Abstract
Microbial levan is an important biopolymer with considerable potential in food and medical applications. Bacillus amyloliquefaciens NK-ΔLP strain can produce high-purity, low-molecular-weight levan, but production is relatively low. To enhance the production of levan, six extracellular protease genes (bpr, epr, mpr, vpr, nprE and aprE), together with the tasA gene (encoding the major biofilm matrix protein TasA) and the pgsBCA cluster (responsible for poly-γ-glutamic acid (γ-PGA) synthesis), were intentionally knocked out in the Bacillus amyloliquefaciens NK-1 strain. The highest levan production (31.1 g/L) was obtained from the NK-Q-7 strain (ΔtasA, Δbpr, Δepr, Δmpr, Δvpr, ΔnprE, ΔaprE and ΔpgsBCA), which was 103% higher than that of the NK-ΔLP strain (ΔpgsBCA) (15.3 g/L). Furthermore, the NK-Q-7 strain also showed a 94.1% increase in α-amylase production compared with NK-ΔLP strain, suggesting a positive effect of extracellular protease genes deficient on the production of endogenously secreted proteins. This is the first report of the improvement of levan production in microbes deficient in extracellular proteases and TasA, and the NK-Q-7 strain exhibits outstanding characteristics for extracellular protein production or extracellular protein related product synthesis.
Microbial levan, one of the two main types of fructan biopolymers, is mainly polymerized via β-(2 → 6) bonds1 and has been isolated from Gram-negative bacteria, Gram-positive bacteria and some fungi2. Levan has many favourable properties, and is used in variety of industrial applications including in foods, cosmetics and pharmaceuticals3,4,5.
Previous work has revealed that microbial levan is synthesized in the medium by the secreted levansucrase (EC: 2.4.1.10) from the sucrose substrate6. Microorganisms synthesize higher-molecular-weight levan at the beginning of fermentation, after which the molecule is hydrolyzed to lower-molecular-weight levan products in the presence of the β-2,6-fructofuranoside linkage-hydrolyzing enzyme, levanase5.
Bacillus amyloliquefaciens NK-1 has the ability to co-produce γ-PGA and levan during fermentation. The pgsBCA genes (responsible for γ-PGA synthesis) deletion strain B. amyloliquefaciens NK-ΔLP can produce high-purity levan, with the highest titer observed being 14 g/L7. The molecular weight of levan obtained from the NK-ΔLP strain is around 5 kDa, which is much lower than other reported levan products8. The Bacillus amyloliquefaciens strain was isolated from fermented food9, thus its levan product was supposed to have the potential to be the dietary supplements10. However, the low levan production by this strain is unable to meet industrial demands. Therefore, further strain improvement through metabolic engineering is required.
Most of the strategies applied thus far to enhance levan production have been limited to medium optimization or fermentation process improvement11,12, and only a few metabolic engineering strategies have been utilized to improve levan production. Senthilkumar et al.13 studied the effect of the disruption of the Zymomonas mobilis extracellular sucrase gene (sacC) on levan production. The sacC gene mutant strain showed three-fold higher levansucrase (SacB) activity than the wild-type strain and the levan titer increased from 15.5 g/L to 21.2 g/L. Similar genetic modification was a performed in Lactobacillus reuteri, demonstrating that disruption of the sucrose phosphorylase gene scrP, (encoding a sucrose hydrolysis enzyme), also increased levan production14. Shida et al.15 studied the effects of disrupting the levanase gene sacC on levan production in Bacillus subtilis 327UH strain. The results showed that there was no difference in the obtained levan yield between the sacC-deficient strain and the wild-type strain; however, the polymerization degree of levan obtained from the sacC-deficient strain was approximately three times higher compared with that from the wild-type strain. Over-expression of the sacB gene is another method for improving levan production. Ananthalakshmy et al.16 expressed the sacB gene by using the pLSD19 plasmid in the sucrase mutant Zymomonas mobilis Zsuc1 strain and found that levan production increased from 5 g/L to 10.7 g/L compared with the wild-type strain.
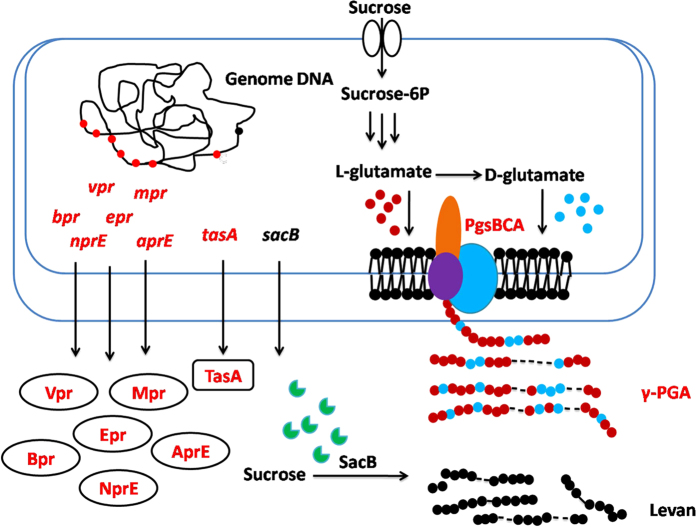
In the current study, we proposed a new strategy for metabolic engineering of the B. amyloliquefaciens NK-1 strain to improve levan production. TasA is the main protein in biofilm matrix17,18. The extracellular matrix surrounds the cells and might block the secretion of levansucrase. Previous works demonstrated that the tasA gene deletion strain could only form deficient biofilm17. The tasA gene was deleted in this work to determine its effects on levan production. Bacillus can produce high level of extracellular proteases to degrade the extracellular protein for cell use19. We speculated that deleting protease genes can expect to find other extracellular proteins more stable. Thus, we decided to delete the extracellular protease genes to improve levan production. In our previous work, we found that NK-1 strain could co-produce γ-PGA and levan simultaneously; moreover, their purification procedures are similar. Thus, pgsBCA genes deletion may increase levan purity as well as its production. A schematic of this proposed genetically engineered metabolic pathway of B. amyloliquefaciens NK-1 is shown in Fig. 1. We aimed to improve levan production by carrying out the above-mentioned three tasks: (1) delete the tasA gene to make the biofilm formation deficient; (2) delete six extracellular proteases genes bpr20,21, epr22, mpr23,24, vpr25, nprE26 and aprE27,28, to decrease the degradation of the levansucrase; and (3) detete the pgsBCA cluster to block the γ-PGA synthesis pathway and obtain a higher purity and yield of the levan product. The final engineered NK-Q-7 strain could produce 31.1 g/L levan in flask, which was 103% higher compared with the production in the NK-ΔLP strain.
Figure 1. Metabolic pathways associated with levan biosynthesis in Bacillus amyloliquefaciens and engineering strategies for levan production.
