BRAFV600E occurs more frequently in other LGG such as, pleomorphic xanthoastrocytomas (60%) [17], gangliogliomas (20–40%) [18,19], diffuse firbrillary astrocytomas (8%) [16]and in approximately 10% of high grade astrocytomas [20]. Thus, activating mutation of BRAF appears to be the most common lesion in intermediate grade astrocytoma. Homozygous deletion of the CDKN2A locus is frequent (~70%) in tumors harboring the BRAFV600E mutation [21]. Recently, Mistry et al reported that low-grade gliomas that harbor BRAFV600E mutations and CDKN2A deletions are at a higher risk of transforming to high-grade gliomas and represent a distinct subtype of secondary high-grade gliomas in children [22]. Findings for BRAF mutation, similar to other tumors with activated BRAF (e.g. melanoma), suggest that activated BRAF may provide a potential drug target [23].
Fanconi anemia (FA) is primarily an autosomal recessive genetic disorder. The FA gene products form a complex that is essential for some forms of DNA damage repair. As a result, the majority of FA patients develop cancer, most often acute myelogenous leukemia, and 90% develop bone marrow failure by age 40. Because of the genetic defect in DNA repair, cells from people with FA are sensitive to cancer chemotherapeutic agents that cause DNA crosslinking, such as mitomycin C. The Fanconi anemia Complementation Group D2 protein (FANCD2) is monoubiquitinated in response to DNA damage, resulting in its localization to nuclear foci with other proteins (BRCA1 and BRCA2 [also known as FANCD1]) involved in homology-directed DNA repair. While FANCD2 deficiency is associated with hypersensitivity to drugs that cross-link DNA [24], bone marrow stromal cells from FANCD2 deficient mice are hypersensitive to ionizing radiation (XRT), whereas hematopoietic progenitor cells are not [25]. In humans adverse events following XRT have been associated with DNA repair disorders (ataxia telangiectasia (A-T) and Nijmegen Breakage (NBS) syndrome), however Fanconi anemia was associated with over half of the reports, suggesting that in humans, deficiency in the Fanconi pathway leads to hypersensitivity to XRT [26]. Indeed, lymphoblastoid cells from Fanconi anemia patients were found to be more sensitive to XRT than lymphoblastoid cells from A-T or NBS patients [26]. Similarly, we reported previously that knockdown of FANCD2 increased the sensitivity of rhabdomyosarcoma cells to XRT [27].
The transcription or translation of FANCD2 appears to be regulated downstream of the TOR complex 1 (TORC1). Guo and colleagues demonstrated that the conditional knockout of mTOR resulted in the loss of FANCD2 mRNA and protein expression in hematopoietic stem and progenitor cells [28]. In hematopietic cells mTOR is thought to regulate DNA damage response through an NF-κB-mediated FANCD2 pathway [29]. We also found that both rapamycin and the TOR kinase inhibitor AZD8055 caused the loss of FANCD2 both in vitro and in rhabdomyosarcoma xenografts, and enhanced XRT sensitivity [27]. In the course of studies with two patient derived xenografts, we noted that inhibition of MEK by selumetinib, suppressed TORC1 signaling only in the context of the BRAFV600E mutation. We were thus interested in determining whether this led to suppression of FANCD2 and hypersensitivity to XRT.
Materials and Methods
Cell lines and xenografts: Previously frozen xenograft tissue was thawed at 37°C and placed in a 10cm petri dish. The tumor pieces were washed in three changes of Hank’s Balanced Salt Solution containing Pen/Strep. The tumor was minced with cross scalpels, and washed in at least four changes of the Hank’s solution followed by allowing the pieces to settle by gravity in a 50 ml tube. The final wash was centrifuged at 50 × g. and the pellet suspended in DMEM/F12 with 10% FBS containing 200units/ml Collagenase 1 (Worthington Biochemical) for 2hr at 37°C. The suspension was plated (37°C, 5% CO2). The cultures were checked every hour until pieces ‘smeared’ when the dish was tilted. The suspensions were transferred to a 50 ml tube and allowed to settle by gravity. Medium was removed and replaced with Astrocyte Basal Medium with serum and supplements (Astrocyte Medium BulletKit, Lonza. Allendale, NJ). Attached cells were passaged when cultures became confluent. Once a continuous cell line (> 20 serial passages) was derived, species determination/contamination was determined by LDH isoenzyme analysis (AuthentiKit System, Innovative Chemistry). BT-40c cells were genotyped by short tandem repeat assay (STR) for comparison to the xenograft tissue, and were found to be identical. DBTRG-05MG [30] and AM38 glioblastoma cells were generously provided by T. Nicolades (University of California, San Francisco). BRAF mutations were confirmed by sequencing, but other authentication was not performed. Cells were used within 6 months of receipt for the experiments reported.
Propagation and treatment of BT40 xenografts: C.B-17 scid−/− female mice (Taconic Farms, Germantown, NY), were used to propagate subcutaneous tumors. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of The Ohio State University (IACUC protocol 2010A00000192-R1 or Nationwide Children’s Hospital (IACUC protocol AR-09-0036). Tumor volumes (cm3) were determined weekly, to determine growth and response, as previously described [31].
Mice were dosed BID with 75mg/kg selumetinib by oral gavage twice daily for 6 weeks (42 days). AZD6244 administration began on day -4. On day 0, X-irradiation treatment (XRT) began and was administered in 2 Gy fractions for 5 consecutive days for a total of 10 Gy irradiation dose using a method previously described [32,33]. After the completion of radiation treatments, mice were dosed once daily on weekends, but continued to be dosed twice daily during the week. Tumor volumes were monitored for up to 23 weeks following all dosing procedures.
