Abstract
Both the hypoxia-inducible factor-1 (HIF-1) and tumor suppressor p53 are involved in the cellular response to hypoxia. How the two transcription factors interact to determine cell fates is less well understood. Here, we developed a network model to characterize crosstalk between the HIF-1 and p53 pathways, taking into account that HIF-1α and p53 are targeted for proteasomal degradation by Mdm2 and compete for binding to limiting co-activator p300. We reported the network dynamics under various hypoxic conditions and revealed how the stabilization and transcriptional activities of p53 and HIF-1α are modulated to determine the cell fate. We showed that both the transrepression and transactivation activities of p53 promote apoptosis induction. This work provides new insight into the mechanism for the cellular response to hypoxia.
Hypoxia, a decrease in oxygen availability, affects both physiological development and tumorigenesis1. A key mediator of the cellular response to hypoxia is the hypoxia-inducible factor-1 (HIF-1), which is a heterodimer of α and β subunits. HIF-1β is constitutively expressed, while HIF-1α is regulated in an oxygen-dependent manner2. HIF-1α is inactive and remains at low levels in normoxia. With enough oxygen available, the hydroxylation of HIF-1α by PHD (prolyl hydroxylase domain protein) promotes its degradation by pVHL (von Hippel-Lindau protein)3, and the hydroxylation of HIF-1α by FIH-1 (factor inhibiting HIF-1) represses its transcriptional activity via preventing the recruitment of co-activator p3004. In hypoxia, both PHD and FIH-1 are deactivated, and thus HIF-1α is stabilized and activated. As a transcription factor, HIF-1 induces expression of target genes such as VEGF (vascular endothelial growth factor), EPO (erythropoiesis) and p215,6. VEGF and EPO regulate adaptive responses to hypoxia, while p21 induces cell-cycle arrest.
The tumor suppressor p53 also mediates the hypoxic response. p53 is kept at basal levels in unstressed cells because of Mdm2-mediated ubiquitination and proteasomal degradation. The p53 response to hypoxia is diverse, depending on the cell type, the degree and duration of hypoxia7. For example, p53 is stabilized via phosphorylation by the ATR (ataxia-telangiectasia mutated and Rad3-related) kinase only under severe hypoxia8. Moreover, high levels of p53 can induce apoptosis in a different manner from that when it is activated by DNA damage9,10. Notably, different mechanisms have been proposed for p53-mediated apoptosis in hypoxia: p53 promotes apoptosis mainly by transrepressing antiapoptotic genes like microRNA(miR)-17-9211,12, or by transactivating proapoptotic genes such as puma (p53-upregulated mediator of apoptosis), fas and bnip3l13,14,15. An issue naturally arises concerning whether these mechanisms are mutually exclusive or can be coordinated in one setting.
There exists an intricate interplay between p53 and HIF-1α. It was proposed that p53 stabilization is dependent on HIF-1α16; HIF-1α may bind to Mdm2, inhibiting Mdm2-dependent degradation of p5317. But HIF-1α accumulates with similar kinetics under mild and severe hypoxia, whereas p53 stabilization occurs only in severe hypoxia8. That is, HIF-1α upregulation is insufficient for p53 stabilization. On the other hand, it was reported that moderate p53 expression results in attenuated transcriptional activity of HIF-1 since p53 and HIF-1α compete for limiting p300, whereas high p53 expression leads to degradation of HIF-1α18. It is necessary to further clarify how the stabilization and transcriptional activities of p53 and HIF-1α are regulated under various hypoxic conditions.
Several mathematic models were developed to characterize the dynamics of the HIF-1 pathway, focusing on the mechanism for HIF-1α activation19,20,21. Few modeling studies explored the role for p53 in hypoxia. It is a challenge to reveal how the interplay between HIF-1α and p53 determines the cellular outcome. Here, we built a network model to characterize crosstalk between HIF-1 and p53 signaling, comprising their upstream modulators and downstream effectors. The analysis of network dynamics reveals that the cell either adapts to hypoxia or commits apoptosis, depending on the intensity of hypoxia. In mild hypoxia, HIF-1α accumulates to evoke transient cell-cycle arrest by inducing p21, whereas p53 remains at low levels. Under severe hypoxia, the accumulation of p53 attenuates HIF-1α activity and suffices to repress the expression of miR-17-92; consequently, BIM is induced to activate Caspase-3 and apoptosis ensues. In anoxia, p53 rises to high levels, transrepressing miR-17-92 and transactivating PUMA synergistically, whereas HIF-1α is degraded and inactivated. Thus, apoptosis can be triggered promptly. This work sheds new light on the mechanisms for the p53-HIF-1α interplay and hypoxia-induced apoptosis.