The red font indicates the genes deleted in this study and the corresponding deficient products. Metabolite symbols: Sucrose-6P, sucrose-6-phosphate; Bpr, bacillopeptidase F; Epr, extracellular serine protease; Mpr, extracellular metalloprotease; Vpr, extracellular serine protease; NprE, extracellular neutral metalloprotease; AprE, extracellular alkaline serine protease; TasA, major biofilm matrix protein; pgsBCA, poly-γ-glutamate synthase.
Results
Construction of marker-less gene deletion mutants
In this work, we sought to improve levan production in a B. amyloliquefaciens strain by deleting the tasA gene, extracellular protease genes and the pgsBCA cluster. For the preferential effect behavioral test on γ-PGA production, we first deleted the tasA gene and then sequentially deleted the six extracellular protease genes bpr, epr, mpr, vpr, nprE and aprE in the NK-1 strain. The target strains were designated NK-P-X (X = 1–7). Next, the γ-PGA synthetase cluster pgsBCA was deleted from the NK-P-X strains, and the resultant γ-PGA-deficient strains were designated NK-Q-X.
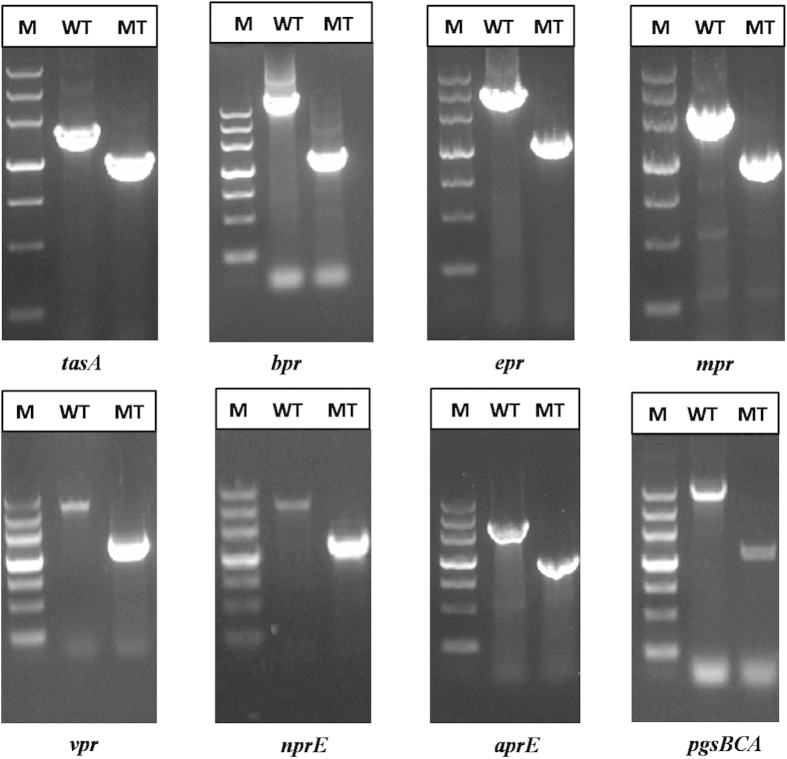
We constructed these gene deletion mutants via a marker-less knockout method. This method is based on using the upp gene, which encodes uracil-phosphoribosyl-transferase, as the counter-selectable marker. The upp cassette and 5-fluorouracil (5-FU) selection were used to identify marker-less gene deletions29. According to the PCR results shown in Fig. 2 and the DNA sequencing results, we confirmed that the gene mutant strains had been successfully constructed.
Figure 2. Confirmation of gene deletions via PCR.
Chromosomal DNA served as the template for amplification. Lane M: DNA marker III; Lane WT: strains amplified with relevant detection primers using B. amyloliquefaciens NK-1 chromosomal DNA as the template; Lane MT: strains amplified with the relevant detection primers using chromosomal DNA from the gene deletion strains’ as the template.
The effects of tasA gene deletion on biofilm formation
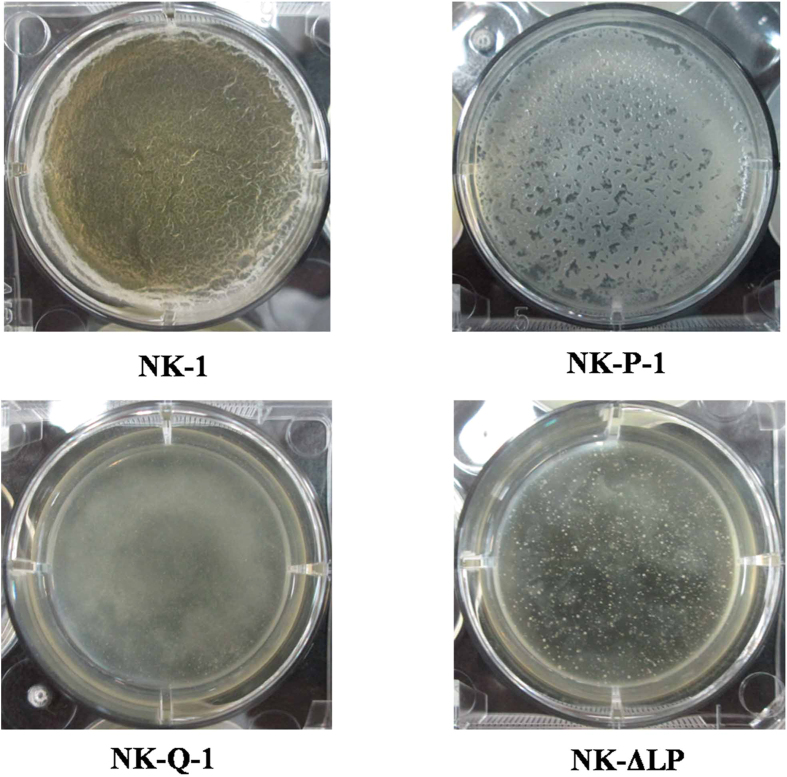
Our previous work showed that B. amyloliquefaciens NK-1 could form structurally complex biofilms30. Genetic, biochemical and cytological evidences suggested that this complex extracellular matrix is mainly composed of TasA and Eps and the absence of TasA or Eps results in a residual matrix17,31. Levan and γ-PGA also contribute to bacterial biofilm formation, cross-linking with other components to make the biofilm complete32,33. Biofilm formation by the NK-1, NK-P-1, NK-Q-1 and NK-ΔLP strains was observed and the results were shown in Fig. 3. As expected, the NK-1 strain was able to form a complete pellicle, whereas the tasA gene deletion NK-P-1 strain could only form a deficient, incomplete pellicle. Unexpectedly, neither of the pgsBCA genes deletion strains (NK-Q-1 and NK-ΔLP) could form a pellicle. This result indicates that γ-PGA is the main component of the biofilm formed by the γ-PGA-producing strain and that the strain with deficient γ-PGA production could not form a biofilm.