Western blotting
Cell lysis, protein extraction and immunoblotting were performed as previously described [34]. Briefly, 20 µg of total sample was resolved on a 4–12% SDS-polyacrylamide gel. Proteins were transferred to a PVDF membrane and immune-detection was performed with specific antibodies. Immunoreactive bands were visualized by using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Rockford, IL) and HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ). Antibodies against PARP (46D11), P-STAT3 (Tyr705) (D3A7), STAT3 (79D7), P-AKT (Ser473) (D9E), AKT, P-ERK1/2 (Thr202/Tyr204) (D13.14.4E), ERK, P-S6 (Ser235/236) (D57.2.2E), S6 (5G10), P-4E-BP1 (Thr37/46) (236B4), 4E-BP1 (53H11), and GAPDH (D16H11) were from Cell Signaling Technology (Danvers, MA); FANCD2 (EPR2302) from Abcam (Cambridge, MA). Horseradish peroxidase-conjugated secondary antibody was from Cell Signaling Technology.
Proliferation and Clonogenic Assays
Initial sensitivity of BT-40, DBTRG-05MG and AM38 cells to selumetinib was determined by exposure to selumetinib (0.01– 50 µM) for 1, 2, and 4 days, stained with alamarBlue® (Life Technologies, Carlsbad, CA), and fluorescence read at an excitation filter setting of 530 nm and an emission filter setting of 590 nm. The fluorescent values of the treated samples versus the untreated samples are plotted as percent viability. Values for each test sample represent the average of quadruplicates. The values displayed are representative of three independent experiments.
For the clonogenic assays, BT-40 cells were cultured in the presence or absence of AZD6244 (100 nM and 1 µM) for 4 days. Colony formation by DBTRG-05MG and AM38 cells was determined at 1 µM. Following treatment, cells were trypsinized, counted with a hemocytometer, and seeded in triplicate at varying densities (100 to 12,800 cells) in 6-well plates. Cells were maintained in 2 ml of total growth media containing DMEM, penicillin (100 U/ml), streptomycin (100 U/ml), 2 mM glutamine, and 20% (v/v) fetal bovine serum. After 14 days in culture, colonies containing in excess of 50 cells were stained with a mixture of 95% methanol and 0.5% crystal violet. The number of colonies was counted using a stereomicroscope. Plating efficiency (PE), number of colonies formed/ number of cells seeded × 100, and surviving fraction (SF), PE of treated sample/ PE of control × 100. The data represents the average and standard deviation of the SF of the 3 replicates.
Results
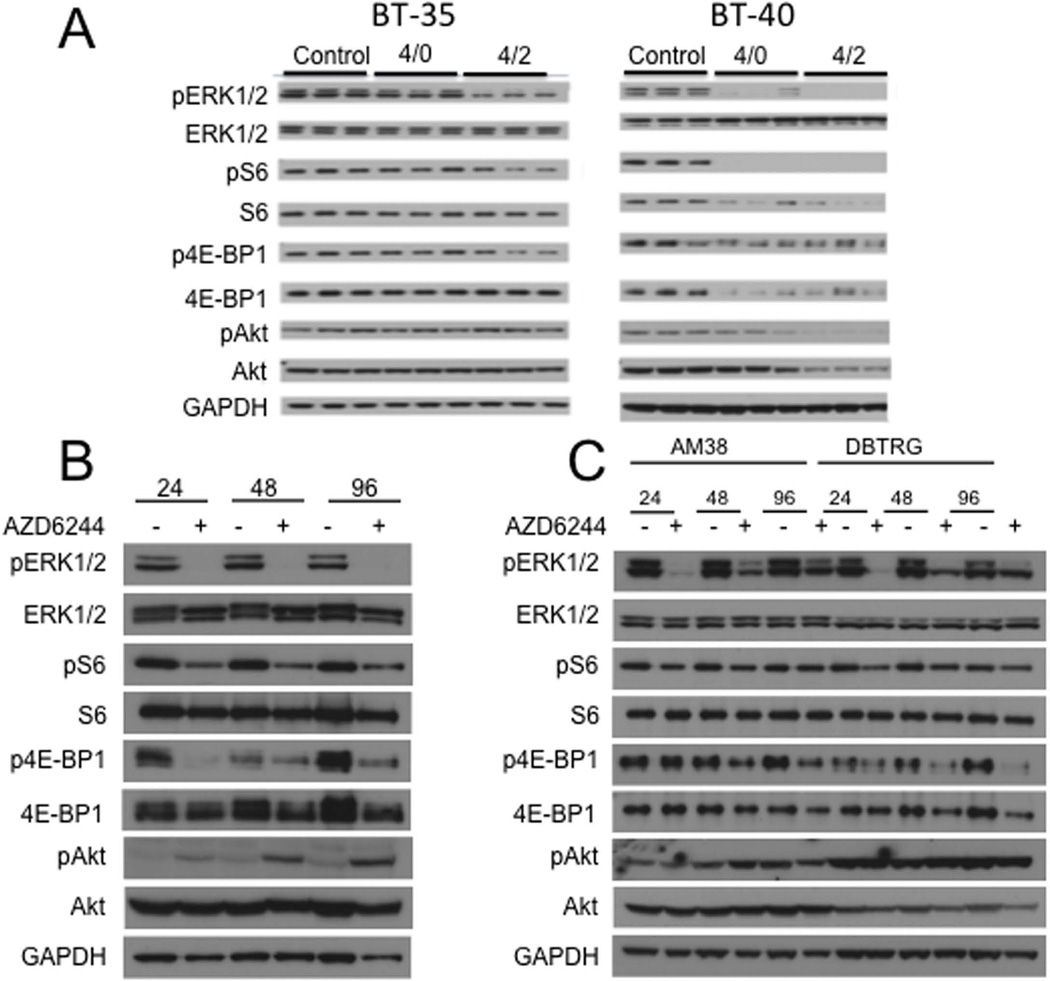
BRAFV600E regulates TORC1 signaling: We reported previously that the BRAFV600E BT-40 astrocytoma xenograft was highly sensitive to the MEK inhibitor selumetinib, whereas the BT-35 astrocytoma with wild type BRAF was intrinsically resistant to the same treatment [35]. Selumetinib, inhibited phosphorylation of the downstream substrates ERK-1 and ERK-2 in both xenograft lines, although suppression of pERK was somewhat greater in BT-40 xenografts (Figure 1A). However, in BT-40 xenografts, TORC1 signaling was attenuated as shown by decreased phospho-4EBP1 and phospho-S6 that was also associated with decreased total protein in BT-40 xenografts. In contrast TORC1 appeared not to be inhibited by selumetinib in the BT-35 xenograft. To investigate this further, we derived a cell line from the BT-40 xenograft. The cell line BT-40c had identical STR analysis to the parental xenograft tissue, and was free from mouse cells as determined by LDH analysis. As with the xenograft, BT-40c cells were heterozygous for the BRAFV600E mutation. Similar to the xenograft data, exposure of BT-40c cells to selumetinib caused a rapid decrease in TORC1 signaling. Notably, phospho-4EBP1 was markedly reduced by 24 hr exposure, and phospho-S6 was also reduced, however total protein levels were unchanged, (Figure 1B). In the cell line, inhibition of TORC1 was associated with feedback phosphorylation of AKT (S473). Inhibition of TORC1 signaling was less marked in two cell lines derived from adult glioblastoma with BRAFV600E mutations, AM38 and DBTRG-MG. In DBTRG-MG cells phospo-4EBP1 was reduced at 48 and 96 hr, whereas the decrease was less evident in AM38 cells (Figure 1C). In contrast, phospho-S6 appeared relatively unaffected in these two adult derived glioblastoma lines.
