Abstract
Objective
To evaluate the outcome of an intraprostatic injection of botulinum toxin-A (BTX-A) in men with refractory chronic prostatitis-associated chronic pelvic-pain syndrome (CP/CPPS) and to compare the efficacy of the transurethral and transrectal routes.
Patients and methods
In an uncontrolled randomised clinical trial conducted in men with refractory CP/CPPS, the patients were classified into two groups according to the route of BTX-A injection; transurethral (group 1, 28 patients) and transrectal ultrasonography-guided (group 2, 35 patients). The chronic prostatitis symptom index (CPSI), maximum urinary flow rate (Qmax) and white blood cell (WBC) count in expressed prostatic secretion (EPS) were measured before and at 3, 6 and 12 months after the injection. A significant clinical improvement (SCI, defined as a reduction of 4 points or a 25% decrease in total CPSI score) was correlated with patient age, prostate volume and symptom duration.
Results
In group 1, the pain and quality-of-life domain scores improved, but statistically significantly only at 6 months. The voiding score improved at all follow-up visits. In group 2 there was a significant improvement in all the CPSI domain scores at all follow-up visits, except for pain, which was insignificantly improved by 12 months. The SCI ratings in groups 1 and 2 were 36%, 79% and 57%, and 49%, 89% and 74% in group 2 at the three follow-up visits, respectively. The Qmax was significantly improved in both groups during the follow-up (except at 12 months in group 1). There was a significant reduction in the mean WBC count in the EPS in patients with inflammatory prostatitis. Both prostate volume and symptom duration were significantly associated with a lower SCI rating.
Conclusion
BTX-A is an available treatment option for patients with refractory CP/CPPS. It is more effective in patients with a small prostate and short symptom duration. The transrectal route provided better results than the transurethral route. More prospective longer term studies are needed.
Abbreviations: BTX-A, botulinum toxin type A; CP/CPPS, chronic prostatitis associated with chronic pelvic pain syndrome; NIH, National Institutes of Health; CPSI, chronic prostatitis symptom index; Qmax, maximum urinary flow rate; WBC, white blood cell; EPS, expressed prostatic secretion; SCI, satisfactory clinical improvement; QoL, quality of life; HPF, high-power field
Keywords: Chronic prostatitis, Pelvic pain syndrome, Botulinum toxin A
Introduction
There has been increasing research into managing the chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), defined ‘as discomfort or pain in the pelvic region for ⩾3 months, with sterile specimen cultures and white blood cell (WBC) counts in prostate-specific specimens, e.g., semen, expressed prostatic secretion (EPS) and urine collected after prostate massage’ [1,2]. Based on previous data, CP is associated with ≈2 million outpatient visits/year in the USA (8% to urologists and 1% to primary-care physicians) with high direct costs for treatment (US$ 4000/patient/year) [3].
According to the USA National Institutes of Health (NIH) classification system, CP/CPPS is ‘prostatitis category III’ [1]. By definition, nonbacterial CP is an inflammatory response in the absence of any detectable causative micro-organism. The exact cause of CP/CPPS (NIH IIIB) is unknown but it might be due to psychological conditions, atypical bacterial infection, immune, neurological, endocrine dysfunction, and dyssynergic voiding [2–10]. As there is no definite cause for CP/CPPS, there is no definite or standard therapy for treatment.
Although the mechanism of action of botulinum toxin-A (BTX-A, produced by the Gram-positive anaerobic bacterium Clostridium botulinum) on prostatic tissues is unclear at the molecular and/or histological levels, it is supposed to affect motor, sensory or glandular function, in addition to an anti-inflammatory action through the modulation of various neurotransmitters released in different kinds of tissue. BTX-A has been assessed for managing voiding disorders due to an overactive bladder, detrusor-external sphincter dyssynergia, or BPH [11]. There are few clinical trials evaluating BTX-A for managing CP/CPPS, and unfortunately most of these trials included few patients or lacked a subjective and objective description of the outcome variables [6–9].
In the present study we aimed to evaluate the results of an intraprostatic injection with BTX-A in patients with refractory CP/CPPS (NIH-III) using clinical, urodynamic and laboratory variables, and compare the transurethral and transrectal routes of injection.
