Abstract
Objective
To evaluate the outcome of the expectant management of ureteric stones and to determine the factors predictive of the spontaneous passage of stones.
Patients and methods
We retrospectively reviewed the medical records of patients who had ureteric stones of ⩽10 mm and who were treated conservatively at our institutions during the period 2008–2013. The stone-passage rate and time, and different clinical, laboratory and radiological variables, were analysed.
Results
In all, 163 patients with ureteric stones were enrolled in the study, of whom 127 (77.9%) passed their stones spontaneously, with a mean (SD) passage time of 24.0 (8.09) days. The cumulative stone-passage rate was 1.6%, 15%, 41.7%, 72.4%, 89.8% and 98.4% at 7, 14, 21, 28, 35 and 42 days from the first presentation, respectively. Patients with a high pain-scale score, stones of ⩽5 mm, a lower ureteric stone, a high white blood cell count and those with absent computed tomography (CT) findings of perinephric fat stranding (PFS) and tissue-rim sign (TRS) had a higher likelihood of spontaneous stone passage. Patients with stones of ⩽5 mm, stones in the lower ureter and those with no PFS had a shorter spontaneous passage time. In a multivariate analysis the absence of PFS and TRS were the only significant predictors for spontaneous stone passage (P < 0.001 and 0.002, respectively).
Conclusions
The spontaneous ureteric stone-passage rate and time varies with different factors. The absence of CT findings of PFS and TRS are significant predictors for stone passage, and should be considered when choosing the expectant management.
Abbreviations: PFS, perinephric fat stranding; RBC, red blood cells; WBC, white blood cells; SPR, spontaneous (stone) passage rate; TRS, tissue-rim sign; UHCT, unenhanced helical CT; BMI, body mass index
Keywords: Predictors, Ureteric calculi, Spontaneous passage
Introduction
The global incidence of urinary stone disease is estimated to be 2–20% [1], whilst the lifetime prevalence of urolithiasis is reported to be 5–12% [2]. The choice between watchful waiting and active management until spontaneous passage is the main problem for the urologist when managing patients with ureteric stones [3]. Although it is documented that almost 60% of ureteric stones could pass spontaneously [4], 40% could not. The period until stone passage, which is supposed to be ≈4 weeks [5], might expose the patient to unwanted complications, like recurrent attacks of renal colic or UTIs. The decision to use expectant treatment depends on various factors, including the size of the stone, its location and patient preference [6]. Therefore, identifying particular predictive factors would aid the urologist in deciding whether to manage the patient conservatively or not. The aim of the present study was to evaluate the outcome of the expectant management of ureteric stones, and to determine the clinical, laboratory and radiological factors that predict spontaneous stone passage.
Patients and methods
We retrospectively reviewed the medical records and imaging studies of adult patients with solitary ureteric stones who presented to the emergency department between 2008 and 2013, and who had decided to be managed expectantly, based on their preference or by our suggestion. As per the protocol of our hospital, all patients presenting with renal colic had a detailed medical history taken, a thorough physical examination, urine analysis, a complete blood count, blood urea and serum creatinine measurement, a plain abdominal X-ray, and unenhanced helical CT (UHCT). The diagnosis of ureteric stones was based on the presence of an unequivocal finding of a stone on UHCT, and the stone size was defined by its largest diameter. Pregnant women, patients with bilateral ureteric stones, a solitary kidney, a stone of ⩾10 mm, moderate or severe hydronephrosis, active UTI, renal insufficiency and those with a comorbidity impeding the use of analgesics, were excluded from expectant management. The primary pain was managed by an intramuscular injection with diclofenac 75 mg and/or tramadol hydrochloride 50–100 mg. If the pain improved the patients were sent home on an oral analgesic and asked to attend a follow-up regularly for 45 days. At the follow-up patients were asked about any symptoms or complications, pain severity and frequency, and if they had seen the stone or stone fragments during urination. Also, urine analysis, a serum creatinine measurement, a plain abdominal X-ray and urinary ultrasonography were done routinely at each follow-up visit. For patients with radiolucent stones, UHCT was done at ≈6 weeks from the first stone episode if the stone was not expelled.