Figure 1.
Inhibition of MEK suppresses TORC1 in the context of mutant BRAF. A. Mice bearing BT35 (BRAF wild type) or BT-40 (BRAFV600E) xenografts were administered selumetinib (75 mg/kg BID). Twelve hours after dose 7 tumors were harvested (designated 4/0) or 2 hr post dose 8 (designated 4/2); B. BT-40c cells were exposed to selumetinib (AZD6244, 1 µM) for the indicated times; C. AM38 and DBTRG-05MG cells were treated as indicated with selumetinib (AZD6244, 1 µM).
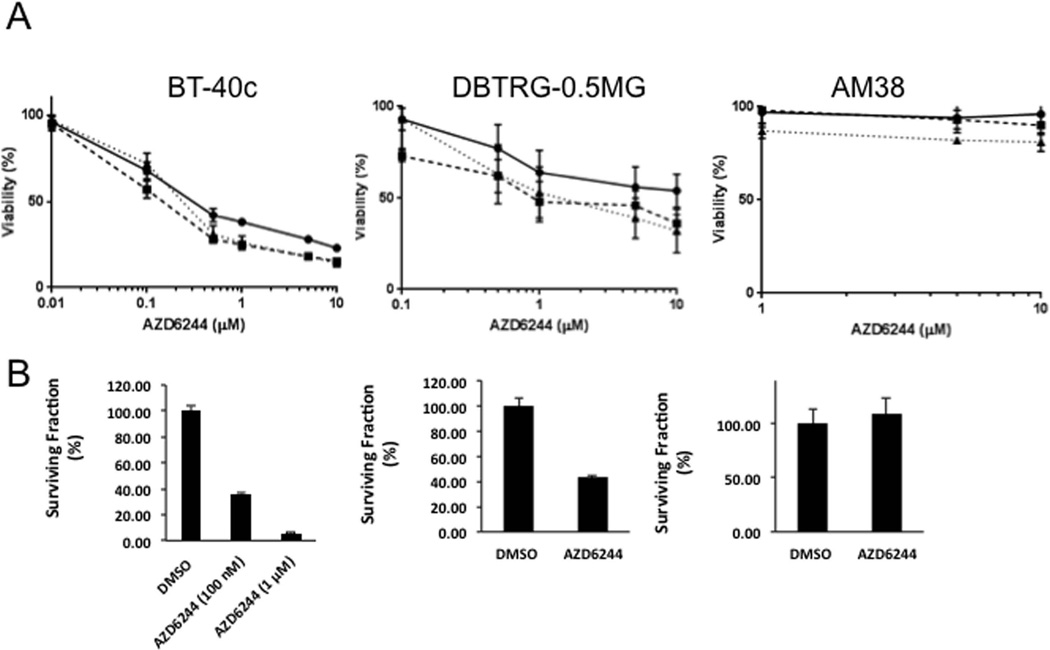
In BT-40 xenografts, selumetinib treatment causes complete tumor regression [35] with regrowth several weeks after termination of treatment, suggesting significant cell killing. We examined whether the response to selumetinib treatment was cytostatic or cytototoxic in the three BRAF-mutant cell lines. As shown in (Figure 2A), proliferation of BT-40 cells was very sensitive to selumetinib (IC50 ~0.2 µM) whereas DBTRG-05MG cells were less sensitive (IC50 ~1 µM) and AM38 cells were quite refractory to selumetinib treatment (IC50 > 10 µM) when exposed for 96 hr. To examine whether decreased proliferation was a consequence of decreased clonogenic capacity, celle were exposed to selumetinib at 0.1 and 1 µM for 4 days, drug was removed and colonies were allowed to form over 14 days in the absence of drug. Selumetinib dramatically reduced clonogenic survival of BT-40c cells at 0.1 µM whereas it had modest activity in reducing colony formation in DBTRG-MG cells, and no effect on the ability of AM381 cells to form colonies at 1 µM (Figure 2B). These results further differentiate the childhood astrocytoma from the adult glioblastoma cell lines, but also support the conjecture that inhibition of TORC1 signaling by MEK inhibitors in the context of BRAF mutation is required for cell death [36].
Figure 2.
