Abstract
Urothelial bladder tumour in childhood is extremely rare, and almost all the reported cases have been low-grade tumours with a favourable outcome. Here we review 57 reports comprising 127 cases, and we report two new cases.
Abbreviations: US, ultrasonography; PUNLMP, papillary urothelial neoplasm of low malignant potential; UC, urothelial carcinoma; TURBT, transurethral resection of the bladder tumour; SEER, surveillance, epidemiology and end results
Keywords: Urothelial, Bladder, Tumour, Childhood
Introduction
Urothelial bladder tumour in childhood is extremely rare [1]. Deming (cited in [2]) reported the first such case, in a patient aged <10 years, in 1924. In 1969, Javadpour and Mostofi [2] identified 40 primary epithelial bladder tumours in the first two decades of life from 10,000 total cases.
A more recent review of previous reports identified 125 patients who were aged <20 years, of whom only 20 were aged <10 years [3], and the origin of such cases was mesodermal; reports on this topic are very limited. These tumours have been shown to have a low grade of malignancy, showing little tendency to recur [4], and have a good prognosis. Here we review previous cases and report two new cases of urothelial bladder tumour.
Methods
We reviewed the databases of PubMed and Hinari for reports in English, searched using the keywords ‘bladder’, ‘transitional cell carcinoma’ and ‘children’. We identified 57 articles [1–57], comprising 127 cases (Table 1). We also describe two new cases.
Table 1.
A review of the 127 cases: All the studies were of level of evidence 5.
| Ref. | Cases/sex | Age (years) | Cell type | Treatment | Outcome |
|---|---|---|---|---|---|
| [1] | 1/M | 4 | High-grade (grade 3), muscle-invasive | TURBT, R after 3 months, | NR at 6 months |
| Papillary UC, stage T2b | Partial cystectomy + chemo | ||||
| [2] | 6/4M/2F | 6–17 | Grade I TCC | TURBT | NR |
| [3] | 8/6M/2F | <18 | Two G1Ta, 1 G1T1, 1 G2T1, and 5 G2Ta | TURBT | NR 8–27 years |
| [4] | 1/M | 10 | Grade I well-differentiated | TURBT | Pending |
| [5] | 12/M | <21 | Low-grade/low-stage | TURBT | 1 patient had 1 R |
| [6] | 1/F | 9 | Grade 1 TCC | TURBT | N/A |
| [7] | 5/M | 11–18 | Low-grade | TURBT | N/A |
| [8] | 7/M + F | <16 | Low-grade | TURBT | NR after 18 months |
| [9] | 1/M | 5 | High-grade | Partial cystectomy | NR after 1 year |
| [10] | 1/F | 16 | High-grade invasive TCC | Radio- and chemotherapy | Died from metastatic disease |
| [12] | 1/F | 9 | Low-grade | TURBT | NR in 4 years |
| [13] | 2/M/F | 15/18 | Superficial TCC | TURBT | N/A |
| [14] | 2/F | 8/9 | Grade 1 stage pTa | TURBT | NR in 4 years |
| [15] | 1/F | 12 | Superficial TCC | TURBT | N/A |
| [16] | 1/M | 10 | Grade 2 TCC with lamina propria invasion | TURBT + Mitomycin | N/A |
| [17] | 1/F | 10 | PUNLMP | TURBT | NR in 9 months |
| [18] | 23/19M/4F | 4–20 | 2 papilloma, 10 PUNLMP, 8 low grade, | TURBT | 3 R in 13 years |
| 3 high grade | |||||
| [19] | 1/M | 12 | Ta grade II | TURBT | NR in 2 months |
| [20] | 1/M | 13 | Low grade | TURBT | N/A |
| [21] | 1/F | 10 | Grade I papillary TCC | TURBT | R in 2 years |
| [22] | 1/M | 18 | Grade 1 TCC | TURBT | NR in 2 years |
| [24] | 2/M | <20 | Grade 1 and 2 | TURBT | N/A |
| [25] | 1/M | 8 | Grade I superficial (pTa) | TURBT | NR in 5 years |