Figure 3. Cell pellicle formation in the B. amyloliquefaciens NK-1, B. amyloliquefaciens NK-P-1, B. amyloliquefaciens NK-Q-1 and B. amyloliquefaciens NK-ΔLP strains.
Cells were cultured at 30 °C for 72 h in MSgg broth in a six-well microtiter dish.
Comparison of γ-PGA production beween B. amyloliquefaciens NK-1 and the mutant NK-P-X strains in flask culture
The effects of the deletion of tasA and the six-extracellular protease genes on γ-PGA production were characterized in this work. The γ-PGA fermentation results obtained from B. amyloliquefaciens NK-1 and the gene deletion mutant strains are shown in Fig. 4. γ-PGA production remained unchanged after deleting the tasA, bpr, epr and mpr genes. However, the NK-P-5 strain, harboring further deletion of the vpr gene, showed increased γ-PGA production. The NK-P-5 strain exhibited the highest γ-PGA yield, which was a 24.2% increase compared with the NK-1 strain, leading to a titer of 4.62 g/L, compared with 3.72 g/L for the control. γ-PGA production was lower in the strains in which the nprE and aprE genes were deleted. γ-PGA production from the NK-P-7 strain was 2.19 g/L, which was 47.4% lower than in the NK-P-5 strain. Moreover, the dry cell weight (DCW) of the NK-P-7 strain was lower than other strains, and this strain was observed to enter the cell decline phase earlier.
Figure 4. γ-PGA fermentation results in the B. amyloliquefaciens NK-1 and mutant NK-P-X strains after 48 h of cultivation.
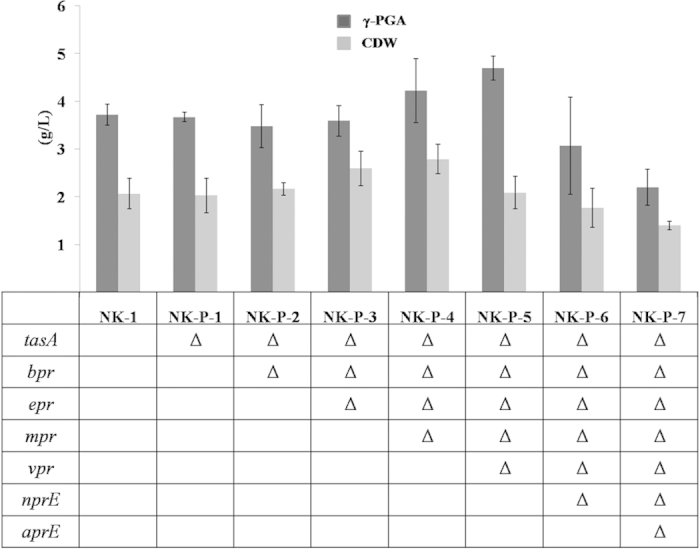
Values represent the means ± SD. Asterisks indicate significant difference from the NK-1 strain (P < 0.05). All cultures were repeated at least five times.
Comparison of levan production between the B. amyloliquefaciens NK-ΔLP and mutant NK-Q-X strains in flask culture
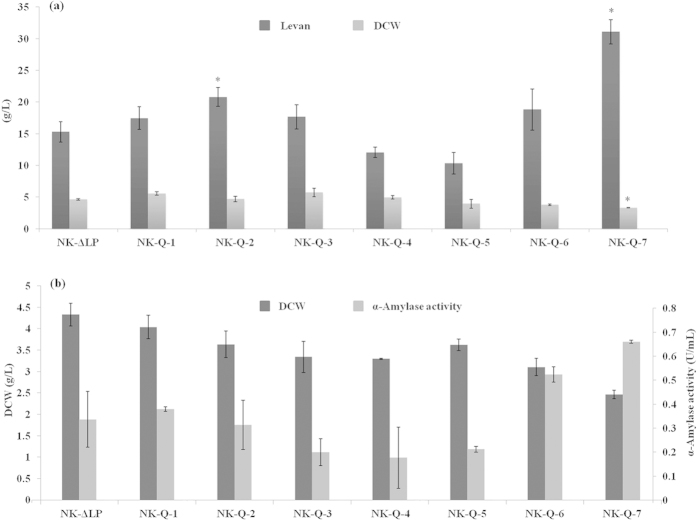
To evaluate the effect of the accumulation of gene-targeted B. amyloliquefaciens mutants on levan production, flask culture of B. amyloliquefaciens NK-ΔLP and the mutant strains was undertaken under identical conditions. We sought to delete the tasA gene for two reasons: (1) disruption of tasA can conserve energy and favor the production of levan via the metabolic flux; (2) Cells in biofilms are embedded in the extracellular matrix, which contains TasA, the major matrix protein. We speculated that by removing TasA, which is known to be bound to cells17, we may influence the export of extracellular levansucrase. Therefore it might have been possible that lack of TasA increases the production of levan. The fermentation results shown in Fig. 5a indicated that the deletion of tasA gene did affect levan production. The NK-Q-1 strain showed a slight 14.4% increase in levan production (17.5 g/L) compared with the NK-ΔLP strain (15.3 g/L).
Figure 5. Levan and α-amylase fermentation results in the B. amyloliquefaciens NK-ΔLP and mutant NK-Q-X strains.
(a) Comparison of levan production and dry cell weights between NK-ΔLP and the mutant strains after 48 h of cultivation. (b) Comparison of α-amylase production and dry cell weights between NK-ΔLP and the mutant strains after 48 h of cultivation. Values represent the means ± SD. Asterisks indicate significant difference from the NK-ΔLP strain (P < 0.05). All cultures were repeated at least five times.
Levan is synthesized in the medium by secreted levansucrase. We reasoned that the lack of extracellular proteases might make the extracellular levansucrase more stable, thereafter improving levan production in the medium. As shown in Fig. 5a, the extracellular protease gene deletion strains NK-Q-2 and NK-Q-7 exhibited levan production increase. Among these strains, NK-Q-7 displayed the highest increase in productivity (31.1 g/L), resulting in 103% improvement of levan production compared with the NK-ΔLP control. The molecular weight of levan obtained from NK-Q-7 strain was 4, 600 Da, and its purity reached 94.1 ± 1.3%. However, not all of the mutant strains showed improved levan production; the NK-Q-4 and NK-Q-5 strains displayed decreases of 21.3% and 32.4% in levan production, respectively.
Comparison of α-amylase production between B. amyloliquefaciens NK-ΔLP and the mutant NK-Q-X strains in flask culture
To evaluate the effect of the gene deletions on extracellular protein production, we also determined the α-amylase production. The results regarding α-amylase production are shown in Fig. 5b. Consistent with the levan fermentation results (Fig. 5a), the NK-Q-7 strains showed significantly increased α-amylase production to approximately 0.66 U/mL of amylase activity, which was 94.1% higher compared with the NK-ΔLP strain.