Selumetinib (AZD6244) suppresses proliferation and colony formation. A Inhibition of proliferation, determined by Alamar Blue assay, following 24 (black circle), 72 (black square) or 96 (black triangle) hr exposure to increasing concentrations of selumetinib. B. colony formation, BT-40, DBTRG-05MG and AM38 cells were exposed to the indicated concentrations of selumetinib for 96 hr. The cells were washed extensively, and plated at colony forming density. Colonies were counted after 14 days. (Mean ± SD, n=3).
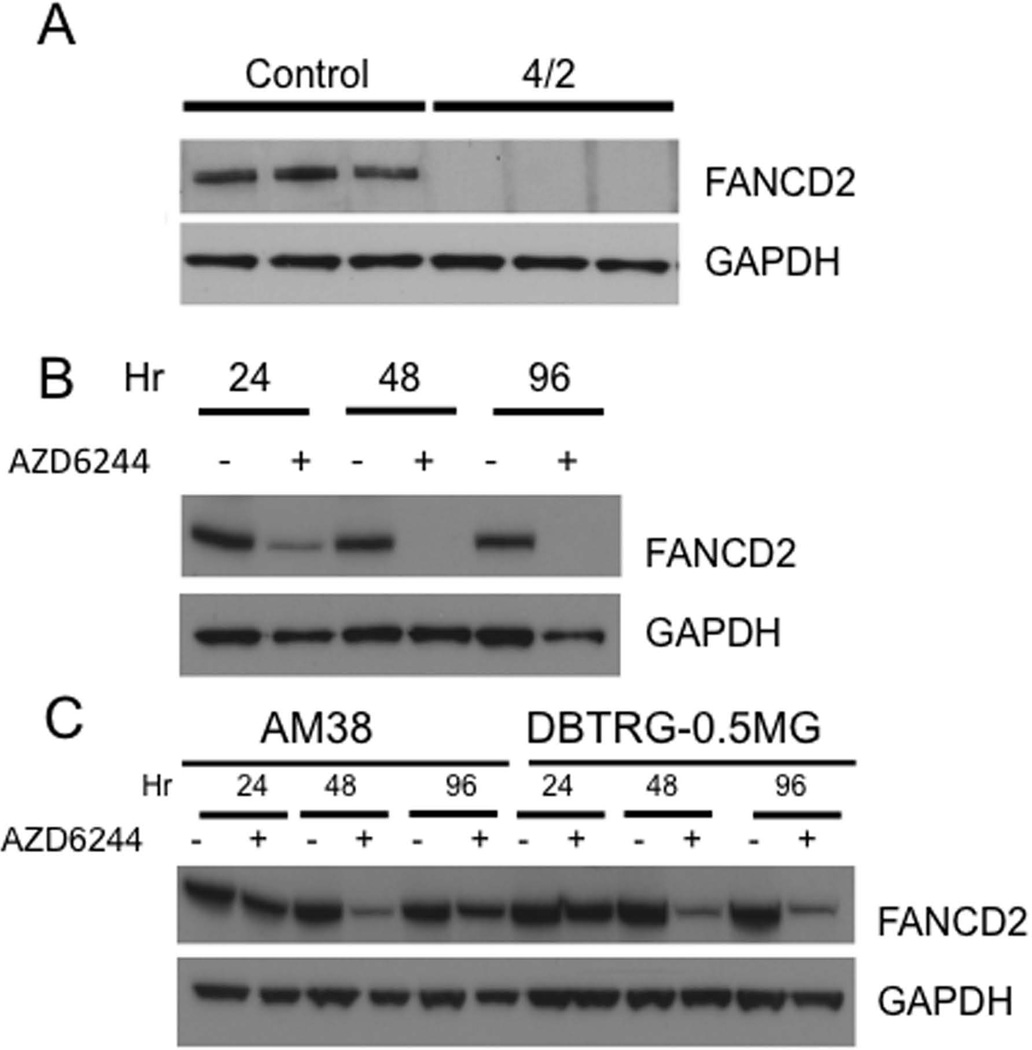
The results support the idea that in BT-40 xenografts, and in the derived cell line, BRAF signaling regulates TORC1 activity, possibly through suppression of the tuberous sclerosis complex, a negative regulator of TORC1 [37,38]. We have shown previously that treatment of mice bearing rhabdomyosarcoma xenografts with the TOR kinase inhibitor, AZD8055, or rapamycin, resulted in a downregulation of the DNA damage repair protein FANCD2 [27]. To determine whether selumetinib treatment reduced FANCD2 in BT-40 xenografts, mice were treated for four days, and tumors harvested 4 hr after the last dose. As shown in Figure 3A, FANCD2 was readily detected in untreated tumors, whereas it was undetectable in tumors from treated mice. In cultured BT-40c cells, selumetinib treatment for 24 hr also led to a substantial decrease in FANCD2 protein, and complete loss of FANCD2 by 48 hr (Figure 3B). Selumetinib also decreased FANCD2 in AM38 and DBTRG-MG cells, although the decrease was maximal at 48 hr and for AM38 cells FANCD2 levels had increased by 96 hr exposure (Figure 3C).
Figure 3.
Inhibition of MEK signaling suppresses FANCD2 in vivo and in vitro. A. Mice bearing BT-40 (BRAFV600E) xenografts were administered selumetinib (75 mg/kg BID). Two 2 hr post dose 8 (designated 4/2) tumors were harvested; B. BT-40c cells were treated as indicated with selumetinib (AZD6244, 1 µM); C. AM38 and DBTRG-0.5MG cells were exposed to selumetinib (AZD6244, 1 µM) for the indicated periods. Mean ± SD (n=3).