The data retrieved included the patients’ characteristics, i.e., age, gender, smoking habit, body mass index (BMI) and pain severity. Also, laboratory data were collected, i.e., urinary pH, urinary white blood cell (WBC) and red blood cell (RBC) count, blood total WBC count, blood urea and serum creatinine levels. Similarly, we collected data on the stone characteristics and reno-ureteric units, i.e., the number, size and level of stone, and state of the pelvi-calyceal system. The CT findings of a tissue-rim sign (TRS) and perinephric fat stranding (PFS) were also recorded. Finally, the spontaneous stone-passage rate (SPR) and time, and need for intervention, were recorded.
The pain severity was calculated using a verbal numerical rating scale from 1 to 10. The ureter was divided into the upper ureter, defined as the segment above the upper border of the sacroiliac joint, the middle ureter, i.e., the segment over the sacroiliac joint, and lower ureter, i.e., the segment below the lower border of the sacroiliac joint. The TRS was considered positive if there was annular soft-tissue attenuation (20–40 Hounsfield units) of ⩾2 mm surrounding the stone. The PFS was considered positive if there were many thick strands of soft-tissue attenuation in the perinephric space.
Spontaneous passage of the stone was considered to have occurred by the patient’s observation of a stone in the urine, and with no primary stone detectable on the imaging study. For patients with a stone-free ureter on the last imaging study, but unnoticed stone expulsion, the date of the last positive stone detection was recorded.
The chi-squared test or linear-by-linear association test was used to compare categorical variables. Student’s t-test or one-way anova was used to compare continuous variables. Univariate and multivariate logistic regression analyses were used to identify factors that affected the spontaneous passage of ureteric stones. The regression analysis results are shown as the odds ratio and 95% CI. In all tests, P < 0.05 was considered to indicate statistical significance.
Results
Over the 5-year period 236 patients were diagnosed as having ureteric stones and were initially managed conservatively. From these we excluded 73 patients who had incomplete medical records, no CT films, who were receiving medication with a known stone expulsive effect, or who were lost to follow-up. The data from the remaining 163 patients were analysed, and they included 105 males (64.4%) and 58 females (35.6%), with a mean (SD) age of 36.4 (12.55) years, a mean BMI of 26.1 (4.28) kg/m2 and a mean (SD, range) pain-scale score of 4.8 (1.82, 3–9). Only eight patients needed hospital admission at their first presentation due to the persistence of pain. The most common stone location was in the lower ureter (47.9%), followed by the middle ureter (30.1%) and upper ureter (22.1%). The mean (SD) stone size was 5.7 (1.84) mm, with 57.1% on the left side and 42.9% on the right side. Hydronephrosis was present in 52 (31.9%) patients, and on UHCT there was PFS in 31 (19%) and TRS in 63 (38.7%) patients.
Of the 163 patients, 127 (77.9%) passed their stones spontaneously, while the remaining 36 did not. Patients who failed to pass their stones were treated by ureteroscopic lithotripsy (18), ESWL (13) and by medical treatment (five). The characteristics of these two groups of patients are summarised in Table 1. Patients who passed their stones spontaneously had a significantly lower BMI, a higher pain-scale score, smaller stones, a stone in the lower or middle ureter, no hydronephrosis, and no PFS or TRS on UHCT.
Table 1.
A comparison of patients according to the success of spontaneous passage.