Patients and methods
The study was conducted as an uncontrolled randomised clinical trial on sexually active patients (with an International Index of Erectile Function-5, IIEF-5, score of ⩾17) with CP/CPPS who were managed during the period from January 2008 to December 2013 in our department. Written consent was obtained from all participants after counselling about the nature of the study, including potential benefits and risks. The study protocol was reviewed and approved by our institutional review board.
Patients selected were those with CP/CPPS (IIIA or B), aged <50 years, with a symptom duration of >2 years and who were refractory to other medications (failed responses to antibiotics, α-blockers and anti-inflammatory agents). Patients were excluded if they had bleeding disorders, associated UTI, urolithiasis, urethral stricture, a low maximum urinary flow rate (Qmax, <10 mL/s), a postvoid residual urine volume of >100 mL, and/or were unfit for BTX-A, having associated neuropathy or emphysema.
The preoperative evaluation included a complete history, and clinical examination, with completion of an Arabic version of the CP symptom index (CPSI) [12]. All patients had a urine sample analysed and cultured before and after prostatic massage, and uroflowmetry. Cystoscopy was done under local anaesthesia as an outpatient procedure for patients with a Qmax of <15 mL/s, to exclude organic obstruction.
The included patients were randomised using a coin-tossing technique into two groups according to the route of BTX-A injection (Botox®, Allergan, Inc., Irvine, CA, USA). The intraprostatic injection with BTX-A was delivered under spinal anaesthesia through an endoscopic transurethral route (group 1) or TRUS-guided route (group 2). The preoperative preparation included overnight fasting, a night enema, oral metronidazole 500 mg three times daily (in group 2) and ciprofloxacin 500 mg twice daily, starting 48 h before to 4 days afterwards.
BTX-A was injected after preparation (100 U dissolved in 3 mL of saline), using a cystoscopic needle (group 1) to deliver 0.5 mL bilaterally just distal to the internal sphincter, the mid-lobe, and just proximal to external urethral sphincter (six sites in all). In group 2, the injection was to the base, mid-gland and prostate apex bilaterally (using a Chiba needle 22 G, guided with TRUS, using a 6.5–7.5 MHz transducer).
The follow-up was planned at 3, 6 and 12 months after the injection, and every visit included a clinical evaluation (including CPSI scoring) and measurement of Qmax. A reduction of ⩾4 points or a 25% decrease in total CPSI score was considered as an objective indicator of a satisfactory clinical improvement (SCI) [13,14]. Also, urine samples taken before and after prostatic massage were analysed at the 6- and 12-month visits.
The collected data were analysed statistically using Student’s paired t-test and the Wilcoxon matched-pairs test (for variables within the same group), Spearman correlation to test the association between variables, and the chi-squared test for qualitative data (number and percentage distributions). For all statistical tests P < 0.05 was taken to indicate statistical significance.
Results
In all, 67 men with CP/CPPS were included, 32 in group 1 and 35 in group 2, although four patients in group 1 were unavailable for follow-up and were excluded from the analysis. Ten patients in group 1 and 15 in group 2 had inflammatory CP/CPPS (type NIH-IIIA). The baseline data are shown in Table 1.
Table 1.
The baseline data of patients in groups 1 and 2.
| Variable | Group 1 | Group 2 | P |
|---|---|---|---|
| Number of patients | 28 | 35 | |
| CP/CPPS IIIA/B | 10/18 | 15/20 | 0.75⁎ |
| Mean (SD) | |||
| Age (years) | 42.0 (5.2) | 41.4 (5.0) | 0.64† |
| Prostate volume | 37.9 (5.14) | 36.4 (4.86) | 0.13† |
| Duration of symptoms (years) | 7.23 (2.13) | 7.58 (3.76) | 0.22† |
| Median (range) WBCs/HPF | 84 (43–93) | 63 (40–89) | 0.06† |
Chi-squared.
Unpaired t-test.
Complications after the procedure included dysuria, which was slightly exacerbated in 12 patients (43%) in group 1 and 17 (49%) in group 2, and all were treated with ciprofloxacin and NSAIDs. Also, haematuria was noted in six (17%) and three (9%) patients in groups 1 and 2, respectively, that resolved spontaneously within a week. Two patients in group 1 (7%) and five in group 2 (14%) developed haematospermia that also resolved spontaneously. None of patients developed de novo sexual dysfunction (no deterioration of the IIEF-5 score) at any of the follow-up visits.
