Abstract
Magnesium sulfate was given to pediatric cardiac surgical patients during cardiopulmonary bypass period in an attempt to reduce the occurrence of postoperative junctional ectopic tachycardia (PO JET). We reviewed our data to evaluate the effect of magnesium on the occurrence of JET and assess a possible relationship between PO JET and procedure complexity. A total of 1088 congenital heart surgeries (CHS), performed from 2005 to 2010, were reviewed. A total of 750 cases did not receive magnesium, and 338 cases received magnesium (25 mg/kg). All procedures were classified according to Aristotle score from 1 to 4. Overall, there was a statistically significant decrease in PO JET occurrence between the two groups regardless of the Aristotle score, 15.3 % (115/750) in non-magnesium group versus 7.1 % (24/338) in magnesium group, P < 0.001. In the absence of magnesium, the risk of JET increased with increasing Aristotle score, P = 0.01. Following magnesium administration and controlling for body weight, surgical and aortic cross-clamp times in the analyses, reduction in adjusted risk of JET was significantly greater with increasing Aristotle level of complexity (JET in non-magnesium vs. magnesium group, Aristotle level 1: 9.8 vs. 14.3 %, level 4: 11.5 vs. 3.2 %; odds ratio 0.54, 95 % CI 0.31–0.94, P = 0.028). Our data confirmed that intra-operative usage of magnesium reduced the occurrence of PO JET in a larger number and more diverse group of CHS patients than has previously been reported. Further, our data suggest that magnesium’s effect on PO JET occurrence seemed more effective in CHS with higher levels of Aristotle complexity.
Keywords: Magnesium, Ectopic junctional tachycardia, Congenital heart surgery, Postoperative arrhythmia
Introduction
Postoperative junctional ectopic tachycardia (PO JET) occurs more frequently after surgical repair of certain types of congenital heart defects [13, 15, 16, 25]. PO JET can impact and complicate the postoperative course significantly delaying recovery from surgery. PO JET may produce unfavorable hemodynamics that prolong the cardiac intensive care unit (CICU) and hospital stay, prolong time on the ventilator, and on rare occasion require the use of extra-corporeal circulatory support (ECMO) as rescue therapy for low cardiac output or adverse effects following the administration of antiarrhythmic medication [1, 4, 16].
The cause of PO PET is multi-factorial. Early studies of PO JET attributed risk to surgery around the AV conduction system or ventricular septal defect closure. More recent studies suggest the importance of surrogate measures of myocardial ischemia, such as aortic cross-clamp time, cardiopulmonary bypass (CPB) time, and PO troponin levels as better predictors for occurrence of PO JET [1, 2, 15].
A previous randomized double-blind controlled trial in a small population of children undergoing congenital heart surgery (99 cases, 30 of whom were controls) showed that the incidence of hypomagnesemia and PO JET following CHS can be reduced by magnesium administration [14]. At our institution, we started administration of magnesium sulfate at the time of rewarming in 2009, after noting a consistent high rate of PO JET occurrence. We present these results herein to extend these observations to a larger, perhaps more representative, patient population that would allow us to evaluate the effects of magnesium on JET occurrence by the complexity of CHS.
We hypothesized that ischemia of the heart during surgery and reperfusion injury following aortic cross-clamp release, despite cardioplegia protection, was the dominant risk factor for PO JET occurrence. We tested (1) whether the routine administration of magnesium after aortic cross-clamp release reduced PO JET occurrence in a larger cohort of CHS patients with considerable more diversity of congenital heart disease types than had been previously reported and (2) evaluated whether there was a relationship between the duration of myocardial ischemic time and the pharmacologic effect of magnesium in reducing the frequency of PO JET occurrence. The implication is that the effect of magnesium on reduction in PO JET would be greater in patients requiring surgical repair of more complex congenital heart defects.
Materials and Methods
Patients
Patients who underwent CHS with CPB at Children’s National Medical Center from 2005 to 2010 were retrospectively reviewed. This included electronic chart, electrocardiograms, and telemetry record review from patients entering the CICU to patients being discharged to determine the occurrence of PO JET. The following clinical data were carefully reviewed and collected: date of surgery, gender, ethnicity, height, weight, body surface area, occurrence of PO JET event, total surgical time, aortic crossing-clamp time, CPB time, dopamine and milrinone usage, length of stay in the CICU, and mortality. The Children’s National Medical Center Institutional Review Board for research on human subjects approved this study involving chart abstracts to assemble a de-identified cohort.