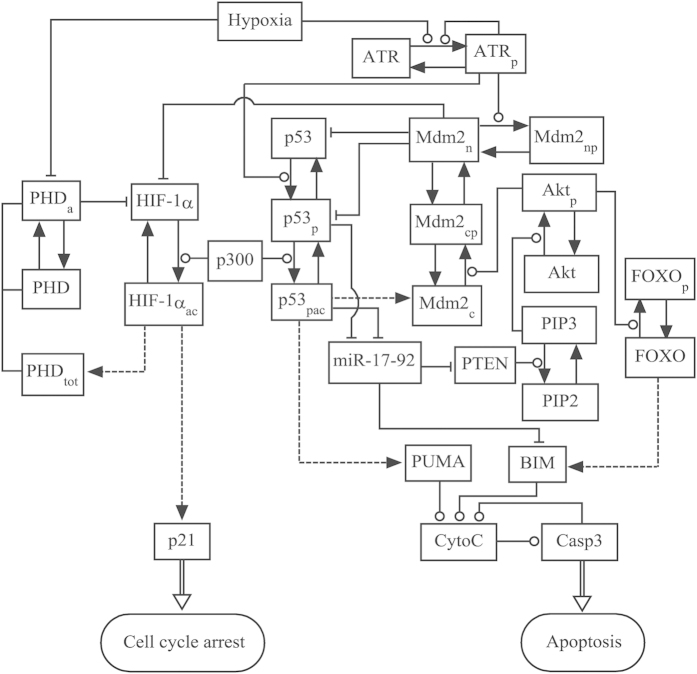
Modeling crosstalk between the HIF-1 and p53 pathways. We constructed an integrated model to explore the cell-fate decision upon hypoxia, characterizing the activation of HIF-1α and p53, their selective expression of target genes, and apoptosis induction (Fig. 1). We focused on the interplay between HIF-1α and p53 under various hypoxic conditions. The key points of the model are presented as follows.
Figure 1. Schematic depiction of the model.
The model characterizes crosstalk between the HIF-1 and p53 pathways upon hypoxia. In hypoxia, HIF-1α is stabilized due to reduced hydroxylation by PHD. Under severe hypoxia, the ATR kinase is activated via auto-phosphorylation upon hypoxia-induced replication arrest, and p53 is further activated by ATR. The shared coactivator p300 is required for the full transcriptional activity of p53 and HIF-1α. HIF-1α evokes transient cell-cycle arrest via inducing p21, whereas p53 can induce apoptosis via transrepressing or/and transactivating target genes. Dashed lines denote the expression of target genes by HIF-1α or p53, while solid arrowed lines represent the transitions between proteins. Circle- and bar-headed lines denote the promotion and inhibition of transition or production, respectively.
Regulation of HIF-1α activity. In normoxia, HIF-1α is kept at low levels due to oxygen-dependent hydroxylation that promotes its degradation by pVHL3. HIF-1α can also be degraded by Mdm2 in a p53-dependent manner22. Under hypoxic conditions, the hydroxylation of HIF-1α drops remarkably, and HIF-1α accumulates. HIF-1α dimerises with HIF-1β in the nucleus. p300/CBP promotes the acetylation of HIF-1α, enhancing its transcriptional activity. In our model, HIF-1α is divided into unacetylated (HIF-1α, inactive) and acetylated (HIF-1αac, active) forms, and their conversion is controlled by p300. The amount of p300 is limited18 and is set to a constant in the standard parameter setting. Because they can bind to p300 at different sites23,24, HIF-1α and p53 compete for limiting p300, the modeling of which is described later. Given the inhibitory effect of acetylation on protein degradation, only unacetylated HIF-1α is degraded through the PHD- or Mdm2-dependent mechanism22. The degradation and (de)acetylation processes are taken as enzyme-catalyzed reactions and assumed to follow the Michaelis-Menten kinetics (see Eqs. 1–2 in Supporting Material).
Activated HIF-1 can induce production of VEGF, EPO, PHD and p21. We explicitly characterize the induction of p21 and PHD with the Hill function (Eqs. 3 and 4), while implicating the roles for VEGF and EPO in the cellular adaptation to hypoxia. The HIF-1-PHD negative feedback promotes the adaptation of cells to mild hypoxia25,26. PHD is divided into inactive (PHD) and active (PHDa) forms, and their conversion depends on oxygen concentration (Eq. 6).
Activation of ATR. In severe hypoxia, the ribonucleotide reductase activity declines, leading to production of single-stranded DNA (ss-DNA) at stalled replication forks. ss-DNA is then coated with replication protein A (RPA), and the ATR-ATRIP (ATR interacting protein) complex is recruited to ss-DNA, which promotes ATR phosphorylation at T198927. ATR phosphorylation enhances its interaction with TopBP1, which further activates the kinase activity of ATR. Thus, the activation of ATR is positively regulated.
ATR is divided into ATR (inactive) and ATRp (active), and their dynamics are described by Eqs. 7–8. The total level of ATR is assumed to be constant since ATR is mainly regulated posttranslationally27. The phosphorylation and dephosphorylation of ATR are characterized by the Michaelis-Menten kinetics28. The parameters are set to ensure that most of ATR is inactive in normoxia or mild hypoxia and ATR is quickly activated under severe hypoxia29.
Regulation of p53 activity. p53 is degraded by Mdm2, which is induced by p53. ATRp inhibits Mdm2 activity by phosphorylating it at Ser40730 and disrupts the p53-Mdm2 interaction by phosphorylating p53 at Ser15, thereby leading to p53 accumulation29. Based on its posttranslational modifications, nuclear p53 is divided into three forms: p53 (unphosphorylated), p53p (phosphorylated), and p53pac (phosphorylated and acetylated). Four forms of Mdm2 are defined here: Mdm2c (cytoplasmic unphosphorylated), Mdm2cp (cytoplasmic phosphorylated), Mdm2n (nuclear unphosphorylated), and Mdm2np (nuclear phosphorylated). For simplicity, we assume that only Mdm2cp can enter the nucleus31. Mdm2np cannot act as an E3 ubiquitin ligase30.