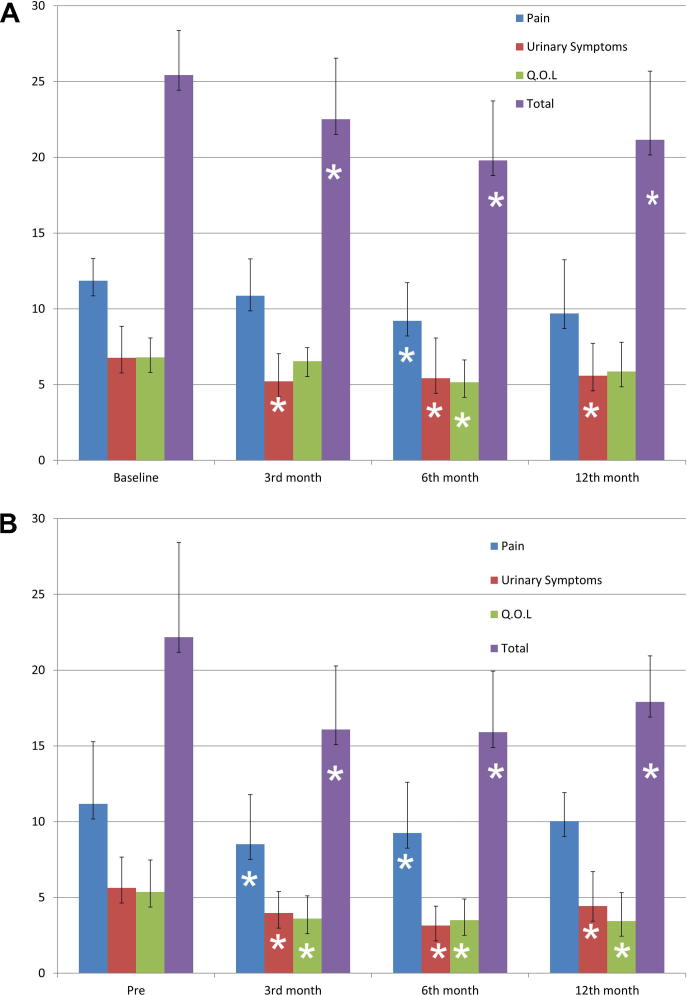
In group 1 the mean scores for the pain and quality-of-life (QoL) domains decreased during the follow-up (up to 12 months) but this was statistically significant only at 6 months. The voiding score domain decreased significantly at all follow-up visits. Based on these changes, the symptom-scale scores and total CPSI scores decreased significantly at all three follow-up visits (Fig. 1A).
Figure 1.
The CPSI domain scores for group 1 (A) and group 2 (B) at the baseline and the three follow-up visits. ∗Significantly different at P < 0.05.
In group 2 the pain, voiding and QoL domain scores were significantly reduced at all three follow-up visits (except the pain domain at 12 months). Consequently the symptom-scale scores and total CPSI scores also decreased significantly at all the follow-up visits (Fig. 1B).
The proportion of patients with a SCI (>4-point reduction in total CPSI score) was 36%, 79% and 57% for group 1 (at the three follow-up visits, respectively) and 49%, 89% and 74% in group 2, respectively, with no statistically significant differences between the groups (P > 0.05).
The correlation between different factors that might affect the outcome, i.e., the NIH class (IIIA or IIIB), baseline CPSI scores, duration of symptoms and TRUS-estimated prostate volume, showed a significant negative correlation between the size of the prostate and percentage of responders at 3 months (rs = −0.34, 95% CI −0.53 to −0.07; P < 0.05) that was more significant at 6 months (r = −0.59, 95% CI −0.73 to −0.41; P < 0.05). There was also a negative correlation between the duration of symptoms and percentage of responders at 6 months (r = −0.31, 95% CI −0.51 to −0.08; P < 0.05). Thus both a smaller prostate and shorter duration of symptoms are predictive of better treatment results.
The Qmax was significantly improved in group 1 at 3 and 6 months (23.5 and 24.9 mL/s, vs. the baseline value of 20.1 mL/s) and insignificantly different at 12 months (22.9 mL/s). In group 2 the Qmax was statistically significantly better at all the follow-up visits (25.5, 25.8 and 24.2 mL/s, vs. 21.1 mL/s before injection).