Determination of extracellular protease activities in the B. amyloliquefaciens NK-Q-X strains
We further analyzed extracellular protease activities in the NK-Q-X strains at the end of stationary phase during the levan fermentation process, and the results are presented in Table 1. The protease activities of NK-ΔLP and NK-Q-1 were comparable, indicating that the deletion of tasA had no effect on cell protease activity. The bpr gene deletion strain NK-Q-2 showed a 38% decrease compared with the NK-ΔLP strain, whereas the NK-Q-3 strain, in which both bpr and epr were deleted, exhibited an approximately a 50% decrease in protease activities. Surprisingly, and in contrast to previous studies, the detected protease activities was unchanged after deletion the mpr and vpr genes. However, the strain without nprE and aprE gene showed further decreases in protease activity; protease activity was decreased by 56% in NK-Q-6 and by a notable 86% in NK-Q-7 compared with the NK-ΔLP strain.
Table 1. Comparison of extracellular protease activity between the Bacillus amyloliquefaciens NK-ΔLP and Bacillus amyloliquefaciens NK-Q-X strains in levan fermentation medium.
| Strains | Extracellular protease activity (%) |
|---|---|
| NK-ΔLP | 100 ± 12.3 |
| NK-Q-1 | 104 ± 1.7 |
| NK-Q-2 | 62.1 ± 3.3 |
| NK-Q-3 | 50.5 ± 1.4 |
| NK-Q-4 | 55.6 ± 17.4 |
| NK-Q-5 | 54.6 ± 2.0 |
| NK-Q-6 | 44.2 ± 0.7 |
| NK-Q-7 | 14.3 ± 1.2* |
All cultures were repeated at least five times.
Asterisks indicate significant difference from the NK-ΔLP strain (P < 0.05).
Discussion
B. amyloliquefaciens NK-1 is derived from the LL3 strain, which was isolated from fermented food (Korea bibimbap paste)9, thus it is safe for human beings. In our previous work, we found that the NK-1 strain could co-produce γ-PGA and levan simultaneously (Fig. 6a). After deleting the pgsBCA cluster, the obtained NK-ΔLP strain could not produce γ-PGA, and the remaining product mainly consisted of levan, at a purity reaching 92.7% (Fig. 6b) 7. From our previously work, we determined that the bacteria could produce levan products of two different molecular weights. Higher-molecular-weight levan is produced at early timepoints and is then hydrolyzed to lower-molecular-weight levan by levanase, and the low-molecular-weight levan is dominant after 48 h of fermentation7. The levan molecular weight obtained from the NK-ΔLP strain was mostly around 5 kDa7, which is significantly lower than other reported levan products8. However, levan production needs to be improved to meet the requirements for industrial application34.
Figure 6. GPC results for γ-PGA and levan products.
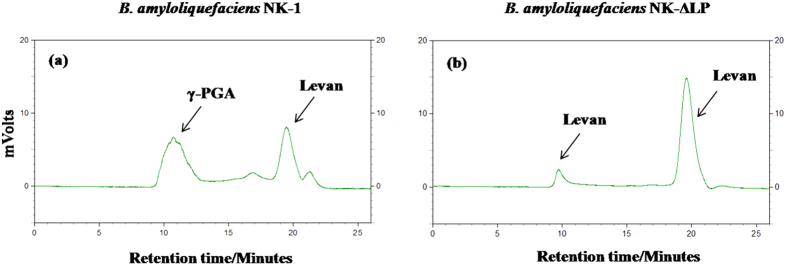
(a) GPC spectrum of γ-PGA obtained from B. amyloliquefaciens NK-1 and (b) GPC spectrum of levan obtained from B. amyloliquefaciens NK-ΔLP.
We deleted the tasA gene from the NK-1 strain, and the resulting NK-P-1 strain could form incomplete biofilms, whereas the tasA and pgsBCA double-deletion NK-Q-1 strain could not form a biofilm (Fig. 3). We further studied the effects of these deletions on levan synthesis. The NK-Q-1 strain showed a 14.4% increase in levan production compared with the NK-ΔLP strain (Fig. 5a). Unlike our expected, the deletion of tasA gene had little effect on levan production.
Bacillus species can produce high levels of extracellular proteases to degrade secreted heterologous proteins19. Many protease-deficient strains have been constructed and display favorable features for the improvement of heterologous protein production. B. subtilis WB600 is a strain that is deficient in six-extracellular-protease (ΔnprE, ΔnprB, ΔaprE, Δepr, Δmpr, Δbpr) and showes improved production of heterologously expressed β-lactamase, streptokinase and the antidigoxin single-chain antibody fragment over the wild-type strain35,36,37. Although many works have focused on the effect of extracellular-protease-deficiency on heterologous proteins, few efforts have been made to study its effect on endogenous protein production. We hypothesized that the extracellular proteases degrade not only misfolded proteins but also degraded proteins (which still exhibit low catalytic activity) or even fully-functional proteins in the end stage of fermentation when nitrogen is limited. Based on this hypothesis, we aimed to improve levan production by knocking out the microbe’s extracellular protease genes. Levan is produced by the secreted levansucrase SacB, and extracellular proteases may affect the amount and activity of SacB to some degree, decreasing levan production. And the extracellular-proteases-deficient strains probably exhibit increased production of extracellular protein including SacB, thereafter increase the production of levan. The NK-1 strain exhibits seven extracellular proteases: Bpr, Epr, Mpr, Vpr, NprE, AprE and WprA38, and we aimed to delete all of these associated genes. However, we failed to delete the wprA gene for unknown reasons. The other six genes were successfully deleted.
The six-gene-deletion NK-Q-7 strain showed the highest levan production, presenting increases of approximately about 102% and 78% compared with the NK-ΔLP and NK-Q-1 strains, respectively. We speculated that the increase of levan production was related to the increase of SacB production, and the deletion of extracellular protease genes improved the production of extracellular protein-SacB. To evaluate our speculation, we further determined the effect of gene deletions on another endogenous product α-amylase (a secreted Bacillus protein). The NK-Q-7 strain showed the highest yield of α-amylase as well, with production being increased by 96% and 74%, compared with the NK-ΔLP and NK-Q-1 strains, respectively. These results demonstrate that the deficiency of extracellular protease genes indeed increase the cells’ production of their own secreted proteins. Despite exhibiting highest production, the DCW of NK-Q-7 was the lowest among these strains. Additionally, more frequent cell lysis was detected in the end stage of fermentation and the number of living cells was 28 ± 9% less than that of the control NK-ΔLP strain. Another interesting phenomenon observed in this strain was the shorter time (24 h) required for the organism to adapt to the high carbon source conditions (in the levan seed culture) compared with other six strains (30–36 h) when transferred from incubation in LB medium. This particular feature might offset the early cell lysis in the end stage of fermentation.