The whole cohort of study patients (1088 subjects) was divided into two groups. Patients operated on between January 2005 and July 2008 did not receive intra-operative magnesium (Group 1, non-Mg group, n = 750). During this time period, a total of 1009 patients underwent surgical repair and were reviewed. One hundred cases were excluded because of ineligibility that included (1) history of preoperative arrhythmia, (2) incomplete or absent medical records, and (3) unclear description or no electrocardiographic evidence of PO JET. Another 159 cases randomly selected were preserved for an on-going study to evaluate risk factors for the occurrence of PO JET, as we already had adequate numbers for this group from the statistic point of view, leaving 750 patients for inclusion in Group 1. Patients operated on after May 2009 until July 2010 were almost universally administered magnesium (Group 2, Mg group, n = 338). During this time period, a total of 369 patients underwent surgical repair and were reviewed. Thirty-one were excluded because of ineligibility based on the above exclusive criteria, leaving 338 patients for inclusion in Group 2. Patients in the Mg group received a single bolus of magnesium sulfate (25 mg/kg) into the CPB circuit at the beginning of rewarming. The cardioplegia solution (Baxter, Deerfield, IL, USA), which contains 0.325 % of magnesium chloride, was given to all the patients as needed according to our standard CPB protocol.
We defined JET for the purposes of this study as a supraventricular arrhythmia (wide or narrow QRS complex—same morphology as in sinus rhythm) with no preceding P wave at a rate that exceeded the normal junctional escape rate for age. The pattern of VA conduction could be either 1:1 VA conduction, VA Wenckebach or dissociated. JET usually exhibited variability in rate at onset or termination of the arrhythmia (warm-up or cool-down) and did not demonstrate sudden onset or termination. The ventricular rate had to be ≥120 bpm. We elected to have a more inclusive definition of JET, so that we could incorporate all cases of JET into our study population. All ECGs, rhythm strips, and Holter monitors were reinterpreted, without regard to treatment group, based on the above criteria to detect JET. If a patient was described in the clinical notes as having JET but there was no electrocardiographic supportive evidence for JET, or if we disagreed with the original ECG interpretation, these patients were removed from the study.
Cardiac Surgical Procedure: Aristotle Score
The Aristotle scoring system was used to assess the complexity level of surgical procedures. The Aristotle basic score is a procedure-adjusted complexity score and only applies to procedures. It is based on the potential for mortality, the potential for morbidity, and the anticipated technical difficulty. The complexity was based on procedures as defined by the Society of Thoracic Surgeons and the European Association of Cardio-Thoracic Surgery International Nomenclature [9]. Four levels of procedural complexity were defined, which matches the basic score range from 1.5 to 15: level 1(1.5–5.9), level 2 (6.0–7.9), level 3 (8–9.9), and level 4 (10.0–15.0). The level of complexity was obtained from our Children’s National Medical Center Cardioaccess Surgical Database, which automatically calculates the basic score of the primary procedures.
Statistical Analysis
Stata 11.2 (StataCorp LP, College Station, TX, 2012) was used for the statistical analyses. At first, contingency table analyses and Pearson’s Chi-square tests were used to evaluate the relationship between two categorical variables, such as risk of JET by Aristotle level. Two-sample median tests also based on the Pearson’s Chi-square were used to compare two groups for normally distributed measurements, such as weight, BSA, surgical time, aortic cross-clamp time, and CPB time. Subsequently, multiple variable logistic regression modeling was implemented to evaluate magnesium’s effect on PO JET, controlling for the factors emerging from simpler analyses that were related to the risk of JET. Logistic models first explored evidence of effect modification by magnesium by adding a cross-product or interaction term between magnesium and Aristotle score. Statistical significance was based on a two-tailed type I error of 0.05 and on 95 % confidence limits.