| [26] | 2/M | <10 | Low-grade | TURBT | N/A |
| [27] | 6/4M/2F | 10–22 | Low-grade and low-stage | TURBT | N/A |
| [28] | 1/M | 8 | Papillary TCC with lymphangiectasia | TURBT | N/A |
| [29] | 1/M | 11 | Low-grade | TURBT | N/A |
| [30] | 1/M | 13 | Low-grade | TURBT | N/A |
| [32] | 1/F | 8 | Grade 1 TCC | TURBT | N/A |
| [33] | 7/M | <20 | Low-grade | TURBT | NR |
| [34] | 1/M | 14 | Papillary noninvasive TCC | TURBT | N/A |
| [35] | 3/M | < 18 | Low-grade | TURBT | Two R |
| [37] | 1/M | <18 | Grade II to III + submucosal invasion | TURBT | NR in 30 months |
| [38] | 1/F | 10 | Papillary carcinoma | TURBT | N/A |
| [40] | 1/M | 16 | Grade I–II | TURBT | NR in 2 years |
| [41] | 2/M | 2–3 | Papillary epithelial tumours | TURBT | N/A |
| [45] | 1/M | 11 | Papillary TCC | TURBT | N/A |
| [46] | 8/5M/3F | 10–20 | Low-grade | TURBT | NR in 7 years |
| [47] | 1/M | <18 | Low-grade | TURBT | N/A |
| [50] | 1/M | 10 | Grade 1, noninvasive TCC | TURBT | NR in 2 years |
| [52] | 2/M | <18 | Low-grade | TURBT | 1 R |
| [53] | 4/2F/2M | 2–18 | Low-grade TCC | TURBT | NR at 3/6/4/1.5 years |
| [54] | 1/F | 16 | Papillary TCC, grade I/Costello syndrome | TURBT | NR in 2 years |
| Present | 2/M | 5–12 | Low-grade, 1 PUNLMP | TURBT | NR in 3 years |
Case 1
A 5-year-old boy from Hadramout, Yemen, presented in 2009 with 1 week of gross haematuria and interrupted urinary stream. There was no significant family, present or previous history. On physical examination at the time of presentation, the patient’s vital signs were stable. The abdomen was not tender and not distended. There were no associated anomalies.
There was no recurrence after a 3-year follow-up using abdominal ultrasonography (US) and cystoscopy, with assessments every 3 months in the first year then every 6 months thereafter. Fig. 1(a–c) shows the histological findings. Sections showed elongated papillary fronds lined by several layers of transitional cells with slightly enlarged crowded nuclei. There was no frank pleomorphism and no stromal invasion. The diagnosis was papillary urothelial neoplasm of low malignant potential (PUNLMP, WHO grade I).
Figure 1.
(a–c) Histological findings at both low (×100) and high (×400) power. Haematoxylin and eosin stain.
Case 2
A 12-year-old boy from Baghdad, Iraq, presented in August 2013 with two recent attacks of painless haematuria and clots. His medical history indicated a previous appendectomy and tonsillectomy. He also had treatment for rheumatic fever. US and pelvic MRI showed a 2 × 1 cm posterior wall tumour. Cystoscopy was first used 3 months earlier in another hospital and revealed a fungating tumour at the left posterolateral wall near the left ureteric orifice. He underwent transurethral resection of the bladder tumour (TURBT), and the histopathology showed a papillary TCC of low grade and no stromal invasion.
In a reassessment, US showed a soft-tissue lesion in the left bladder wall (Fig. 2). Cystoscopy (Fig. 3) showed a villous lesion above the left ureteric orifice of ≈ 2 × 2 cm in diameter. The tumour was resected (TURBT) and the bed cauterised.
Figure 2.

An ultrasonogram showing the soft-tissue lesion in the left bladder wall.
Figure 3.
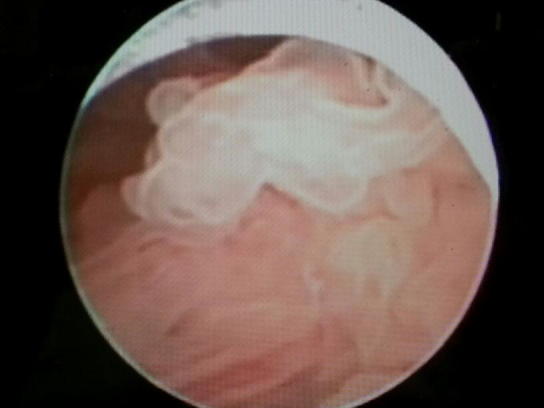
A cystoscopic view of a villous lesion above the left ureteric orifice.