The results of the urine analyses at 6 and 12 months showed that none of the patients with NIH-IIIB prostatitis in both groups developed >10 inflammatory cells/high-power field (HPF) in the EPS. However, there was a significant reduction in the mean WBC count in the EPS (in both groups) after BTX-A injection at 6 months (median 40 and 38, vs 84 and 63 WBCs/HPF for both groups, respectively) and at 12 months (median 39 and 41 WBCs/HPF).
Discussion
The management of refractory CP/CPPS is frustrating and disappointing both for patients and physicians. Many treatment protocols have been assessed previously, e.g., α-blocker therapy and antimicrobial therapy, but have failed to provide an acceptable outcome [15–21], and the search for other drug regimens for such patients has gained favour among physicians [22,23].
To our knowledge there are few clinical trials evaluating the role of BTX-A in patients with CP/CPPS, and only one was designed as a controlled study. These studies used different routes of injections (transurethral, transrectal and transperineal), different doses of BTX-A (30–200 U) and different outcome variables (uroflowmetry, visual pain analogue score, pelvic floor tenderness, Global Response Assessment and total CPSI) [24–29].
Compared to previous studies, we did not use the transperineal approach because of concerns about drug leakage outside the prostate, as localising the injection within the prostate is sometimes difficult. In both groups we used six injection sites that included the prostatic glands, and external and internal sphincters.
The transurethral injection with BTX-A was associated with an improvement in the CPSI pain domain score that was statistically significant at 6 months (similar to the QoL domain). There was also a significant improvement in the voiding domain score at all follow-up visits that was considered to be the permanent factor reducing the total CPSI. Group 2 (transrectal BTX-A) showed an early significant improvement in the pain and voiding domains of the CPSI. Similar to group 2, the pain score increased again, but not significantly, at 12 months.
Many studies show that BTX-A relieves pain in animal and non-genitourinary pain models (myofascial pain, cervical dystonia, neuropathic pain, etc.) and this could be applicable to CPPS [30]. In addition, pain, which is a major constituent of interstitial cystitis, was improved by a detrusor injection with BTX-A [31]. In rat models of prostatic pain, the pain-response pathways were reduced significantly by BTX-A, mainly that of cyclo-oxygenase-2 [32].
The pain-relief effect of BTX-A might result from its muscle-relaxing effect. Hence, in the studies by Maria et al. [24] and Zermann et al. [25], the BTX-A injection was directed to the region of the external urethral sphincter. However, other studies showed that BTX-A has a direct effect on pain through the modulation of pain neurotransmitters [32,33].
Regarding the voiding domains, BTX is thought to block pre-synaptic acetylcholine release at the neuromuscular junction, resulting in temporary chemo-denervation and muscle relaxation. Also, BTX inhibits the release of noradrenaline, suggesting a potential role in smooth muscle relaxation of the lower urinary tract [34].
The maximum percentage of responders (>4-point reduction in their total CPSI) after BTX-A injection was at 6 months (79% and 89% in groups 1 and 2, respectively). This delayed maximal response could be because the anti-nociceptive properties of BTX-A usually take longer to be clinically evident [31].
The statistical correlation showed that smaller prostates were associated with a better clinical improvement. This correlation was evident at 3 months and clinically significant at 6 months. This effect was described previously in a clinical study, treating BPH with BTX-A injections, but a high BTX-A dose was required to induce a significant tissue apoptosis, that is not necessary for managing CP/CPPS [35]. Another significant factor identified was the duration of symptoms, such that patients with a shorter duration had a better improvement in their pain and voiding scores after injection. Two patients who had no pain (zero score) at 6 months had a mean symptom duration of 2 and 3 years only.
For uroflowmetry, there was an increase in Qmax associated with the improvement in the voiding score domain. However, in group 1 the significant improvement in voiding score at 12 months was not associated with a significant improvement in the uroflowmetric pattern.
In conclusion, although the mechanism of action of BTX-A is not completely understood, it is an available, safe and tolerable treatment option for patients with refractory CP/CPPS. It is more effective for patients with a small prostate and short duration of symptoms. In terms of improving the CPSI, the transrectal route gave better results than the transurethral route. A further longer term evaluation, blind and/or controlled studies are required to validate these early results.
Conflict of interest
None.
Source of funding
None.