We also characterized the extracellular protease activity of the NK-Q-X strains in levan fermentation medium. Consistent with our hypothesis, the extracellular protease activity of the NK-Q-7 strain showed a dramatic decrease to approximately 14.3% of that in the NK-1 strain (Table 1). However, this extracellular protease activity was still higher than in other previously reported strains, such as B. subtilis GB2054 (with inactivation of two extracellular proteases, NprE and Apr, and no detectable extracellular protease activity)39 and B. subtilis WB700 (with inactivation of seven extracellular proteases, NprE, NprB, AprE, Epr, Mpr, Bpr and Vpr, and decreased extracellular protease activity to approximately 0.14% of that in the wild-type strain)40. This difference may be attributable to the applied cell culture conditions. However, not all of the mutant strains showed increased levan production. For example, the NK-Q-4 and NK-Q-5 strains were deficient in extracellular protease activity, yet showed reduced levan production. The mpr and vpr gene deletions might be harmful to levan production.
As the production of TasA and extracellular proteases consumes a great deal of energy, we also studied the effect of these gene deletions on γ-PGA production. However, their deletions had little effect on γ-PGA production, and only the NK-P-5 strain exhibited a slight increase in γ-PGA production (Fig. 4).
In summary, we proposed a new strategy for improving levan production in this work. We deleted the tasA gene, six extracellular protease genes and the pgsBCA cluster. The resulting NK-Q-7 strain produced 31.1 g/L levan, which was 103% higher than the production in the NK-ΔLP control strain. The NK-Q-7 also yielded 96% more α-amylase. These results indicate that NK-Q-7 strain could be utilized as a candidate cell factory for secreted protein production or secreted protein related product synthesis.
Methods
Strains, plasmids and growth conditions
All of the strains and plasmids used in this work are listed in Table 2. The B. amyloliquefaciens NK-1 strain was a derivative of the LL3 strain with the endogenous plasmid pMC1 and the upp gene deleted41,42. The B. amyloliquefaciens NK-ΔLP strain was a derivative of the NK-1 strain with the pgsBCA cluster deleted. Escherichia coli DH5α was used for plasmid propagation and transformation. The dam-and dcm- deficient E. coli strain GM2163 was used for plasmid demethylation.
Table 2. Strains and plasmids used in this study.
| Strains and plasmids | Relevant genotype and characteristics | Source |
|---|---|---|
| Strains | ||
| B. amyloliquefaciens LL3 | wild type | (9) |
| B. amyloliquefaciens NK-ΔLP | NK-1 derivative, ΔpgsBCA | This lab |
| B. amyloliquefaciens NK-1 | LL3 derivative, ΔpMC1, Δupp | (42) |
| B. amyloliquefaciens NK-P-1 | NK-1 derivative, ΔtasA | This work |
| B. amyloliquefaciens NK-P-2 | NK-1 derivative, ΔtasA, Δbpr | This work |
| B. amyloliquefaciens NK-P-3 | NK-1 derivative, ΔtasA, Δbpr, Δepr | This work |
| B. amyloliquefaciens NK-P-4 | NK-1 derivative, ΔtasA, Δbpr, Δepr, Δmpr | This work |
| B. amyloliquefaciens NK-P-5 | NK-1 derivative, ΔtasA, Δbpr, Δepr, Δmpr, Δvpr | This work |
| B. amyloliquefaciens NK-P-6 | NK-1 derivative, ΔtasA, Δbpr, Δepr, Δmpr, Δvpr, ΔnprE | This work |
| B. amyloliquefaciens NK-P-7 | NK-1 derivative, ΔtasA, Δbpr, Δepr, Δmpr, Δvpr, ΔnprE, ΔaprE | This work |
| B. amyloliquefaciens NK-Q-1 | NK-P-1 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-2 | NK-P-2 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-3 | NK-P-3 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-4 | NK-P-4 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-5 | NK-P-5 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-6 | NK-P-6 derivative, ΔpgsBCA | This work |
| B. amyloliquefaciens NK-Q-7 | NK-P-7 derivative, ΔpgsBCA | This work |
| E. coli DH5α | F−, φ80dlacZΔM1, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rk−, mk+), phoA, supE44, λ− thi-1, gyrA96, relA1 | This lab |
| E. coli GM2163 | F−, ara-14 leuB6 thi-1 fhuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsL 136 (Strr) xyl-5 mtl-1 dam13::Tn9 (Camr) dcm-6 mcrB1 hsdR2 mcrA | This lab |
| Plasmids | ||
| pKSV7 | Shuttle vector, temperature-sensitive (ts) replication origin, Ampr (gram-negative) and Cmr (gram-positive) | (45) |
| p-KSU | pKSV7-derivation with upp gene | (46) |
| pKSV7-△LP | p-KSU-derivation with deletion fragment of pgs operon | This lab |
| pKSV7-△tasA | p-KSU-derivation with deletion fragment of tasA | This work |
| pKSV7-△bpr | p-KSU-derivation with deletion fragment of bpr | This work |
| pKSV7-△epr | p-KSU-derivation with deletion fragment of epr | This work |
| pKSV7-△mpr | p-KSU-derivation with deletion fragment of mpr | This work |
| pKSV7-△vpr | p-KSU-derivation with deletion fragment of vpr | This work |
| pKSV7-△nprE | p-KSU-derivation with deletion fragment of nprE | This work |
| pKSV7-△aprE | p-KSU-derivation with deletion fragment of aprE | This work |
For routine strain construction and maintenance, the B. amyloliquefaciens and E. coli strains were grown at 37 °C in Luria-Bertani (LB) medium. To produce γ-PGA, the B. amyloliquefaciens strains were cultured at 37 °C and 180 rpm, for 48 h in γ-PGA fermentation medium43. To produce levan, B. amyloliquefaciens strains were cultured at 37 °C and 180 rpm, for 48 h in levan fermentation medium (pH 6.0), which containing 250.9 g/L sucrose, 2.6 g/L urea, 0.62 g/L MgSO4, 8.16 g/L KH2PO4, 18.24 g/L K2HPO4·3H2O, 1 mM FeSO4, 1 mM CaCl2, 1 mM MnSO4 and 1 mM ZnCl2. To produce α-amylase, the B. amyloliquefaciens strains were cultured at 37 °C and 180 rpm, for 48 h in α-amylase fermentation medium (pH 7.0), containing 10 g/L soluble starch, 2 g/L tryptone, 1 g/L KH2PO4, 2.5 g/L Na2HPO4, 2 g/L (NH4)2SO4, 1 g/L NaCl, 0.05 g/L MgSO4·7H2O and 0.05 g/L CaCl244.
The concentrations of the antibiotics used in this work were as follow: 100 μg/mL ampicillin, 5 μg/mL chloramphenicol. The final concentration of 5-FU was 100 μg/mL.