Results
Study Population
A cohort of 1088 patients undergoing congenital heart surgery with CPB were collected for this study and divided into two groups. The demographics, preoperative, and intra-operative variables of both groups are shown in Table 1. Age, body weight, BSA, and total surgical time were significantly higher in the Mg group than in the non-Mg group. Aortic cross-clamp time was shorter in the Mg group. There were no significant differences between the Mg and non-Mg groups in terms of other study variables—CPB time, gender, ethnicity, length of CICU stay, and hospital mortality.
Table 1.
Demographic and baseline clinical data for patients in the non-magnesium and magnesium study groups
| Variables | Non-Mg group (n = 750) Group 1 | Mg group (n = 338) Group 2 | P value |
|---|---|---|---|
| Age (days) | |||
| Median (min, max) | 144 (1, 13369) | 196 (0.5, 17431) | 0.003a |
| Weight (kg) | |||
| Median (min, max) | 5.5 (1.4, 94) | 6.3 (2.0, 103) | 0.003a |
| BSA (m2/kg) | |||
| Median (min, max) | 0.31 (0.13, 1.94) | 0.34 (0.16, 2.24) | 0.018a |
| Gender [N (%)] | |||
| Male | 406 (54) | 177 (52) | 0.589b |
| Female | 344 (46) | 161 (48) | |
| Ethnicity [N (%)] | |||
| Caucasian | 329 (43.9) | 146 (43.2) | |
| African-American | 189 (25.2) | 101 (29.9) | |
| African | 21 (2.8) | 1 (0.3) | |
| Hispanic | 103 (13.7) | 44 (13.0) | |
| Asian | 32 (4.3) | 12 (3.6) | |
| Middle eastern | 76 (10.1) | 34 (10.1) | |
| Total surgical time (min) | |||
| Median (min, max) | 190 (105, 619) | 205 (105, 470) | <0.001a |
| Aortic CC time (min) | |||
| Median (min, max) | 43 (7, 159) | 41 (9, 146) | 0.041a |
| CPB time (min) | |||
| Median (min, max) | 84 (14, 324) | 83 (24, 263) | 0.403a |
| CICU stay (days) | |||
| Median (min, max) | 4 (1, 212) | 3 (1, 198) | 0.458c |
| Surgical mortality [N (%)] | 40 (5.3) | 12 (3.6) | 0.247d |
Median test
Pearson’s Chi-square test
Survival analysis controlled for magnesium, Aristotle score, magnesium × Aristotle score, body surface area, surgery time, aortic cross-clamp time, dopamine administration, milrinone administration
Logistic model controlled for the same variables as CICU stay
The average maximum heart rate during PO JET in the magnesium and non-magnesium groups was the following: 183 ± 21.9 versus 181 ± 21.8 bpm, P = 0.68.
Aristotle Scoring System
The Aristotle basic score for the surgical procedures performed during this time period ranged from 1.5 to 15 and was classified into four levels of complexity. The distribution of surgical procedures classified by the Aristotle score was similar between the two study groups, Fig. 1, P = 0.058.
Fig. 1.