PROSTATIC DISORDERS
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Fall M., Baranowski A.P., Elneil S., Engeler D., Hughes J., Messelink E.J. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57:35–48. doi: 10.1016/j.eururo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Mardh P., Colleen S., Holmquist B. Chlamydia in chronic prostatitis. Br Med J. 1972;4:361. doi: 10.1136/bmj.4.5836.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weidner W., Diemer T., Huwe P., Rainer H., Ludwig M. The role of Chlamydia trachomatis in prostatitis. Int J Antimicrob Agents. 2002;19:466–470. doi: 10.1016/s0924-8579(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 4.Ouzounova-Raykova V., Ouzounova I., Mitov I.G. May Chlamydia trachomatis be an aetiological agent of chronic prostatic infection? Andrologia. 2010;42:176–181. doi: 10.1111/j.1439-0272.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 5.Ohkawa M., Yamaguchi K., Tokunaga S., Nakashima T., Fujita S. Ureaplasma urealyticum in the urogenital tract of patients with chronic prostatitis or related symptomatology. Br J Urol. 1993;72:918–921. doi: 10.1111/j.1464-410x.1993.tb16297.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1–8. doi: 10.1136/sti.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickel J.C., Downey J., Clark J., Casey R.W., Pammerville P.J., Barkin J. Levofloxacin for chronic prostatitis/chronic pelvic pain syndrome in men: a randomized placebo-controlled multicenter trial. Urology. 2003;62:614–617. doi: 10.1016/s0090-4295(03)00583-1. [DOI] [PubMed] [Google Scholar]
- 8.Wenninger K., Heiman J.R., Rothman I., Berghuis J.P., Berger R.E. Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol. 1996;155:965–968. [PubMed] [Google Scholar]
- 9.Gatenbeck L., Aronsson A., Dahlgren S., Johansson B., Stromberg L. Stress stimuli-induced histopathological changes in the prostate: an experimental study in the rat. Prostate. 1987;11:69–76. doi: 10.1002/pros.2990110109. [DOI] [PubMed] [Google Scholar]
- 10.Doble A., Walker M.M., Harris J.R., Taylor-Robinson D., Witherow R.O. Intraprostatic antibody deposition in chronic abacterial prostatitis. Br J Urol. 1990;65:598–605. doi: 10.1111/j.1464-410x.1990.tb14827.x. [DOI] [PubMed] [Google Scholar]
- 11.Parker J., Buga S., Sarria J.E., Spiess P.E. Advancements in the management of urologic chronic pelvic pain: what is new and what do we know? Curr Urol Rep. 2010;11:286–291. doi: 10.1007/s11934-010-0121-9. [DOI] [PubMed] [Google Scholar]
- 12.El-Nashaar A., Fathy A., Zeedan A., Al-Ahwany A., Shamloul R. Validity and reliability of the Arabic version of the National Institutes of Health chronic prostatitis symptom index. Urol Int. 2006;77:227–231. doi: 10.1159/000094814. [DOI] [PubMed] [Google Scholar]
- 13.Propert K.J., Litwin M.S., Wang Y., Alexander R.B., Calhoun E., Nickel J.C. Responsiveness of the National Institutes of Health chronic prostatitis symptom index (NIH-CPSI) Qual Life Res. 2006;15:299–305. doi: 10.1007/s11136-005-1317-1. [DOI] [PubMed] [Google Scholar]
- 14.Nickel J.C. Treatment of chronic prostatitis/chronic pelvic pain syndrome. Int J Antimicrob Agents. 2008;31(Suppl. 1):S112–S116. doi: 10.1016/j.ijantimicag.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickel J.C., Downey J., Johnston B., Clark J. Predictors of patient response to antibiotic therapy for the chronic prostatitis/chronic pelvic pain syndrome: a prospective multicenter clinical trial. J Urol. 2001;165:1539–1544. [PubMed] [Google Scholar]
- 16.Erickson B.A., Schaeffer A.J., Van Le B. Chronic prostatitis. Clin Evid (Online) 2008 May 22 pii: 1802. [PMC free article] [PubMed] [Google Scholar]
- 17.Dahm P., Dmochowski R.R. Treatment of chronic prostatitis/chronic pelvic pain syndrome. In: Nickel J.C., editor. Evidence-based urology. Wiley-Blackwell; 2010. pp. 112–116. [Google Scholar]