The coactivator p300 is required for the full transcriptional activity of p53 and HIF-1. Their competition for binding to p300 is characterized by the Michaelis-Menten kinetics with competitive inhibition (see Eqs. 1–2 and 10–11). The acetylation rate of p53p (or HIF-1α) is an increasing function of its own concentration, and is a decreasing function of the level of HIF-1α (or p53p). On the other hand, p53, p53p and HIF-1α are targeted for degradation by Mdm2. The involved competition is also characterized by the Michaelis-Menten kinetics (Eqs. 1, 9 and 10). Notably, Mdm2-mediated degradation of p53pac is neglected since its dual modifications block their interaction. p53-induced expression of target genes, including mdm2 and puma, is all characterized by the Hill function, and the Hill coefficient is set to 4 given the p53 tetramer acts as a transcription factor.
Mdm2c can be phosphorylated by Akt, promoting its nuclear entry32. Akt is activated via phosphorylation by PIP3 (phosphatidylinositol 3,4,5-trisphosphate), which is dephosphorylated by PTEN (phosphatase and tensin homolog) into PIP2 (phosphatidylinositol-4, 5-bisphosphate). The phosphorylation and dephosphorylation of Mdm2, Akt and PIP2/3 are all characterized by the Michaelis-Menten kinetics (Eqs. 12–15, 17 and 19). The total amount of Akt and that of PIP2 and PIP3 are separately assumed to be constant, similar to ref. 33.
Induction of apoptosis by p53. Different mechanisms have been proposed for p53-induced apoptosis in response to hypoxia. On one hand, repression of miR-17-92 by p53 is important for apoptosis induction since this relives some proapoptotic genes such as pten and bim from the inhibition by miR-17-9212,34. FOXO (forkhead box protein class O) is a typical transcription factor of bim, and its transcriptional activity is inhibited indirectly by Akt via phosphorylation35. On the other hand, p53 also induces apoptosis by directly transactivating some proapoptotic genes such as puma and fas13,14.
Here, we assume that p53 indirectly upregulates BIM and PTEN by transrepressing miR-17-92 in severe hypoxia or anoxia and directly induces PUMA in anoxia. There are two forms of FOXO: FOXO (active) and FOXOp (phosphorylated, inactive), and Akt promotes the conversion from FOXO to FOXOp. High expression of either BIM or PUMA promotes the release of cytochrome c (CytoC) from mitochondria, resulting in the activation of Caspase-3 (Casp3)36. A positive feedback exists between CytoC release and Casp3 activation37. The dynamics of CytoC and Casp3 are characterized in a similar manner to that in ref. 9 (Eqs. 26 and 27). The sustained activation of Casp3 is taken as the marker of apoptosis here.
Results
Overview of the dynamics of the HIF-1α and p53 pathways
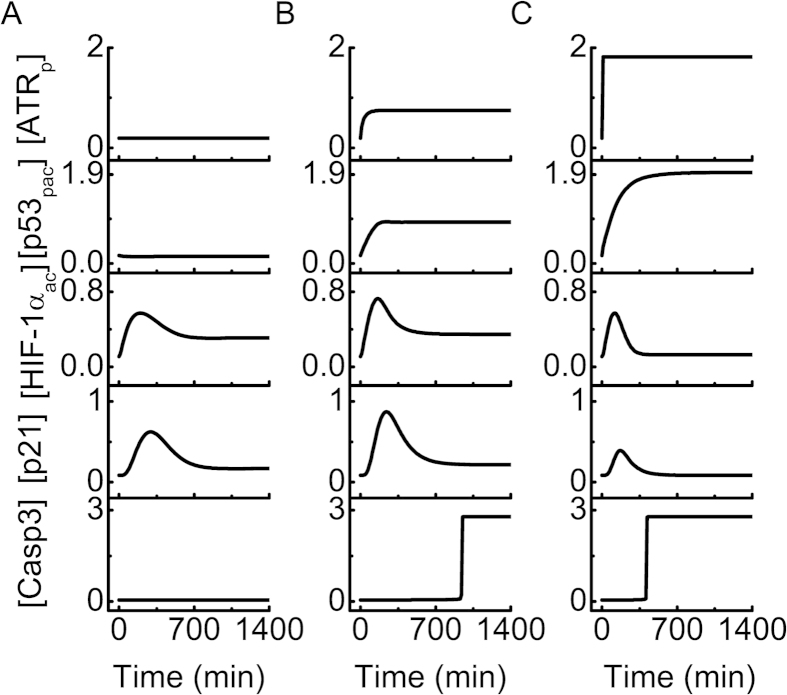
To illustrate the typical dynamics of the HIF-1α and p53 pathways, we present the temporal evolution of protein concentrations under various hypoxic conditions in Fig. 2 ([…] denotes the concentration of each component throughout the paper). In mild hypoxia (2% O2), [p53pac] is at a basal level because ATR is inactivated, and thus Casp3 remains inactive (Fig. 2A). [HIF-1αac] gradually rises and drops to an intermediate level, since PHD is induced by HIF-1 to promote the degradation of HIF-1α (see Supplemental Fig. S1). p21 is induced by HIF-1 rather than p53 to trigger cell-cycle arrest. Consequently, the cell survives and adapts to mild hypoxia.
Figure 2. Overview of the network dynamics under different hypoxic conditions.
Temporal evolution of the levels of ATRp, p53pac, HIF-1αac, p21 and Casp3 in mild hypoxia (2% O2, (A)), severe hypoxia (0.02% O2, (B)), or anoxia (0% O2, (C)).