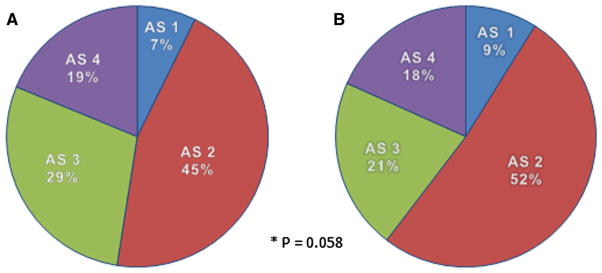
Aristotle score distribution in the two study groups. a Case distribution in non-magnesium group. b Case distribution in magnesium group. AL aristotle level. See text for explanation
Effect of Magnesium on the Incidence of PO JET
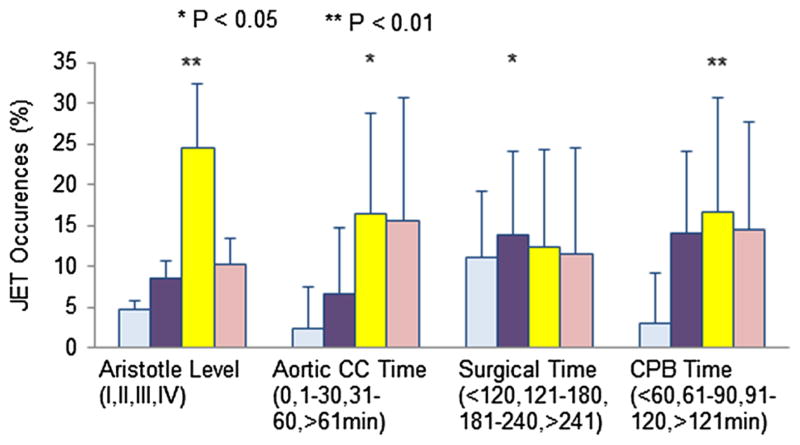
The overall incidence of PO JET was significantly reduced in patients receiving magnesium (7.1 % in the Mg group, vs. 15.3 % in the non-Mg group, P < 0.001). Reduction in PO JET seemed significantly greater with increasing Aristotle level of complexity (Table 2, odds ratio 0.49, 95 % CI 0.29–0.85 P = 0.01). A higher incidence of JET was noticed with increasing surgical time (P = 0.018), aortic cross-clamp time (P = 0.017), CPB time (P < 0.001), and Aristotle score (P = 0.01, Fig. 2). In the non-Mg group, univariate analysis revealed that the risk of PO JET increased with increasing Aristotle score (1.8–14.98 %, Table 2).
Table 2.
Relationship between Aristotle score and occurrence of PO JET in non-magnesium and magnesium groups
| Frequency of JET occurrence (%) at different aristotle levels
|
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Non-Mg group | 1.82 (1/55) | 9.73 (33/339) | 27.91 (60/215) | 14.89 (21/141) |
| Mg group | 10.00 (3/30) | 6.32 (11/174) | 13.89 (10/72) | 0.00(0/62) |
P = 0.01 (non-magnesium vs. magnesium group)
Fig. 2.
Univariate predictors for postoperative JET occurrence. Illustrated in this figure is the frequency of postoperative JET occurrence over different a Aristotle levels (1–4), b aortic cross-clamp times (from 0 to >61 min), c surgical times (from ≤2 to >4 h), and d CPB times (from ≤60 min to >2 h). CC crossing clamp. See text for explanation
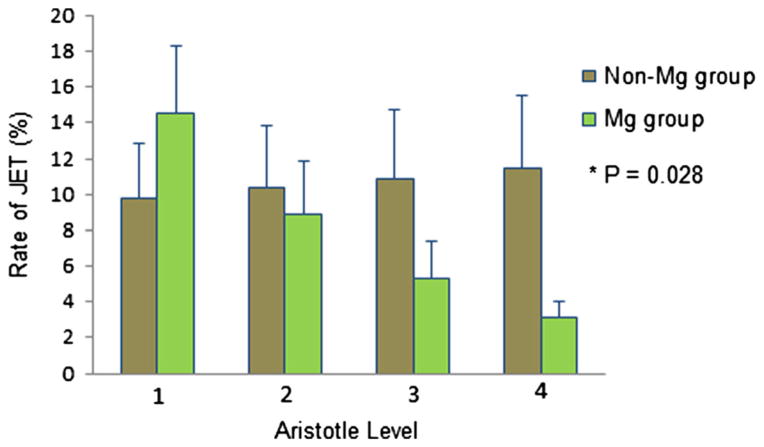
Multivariate analysis revealed that the risk of PO JET increased slightly with increasing Aristotle score in the non-Mg group (9.8–11.48 %). However, in the Mg group, there was a significant reduction in PO JET risk from 14.5 to 3.2 % across Aristotle levels 1 to 4. (P = 0.028, Fig. 3; Table 3) when controlling for the following factors—body surface area, surgical and aortic cross-clamp time, use of dopamine and milrinone (risk factors known to be associated with the occurrence of PO JET).
Fig. 3.
Adjusted rate of postoperative JET occurrence by treatment group (multivariate analysis). Relationship between Aristotle level and occurrence of PO JET in the two study groups (non-magnesium and magnesium) after the analysis was controlled for BSA, surgery time, aortic cross-clamp time, and dopamine and milrinone administration. See text for explanation
Table 3.