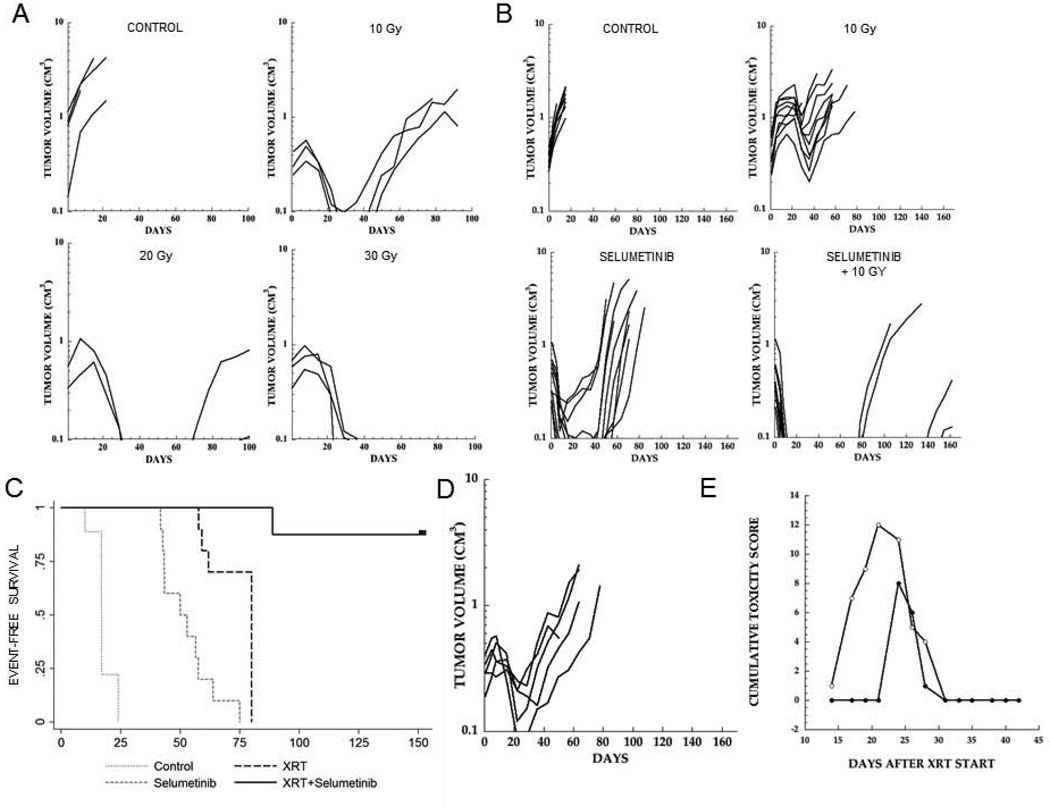
Inhibition of TORC1 has been associated with increased sensitivity to XRT [39], and it was considered that rapamycin-induced G1 arrest could contribute to the sensitizing effects. We also showed that AZD8055 could enhance the effect of XRT, and that FANCD2 was regulated downstream of TORC1 in part by S6K1/2 activity [27,40]. It was therefore of interest to determine whether selumetinib could enhance daily fractionated XRT. In a pilot study to define the radiosensitivity of BT-40 xenografts, tumor-bearing mice were irradiated (2 Gy fractions) daily to a total dose of 10, 20 or 30 Gy, as previously described [32]. As shown in Figure 4A, BT-40 xenografts were relatively sensitive to XRT, with tumor regression below a volume of 0.1 cm3 at all dose levels. After 10 Gy all tumors recurred between 4 and 7 weeks, whereas after 20 Gy 2 of 2 tumors regrew with recurrences at 10 and 13 weeks, whereas no tumors regrew at 30 Gy (n=3) during the period of observation (100 days) (Figure 4A). We thus chose a total dose of 10 Gy XRT to examine with and without selumetinib treatment. Mice bearing BT-40 xenografts were treated for 4 days, to reduce FANCD2 levels, before starting XRT. As shown in Figure 4B, untreated (control) tumors showed consistent progressive growth. XRT (10 Gy) alone caused some tumor regressions with subsequent regrowth. For control tumors, median time to event was 17 days, whereas for XRT alone it was 80 days (P<0.0001). Selumetinib treatment induced complete regression of most tumors with progression starting around week 5 of the 6-week treatment period, consistent with previous data [35,41], with median time to event being 51 days (P<0.0001 vs control). In contrast to XRT or selumetinib administered as single agents, the combination caused complete regression of all tumors, with only 4 of 10 (40%) recurrent tumors within the 23 week observation period, and significantly better than radiation or selumetinib alone (P<0.0001 for both comparisons). Kaplan-Meier estimates for event-free survival for each group are presented (Figure 4C). Because XRT was delayed during the 4-day pretreatment of mice with selumetinib (to reduce FANCD2), tumors at the start of XRT were larger than in the selumetinib alone arm. To test the possible effect of size, a further group of mice with smaller tumors was added. The response to XRT was similar with some tumors regressing, and median day to event being 71 days (Figure 4D). Using an index of skin toxicity previously reported [40], there was a slight decrease in the cumulative toxicity in the combination treatment arm of this study compared to 10 Gy XRT alone (Figure 4E).
Figure 4.
Selumetinib synergizes with XRT in BT-40 xenografts. A. Pilot dose ranging study. Mice bearing subcutaneous BT-40 xenografts were irradiated to a total of 10, 20 or 30 Gy in 2 Gy daily fractions (5 days/week). B. Based on the pilot data, mice received a total of 10 Gy, selumetinib (75 mg/kg BID×42) or the combination of XRT + selumetinib. Tumor volumes were calculated as described in Materials and Methods. Individual tumor growth curves are shown. C. Kaplan-Meier plots for event-free survival; D. An additional group of BT-40 bearing mice was irradiated (10 Gy) when tumors were smaller; E. Cumulative toxicity scores for 10 Gy XRT (open black circle) or selumetinib + 10 Gy XRT (closed black circle) (n=10 mice per group).