The histopathology showed fragments of ulcerated bladder mucosa with intense mixed acute and chronic inflammatory infiltrate (Fig. 4a–c). There was one small fragment of papillary urothelium with enlarged crowded nuclei, consistent with low-grade papillary urothelial carcinoma (UC). There was no stromal invasion. The child is currently asymptomatic and follow-up cystoscopy is scheduled.
Figure 4.
(a–c) Low- (×100) and high-power (×400) views showing the histopathology in sections with fragments of ulcerated bladder mucosa with intense mixed acute and chronic inflammatory infiltrate. There was one small fragment of papillary urothelium with enlarged crowded nuclei, consistent with low-grade papillary urothelial carcinoma. There was no stromal invasion. Haematoxylin and eosin stain.
Discussion
True urinary bladder UC in children is very unusual [50], although there are occasional cases. In 1969, Javadpour and Mostofi [2] identified 40 primary epithelial bladder tumours in patients in the first two decades of life, from 10,000 total cases. Most cases [34] were papillary, in patients aged 6–20 years, and who presented with gross haematuria. In the first decade of life, primary bladder carcinoma was suggested to be even more uncommon [10], with 30% of cases in children aged ⩽10 years [49].
Williamson et al. [50] warned that the diagnosis of urothelial tumours in children might be delayed. Urologists might be reluctant to evaluate these patients. The biological behaviour of the tumour must also be considered; the tumour might be of low grade but a follow-up is needed, especially in cases of multiple tumours.
Molecular studies suggest that tumours in children differ in their pathways from those in adult patients. Fine et al. [19] reported a series of 23 urothelial neoplasms in patients aged ⩽20 years. They confirmed that these tumours were of low grade and had a low risk of recurrence.
Alanee and Shukla [49] identified 140 cases of bladder cancer, with PUNLMP and rhabdomyosarcoma comprising 50.7% and 36.4% of the tumours, respectively. Between 1973 and 2003 the incidence of bladder malignancies increased significantly. The conditional survival calculated for 1 and 2 years after diagnosis was 93.6% and 97.5%. Fifty-one cases of embryonal rhabdomyosarcoma were identified. Reviewing the Surveillance, Epidemiology and End Results (SEER) database, they found that bladder cancer in children aged <18 years was rare, with only 140 patients registered in the SEER database during the period assessed. The results were consistent with previous multi-institutional studies for the higher incidence of PUNLMP in children. They also confirmed a high incidence of these tumours in males (male: female ratio, 3:1).
In the present review we excluded those patients with PUNLMP in the series reported by Alanee and Shukla [49], as there is controversy about the diagnosis of PUNLMP and its accuracy in the SEER registry. The term PUNLMP was only introduced in 2004. In the same study, the survival curves should be considered carefully, as the addition of higher-grade tumours is a limiting factor. Furthermore, based on SEER analysis, there might have been errors in reporting one or more parameters of the malignancies [49].
Data on the molecular characteristics and clinical behaviour of bladder tumours in children are less well understood than those of adult/elderly patients [50,51].
As for the follow-up assessments, CT and virtual cystoscopy are much less invasive [52]. For practical purposes [53–55] patients with PUNLMP should be treated similarly to patients with low-grade, noninvasive UC. Molecular grading of these tumours can be used to further assess their biological potential [56].
Because of the low but definite risk of recurrence and grade progression, an appropriate clinical follow-up of patients with primary PUNLMP is warranted [57]. The 1998 WHO/ISUP classification of urothelial neoplasms can be applied reproducibly by pathologists, with a moderate level of agreement. PUNLMP lesions have a more sedate clinical behaviour than UCs but the hazard of recurrence and progression remains, and thus a follow-up is important [57].
In the present report we included articles that were registered after 2004, as was one of the present cases (PUNLMP), but cases of PUNLMP before 2004 were not included.