Logistic multivariate model for prediction of PO JET occurrence
| Variables | Odds ratio (95 % CI) | P value |
|---|---|---|
| Magnesium | 2.88 (0.70–11.83) | 0.142 |
| Aristotle score | 1.06 (0.82–1.37) | 0.650 |
| Magnesium × Aristotle score | 0.54 (0.31–0.94) | 0.028 |
| BSA | 0.51 (0.20–1.33) | 0.170 |
| Surgery time (min) | 0.999 (0.995–1.002) | 0.489 |
| Aortic cross-clamp | 4.12 (1.26–13.49) | 0.019 |
| Dopamine administration | 2.04 (1.25–3.32) | 0.004 |
| Milrinone administration | 2.98 (1.93–4.58) | <0.001 |
Relationship Between Aristotle Score and Measures of Myocardial Ischemia
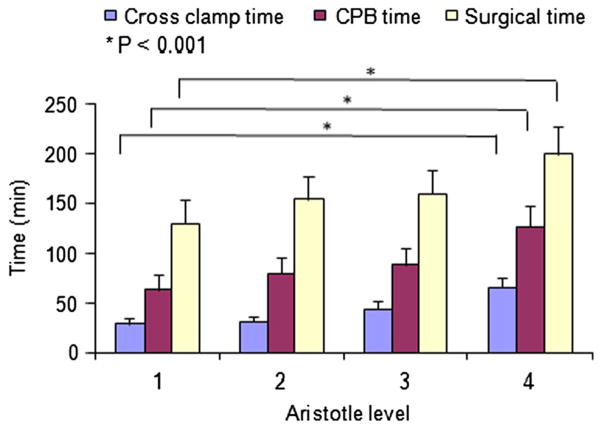
We further studied whether there was a relationship between either the aortic cross-clamp time or the length of CPB time versus the Aristotle score. Our data showed that aortic cross-clamp time and the CPB time increased with increasing level of the Aristotle score, (Fig. 4, P < 0.001). In addition, aortic cross-clamp during CPB played a significant role in the risk of occurrence of PO JET (odds ratio 4.12, 95 % CI 1.26–13.49, P = 0.019).
Fig. 4.
Distribution of aortic cross-clamp times, cardiopulmonary bypass times, and surgical times separated into different Aristotle levels. See text for explanation
Other Risk Factors for Occurrence of PO JET
Postoperative administration of dopamine and milrinone increased the risk of occurrence of PO JET in the total surgical group odds ratio 1.81, 95 % CI 1.10–2.96, P = 0.018 for dopamine; odds ratio 2.85, 95 % CI 1.84–4.41 P < 0.001 for milrinone.
Preoperative total serum magnesium levels (ionized Mg data not available in this retrospective study) were lower in patients who developed PO JET (1.82 ± 0.24 for JET vs. 1.97 ± 0.26 mg/dl for non-JET, P = 0.022). Serum magnesium levels increased in the total group from 1.95 ± 0.27 to 3.02 ± 0.76 mg/dl after magnesium was given. However, the magnesium levels were higher in patients who still developed PO JET (3.31 ± 0.88 for JET vs. 2.99 ± 0.78 mg/dl for non-JET, P = 0.02), suggesting a complex relationship to total serum magnesium levels.
There were statistically significant differences between non-JET and JET patients regarding patient age at time of surgery [201 vs. 66 days (P = 0.04)], body weight [6.67 vs. 5.00 kg, (P = 0.03)], and length of CICU stay time [3 vs. 12 days (P < 0.01)].
Impact of Magnesium on CICU Stay and Mortality
The length of stay in the CICU was 4 and 3 days in the non-Mg and Mg groups, respectively (P = 0.458), and the surgical mortality was 5.3 % in the non-Mg group and 3.6 % in Mg groups (P = 0.247), indicating no difference.