| Mean (SD) or n (%) variable | Stone passage |
P | |
|---|---|---|---|
| Yes | No | ||
| No. of patients | 127 | 36 | |
| Age (years) | 35.4 (12.40) | 39.8 (12.64) | 0.063 |
| Gender | |||
| M | 81 (63.8) | 24 (66.7) | 0.749 |
| F | 46 (36.2) | 12 (33.3) | |
| BMI (kg/m2) | 25.7 (4.23) | 27.6 (4.16) | 0.016 |
| Smoking | |||
| No | 86 (67.7) | 25 (69.4) | 0.844 |
| Yes | 41 (32.3) | 11 (30.6) | |
| Pain scale score | 4.98 (1.81) | 4.31 (1.77) | 0.048 |
| Side of stone | |||
| Right | 51 (40.2) | 19 (52.8) | 0.177 |
| Left | 76 (59.8) | 17 (47.2) | |
| Level of stone | |||
| Upper | 12 (9.4) | 24 (66.7) | <0.001 |
| Middle | 40 (31.5) | 9 (25) | |
| Lower | 75 (59.1) | 3 (8.3) | |
| Size of stone, mm | 5.13 (1.43) | 7.66 (1.77) | <0.001 |
| Radio-opaque stone | |||
| No | 45 (35.4) | 15 (41.7) | 0.494 |
| Yes | 82 (64.6) | 21 (58.3) | |
| Hydronephrosis | |||
| No | 95 (74.8) | 16 (44.4) | 0.001 |
| Yes | 32 (25.2) | 20 (55.6) | |
| Gross haematuria | |||
| No | 70 (55.1) | 24 (66.7) | 0.216 |
| Yes | 57 (44.9) | 12 (33.3) | |
| Urine pH | 6.17 (0.61) | 6.29 (0.64) | 0.287 |
| Urinary | |||
| RBC count (/hpf) | 22.4 (12.27) | 21.9 (12.73) | 0.844 |
| WBC count (/hpf) | 9.87 (4.48) | 10.81 (5.73) | 0.304 |
| Serum | |||
| WBC count (/hpf) | 9949 (3036.1) | 8644 (2149.3) | 0.017 |
| Creatinine, μmol/L | 103.4 (22.53) | 104.6 (9.69) | 0.755 |
| PFS | |||
| No | 121 (95.3) | 11 (30.6) | <0.001 |
| Yes | 6 (4.7) | 25 (69.4) | |
| TRS | |||
| No | 94 (74) | 6 (16.7) | <0.001 |
| Yes | 33 (26) | 30 (83.3) | |
/hpf, per high-power field.
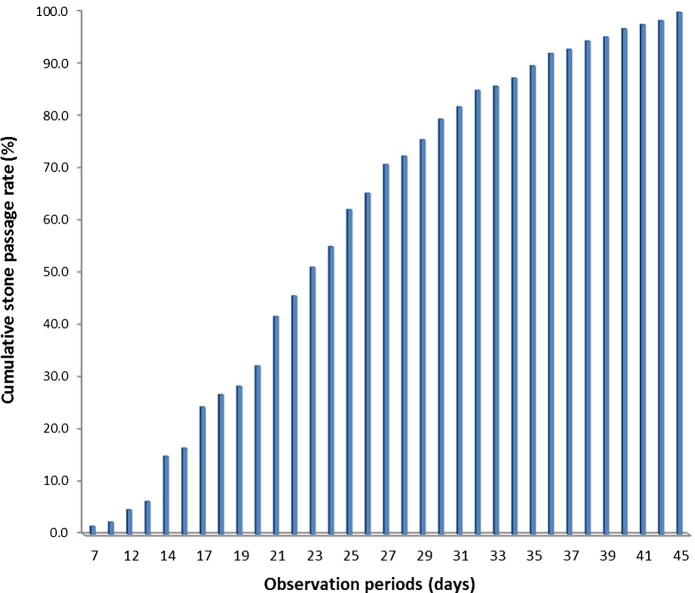
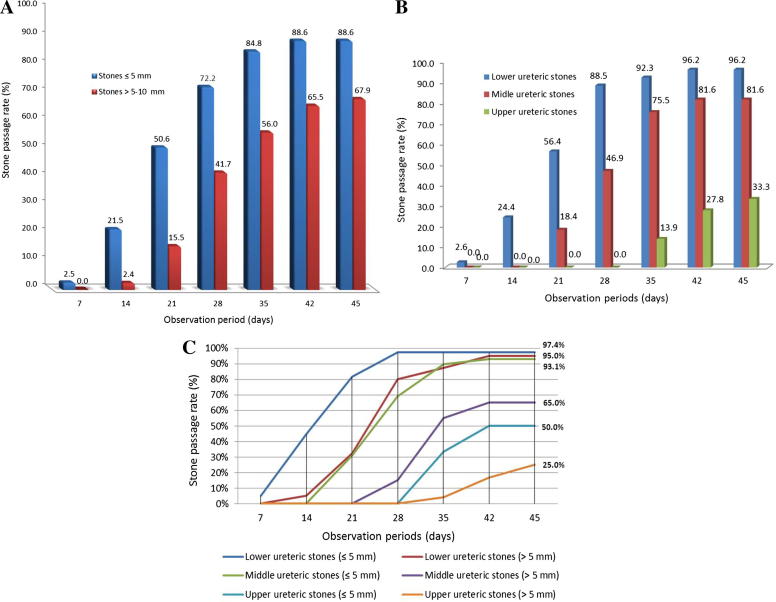
The cumulative SPR was 1.6%, 15%, 41.7%, 72.4%, 89.8% and 98.4% at 7, 14, 21, 28, 35 and 42 days from the first presentation, respectively (Fig. 1). The SPR was 88.6% for stones of ⩽5 mm and 67.9% for stones of 5–10 mm (P = 0.001). According to the level of the stone, the SPR was 33.3% for stones in the upper ureter, 81.6% in the middle ureter and 96.1% in the lower ureter (P < 0.001; Scheffe posthoc test P < 0.001) for the three stone location groups. The SPRs, according to the size and location of stones, during the observation period are shown in Fig. 2(A–C).