DNA manipulation and plasmid construction
To construct the gene deletion vectors, the temperature-sensitive pKSU plasmid was used, which was derived from the pKSV7 plasmid45,46. The upp expression cassette was ligated to the pKSV7 plasmid as a reverse selection marker. All of the oligonucleotide primers used in this work are listed in Table 3. We decided to delete eight genes: tasA, aprE, bpr, epr, mpr, nprE, vpr and pgsBCA. The upstream and downstream fragments of the genes targeted for deletion were amplified with PrimeSTAR HS DNA polymerase (Takara Bio, Japan) using the primers N-SF/N-SR and N-XF/N-XR, respectively (N represents the relevant gene name). The obtained upstream and downstream DNA fragments were joined via overlap-PCR. The combined fragments were first ligated to the pMD19-T simple vector (Takara Bio, Japan). All of the constructed plasmids were verified through DNA sequencing (BGI, China). They were then restricted by endonucleases Sal I and BamH I and ligated into the pKSU vector digested with the same enzymes, respectively. Finally the generated gene deletion plasmids were designated pKSV7-ΔN.
Table 3. Primers used in this study.
| Primers | Sequence (5′–3′) |
|---|---|
| tasA-SF | CCCGGATCCACTCTCAAAATACATCAGACAAATAG |
| tasA-SR | CGTTCAGGAACGTTCTTGCTTTTTTGCTGTCTAATGTTTC |
| tasA-XF | ACAGCAAAAAAGCAAGAACGTTCCTGAACGATAATACATC |
| tasA-XR | GGGGTCGACGAATTTTTTCGCATGTTCAAACATT |
| tasA-SS | GACTGACGTCATGAGCTGCTGGGTTTTT |
| tasA-XX | CCAAGTTCTTTTTCACCGGGAACGCC |
| bpr-SF | CCCCGGATCCTAACGCCCTTAAAACGAAATCT |
| bpr-SR | TTATTTTTCACATTTCTTTTTCTTTTTCATAGTCTGCCTC |
| bpr-XF | ATGAAAAAGAAAAAGAAATGTGAAAAATAACAAGAC |
| bpr-XR | CCCCGTCGACTTACTGAACGTCACTCATATC |
| bpr-SS | TAGACACGTATTTTCAGCGTGATCC |
| bpr-XX | GCTCGGAGGCTATTCAGTTGCGTAT |
| epr-SF | CGCGGATCCCCAGGGATGGACAAGAAC |
| epr-SR | TAAGCGCTCGTATTCGTTCTCGTTACTGCAGG |
| epr-XF | CAGTAACGAGAACGAATACGAGCGCTTATTGG |
| epr-XR | AGGCGTCGACAAAGCGGAGGAGAAATACAG |
| epr-SS | GCGGGTTTATCCTGTTCTTAATCGG |
| epr-XX | GGCACCGTTATTTTCTACAGCCTGG |
| mpr-SF | CCCGGATCCACTCTCAAAATACATCAGACAAATAG |
| mpr-SR | CGTTCAGGAACGTTCTTGCTTTTTTGCTGTCTAATGTTTC |
| mpr-XF | ACAGCAAAAAAGCAAGAACGTTCCTGAACGATAATACATC |
| mpr-XR | GGGGTCGACGAATTTTTTCGCATGTTCAAACATT |
| mpr-SS | GACTGACGTCATGAGCTGCTGGGTTTTT |
| mpr-XX | CCAAGTTCTTTTTCACCGGGAACGCC |
| vpr-SF | CCCCGGATCCTAACGCCCTTAAAACGAAATCT |
| vpr-SR | TTATTTTTCACATTTCTTTTTCTTTTTCATAGTCTGCCTC |
| vpr-XF | ATGAAAAAGAAAAAGAAATGTGAAAAATAACAAGAC |
| vpr-XR | CCCCGTCGACTTACTGAACGTCACTCATATC |
| vpr-SS | TAGACACGTATTTTCAGCGTGATCC |
| vpr-XX | GCTCGGAGGCTATTCAGTTGCGTAT |
| nprE-SF | CCCCGGATCCTAACGCCCTTAAAACGAAATCT |
| nprE-SR | TTATTTTTCACATTTCTTTTTCTTTTTCATAGTCTGCCTC |
| nprE-XF | ATGAAAAAGAAAAAGAAATGTGAAAAATAACAAGAC |
| nprE-XR | CCCCGTCGACTTACTGAACGTCACTCATATC |
| nprE-SS | TAGACACGTATTTTCAGCGTGATCC |
| nprE-XX | GCTCGGAGGCTATTCAGTTGCGTAT |
| aprE-SF | CCCCGGATCCTAACGCCCTTAAAACGAAATCT |
| aprE-SR | TTATTTTTCACATTTCTTTTTCTTTTTCATAGTCTGCCTC |
| aprE-XF | ATGAAAAAGAAAAAGAAATGTGAAAAATAACAAGAC |
| aprE-XR | CCCCGTCGACTTACTGAACGTCACTCATATC |
| aprE-SS | TAGACACGTATTTTCAGCGTGATCC |
| aprE-XX | GCTCGGAGGCTATTCAGTTGCGTAT |
The restriction enzyme cleavage sites are underlined.
Construction of gene knockout mutant strains
To carry out multiple gene deletions in a single strain, a modified marker-less gene deletion method was used to construct the gene knockout mutant strains29,46. The primers N-SS and N-XX (N represents the relevant gene name) were used to determine the gene deletion mutants via PCR. We deleted the tasA, bpr, epr, mpr, vpr, nprE and aprE genes in turn from the NK-1 strain. The gene disruption mutants were designated B. amyloliquefaciens NK-P-1, NK-P-2, NK-P-3, NK-P-4, NK-P-5, NK-P-6 and NK-P-7, respectively. We further deleted the pgsBCA cluster (for γ-PGA synthesize) from the NK-P-X strains (X represents the numbers 1–7). The resulting seven γ-PGA-deficient strains were designated NK-Q-X.
Production of γ-PGA, levan and α-amylase in flask culture
For γ-PGA production, single colonies of the B. amyloliquefaciens NK-P-X strains were transferred to 50 mL of γ-PGA fermentation medium. After 18 h of incubation at 37 °C and 180 rpm, l mL of the cultures was transferred to 100 mL of fermentation medium in shaking flasks and then fermented for 48 h.
The B. amyloliquefaciens NK-Q-X strains were used for levan and α-amylase production. As the cell growth rates in the levan fermentation medium and the α-amylase fermentation medium were lower than in the γ-PGA fermentation medium, the seed culture times were extended to 24 h–40 h. All cultures were repeated at least five times.