Discussion
PO JET is one of the most common arrhythmias occurring after congenital heart surgery and can have a significant negative impact on cardiac function in the early postoperative period. The occurrence of PO JET can result in a longer mechanical ventilation time, longer length of CICU stay, and even higher surgical mortality rate as described in the literature [1, 2, 16], and as our group has previously reported [16]. The data we report herein in the largest cohort of CHS patients with a broad diversity of congenital heart disease types (greater than 1000 patients) supported previous findings that magnesium can decrease the occurrence of PO JET [5, 14]. Our data showed that magnesium significantly reduced the incidence of PO JET in patients receiving magnesium after aortic clamp release (7.1 % in the Mg group, vs. 15.3 % in the non-Mg group, a 54 % decrease). Through analysis of this very large cohort of CHS patients, we found that the effect of magnesium in reducing PO JET occurrence seemed greater in patients having more complex CHS with higher Aristotle scores. Even though there were no significant differences in the length of CICU stay or surgical mortality rate between the non-Mg and Mg group, CICU length of stay was much longer in PO JET patients despite magnesium administration (12 vs. 3 days).
Our previous study, which aimed at identifying risk factors for the occurrence of PO JET, revealed that prolonged aortic cross-clamp time, longer CPB time, younger age, lower body weight, and inotrope usage were associated with the occurrence of PO JET [7, 16]. The current study also suggests that aortic cross-clamp, which induces ischemia and reperfusion injury, is a strong risk factor associated with the occurrence of PO JET. Our animal studies confirm that JET can be caused by prolonged ischemia and reperfusion injury to the myocytes and conduction system [17]. Human studies have demonstrated elevated CPK-MB and troponin levels to be associated with an increased likelihood of PO JET [1, 15]. Reperfusion injury can result from reactive oxygen species and free radical release in myocytes and mitochondria. Intracellular disruption of mitochondria and sarcoplasmic reticulum function increases intracellular calcium levels, impairing diastolic relaxation, and results in dysfunction of the conduction system [12]. These physiologic disturbances in turn can enhance automaticity or result in triggered activity from an ectopic focus within the atrioventricular node or His bundle [10, 21], particularly in high catecholamine states (which occurs following milrinone and dopamine administration).
Magnesium has been successfully used in the prophylaxis and treatment of a variety of cardiac arrhythmias [11, 19, 22]. Magnesium is one of the more important intra-cellular cations and a cofactor for many of the enzymatic reactions involved in mitochondrial energy metabolism. As a natural calcium antagonist, magnesium decreases inward calcium current via the calcium channels and stabilizes the membrane potential by facilitation of potassium entry into the cells. Magnesium increases the relative refractory period and decreases the vulnerable period, reducing the risk of reentry [3, 12, 18]. Imura H et al. [8] showed that magnesium rescues the heart following ischemia, by reducing intracellular calcium, which in turn, would aid metabolic recovery. Using a Langendorff rat model of hypoxia-re-oxygenation injury, Sato et al. demonstrated that high intra-myocardial levels of magnesium prevented intracellular calcium overload, but also facilitated the opening of mitochondrial KATP channels [20, 23], preserving resting membrane potential. In another rat model study, magnesium pretreatment played a cardio-protective role in ischemia-reperfusion injury, possibly by inhibiting the upregulation of P-selectin expression [24].
Despite very few studies showing a correlation between preoperative or postoperative serum hypomagnesemia and JET occurrence, including our own data, magnesium has nevertheless been used successfully for prophylaxis and treatment of postoperative JET [6]. Two randomized clinical trials with small cohorts showed that the incidence PO of JET was significantly reduced with magnesium supplement and this effect seemed dose-related [9, 14]. In one of the trials with a total study population size of 99 patients, Manrique found that the incidence of PO JET was reduced from 17.9 to 6.7 %, when 25 mg/kg of magnesium was given, and was further reduced to 0 % with 50 mg/kg of magnesium [14]. The current study corroborates these previous observations in a very large cohort (1088) of CHS patients. The importance of preoperative or postoperative hypomagnesium is still unclear, since in our study, despite a higher magnesium level post-op some patients still developed JET. In fact, the postoperative group that developed JET had a higher serum magnesium level than the postoperative group that did not. In our study, giving magnesium sulfate (25 mg/kg) at the beginning of warming seemed to significantly reduce the incidence of PO JET. Further, we noted that magnesium was more effective in CHS with a higher level of Aristotle complexity, from level 2 and above, and had little protective effect in CHS classified as Aristotle level of complexity 1. As the Aristotle level of complexity increases, aortic cross-clamp time becomes longer, leading to the potential for more ischemia and reperfusion injury to the myocardium and conduction system after aortic de-clamping. We speculate that magnesium may lessen the effects of ischemia and reperfusion injury and retraction in more complicated CHS with longer cross-clamp times by the above-discussed mechanisms.