Discussion
Radiation resistance has been extensively studied in the context of oncogenic Ras [42], although there is little information as to whether activation of BRAF confers radiation resistance in a similar manner. Radiation resistance induced by oncogenic Ras appears to be mediated through activation of the PI3K/Akt pathway, as inhibitors of PI3K, but not rapamycin, MEK or p38 were able to selectively radiosensitize in the presence of oncogenic Ras. Sensitization to radiation by trastuzumab or cituximab also appears related to inhibition of PI3K signaling by these antibodies [43–45]. Similarly, Ras-mediated radiation resistance was not linked to MAP kinase activation in bladder carcinoma cell lines [46]. Thus, the majority of studies indicate that radiation resistance is mediated by activation of the PI3K/AKT pathway. In the present study, we examined the effect of MEK inhibition of phosphorylation of downstream MAP kinases and TORC1 signaling. Inhibition of MEK by selumetinib inhibited ERK phosphorylation in BT-35 and BT-40 astrocytoma xenografts, but only in the BT-40 BRAF-mutant xenograft did inhibition of MEK significantly down regulate TORC1 signaling. In vitro, inhibition of MEK dramatically reduced clonogenic survival in BT-40c cells, derived from the xenograft, whereas it had less effect on survival in two BRAF(V600E) mutant glioblastoma lines derived from adults. In these three cell lines, loss of clonogenic survival appeared to relate to the ability of selumetinib to inhibit TORC1 signaling, with greatest inhibition in the BT-40c cell line, and least effect on TORC1 signaling in the AM38 line correlating with maintenance of colony formation.
In mice, conditional knockout of mTOR results in the loss of the Fanconi anemia group D2 (FANCD2) mRNA and protein expression in hematopoietic stem and progenitor cells [47]. Loss of FANCD2 resulted in a significant gain of DNA double-strand breaks and cell sensitivity when exposed to DNA cross-linking chemotherapeutic agents. Consistent with these results, inhibition of TOR kinase by AZD8055 or TORC1 signaling by rapamycin resulted in a loss of FANCD2 in sarcoma xenografts or cell lines, resulting in increased sensitivity to ionizing radiation [27]. We were therefore interested in determining whether down-regulation of FANCD2 as a consequence of MEK inhibition would sensitize tumors to fractionated XRT. In the pilot study designed to assess the sensitivity to XRT of BT-40 xenografts we found that a dose of 10 Gy, given as daily 2 Gy fractions induced complete regression of tumors with consistent regrowth between 30 and 50 days following XRT. Higher doses resulted in fewer tumor recurrences following complete regression during the period of observation (100 days). Selumetinib induced complete regression of most BT-40 xenografts, consistent with our previous study [35] with tumor regrowth occurring after completion of the 6-week treatment. For combination treatment, selumetinib was administered for 4 days to reduce FANCD2 levels prior to starting XRT. XRT (10 Gy) induced partial regression with subsequent regrowth, whereas combined with selumetinib there were only four tumor recurrences (2 at 80 and 2 at 134 days) with 60% of mice remaining tumor-free at termination of the experiment (day 160). Thus, 10 Gy XRT combined with selumetinib appears at least equivalent to 20 Gy XRT, based on the pilot study.
Selumetinib has been shown to enhance the effect of XRT both in vitro and in vivo [48–50], although mechanisms to account for these effects vary. In pancreatic carcinoma cells, selumetinib treatment increased mitotic catastrophe, possibly by altering DNA damage checkpoint response mediated by Chk1 [48]. In NSCLC and other carcinoma cell lines it was proposed that selumetinib enhances radiation sensitivity by downregulating survival and growth signals mediated by EGFR ligands [49]. Selumetinib also enhanced XRT against lung and colorectal cancer cell lines in vitro, and reduced hypoxia-inducible factor 1α transcription under conditions of hypoxia [50]. Selumetinib reduced vascular perfusion and hypoxic fraction as determined by pimonidazole binding, an effect exacerbated in tumors treated with XRT and selumetinib. In our study, there was a rapid response to selumetinib, with most tumors regressing completely within 7–14 days. We gave 4 days of treatment with selumetinib prior to initiating XRT treatment, as previously we had determined that FANCD2 was depleted by this time. However, in vitro, selumetinib treatment rapidly depleted FANCD2, hence the 4-day pretreatment may not be necessary to enhance XRT. Shannon et al., [50] found equal potentiation of XRT whether it was given during the first 5 days or last 5 days of selumetinib treatment in mouse tumor models, whereas other studies suggest that pretreatment with MEK inhibitors seems to be important for potentiating XRT [48,51]. Selumetinib was shown to reduce levels of vascular endothelial growth factor (VEGF) that protects endothelial cells from lethal effects of radiation [52,53], and we have shown that suppression of TORC1 signaling results in reduced tumor cell-derived VEGF, at least in some pediatric cancer lines [54]. Thus, the exact mechanism for synergy between selumetinib and XRT in the BT-40 model may be complex, and requires additional study.
One concern over combining MEK inhibition with XRT is the potential to increase normal tissue toxicity, in this case brain tissue, causing enhanced radiation-induced necrosis. In the study reported here, there was no increase in local XRT-induced skin toxicity when selumetinib was combined with XRT, or loss of body weight (not shown) suggesting that radiation enhancement may be tumor-specific. Conceptually, suppression of TORC1 signaling resulting in downregulation of FANCD2 should be context specific, occurring only in mutant BRAF tumor cells when MEK is inhibited. Further, while the MAP kinase pathway is activated in several brain pathologies, and can be activated by exogenous ligands, phospho-ERK levels are very low in normal brain [55,56]. A recent report from the Pediatric Brain Tumor Consortium indicates that selumetinib has promising clinical activity in children with low-grade glioma [57]. As children with low-grade tumors often receive XRT either upfront (in older patients) or after recurrence, and many harbor BRAF aberrations, suppression of MEK in the context of activated BRAF may selectively sensitize tumor tissue to XRT, allowing more effective tumor control, or allowing reduced doses of XRT thus reducing radiation-induced late effects. In addition, these data also support the use of a selumetinib as a radiosensitizer at diagnosis in the small subset of children with high-grade glioma who harbor a BRAF V600E mutations [20].