Enzyme assays
α-amylase activity was measured via a modified dinitrosalicylic acid (DNS) method, which was based on the amount of reducing sugars released from soluble starch47. A mixture (pH 5.9) containing 1% soluble starch and 0.05 M sodium citrate buffer was heated at 40 °C for 10 min in a water bath. Then, 0.1 mL of culture extract was added to the substrate and incubation was continued at 40 °C for 30 min with gentle shaking. The reaction was subsequently stopped by adding 2 mL of the DNS reagent, after which the reaction was heated at 100 °C for 10 min, and its absorbance was measured at 540 nm. A glucose standard curve was used to determine α-amylase activity. One unit (U) of α-amylase activity was defined as the amount of enzyme that liberated one micromole of reducing sugar in one minute under the assay conditions.
Protease activity in the supernatant of the levan fermentation medium at the end of the stationary phase was measured via a modified casein digestion method48. One milliliter of 1% casein solution in 0.2 M Tris buffer (pH 8.5) was incubated with 1 mL of test culture for 30 min at 37 °C. The reaction was stopped by the addition of 2 mL of 10% trichloroacetic acid (TCA) solution. After centrifugation at 1000 rpm for 5 min, 1 mL of the supernatant was reacted with the Folin-reagent (Dingguo, China). The protease activity was determined from the absorbance of the reaction at 680 nm. The protease activity determined for NK-ΔLP in levan fermentation medium was defined as 100%.
Biofilm formation
For the analysis of pellicle formation, the NK-1, NK-P-1, NK-Q-1 and NK-ΔLP strains were cultured on LB agar for 18 h and subsequently incubated in LB broth to an OD600 of 1.0. Next, 10 μL aliquots of the cultures were added to 10 mL of MSgg broth17 in a six-well microtitre dish. The dishes were incubated at 30 °C for 72 h without agitation, and the pellicles that formed were photographed by a digital camera equipped with a close-up lens (Canon, Tokyo, Japan).
Analytical procedures
The optical density (OD) of the cultures was measured with a SHIMADZU UV-1800 spectrophotometer (Kyoto, Japan). The dry cell weight was determined from 100 mL of broth, and the cells were harvested via centrifugation and dried at 50 °C for 24 h to a constant weight after washed with distilled water. γ-PGA was purified by a previously described method9,49. The procedure for the purification of levan was similar to that for γ-PGA, with the exception of the use a 3500 MW dialysis bag following the precipitated of levan with four-fold volumes the cold ethanol. The molecular weight of the levan was determined by a gel permeation chromatography (GPC) system42. An Alltech system controller (Alltech Associates Inc., US) with a Shodex KW804 column (Showa Denko KK, Japan) and a refractive index (RI) detector (Schambeck SFD GmbH, Germany) were used. 0.25 mol/L NaNO3 was used as the mobile phase with a flow rate of 0.6 mL/min. Shodex Pullulan-82 standards were used to construct the calibration curve. To measure the levan product purity, 50 mg levan product was hydrolyzed by 6 mol/L HCl at 100 °C for 15 min. The optical density at 291 nm was measured by a SHIMADZU UV-1800 spectrophotometer (Kyoto, Japan) to determine the concentration of fructose in the hydrolyzate50. The fructose standards were used to construct the calibration curve. The number of the monosaccharide (N) in levan product was defined as: 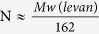 The purity of levan (%) = fructose concentration
The purity of levan (%) = fructose concentration 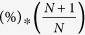 . The living cells were detected by the optical microscope (XSP-8CA, Shanghai, China) and the methylene blue dye after 48 h of cultivation.
. The living cells were detected by the optical microscope (XSP-8CA, Shanghai, China) and the methylene blue dye after 48 h of cultivation.
Additional Information
How to cite this article: Feng, J. et al. Recruiting a new strategy to improve levan production in Bacillus amyloliquefaciens. Sci. Rep. 5, 13814; doi: 10.1038/srep13814 (2015).
Acknowledgments
This work was supported by the National Key Basic Research Program of China (“973” -Program) 2012CB725204, the National Key Technology Support Program 2015BAD16B04, the Natural Science Foundation of China Grant Nos. 31470213 and 31170030, and the Project of Tianjin, China (13JCZDJC27800, 13JCYBJC24900, 13TXSYJC40100 and 14ZCZDSF00009) and the Ph.D. Candidate Research Innovation Fund of Nankai University.
Footnotes
Author Contributions J.F. and C.J.S. designed the research. J.F., Y.Y.G. and Y.F.Q. performed the research. J.F., W.Z., W.X.G., C.Y. and S.F.W. analyzed the data. J.F. and M.F.C. wrote the paper. All of the authors reviewed the manuscript.
References
- Han Y. W. Microbial levan. Adv. Appl. Microbiol. 35, 171–194 (1990). [DOI] [PubMed] [Google Scholar]
- Velázquez-Hernández M. L. et al. Microbial fructosyltransferases and the role of fructans. J. Appl. Microbiol. 106, 1763–1778 (2009). [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. et al. In vitro digestibility and fermentability of levan and its hypocholesterolemic effects in rats. J. Nutr. Biochem. 10, 13–18 (1999). [DOI] [PubMed] [Google Scholar]
- Yoo S. H., Yoon E. J., Cha J. & Lee H. G. Antitumor activity of levan polysaccharides from selected microorganisms. Int. J. Biol. Macromol. 34, 37–41 (2004). [DOI] [PubMed] [Google Scholar]
- Shih I. L., Chen L. D. & Wu J. Y. Levan production using Bacillus subtilis natto cells immobilized on alginate. Carbohyd. Polym. 82, 111–117 (2010). [Google Scholar]
- Dedonder R. Levansucrase from Bacillus subtilis. Method. Enzymol. 8, 500–505 (1966). [Google Scholar]
- Feng J. et al. Construction of a Bacillus amyloliquefaciens strain for high purity levan production. FEMS. Microbiol. Lett. (2015). 10.1093/femsle/fnv079 [DOI] [PubMed] [Google Scholar]
- Ghazi I. et al. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 128, 204–211 (2007). [DOI] [PubMed] [Google Scholar]
- Cao M. F. et al. Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour. Technol. 102, 4251–4257 (2011). [DOI] [PubMed] [Google Scholar]
- Huang M. Y. et al. High-yield levan produced by Bacillus licheniformis FRI MY-55 in high-sucrose medium and its prebiotic effect. J. Pure. Appl. Microbio. 7, 1585–1599 (2013). [Google Scholar]
- Silbir S., Dagbagli S., Yegin S., Baysal T. & Goksungur Y. Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohyd. Polym. 99, 454–461 (2014). [DOI] [PubMed] [Google Scholar]
- Wu F. C., Chou S. Z. & Shih I. L. Factors affecting the production and molecular weight of levan of Bacillus subtilis natto in batch and fed-batch culture in fermenter. J. Taiwan. Inst. Chem. E. 44, 846–853 (2013). [Google Scholar]
- Senthilkumar V., Rameshkumar N., Busby S. J. W. & Gunasekaran P. Disruption of the Zymomonas mobilis extracellular sucrose gene (sacC) improves levan production. J. Appl. Microbiol. 96, 671–676 (2004). [DOI] [PubMed] [Google Scholar]