Study Limitations
This study bears all the limitations of an historical retrospective chart review, such as missing data, hard to control bias, confounders, lack of randomization, and no blinding. All the data were from records and charts. Only patients with electrocardiographic confirmation of JET were included. Therefore, some patients who might have had JET based on the clinician’s judgment of the cardiac rhythm on telemetry were excluded, leading to an underestimation of the frequency of PO JET.
Since our study is a comparison of preintervention and postintervention (magnesium administration), it is not possible to exclude some other changes in surgical, intra-operative, or postoperative management that might have affected the occurrence of PO JET in Group 2. In the future, we will analyze the effect of a higher dose of magnesium (≥50 mg/kg) on postoperative JET in order to see whether a dose–effect relationship exists, which would further strengthen the causality of magnesium in reducing PO JET occurrence. We also need to investigate in more detail why intra-operative administration of magnesium at the time of aortic cross-clamp release had a greater preventive effect in patients undergoing more complex CHS procedures.
Conclusions
Evidence is presented that magnesium given intra-operatively at the time of rewarming seems associated with a sizable reduction (54 %) in the risk of PO JET in a very large cohort of diverse CHS patients. The effect of magnesium appeared larger in CHS procedures with greater surgical complexity and in procedures with higher Aristotle scores. Our data suggest that the higher the Aristotle score, the larger the effect of magnesium in reducing the occurrence of PO JET. Magnesium may not benefit patients undergoing simpler CHS procedure with an Aristotle score below level 2.
Acknowledgments
The authors thank Sara Badia, MD, for participating in data collection and recording.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Andreasen JB, Johnsen SP, Ravn HB. Junctional ectopic tachycardia after surgery for congenital heart disease in children. Intensive Care Med. 2008;34:895–902. doi: 10.1007/s00134-007-0987-2. [DOI] [PubMed] [Google Scholar]
- 2.Batra AS, Chun SD, Johnson TR, et al. A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatr Cardiol. 2006;27:51–55. doi: 10.1007/s00246-005-0992-6. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238:163–179. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 4.Dodge-Khatami A, Miller OI, Anderson RH, Gil-Jaurena JM, Goldman AP, de Leval MR. Impact of junctional ectopic tachycardia on postoperative morbidity following repair of congenital heart defects. Eur J Cardiovasc Surg. 2002;21:255–259. doi: 10.1016/s1010-7940(01)01089-2. [DOI] [PubMed] [Google Scholar]
- 5.Dorman BH, Sade RM, Burnette JS, et al. Magnesium supplementation in the prevention of arrhythmias in pediatric patients undergoing surgery for congenital heart defects. Am Heart J. 2000;139:522–528. doi: 10.1016/s0002-8703(00)90097-8. [DOI] [PubMed] [Google Scholar]
- 6.Gu WJ, Wu ZJ, Wang PF, Aung LH, Yin RX. Intravenous magnesium prevents atrial fibrillation after coronary artery bypass grafting: a meta-analysis of 7 double-blind, placebo-controlled, randomized clinical trials. Trials. 2012;13:41–49. doi: 10.1186/1745-6215-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman TM, Bush DM, Wernovsky G, et al. Postoperative junctional ectopic tachycardia in children: incidence, risk factors, and treatment. Ann Thorac Surg. 2002;74:1607–1611. doi: 10.1016/s0003-4975(02)04014-6. [DOI] [PubMed] [Google Scholar]