A univariate analysis [51] showed that high Ki67 expression and low cyclin D1 immunohistological expression were associated with a greater risk of recurrence in the ‘young’ group, while reduced p27Kip1 expression and p53 overexpression were not. By contrast, reduced p27Kip1 expression correlated with a greater risk of recurrence in elderly patients, suggesting that distinct molecular pathways might be involved in the development and progression of these tumours.
In the hereditary nonpolyposis colorectal cancer syndrome, there is a predisposition to urinary tract involvement [51], but there could be an incidence of urothelial tumours in Costello syndrome [22].
It appears that multiple tumours might be more likely to recur, and thus a periodic follow-up is mandatory. Cystoscopy in children is an invasive procedure that requires general anaesthesia, but it cannot be replaced by US and urine cytology. The present study has limitations, because it was retrospective and many abstracts were not available.
Funding
None.
Conflict of interest
None.
PEDIATRIC UROLOGY
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Neogi S., Kariholu P.L., Dhakre G., Gupta V., Agarwal N., Bhadani P. Malignant urothelial carcinoma of urinary bladder in a young child: a rare case report. Urology. 2013;81:888–890. doi: 10.1016/j.urology.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Javadpour N., Mostofi F.K. Primary epithelial tumors of the bladder in the first decade of life. J Urol. 1969;101:706–710. doi: 10.1016/s0022-5347(17)62407-8. [DOI] [PubMed] [Google Scholar]
- 3.Lerena J., Krauel L., García-Aparicio L., Vallasciani S., Suñol M., Rodó J. Transitional cell carcinoma of the bladder in children and adolescents: six-case series and review of the literature. J Ped Urol. 2010;6:481–485. doi: 10.1016/j.jpurol.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Bujons A., Caffaratti J., Maria Garat J., Villavicencio H. Long-term follow-up of transitional cell carcinoma of the bladder in childhood. J Ped Urol. 2013;20:1–4. doi: 10.1016/j.jpurol.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Quillin S.P., McAlister W.H. Transitional cell carcinoma of the bladder in children: radiologic appearance and differential diagnosis. Urol Radiol. 1991;13:107–109. doi: 10.1007/BF02924601. [DOI] [PubMed] [Google Scholar]
- 6.Benson R.C., Jr., Tomera KM., Kelalis PP. Transitional cell carcinoma of the bladder in children and adolescents. J Urol. 1983;130:54–55. doi: 10.1016/s0022-5347(17)50950-7. [DOI] [PubMed] [Google Scholar]
- 7.Yarmohammad A., Ahmadnia H., Asl Zare M. Transitional cell carcinoma in children. report of a case and review of the literature. Urology. 2005;2:120–121. [PubMed] [Google Scholar]
- 8.Hoenig D.M., McRae S., Chen S.C., Diamond D.A., Rabinowitz R., Caldamone A.A. Transitional cell carcinoma of the bladder in the pediatric patient. J Urol. 1996;156:203–205. [PubMed] [Google Scholar]
- 9.Huppman A.R., Pawel B.R. Polyps and masses of the pediatric urinary bladder: a 21-year pathology review. Pediatric Dev Pathol. 2011;14:438–444. doi: 10.2350/11-01-0958-OA.1. [DOI] [PubMed] [Google Scholar]