Under severe hypoxia (0.02% O2), ATR is partly activated, and [p53pac] rises to an intermediate level (Fig. 2B). As a result, [HIF-1αac] first rises and then drops to a moderate level since p53p and HIF-1α compete for binding to limiting p300. p53pac represses the expression of miR-17-92 to relieve the inhibition of BIM expression, and [BIM] rises gradually to a relatively high level (see Supplemental Fig. S2). Since [p53pac] is not high enough, PUMA only accumulates to a low level. Consequently, Casp3 is activated mainly by BIM around 16 h and apoptosis ensues. These results are consistent with the experimental report that p53 can induce apoptosis via transrepressing antiapoptotic genes12,34.
Under anoxia (0.0% O2), ATR is fully activated and [p53pac] rises quickly to a high level (Fig. 2C). Because HIF-1α is degraded by Mdm2 and p300 is mostly associated with p53, [HIF-1αac] returns to its basal level after a transient. Both [BIM] and [PUMA] rise to high levels, and Casp3 is activated around 6 h, which remarkably advances the induction of apoptosis.
The above results indicate that the cellular outcome depends on the extent of hypoxia (note that the hypoxic state is maintained persistently here). In mild hypoxia, [HIF-1αac] settles at an intermediate level, and the cell adapts to the stress after a transient cell-cycle arrest. Under severe hypoxia, the accumulation of p53pac suffices to elevate BIM via downregulating miR-17-92, and apoptosis is induced. In anoxia, the stabilization and transcriptional activity of p53 are markedly enhanced, in sharp contrast to those of HIF-1α. Both transcriptional repression and activation of target genes by p53 are triggered to expedite apoptosis induction, which awaits experimental validation. Notably, the competition for binding to limiting p300 is a key factor modulating the dynamics and activities of p53 and HIF-1α.
Dynamics of HIF-1α and p21
To investigate the dynamics of HIF-1α and p21 systematically, we plot the heat maps of [HIF-1αac] and [p21] as a function of oxygen concentration and time (Fig. 3). In normoxia (above 3% O2), [HIF-1αac] remains at basal levels since most of HIF-1α is degraded (Fig. 3A). In mild hypoxia (between 1.1% and 3% O2), [HIF-1αac] gradually rises and drops due to PHD-dependent degradation. In moderate hypoxia (between 0.03% and 1.1% O2), [HIF-1αac] rises to different plateau levels, which can be as high as 1.6. p21 dynamics closely follow the dynamics of HIF-1αac (Fig. 3B). These results suggest that the cell undergoes a transient cell-cycle arrest in mild hypoxia, or becomes senescent under moderate hypoxia because of sustained p21 induction. It would be interesting to test the occurrence of senescence.
Figure 3. Dynamics of HIF-1αac and p21.
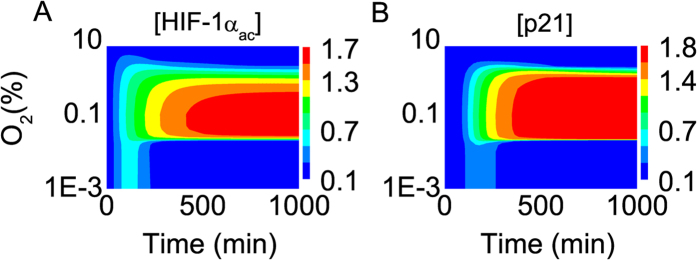
Color-coded concentrations of (A) HIF-1αac and (B) p21 as a function of the logarithm of oxygen concentration and time.
In severe hypoxia (below 0.03% O2), HIF-1α first accumulates because of PHD deactivation; then, p53 competes with HIF-1α for binding to p300, leading to a reduction in HIF-1αac levels and inhibition of HIF-1α activity. In anoxia, p53 competitively binds to p300 and Mdm2 targets HIF-1α for degradation; thus, the HIF-1αac level drops markedly, and rather low levels of p21 are induced. These results are consistent with the experimental observation that Mdm2 promotes HIF-1α degradation in a p53-dependent way22. Together, HIF-1α is partly inactivated under severe hypoxia and is degraded in anoxia.
p53 dynamics and apoptosis induction under severe hypoxia
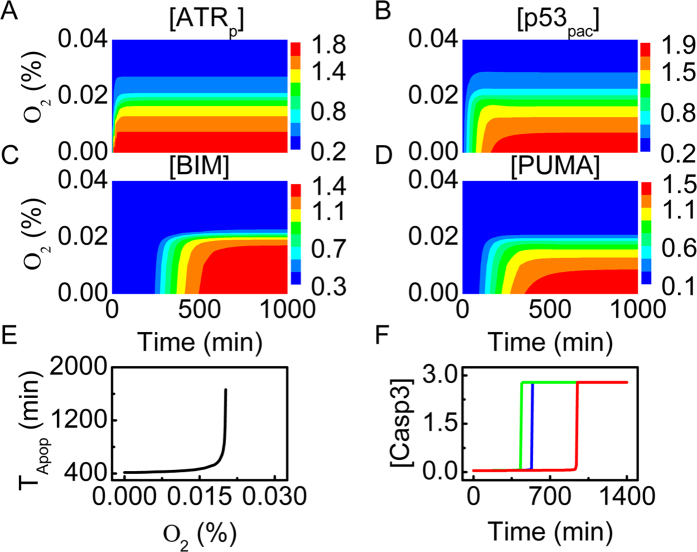
Under severe hypoxia, ATR is activated, and thus p53 is stabilized by phosphorylation. For oxygen concentration below 0.025%, [ATRp] quickly rises to plateau levels (Fig. 4A). ATR is partly activated when oxygen concentration is between 0.01% and 0.025%, or is fully activated when oxygen concentration is below 0.01%. Compared with ATRp, p53pac accumulates more slowly. The steady-state level of p53pac rises with decreasing oxygen concentration (Fig. 4B). Of note, we can compare the simulation results with the experimental observations in ref. 8; for comparison, the measured p53 expression level is divided by its maximum (see Supplemental Fig. S3). p53pac accumulates markedly only in severe hypoxia or anoxia, consistent with the experimental result, and the simulation results reproduce the key features of p53 dynamics in response to hypoxia.