Acknowledgement
This work was supported by USPS awards CA169368 and CA77776 from the National Cancer Institute.
Dr. Paul Smith is an employee of Astrazeneca.
Footnotes
Conflict of Interest. Other authors have no known conflicts.
References
- 1.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17(9):503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, Repka MX, Cohen KJ, Burger PC. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 4.Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68(6):1548–1554. doi: 10.1227/NEU.0b013e318214a66e. discussion 1554–1545. [DOI] [PubMed] [Google Scholar]
- 5.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27(22):3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, Evans DG. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 8.Donahue B. Short- and long-term complications of radiation therapy for pediatric brain tumors. Pediatr Neurosurg. 1992;18(4):207–217. doi: 10.1159/000120664. [DOI] [PubMed] [Google Scholar]
- 9.Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, Dalton J, Zambetti GP, Ellison DW, Kun LE, Gajjar A, Gilbertson RJ, Fuller CE. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25(6):682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 10.Gajjar A, Bhargava R, Jenkins JJ, Heideman R, Sanford RA, Langston JW, Walter AW, Kuttesch JF, Muhlbauer M, Kun LE. Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg. 1995;83(1):67–71. doi: 10.3171/jns.1995.83.1.0067. [DOI] [PubMed] [Google Scholar]
- 11.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SE, White E, Eden C, Hogg T, Northcott P, Mack S, Neale G, Wang YD, Coyle B, Atkinson J, DeWire M, Kranenburg TA, Gillespie Y, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. Journal of neuropathology and experimental neurology. 2008;67(9):878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 16.Penman CL, Faulkner C, Lowis SP, Kurian KM. Current Understanding of BRAF Alterations in Diagnosis, Prognosis, and Therapeutic Targeting in Pediatric Low-Grade Gliomas. Frontiers in oncology. 2015;5:54. doi: 10.3389/fonc.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR, Batchelor TT, Ligon KL, Iafrate AJ, Ligon AH, Louis DN, Santagata S. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappe C, Padovani L, Scavarda D, Forest F, Nanni-Metellus I, Loundou A, Mercurio S, Fina F, Lena G, Colin C, Figarella-Branger D. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013;23(5):574–583. doi: 10.1111/bpa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 20.Nicolaides TP, Li H, Solomon DA, Hariono S, Hashizume R, Barkovich K, Baker SJ, Paugh BS, Jones C, Forshew T, Hindley GF, Hodgson JG, Kim JS, Rowitch DH, Weiss WA, Waldman TA, James CD. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17(24):7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger MS, Ji H, Gutmann DH, James CD. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70(2):512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, Stavropoulos J, Alon N, Pole JD, Ray PN, Navickiene V, Mangerel J, Remke M, Buczkowicz P, Ramaswamy V, Guerreiro Stucklin A, Li M, Young EJ, Zhang C, Castelo-Branco P, et al. BRAF Mutation and CDKN2A Deletion Define a Clinically Distinct Subgroup of Childhood Secondary High-Grade Glioma. J Clin Oncol. 2015;33(9):1015–1022. doi: 10.1200/JCO.2014.58.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439(7074):358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy RD, D'Andrea AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. J Clin Oncol. 2006;24(23):3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- 25.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Wipf P, Parmar K, Greenberger JS. Radiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2(−/−)) mice. Radiation research. 2014;181(1):76–89. doi: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. International journal of radiation oncology, biology, physics. 2009;74(5):1323–1331. doi: 10.1016/j.ijrobp.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen C, Oswald D, Phelps D, Cam H, Pelloski CE, Pang Q, Houghton PJ. Regulation of FANCD2 by the mTOR pathway contributes to the resistance of cancer cells to DNA double-strand breaks. Cancer Res. 2013;73(11):3393–3401. doi: 10.1158/0008-5472.CAN-12-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo F, Zhang S, Grogg M, Cancelas JA, Varney ME, Starczynowski DT, Du W, Yang JQ, Liu W, Thomas G, Kozma S, Pang Q, Zheng Y. Mouse gene targeting reveals an essential role of mTOR in hematopoietic stem cell engraftment and hematopoiesis. Haematologica. 2013;98(9):1353–1358. doi: 10.3324/haematol.2012.080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo F, Li J, Du W, Zhang S, O'Connor M, Thomas G, Kozma S, Zingarelli B, Pang Q, Zheng Y. mTOR regulates DNA damage response through NF-kappaB-mediated FANCD2 pathway in hematopoietic cells. Leukemia. 2013;27(10):2040–2046. doi: 10.1038/leu.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse CA, Mitchell DH, Kleinschmidt-DeMasters BK, Franklin WA, Morse HG, Spector EB, Lillehei KO. Characterization of a continuous human glioma cell line DBTRG-05MG: growth kinetics, karyotype, receptor expression, and tumor suppressor gene analyses. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 1992;28A(9–10):609–614. doi: 10.1007/BF02631035. [DOI] [PubMed] [Google Scholar]
- 31.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 32.Kaplon R, Hadziahmetovic M, Sommerfeld J, Bondra K, Lu L, Leasure J, Nguyen P, McHugh K, Li N, Chronowski C, Sebastian N, Singh M, Kurmasheva R, Houghton P, Pelloski CE. The application of radiation therapy to the Pediatric Preclinical Testing Program (PPTP): results of a pilot study in rhabdomyosarcoma. Pediatr Blood Cancer. 2013;60(3):377–382. doi: 10.1002/pbc.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L, Bondra K, Gupta N, Sommerfeld J, Chronowski C, Leasure J, Singh M, Pelloski CE. Using NanoDot dosimetry to study the RS 2000 X-ray biological irradiator. International journal of radiation biology. 2013;89(12):1094–1099. doi: 10.3109/09553002.2013.817703. [DOI] [PubMed] [Google Scholar]