- 10.Korrect G.S., Minevich E.A., Sivan B. High-grade transitional cell carcinoma of the pediatric bladder. J Ped Urol. 2012;8:e36–e38. doi: 10.1016/j.jpurol.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Ghousheh A.I., Durkee C.T., Groth T.W. Advanced transitional cell carcinoma of the bladder in a 16-year-old girl with Hinman syndrome. Urology. 2012;80:1141–1143. doi: 10.1016/j.urology.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 12.Compérat E., Camparo P., Larré S., Roupret M., Neuzillet Y, Pignot G. Prog Urol. 2013;23:171–175. doi: 10.1016/j.purol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Campo G., Giannarini G., Pomara G., Manassero F., Pistolesi D., Selli C. Bladder papilloma in a 9-year-old girl: a case report. Minerva Pediatr. 2012;64:361–363. [PubMed] [Google Scholar]
- 14.Ruiz E., Alarcón Caba M., Toselli L., Moldes J., Ormaechea M., de Badiola F. Transitional cell carcinoma of the bladder in adolescents: a diagnosis to bear in mind] Arch Argent Pediatr. 2009;107:49–52. doi: 10.1590/S0325-00752009000100011. [DOI] [PubMed] [Google Scholar]
- 15.Patel R., Tery T., Ninan G.K. Transitional cell carcinoma of the bladder in first decade of life. Pediatr Surg Int. 2008;24:1265–1268. doi: 10.1007/s00383-008-2251-4. [DOI] [PubMed] [Google Scholar]
- 16.Dowling C.R., Reddihough D., Smith P., Webb N., McNeill R., Clouston D. Transitional cell carcinoma in the paediatric population. Be aware of unusual aetiologies. J Paediatr Child Health. 2007;43:773–775. doi: 10.1111/j.1440-1754.2007.01218.x. [DOI] [PubMed] [Google Scholar]
- 17.Gülpinar O., Soygür T., Baltaci S., Akand M., Kankaya D. Transitional cell carcinoma of bladder with lamina propria invasion in a 10-year-old boy. Urology. 2006;68:2041–2043. doi: 10.1016/j.urology.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 18.Alam S., Goebel J., Pacheco M.C., Sheldon C. Papillary urothelial neoplasm of low malignant potential in a pediatric renal transplant recipient (PUNLMP): a case report. Pediatr Transplant. 2007;11:680–682. doi: 10.1111/j.1399-3046.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- 19.Fine S.W., Humphrey P.A., Dehner L.P., Amin M.B., Epstein J.I. Urothelial neoplasms in patients 20 years or younger: a clinicopathological analysis using the World Health Organization 2004 bladder consensus classification. J Urol. 2005;174:1976–1980. doi: 10.1097/01.ju.0000176801.16827.82. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez A., Burday D., Sexton W., Ahmad N., Pow-Sang J.M. Urothelial carcinoma in a child. Arch Esp Urol. 2005;58:473–475. doi: 10.4321/s0004-06142005000500017. [DOI] [PubMed] [Google Scholar]
- 21.Kilic N., Turkel T., Balkan E., Sevinir B. Transitional cell carcinoma of the bladder presenting after blunt abdominal trauma: a very rare occurrence in childhood. Int J Urol. 2005;12:316–318. doi: 10.1111/j.1442-2042.2005.01035.x. [DOI] [PubMed] [Google Scholar]
- 22.Urakami S., Igawa M., Shiina H., Shigeno K., Kikuno N., Yoshino T. Recurrent transitional cell carcinoma in a child with the Costello syndrome. J Urol. 2002;168:1133–1134. doi: 10.1016/S0022-5347(05)64609-5. [DOI] [PubMed] [Google Scholar]
- 23.Agarwala S., Hemal A.K., Seth A., Gupta A.K., Bhatnagar V., Mitra D.K. Transitional cell carcinoma of the urinary bladder following exposure to cyclophosphamide in childhood. Eur J Pediatr Surg. 2001;11:207–210. doi: 10.1055/s-2001-15486. [DOI] [PubMed] [Google Scholar]
- 24.Mateos Blanco J., Santamaría Ossorio JI., Pimentel Leo JJ., Sanjuán Rodríguez S. Transitional-cell bladder tumor in childhood. Cir Pediatr. 1999;12:168–170. [PubMed] [Google Scholar]
- 25.Kawaguchi T., Hashimoto Y., Kobayashi H., Kudoh S., Takahashi S., Yanagisawa T. A clinical study of bladder cancer in adolescent patients. Nippon Hinyokika Gakkai Zasshi. 1999;90:614–618. doi: 10.5980/jpnjurol1989.90.614. [DOI] [PubMed] [Google Scholar]