- Januana S. et al. Functional characterization of sucrose phosphorylase and scrR, a regulator of sucrose metabolism in Lactobacillus reuteri. Food. Microbiol. 36, 432–439 (2013). [DOI] [PubMed] [Google Scholar]
- Shida T., Mukaijo K., Ishikawa S., Yamamoto H. & Sekiguchi J. Production of long-chain levan by a sacC insertional mutant form Bacillus subtilis 327UH. Biosci. Biotechnol. Biochem. 66, 1555–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Ananthalakshmy V. K. & Gunasekaran P. Overproduction of levan in Zymomonas mobilis by using cloned sacB gene. Enzyme. Microb. Tech. 25, 109–115 (1999). [Google Scholar]
- Branda S. S., Chu F., Kearns D. B., Loslck R. & Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59, 1229–1238 (2006). [DOI] [PubMed] [Google Scholar]
- Romero D., Aguilar C., Losick R. & Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA. 107, 2230–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westers L., Westers H. & Quax W. J. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim. Biophys. Acta. 1694, 299–310 (2004). [DOI] [PubMed] [Google Scholar]
- Sloma A. et al. Bacillopeptidase F of Bacillus subtilis: purification of the protein and cloning of the gene. J. Bacteriol. 172, 5520–5521 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. C. et al. Cloning, genetic organization, and characterization of a structural gene encoding bacillopeptidase F from Bacillus subtilis. J. Biol. Chem. 265, 6845–6850 (1990). [PubMed] [Google Scholar]
- Sloma A., Ally A., Ally D. & Pero J. Gene encoding a minor extracellular protease in Bacillus subtilis. J. Bacteriol. 170, 5557–5563 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloma A. et al. Gene encoding a novel extracellular metalloprotease in Bacillus subtilis. J. Bacteriol. 172, 1024–1029 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufo G. A. Jr., Sullivan B. J., Sloma A. & Pero J. Isolation and characterization of a novel extracellular metalloprotease from Bacillus subtilis. J. Bacteriol. 172, 1019–1023 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloma A. et al. Cloning and characterization of the gene for an additional extracellular serine protease of Bacillus subtilis. J. Bacteriol. 173, 6889–6895 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L., Wu X. C. & Wong S. L. Cloning and expression of a novel protease gene encoding an extracellular neutral protease from Bacillus subtilis. J. Bacteriol. 173, 6364–6372 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L. & Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an in vitro-derived deletion mutation. J. Bacteriol. 158, 411–418 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L., Price C. W., Goldfarb D. S. & Doi R. H. The subtilisin E gene of Bacillus subtilis is transcribed from a sigma 37 promoter in vivo. Proc. Natl. Acad. Sci. USA 81, 1184–1188 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller K. L., Bender K. S. & Wall J. D. Development of a markerless genetic exchange system for Desulfovibrio vulgaris hildenborough and its use in generating a strain with increased transformation efficiency. Appl. Environ. Microbiol. 75, 7682–7691 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. et al. Metabolic engineering of Bacillus amyloliquefaciens for poly-gamma-glutamic acid (γ-PGA) overproduction. Microb. Biotechnol. 7, 446–455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda S. S. et al. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J. Bacteriol. 186, 3970–3979 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley N. R. & Lazazzera B. A. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-γ-DL-glutamic acid production and biofilm formation. Mol. Microbiol. 57, 1143–1158 (2005). [DOI] [PubMed] [Google Scholar]
- Dogsa I., Brloznik M., Stopar D. & Mandic-Mulec I. Exopolymer diversity and the role of levan in Bacillus subtilis biofilms. PLoS One. 8, e62044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth R., Reddy C. H. S. S. S., Siddartha G., Ramaiah M. J. & Uppuluri K. B. Review on production, characterization and application of microbial levan. Carbohyd. Polym. 120, 102–114 (2015). [DOI] [PubMed] [Google Scholar]
- Wong S. L., Ye R. & Nathoo S. Engineering and production of streptokinase in a Bacillus subtilis expression-secretion system, Appl. Environ. Microbiol. 60, 517–523 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. C., Lee W., Tran L. & Wong S. L. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J. Bacteriol. 173, 4952–4958 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. C., Ng S. C., Near R. I. & Wong S. L. Efficient production of a functional single-chain antidigoxin antibody via an engineered Bacillus subtilis expression-secretion system, Nat. Biotechnol. 11, 71–76 (1993). [DOI] [PubMed] [Google Scholar]
- Margot P. & Karamata D. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall-associated protease. Microbiology. 142, 3437–3444 (1996). [DOI] [PubMed] [Google Scholar]
- Kawamura F. & Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 160, 442–444 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. C., Qureshi M. H. & Wong S. L. Secretory production and purification of functional full-length streptavidin from Bacillus subtilis. Protein Expr. Purif. 24, 348–356 (2002). [DOI] [PubMed] [Google Scholar]
- Geng W. T. et al. Complete genome sequence of Bacillus amyloliquefaciens LL3, which exhibits glutamic acid-independent production of poly-γ-glutamic acid. J. Bacteriol. 193, 3393–3394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. et al. Functions of poly-gamma-glutamic acid (γ-PGA) degradation genes in γ-PGA synthesis and cell morphology maintenance. Appl. Microbiol. Biot. 98, 6397–6407 (2014). [DOI] [PubMed] [Google Scholar]
- Feng J. et al. Curing the plasmid pMC1 from the poly (γ-glutamic acid) producing Bacillus amyloliquefaciens LL3 strain using plasmid incompatibility. Appl. Biochem. Biotechnol. 171, 532–542 (2013). [DOI] [PubMed] [Google Scholar]
- Asgher M., Javaid Asad M., Rahman S. U. & Legge R. L. A thermostable a-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J. Food. Eng. 79, 950–955 (2007). [Google Scholar]
- Smith K. & Youngman P. Use a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 74, 705–711 (1992). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Chromosome integration of the Vitreoscilla hemoglobin gene (vgb) mediated by temperature-sensitive plasmid enhances γ-PGA production in Bacillus amyloliquefaciens. FEMS. Microbiol. Lett. 343, 127–134 (2013). [DOI] [PubMed] [Google Scholar]
- Miller G. Use of dinitosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31, 426–428 (1959). [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J. Appl. Bact. 33, 207–219 (1970). [DOI] [PubMed] [Google Scholar]
- Goto A. & Kunioka M. Biosynthesis and hydrolysis of Poly(γ-glutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotech. Bioch. 56, 1031–1035 (1992). [DOI] [PubMed] [Google Scholar]
- Zhan D. D. Determination of fructose in fruit juice based on UV-Photometric method. J. Qiongzhou. Univ. 10, 23–27 (2003). [Google Scholar]