- 8.Imura H, Lin H, Griffiths EJ, Suleiman MS. Controlled hyperkalemic reperfusion with magnesium rescues ischemic juvenile hearts by reducing calcium loading. J Thorac Cardiovasc Surg. 2011;141:1529–1537. doi: 10.1016/j.jtcvs.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Lacour-Gayet F, Clarke D, Jacobs J, et al. The Aristotle committee. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–924. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Lau EW. Infraatrial supraventricular tachycardias: mechanisms, diagnosis, and management. Pacing Clin Electrophysiol. 2008;31:490–498. doi: 10.1111/j.1540-8159.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Ghimire S, Kim EY. Magnesium supplementation reduces postoperative arrhythmias after cardiopulmonary bypass in pediatrics: a meta-analysis of randomized controlled trials. Pediatr Cardiol. 2013;34:1396–1403. doi: 10.1007/s00246-013-0658-8. [DOI] [PubMed] [Google Scholar]
- 12.Longo DL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud AB, Tantawy AE, Kouatli AA, Baslaim GM. Propranolol: a new indication for an old drug in preventing postoperative junctional ectopic tachycardia after surgical repair of tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2008;7:184–197. doi: 10.1510/icvts.2007.160945. [DOI] [PubMed] [Google Scholar]
- 14.Manrique AM, Arroyo M, Lin Y, et al. Magnesium supplementation during cardiopulmonary bypass to prevent junctional ectopic tachycardia after pediatric surgery: a randomized study. J Thorac Cardiovasc Surg. 2010;139:162–169. doi: 10.1016/j.jtcvs.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 15.Mildh L, Hiippala A, Rautiainen P, Pettila V, Sairanen H, Happonen JM. Junctional ectopic tachycardia after surgery for congenital heart disease: incidence, risk factors and outcome. Eur J Cardiothorac Surg. 2011;39:75–80. doi: 10.1016/j.ejcts.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Moak JP, Arias P, Kaltman JR, et al. Postoperative junctional ectopic tachycardia: risk factors for occurrence in the modern surgical era. Pacing Clin Electrophysiol. 2013;36:1156–1168. doi: 10.1111/pace.12163. [DOI] [PubMed] [Google Scholar]
- 17.Moak JP, Mercader MA, He D, et al. Newly created animal model of human postoperative junctional ectopic tachycardia. J Thorac Cardiovasc Surg. 2013;146:212–221. doi: 10.1016/j.jtcvs.2012.08.068. [DOI] [PubMed] [Google Scholar]
- 18.Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002;28:667–679. doi: 10.1007/s00134-002-1281-y. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen HS, Suenson M, McNair P, Nørregård P, Balslev S. Magnesium infusion reduces the incidence of arrhythmias in acute myocardial infarction: a double-blind placebo-controlled study. Clin Cardiol. 1987;10:351–356. doi: 10.1002/clc.4960100610. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Watanabe M, Kishiro M, Nagata S, Shimizu T, Okada T. Intracellular magnesium and tolerance against hypoxia in immature rat heart. Pediatr Int. 2010;52:773–777. doi: 10.1111/j.1442-200X.2010.03172.x. [DOI] [PubMed] [Google Scholar]
- 21.Stiles MK, Sanders P, Disney P, et al. Differential effects of intravenous magnesium on atrioventricular node conduction in supraventricular tachycardia. Am J Cardiol. 2007;100:1249–1253. doi: 10.1016/j.amjcard.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 22.Sueta CA, Clarke SW, Dunlap SH, et al. Effect of acute magnesium administration on the frequency of ventricular arrhythmia in patients with heart failure. Circulation. 1994;89:660–666. doi: 10.1161/01.cir.89.2.660. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M, Wu JR, Li SS, Li CZ, Okada T. Mechanisms of cardioprotective effects of magnesium on hypoxia-reoxygenation-induced injury. Exp Clin Cardiol. 2004;9:181–185. [PMC free article] [PubMed] [Google Scholar]
- 24.Ying SQ, Fang L, Xiang MX, Xu G, Shan J, Wang JA. Protective effects of magnesium against ischaemia-reperfusion injury through inhibition of P-selectin in rats. Clin Exp Pharmacol Physiol. 2007;34:1234–1239. doi: 10.1111/j.1440-1681.2007.04697.x. [DOI] [PubMed] [Google Scholar]
- 25.Zampi JD, Hirsch JC, Gurney JG, et al. Junctional ectopic tachycardia after infant heart surgery: incidence and outcomes. Pediatr Cardiol. 2012;33:1362–1369. doi: 10.1007/s00246-012-0348-y. [DOI] [PubMed] [Google Scholar]