Figure 4. Protein dynamics and apoptosis induction under severe hypoxia.
(A–D) Color-coded concentrations of ATRp (A), p53pac (B), BIM (C) and PUMA (D) as a function of oxygen concentration and time. (E) The timing of Casp3 activation versus oxygen concentration. (F) Time courses of [Casp3] with oxygen concentration at 0.01% (green), 0.018% (blue), or 0.02% (red).
Activated p53 represses miR-17-92 expression to upregulate BIM; [BIM] rises to distinct levels depending on oxygen concentration (Fig. 4C). In contrast, only when oxygen concentration is below 0.01% can [PUMA] rise to relatively high levels (Fig. 4D), consistent with the experimental observation13. Thus, the transrepression activity of p53 is activated in severe hypoxia, while its transactivation activity is further evoked under anoxia.
Either BIM or PUMA activates Casp3 to trigger apoptosis in hypoxia. The timing of Casp3 activation, TApop, versus oxygen concentration is shown in Fig. 4E. Casp3 can be activated around 400 min if oxygen concentration is below 0.015%; otherwise, TApop rises quickly with increasing oxygen concentration. Apoptosis cannot be induced when oxygen concentration is above 0.022%. As seen in Fig. 4F, the timing of Casp3 activation exhibits a significant delay with increasing oxygen concentration. Together, the cell becomes more sensitive to apoptosis with aggravating hypoxia.
Interplay between HIF-1α and p53 under anoxia
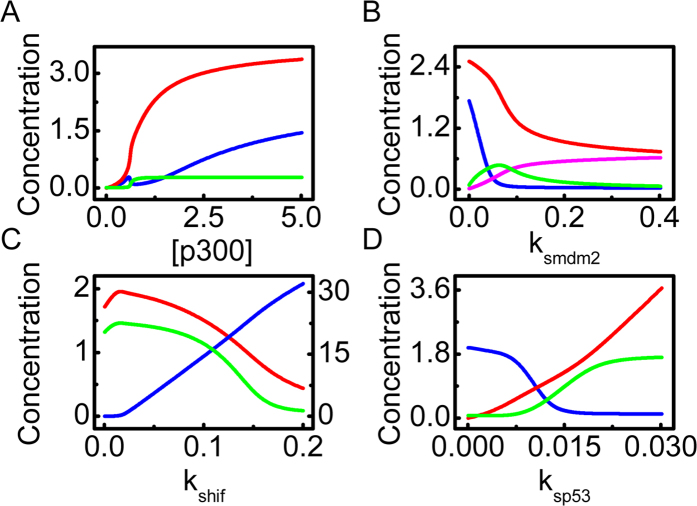
As mentioned above, p53 and HIF-1α compete for limiting p300 to activate their transcriptional activity and are targeted for degradation by Mdm2. Here, we explore their interplay under anoxia. We first investigate how the p300 level influences the steady-state concentrations of proteins (Fig. 5A). With increasing [p300], [p53pac] continuously rises toward saturation. [Mdm2n] remains at a low level over a wide range since Mdm2np predominates in the nucleus. Intriguingly, [HIF-1αac] first rises, then drops, and rises again with increasing [p300]. Since ATR is fully activated and [p53p] is large enough under anoxia, increasing [p300] leads to enhancement of p53 acetylation. When [p300] is very low, the level of unacetylated HIF-1α is sufficiently high, and [HIF-1αac] rises with increasing [p300]. When [p300] further rises, [p53pac] is elevated and HIF-1α is markedly degraded by p53-induced Mdm2, leading to a drop in [HIF-1αac]. If p300 is in excess, more HIF-1α is acetylated, resulting in a continuous rise in [HIF-1αac] with increasing [p300]. The intricate effect of p300 level on the acetylation of HIF-1α is worth testing experimentally.
Figure 5. Interplay between HIF-1α and p53 in anoxia.
(A) Bifurcation diagrams of [p53pac] (red), [HIF-1αac] (blue) and [Mdm2n] (green) versus the amount of p300. (B) Bifurcation diagrams of [p53pac] (red), [HIF-1αac] (blue), [Mdm2c] (green) and [Mdm2n] (pink) versus the p53-induced production rate of Mdm2, ksmdm2. (C) Bifurcation diagrams of [HIF-1αac] (blue, on the right axis), [p53pac] (red) and [PUMA] (green) versus the production rate of HIF-1α, kshif. (D) Bifurcation diagrams of [HIF-1αac] (blue), [p53pac] (red) and [PUMA] (green) versus the production rate of p53, ksp53.