- 34.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69(19):7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Neale G, Keir ST, Carol H, Lock R, Phelps D, Kang MH, Reynolds CP, Maris JM, Billups C, Smith MA. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55(4):668–677. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran RB, Rothenberg SM, Hata AN, Faber AC, Piris A, Nazarian RM, Brown RD, Godfrey JT, Winokur D, Walsh J, Mino-Kenudson M, Maheswaran S, Settleman J, Wargo JA, Flaherty KT, Haber DA, Engelman JA. TORC1 suppression predicts responsiveness to RAF and MEK inhibition in BRAF-mutant melanoma. Sci Transl Med. 2013;5(196):196ra198. doi: 10.1126/scitranslmed.3005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16(1):29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and-2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. The Journal of biological chemistry. 2003;278(39):37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 39.Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62(24):7291–7297. [PubMed] [Google Scholar]
- 40.Singh M, Leasure JM, Chronowski C, Geier B, Bondra K, Duan W, Hensley LA, Villalona-Calero M, Li N, Vergis AM, Kurmasheva RT, Shen C, Woods G, Sebastian N, Fabian D, Kaplon R, Hammond S, Palanichamy K, Chakravarti A, Houghton PJ. FANCD2 Is a Potential Therapeutic Target and Biomarker in Alveolar Rhabdomyosarcoma Harboring the PAX3-FOXO1 Fusion Gene. Clin Cancer Res. 2014;20(14):3884–3895. doi: 10.1158/1078-0432.CCR-13-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bid HK, Kibler A, Phelps DA, Manap S, Xiao L, Lin J, Capper D, Oswald D, Geier B, DeWire M, Smith PD, Kurmasheva RT, Mo X, Fernandez S, Houghton PJ. Development, characterization, and reversal of acquired resistance to the MEK1 inhibitor selumetinib (AZD6244) in an in vivo model of childhood astrocytoma. Clin Cancer Res. 2013;19(24):6716–6729. doi: 10.1158/1078-0432.CCR-13-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, McKenna WG. The Ras radiation resistance pathway. Cancer research. 2001;61(10):4278–4282. [PubMed] [Google Scholar]
- 43.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Molecular cancer therapeutics. 2003;2(11):1113–1120. [PubMed] [Google Scholar]
- 44.Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. International journal of radiation oncology, biology, physics. 2003;57(1):246–254. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 45.Liang K, Jin W, Knuefermann C, Schmidt M, Mills GB, Ang KK, Milas L, Fan Z. Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy. Molecular cancer therapeutics. 2003;2(4):353–360. [PubMed] [Google Scholar]
- 46.Gupta AK, Bernhard EJ, Bakanauskas VJ, Wu J, Muschel RJ, McKenna WG. RAS-Mediated radiation resistance is not linked to MAP kinase activation in two bladder carcinoma cell lines. Radiation research. 2000;154(1):64–72. doi: 10.1667/0033-7587(2000)154[0064:rmrrin]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Guo F, Lie J, Du W, Zhang S, Liu W, Thomas G, Kozma S, Pang Q, Zheng Y. mTOR Regulates DNA Damage Response of Hematopoietic Stem and Progenitor Cells Through Modulation of Fanconi Anemia Core Complex. 53rd ASH Annual Meeting and Exposition. 2011 [Google Scholar]
- 48.Chung EJ, Brown AP, Asano H, Mandler M, Burgan WE, Carter D, Camphausen K, Citrin D. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15(9):3050–3057. doi: 10.1158/1078-0432.CCR-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung EJ, Hudak K, Horton JA, White A, Scroggins BT, Vaswani S, Citrin D. Transforming growth factor alpha is a critical mediator of radiation lung injury. Radiation research. 2014;182(3):350–362. doi: 10.1667/RR13625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon AM, Telfer BA, Smith PD, Babur M, Logie A, Wilkinson RW, Debray C, Stratford IJ, Williams KJ, Wedge SR. The mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res. 2009;15(21):6619–6629. doi: 10.1158/1078-0432.CCR-08-2958. [DOI] [PubMed] [Google Scholar]
- 51.Hamed H, Hawkins W, Mitchell C, Gilfor D, Zhang G, Pei XY, Dai Y, Hagan MP, Roberts JD, Yacoub A, Grant S, Dent P. Transient exposure of carcinoma cells to RAS/MEK inhibitors and UCN-01 causes cell death in vitro and in vivo. Mol Cancer Ther. 2008;7(3):616–629. doi: 10.1158/1535-7163.MCT-07-2376. [DOI] [PubMed] [Google Scholar]
- 52.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–3378. [PubMed] [Google Scholar]
- 53.Gupta VK, Jaskowiak NT, Beckett MA, Mauceri HJ, Grunstein J, Johnson RS, Calvin DA, Nodzenski E, Pejovic M, Kufe DW, Posner MC, Weichselbaum RR. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8(1):47–54. doi: 10.1097/00130404-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Kurmasheva RT, Harwood FC, Houghton PJ. Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors. Mol Cancer Ther. 2007;6(5):1620–1628. doi: 10.1158/1535-7163.MCT-06-0646. [DOI] [PubMed] [Google Scholar]
- 55.Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13(4):473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swatton JE, Sellers LA, Faull RL, Holland A, Iritani S, Bahn S. Increased MAP kinase activity in Alzheimer's and Down syndrome but not in schizophrenia human brain. The European journal of neuroscience. 2004;19(10):2711–2719. doi: 10.1111/j.0953-816X.2004.03365.x. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee A, Jackaki R, Thomas AO, Wu S, Nicoladies T. A phase 1 study of AZD6244 in children with recurrent or refractory low-grade gliomas: A Pediatric Brain Tumor Consortium report. J Clinical Oncology. 2014;32.5s abstract 10065. [Google Scholar]