- 26.Serrano-Durbá A., Domínguez-Hinarejos C., Reig-Ruiz C., Fernández-Córdoba M., García-Ibarra F. Transitional cell carcinoma of the bladder in children. Scand J Urol Nephrol. 1999;33:73–76. [PubMed] [Google Scholar]
- 27.Muñoz-Delgado Salmerón J., Fernández Arjona M., Shihadeh S., De la Fuente Trabado M., García Estevez J.A. Bladder urothelial carcinoma in patients under 10 years of age: report of 2 new cases. Actas Urol Esp. 1997;21:57–59. [PubMed] [Google Scholar]
- 28.Yusim I., Lismer L., Greenberg G., Haomud K., Kaneti J. Carcinoma of the bladder in patients under 25 years of age. Scand J Urol Nephrol. 1996;30:461–463. doi: 10.3109/00365599609182324. [DOI] [PubMed] [Google Scholar]
- 29.Curtis M., Schned A., Hakim S., Cendron M. Papillary transitional cell carcinoma of the bladder with lymphangiectasia in an 8-year-old boy. J Urol. 1996;156:202. [PubMed] [Google Scholar]
- 30.Sánchez Diaz A., de Nova Sánchez E., Fernandez Puentes J.C., García López F., Llorens Martínez F., Orduña Domingo A. Transitional cell tumor of the bladder in children: report of a case. Actas Urol Esp. 1995;19:642–645. [PubMed] [Google Scholar]
- 31.Laurenti C., De Dominicis C., Mattioli D., Rocchegiani A., Franco G., Dal Forno S., Iori F. Transitional cell neoplasm of the bladder in childhood: presentation of a clinical case. Arch Esp Urol. 1993;46:51–54. [PubMed] [Google Scholar]
- 32.Pastor Guzmán J.M., Salinas Sánchez AS., Hernández Millán I., Martínez Martín M., Cañamares Pabolaza L., Virseda Rodríguez JA. Transitional cell tumor of the bladder in childhood. Actas Urol Esp. 1992;16:524–526. [PubMed] [Google Scholar]
- 33.Wilson-Storey D., Allen A.E., Variend S. Transitional cell papillary bladder neoplasm in a girl: an unusual presentation. J Pediatr Surg. 1992;27:113–114. doi: 10.1016/0022-3468(92)90123-o. [DOI] [PubMed] [Google Scholar]
- 34.Madgar I., Goldwasser B., Nativ O., Hanani Y., Jonas P. Long-term followup of patients less than 30 years old with transitional cell carcinoma of bladder. J Urol. 1988;139:933–934. doi: 10.1016/s0022-5347(17)42721-2. [DOI] [PubMed] [Google Scholar]
- 35.Rayner R.J., Watson A.R., Bishop M.C. Haematuria in an adolescent due to bladder carcinoma. Eur J Pediatr. 1988;147:328–329. doi: 10.1007/BF00442709. [DOI] [PubMed] [Google Scholar]
- 36.Paduano L., Chiella E. Primary epithelial tumors of the bladder in children. J Urol. 1988;139:794–795. doi: 10.1016/s0022-5347(17)42640-1. [DOI] [PubMed] [Google Scholar]
- 37.Madgar I., Nativ O., Hanani Y., Jonas P. Transitional cell carcinoma of the bladder in children under ten years of age. A case report. Eur Urol. 1988;14:216–217. doi: 10.1159/000472941. [DOI] [PubMed] [Google Scholar]
- 38.Oesterling J.E., Epstein J.I., Gearhart J.P. Transitional cell carcinoma of the bladder in an adolescent with Turner’s syndrome. J Urol. 1987;137:398–400. doi: 10.1016/s0022-5347(17)44047-x. [DOI] [PubMed] [Google Scholar]
- 39.Lalmand B., Avni E.F., Simon J., Verhest A., Schulman C.C., Struyven J. Transitional cell papillary carcinoma of the bladder in a child. Pediatr Radiol. 1987;17:77–79. doi: 10.1007/BF02386604. [DOI] [PubMed] [Google Scholar]
- 40.van der Vaeren D., Hennebert P., van Cangh P.J. Epithelial tumors of the bladder in children. Report of 2 cases. Acta Urol Belg. 1980;48:459–463. [PubMed] [Google Scholar]
- 41.Mauermayer W., Tauber R., Steuer G. Epithelial tumors of the urinary bladder in the first twenty years of life (author’s transl) Urologe A. 1977;16:286–289. [PubMed] [Google Scholar]