We further probe the role for Mdm2-mediated degradation of p53 and HIF-1α. With increasing the p53-induced production rate of Mdm2, ksmdm2, [HIF-1αac] first drops fast and then remains at basal levels because of enhanced degradation of HIF-1α by Mdm2 (Fig. 5B). In contrast, [p53pac] falls at a slower rate and always remains above an intermediate level. At large ksmdm2, Mdm2n predominates over Mdm2c since [p53pac] is not sufficiently high to transrepress miR-17-92 markedly, which promotes the nuclear entry of Mdm2. Collectively, Mdm2 overexpression inhibits the transcriptional activities of both HIF-1 and p53.
The expression level of HIF-1α has a marked effect on the activities of HIF-1α and p53. The amount of HIF-1α depends on its production rate kshif. For very low kshif, [p53pac] remains at high levels, whereas there is little amount of HIF-1αac (Fig. 5C). When kshif is further increased, more HIF-1α is produced and [HIF-1αac] gradually rises, leading to a drop in [p53pac] because of their competition for limiting p300. Since PUMA expression is p53-dependent, [PUMA] shows the similar dynamics to [p53pac]. Thus, p53 cannot be activated to induce apoptosis even under anoxia when HIF-1α is overexpressed.
Similarly, the p53 expression level affects the activities of HIF-1α and p53. With increasing the rate constant of p53 production, ksp53, [p53pac] rises continuously, whereas [HIF-1αac] drops to basal levels (Fig. 5D). As a result, [PUMA] gradually rises toward saturation at large ksp53. Of note, [HIF-1αac] remains at high levels with ksp53=0, and apoptosis cannot be induced even in anoxia. This result is consistent with the fact that cells with p53 mutation are resistant to proapoptotic stimuli38. Together, the competition between p53 and HIF-1α for p300 and Mdm2 plays a key role in the modulation of their stabilization and transcriptional activity.
Effect of miR-17-92 repression on apoptosis induction
In addition to repression of FOXO-induced BIM expression, miR-17-92 also indirectly suppresses FOXO and p53 activities by inhibiting PTEN expression30. Here, we clarify the significance of miR-17-92 inhibition by p53 in hypoxia-induced apoptosis. Note that the parameter jsmir characterizes the repression strength by p53: the larger jsmir, the weaker the repression becomes (see Eq. 21).
As shown in the bifurcation diagrams, [PTEN] and [BIM] remain at relatively high levels for jsmir ≤ 1, whereas [miR-17-92] is at low levels (Fig. 6A). In contrast, [PTEN] and [BIM] stay at low levels once jsmir > 1, whereas [miR-17-92] gradually rises with increasing jsmir. This suggests that the effective transrepression of miR-17-92 by p53 is indispensable for apoptosis induction under severe hypoxia, which awaits experimental validation.
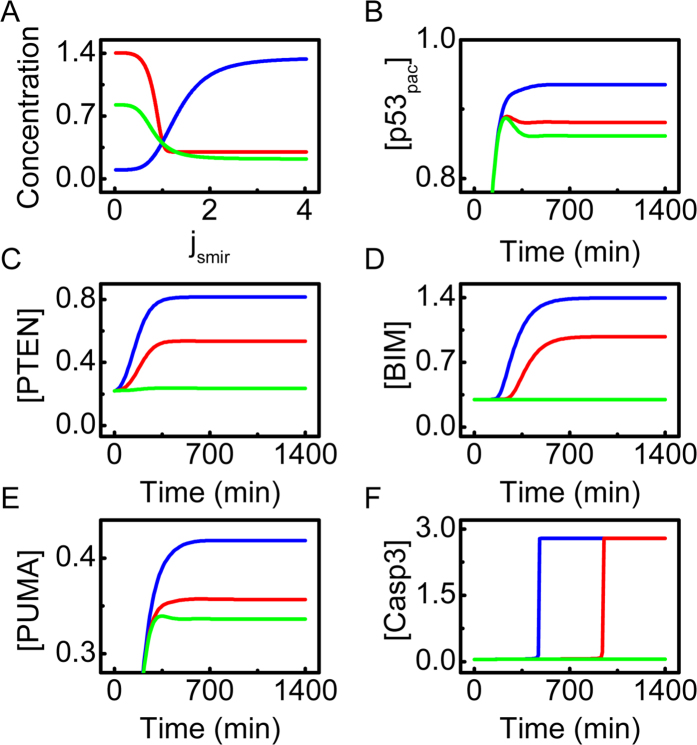
Figure 6. Effect of miR-17-92 on apoptosis induction under severe hypoxia (0.02% O2).
(A) Bifurcation diagrams of [PTEN] (green), [BIM] (red) and [miR-17-92] (blue) as a function of jsmir. (B–F) Time courses of [p53pac] (B), [PTEN] (C), [BIM] (D), [PUMA] (E) and [Casp3] (F) with jsmir = 0.3 (blue), 0.8 (red), or 2.0 (green).
We further show the temporal evolution of protein concentrations for different values of jsmir in Fig. 4B–F. At jsmir = 0.3, [PTEN] reaches a high level, promoting the accumulation of p53. High expression of BIM and PUMA activates Casp3 to trigger apoptosis promptly. If jsmir is increased to 0.8, the expression of PTEN and BIM is reduced, and the levels of p53pac and PUMA are downregulated. Consequently, Casp3 is activated with a marked delay. At jsmir = 2.0, the repression of miR-17-92 expression by p53 becomes very weak, and apoptosis cannot be induced. Collectively, the transrepression of miR-17-92 is significant for apoptosis induction under severe hypoxia.