- 18.Leskinen M., Lukkarinen O., Marttila T. Effects of finasteride in patients with inflammatory chronic pelvic pain syndrome: a double-blind, placebo-controlled, pilot study. Urology. 1999;53:502–505. doi: 10.1016/s0090-4295(98)00540-8. [DOI] [PubMed] [Google Scholar]
- 19.Doble A. An evidence-based approach to the treatment of prostatitis: is it possible? Curr Urol Rep. 2000;1:142–147. doi: 10.1007/s11934-000-0049-6. [DOI] [PubMed] [Google Scholar]
- 20.Nickel J.C., Sorensen R. Transurethral microwave thermotherapy for nonbacterial prostatitis: a randomized double-blind sham controlled study using new prostatitis specific assessment questionnaires. J Urol. 1996;155:1950–1954. doi: 10.1016/s0022-5347(01)66056-7. [DOI] [PubMed] [Google Scholar]
- 21.Peehl D.M., Wong S.T., Rubin J.S. KGF and EGF differentially regulate the phenotype of prostatic epithelial cells. Growth Regul. 1996;6:22–31. [PubMed] [Google Scholar]
- 22.Zvara P., Folsom J.B., Plante M.K. Minimally invasive therapies for prostatitis. Curr Urol Rep. 2004;5:320–326. doi: 10.1007/s11934-004-0060-4. [DOI] [PubMed] [Google Scholar]
- 23.El-Hakim A. Chronic prostatitis/chronic pelvic pain syndrome: is there a role for local drug infiltration therapy? J Endourol. 2004;18:227–231. doi: 10.1089/089277904773582804. [DOI] [PubMed] [Google Scholar]
- 24.Maria G., Destito A., Lacquaniti S., Bentivoglio A.R., Brisinda G., Albanese A. Relief by botulinum toxin of voiding dysfunction due to prostatitis. Lancet. 1998;352:625. doi: 10.1016/S0140-6736(05)79580-5. [DOI] [PubMed] [Google Scholar]
- 25.Zermann D.H., Ishigooka M., Schubert J., Schmidt R.A. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur Urol. 2000;38:393–399. doi: 10.1159/000020314. [DOI] [PubMed] [Google Scholar]
- 26.Shin S., Park D. Multi-regional injections of low-dose botulinum toxin A for men with chronic pelvic pain syndrome (abstract 104) J Urol. 2006;175(Suppl. 4) [Google Scholar]
- 27.Gottsch H.P., Yang C.C., Berger R.E. A review of botulinum toxin use for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:265–270. doi: 10.1007/s11934-010-0118-4. [DOI] [PubMed] [Google Scholar]
- 28.Bschleipfer T., Wagenlehner F.M., Weidner W. Intraprostatic botulinum toxin A injection in chronic prostatitis and chronic pelvic pain syndrome (CP/CPPS) Urol A. 2007;46:1030–1032. doi: 10.1007/s00120-007-1384-8. [DOI] [PubMed] [Google Scholar]
- 29.Gottsch H.P., Yang C.C., Berger R.E. A pilot study of botulinum toxin A for male chronic pelvic pain syndrome. Scand J Urol Nephrol. 2011;45:72–76. doi: 10.3109/00365599.2010.529820. [DOI] [PubMed] [Google Scholar]
- 30.Jeynes L.C., Gauci C.A. Evidence for the use of botulinum toxin in the chronic pain setting – a review of the literature. Pain Pract. 2008;8:269–276. doi: 10.1111/j.1533-2500.2008.00202.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith C.P., Radziszewski P., Borkowski A., Somogyi G.T., Boone T.B., Chancellor M.B. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology. 2004;64:871–875. doi: 10.1016/j.urology.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 32.Chuang Y.C., Yoshimura N., Huang C.C., Wu M., Chiang P.H., Chancellor M.B. Intraprostatic botulinum toxin a injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J Urol. 2008;180:742–748. doi: 10.1016/j.juro.2007.07.120. [DOI] [PubMed] [Google Scholar]
- 33.Chuang Y.C., Yoshimura N., Huang C.C., Chiang P.H., Chancellor M.B. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- 34.Lazzeri M. Repeated botulinum toxin injections: a new answer for further questions. Eur Urol. 2007;52:1571–1573. doi: 10.1016/j.eururo.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Chuang Y.C., Chancellor M.B. The application of botulinum toxin in the prostate. J Urol. 2006;176:2375–2382. doi: 10.1016/j.juro.2006.07.127. [DOI] [PubMed] [Google Scholar]