- 42.Handy P.C., Pai M.G., Budihal M.R., Kaulgud S.R. Carcinoma of the bladder in young children: report of 2 cases. J Urol. 1975;113:264–265. doi: 10.1016/s0022-5347(17)59458-6. [DOI] [PubMed] [Google Scholar]
- 43.Castellanos R.D., Wakefield P.B., Evans A.T. Carcinoma of the bladder in children. J Urol. 1975;113:261–263. doi: 10.1016/s0022-5347(17)59457-4. [DOI] [PubMed] [Google Scholar]
- 44.Makler S., Saenz C.A. Papillary carcinoma in a child. Rev Argent Urol Nefrol. 1970;39:228–230. [PubMed] [Google Scholar]
- 45.Rossi M.B., Wogalter H., Spatz M. Papillary transitional cell tumor of bladder in a 5-year-old boy. J Urol. 1967;97:88–89. doi: 10.1016/S0022-5347(17)62985-9. [DOI] [PubMed] [Google Scholar]
- 46.Johnson A.J., Taylor J.N. Papillary tumor of bladder in a twelve-year-old boy. J Urol. 1962;87:869–870. doi: 10.1016/S0022-5347(17)65059-6. [DOI] [PubMed] [Google Scholar]
- 47.Bujons A., Caffaratti J., Garat J.M., Villavicencio H. Long-term follow-up of transitional cell carcinoma of the bladder in childhood. J Pediatr Urol. 2014;10:167–170. doi: 10.1016/j.jpurol.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 48.DeLair S.M., White R.W., Kurzrock E.A. Secondary transitional cell carcinoma and nitrogen mustard treatment. Urology. 2005;65:1226–1227. doi: 10.1016/j.urology.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Alanee S., Shukla A.R. Bladder malignancies in children aged <18 years. Results from the surveillance, epidemiology end results database. BJU Int. 2009;106:557–560. doi: 10.1111/j.1464-410X.2009.09093.x. [DOI] [PubMed] [Google Scholar]
- 50.Williamson S.R., Lopez-Beltran A., MacLennan G.T., Montironi R., Cheng L. Unique clinicopathologic and molecular characteristics of urinary bladder tumors in children and young adults. Urol Oncol Semin Orig Invest. 2013;31:414–426. doi: 10.1016/j.urolonc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Keetch D.W., Manley C.B., Catalona W.J. Transitional cell carcinoma of bladder in children and adolescents. Urology. 1993;42:447–449. doi: 10.1016/0090-4295(93)90383-l. [DOI] [PubMed] [Google Scholar]
- 52.Gabr A.H., Elbadry M., Elsherief A., Tawfiek R. Computed tomography-virtual cystoscopy in the evaluation of a bladder mass: could it replace standard conventional cystoscopy? Arab J Urol. 2013;11:369–374. doi: 10.1016/j.aju.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanase M., Tsukamoto T., Kumamoto Y., Takagi Y., Mikuma N., Iwasawa A. Transitional cell carcinoma of the bladder or renal pelvis in children. Eur Urol. 1991;19:312–314. doi: 10.1159/000473649. [DOI] [PubMed] [Google Scholar]
- 54.Khasidy L.R., Khashu B., Mallett E.C., Kaplan G.W., Brock W.A. Transitional cell carcinoma of bladder in children. Urology. 1990;35:142–144. doi: 10.1016/0090-4295(90)80063-s. [DOI] [PubMed] [Google Scholar]
- 55.Gripp K.W., Scott C.I., Jr., Nicholson L., Figueroa T.E. Second case of bladder carcinoma in a patient with Costello syndrome. Am J Med Genet. 2000;90:256–259. doi: 10.1002/(sici)1096-8628(20000131)90:3<256::aid-ajmg16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 56.Jones T.D., Cheng L. Papillary urothelial neoplasm of low malignant potential: evolving terminology and concepts. J Urol. 2006;175:1995–2003. doi: 10.1016/S0022-5347(06)00267-9. [DOI] [PubMed] [Google Scholar]
- 57.Lee T.K., Chaux A. Papillary urothelial neoplasm of low malignant potential of the urinary bladder: clinicopathologic and outcome analysis from a single academic center. Human Pathol. 2011;42:1799–1803. doi: 10.1016/j.humpath.2011.03.006. [DOI] [PubMed] [Google Scholar]