Discussion
In this work, we provide a coherent picture of cell-fate decision under hypoxic conditions. Under normoxia, both HIF-1α and p53 are at very low levels. In mild hypoxia, p53 still remains inactive, whereas HIF-1α first accumulates to induce transient cell-cycle arrest and then settles at moderate levels because of the cellular adaptation to hypoxia. Under moderate hypoxia, HIF-1αac expression is sufficiently high, and p21 induction is maintained to trigger cellular senescence. In severe hypoxia, p53 accumulation attenuates HIF-1α activity, and p53 transrepresses antiapoptotic genes like miR-17-92 to relieve inhibition of proapoptotic genes like BIM, thereby inducing apoptosis. Under anoxia, high levels of p53 lead to HIF-1α degradation, and p53 transrepresses and transactivates target genes to remarkably advance apoptosis induction. Such a decision mechanism seems plausible, either promoting cellular adaptation to mild stress or inducing cell death after severe stress.
The interplay between p53 and HIF-1α plays a key role in the hypoxic response. Our results are consistent with experimental observations: moderate p53 expression attenuates HIF-1α activity, whereas high p53 expression eliminates HIF-1α activity39. Notably, HIF-1α is overexpressed in most cancers40, and thus developing HIF-1α inhibitors to prevent the adaptation of tumor cells to hypoxia may be a promising strategy for anticancer41. Our results suggest that reactivation of p53 could contribute to inhibition of HIF-1α activity.
The mechanism for p53 stabilization is somehow controversial in the literature. Previously, it was reported that p53 was stabilized by HIF-1α16. Later, this mechanism was challenged by the report that p53 accumulated only in severe hypoxia8. Thus, HIF-1α alone cannot stabilize p53. Rather, it was found that ATR contributes to p53 stabilization in severe hypoxia when replication arrest occurs. Here, we further show that the competition between p53 and HIF-1α for limiting p300 not only determines their transcriptional activity, but also regulates their abundance because p300-mediated acetylation prevents Mdm2-mediated protein degradation. p53 stabilization is enhanced when more Mdm2 is associated with unacetylated HIF-1α.
Our work may reconcile the contradictory reports on the mechanism for hypoxia-induced apoptosis11,12,13. We propose that depending on the severity of hypoxia, p53 may suppress the expression of antiapoptotic genes like miR-17-92, or inhibit miR-17-92 expression and activate PUMA expression synergistically to induce apoptosis. Accordingly, it takes a relatively long time to evoke apoptosis under severe hypoxia, or apoptosis is induced promptly after exposure to anoxia. Such an action mode of p53 is of functional significance, allowing for cellular adaptation to the hypoxic environment or eliminating cells promptly under strongly anoxic conditions. It would be interesting to experimentally test that.
We showed that effective repression of miR-17-92 expression is required for apoptosis induction under severe hypoxia. miR-17-92 itself can inhibit the production of BIM and PTEN34. Conversely, p53 transrepresses miR-17-92 to elevate BIM and PTEN expression12. BIM can induce apoptosis42, while PTEN contributes to p53 stabilization by sequestering Mdm2 in the cytoplasm32. It is worthy to explore whether other microRNAs induced by p53, such as miR-34a, contribute to apoptosis induction in hypoxia.
Finally, it is worth noting that the cellular response to hypoxia is extremely complicated. Here, we took a simplified approach to characterize the crosstalk between HIF-1α and p53 signaling by focusing on their competition in stabilization and transcriptional activity. We probed the effect of the severity of hypoxia on cell fate, while omitting the influence of the duration of hypoxia. Some other factors were also ignored, such as complex relationships between HIF-1, p53 and pVHL7 and different phosphorylation modifications of p53. Definitely, adding more facets of signal transduction to the model should shed new light on the hypoxic response.
Methods
The details of the model are presented in the Supporting Material. The concentration of each component is represented by a dimensionless state variable. The temporal evolution of dynamic systems is governed by ordinary differential equations, which are presented in Supplemental Method. All the initial values of variables are their lower steady-state values under normoxia and are listed in Supplemental Table S1. The standard parameter values are listed in Supplemental Table S2. Some parameters are set based on experimental measurements or known facts, while others are estimated by comparing the simulation results with experimental data. The unit of time is minutes, while the units of parameters are determined such that the concentrations of proteins are dimensionless. For simplicity, we do not indicate the units of the parameters explicitly. The rate equations are numerically solved using Oscill8.
The robustness of protein concentrations to parameter variations is also analyzed, and the results are presented in Supplemental Table S3. The sensitivity of steady-state levels of 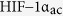 and p53pac to parameter variations is calculated when the parameter values are varied by ±10% with respect to their standard values. These concentrations are only sensitive to the parameters related to HIF-1α regulation, Mdm2-mediated protein degradation, or p53 production. Overall, our simulation results are robust to changes in most parameters, and we provide a model for effectively exploring the interplay between p53 and
and p53pac to parameter variations is calculated when the parameter values are varied by ±10% with respect to their standard values. These concentrations are only sensitive to the parameters related to HIF-1α regulation, Mdm2-mediated protein degradation, or p53 production. Overall, our simulation results are robust to changes in most parameters, and we provide a model for effectively exploring the interplay between p53 and 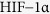 in response to hypoxia.
in response to hypoxia.
Additional Information
How to cite this article: Zhou, C.-H. et al. Modeling the interplay between the HIF-1 and p53 pathways in hypoxia. Sci. Rep. 5, 13834; doi: 10.1038/srep13834 (2015).
Supplementary Material
Acknowledgments
This work was supported by the 973 program of China (No. 2013CB834104), National Natural Science Foundation of China (Nos. 11175084, 11204126 and 31361163003), and PAPD.
Footnotes
Author Contributions X.P.Z. and F.L. designed research; C.H.Z. performed numerical simulations; C.H.Z., X.P.Z., F.L. and W.W. analyzed data; C.H.Z., X.P.Z. and F.L. wrote the paper; F.L. coordinated the research. All authors reviewed the manuscript.
References
- Semenza G. L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 20, 51–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. L., Jiang B. H., Rue E. A. & Semenza G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292, 464–468 (2001). [DOI] [PubMed] [Google Scholar]
- Lando D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003). [DOI] [PubMed] [Google Scholar]
- Greer S. N., Metcalf J. L., Wang Y. & Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 31, 2448–2460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermeus A. & Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2, e164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E. M., Denko N. C., Dorie M. J., Abraham R. T. & Giaccia A. J. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22, 1834–1843 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. P., Liu F. & Wang W. Two-phase dynamics of p53 in the DNA damage response. Proc. Natl. Acad. Sci. USA 108, 8990–8995 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman J. S. & Lowe S. W. Control of apoptosis by p53. Oncogene 22, 9030–9040 (2003). [DOI] [PubMed] [Google Scholar]
- Koumenis C. et al. Regulation of p53 by hypoxia: Dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21, 1297–1310 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. L. et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 28, 2719–2732 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wang Z., Kinzler K. W., Vogelstein B. & Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 100, 1931–1936 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. et al. Hypoxia induces p53-dependent transactivation and Fas/CD95-dependent apoptosis. Cell Death Differ. 14, 411–421 (2006). [DOI] [PubMed] [Google Scholar]
- Fei P. et al. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6, 597–609 (2004). [DOI] [PubMed] [Google Scholar]
- An W. G. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392, 405–408 (1998). [DOI] [PubMed] [Google Scholar]
- Chen D., Li M., Luo J. & Gu W. Direct interactions between HIF-1α and Mdm2 modulate p53 function. J. Biol. Chem. 278, 13595–13598 (2003). [DOI] [PubMed] [Google Scholar]
- Schmid T., Zhou J., Köhl R. & Brüne B. p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1). Biochem. J. 380, 289–295 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutub A. A. & Popel A. S. A computational model of intracellular oxygen sensing by hypoxia-inducible factor HIF1α. J. Cell Sci. 119, 3467–3480 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B., Novák B. & Schofield C. J. Hypoxia-dependent sequestration of an oxygen sensor by a widespread structural motif can shape the hypoxic response - a predictive kinetic model. BMC Syst. Biol. 4, 139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. K. et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J. Cell Sci. 126, 1454–1463 (2013). [DOI] [PubMed] [Google Scholar]
- Ravi R. et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 14, 34–44 (2000). [PMC free article] [PubMed] [Google Scholar]
- Arany Z. et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 93, 12969–12973 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avantaggiati M. L. et al. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89, 1175–1184 (1997). [DOI] [PubMed] [Google Scholar]
- D’Angelo G., Duplan E., Boyer N., Vigne P. & Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: A feedback mechanism that limits HIF-1 responses during reoxygenation. J. Biol. Chem. 278, 38183–38187 (2003). [DOI] [PubMed] [Google Scholar]
- Stiehl D. P. et al. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels: Evidence for an autoregulatory oxygen-sensing system. J. Biol. Chem. 281, 23482–23491 (2006). [DOI] [PubMed] [Google Scholar]
- Liu S. et al. ATR autophosphorylation as a molecular switch for checkpoint activation. Mol. Cell 43, 192–202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko B. N. Cell-signalling dynamics in time and space. Nat. Rev. Mol. Cell Biol. 7, 165–176 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E. M., Dorie M. J. & Giaccia A. J. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 278, 12207–12213 (2003). [DOI] [PubMed] [Google Scholar]
- Shinozaki T., Nota A., Taya A. & Okamoto K. Functional role of Mdm2 phosphorylation by ATR in attenuation of p53 nuclear export. Oncogene 22, 8870–8880 (2003). [DOI] [PubMed] [Google Scholar]
- Mayo L. D. & Donner D. B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98, 11598–11603 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. D. & Cantley L. C. AKT/PKB signaling: Navigating downstream. Cell 129, 1261–1274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee K. B. & Aguda B. D. Akt versus p53 in a network of oncogenes and tumor suppressor genes regulating cell survival and death. Biophys. J. 91, 857–865 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 9, 405–414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloet D. E. A. & Burgering B. M. T. The PKB/FOXO switch in aging and cancer. BBA-Mol. Cell Res. 1813, 1926–1937 (2011). [DOI] [PubMed] [Google Scholar]
- Cory S. & Adams J. M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2, 647–56 (2002). [DOI] [PubMed] [Google Scholar]
- Kirsch D. G. et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 274, 21155–21161 (1999). [DOI] [PubMed] [Google Scholar]
- Levine A. J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997). [DOI] [PubMed] [Google Scholar]
- Schofield C. J. & Ratcliffe P. J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 (2004). [DOI] [PubMed] [Google Scholar]
- Talks K. L. et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411–421 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Choi H. K. & Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 49, 24–40 (2012). [DOI] [PubMed] [Google Scholar]
- Bouillet P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.