Figure 1.
The cumulative SPR during the observational period.
Figure 2.
The SPR during the observational period of: (A) stones of <5 mm, and >5–10 mm; (B) of upper, middle and lower ureteric stones; (C) according to stone size and location.
The overall mean (SD) spontaneous passage time was 24.1 (8.09) days; for stones of ⩽5 and 5–10 mm it was 21.3 (7.38) and 27.5 (7.62) days, respectively, and for stones in the upper, middle and lower ureter it was 37.2 (5.17), 27.7 (5.76) and 20.4 (6.57) days, respectively (Spearman’s rho = 0.418 for size and 0.601 for location; both P < 0.001). The spontaneous passage time according to different variables is summarised in Table 2.
Table 2.
The spontaneous passage time according to different variables.
| Variable | Mean (SD) passage time (days) | P |
|---|---|---|
| Side of stone | 0.828 | |
| Right | 24.3 (7.76) | |
| Left | 23.9 (8.34) | |
| Level of stone | <0.001 | |
| Upper ureter | 37.2 (5.17) | |
| Middle ureter | 27.1 (5.76) | |
| Lower ureter | 20.4 (6.57) | |
| Size of stone (mm) | <0.001 | |
| ⩽5 | 21.3 (7.38) | |
| >5–10 | 27.5 (7.62) | |
| Radio-opaque stone | 0.581 | |
| No | 24.6 (8.18) | |
| Yes | 23.8 (8.07) | |
| Hydronephrosis | 0.118 | |
| No | 23.4 (7.60) | |
| Yes | 26.0 (9.24) | |
| PFS | 0.020 | |
| No | 23.7 (7.91) | |
| Yes | 31.5 (8.71) | |
| TRS | 0.051 | |
| No | 23.2 (7.95) | |
| Yes | 26.4 (8.13) | |
For predictors of spontaneous passage, the significant factors identified on univariate analysis were the serum WBC count, location of stone, size of stone, hydronephrosis, PFS and TRS. Patients with a high serum WBC count (P = 0.006), lower ureteric stones (P < 0.001), no hydronephrosis (P < 0.001), no PFS (P < 0.001), and no TRS (P < 0.001) had a higher likelihood of spontaneous stone passage. On the multivariate analysis only absent PFS (P < 0.001) and no TRS (P = 0.002) were significant (Table 3).
Table 3.
Univariate and multivariate analyses of the factors predicting the spontaneous passage of ureteric stones.
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age | 0.65 (0.31–1.37) | 0.26 | – | |
| Sex | 0.88 (0.40–1.92) | 0.85 | – | |
| BMI | 0.52 (0.24–1.14) | 0.13 | – | |
| Smoking | 1.08 (0.49–2.41) | 0.51 | – | |
| Pain scale | 0.23 (0.03–1.82) | 0.19 | – | |
| Haematuria | 0.61 (0.28–1.33) | 0.25 | – | |
| Urine pH | 0.49 (0.23–1.04) | 0.08 | – | |
| Urine WBC count | 0.67 (0.21–2.10) | 0.6 | – | |
| Serum WBC count | 2.91 (1.28–6.59) | 0.006 | 0.22 (0.04–1.1) | 0.06 |
| Serum creatinine level | 1.31 (0.62–2.76) | 0.57 | – | |
| Radio-opaque stone | 0.77 (0.36–1.64) | 0.59 | – | |
| Level of stone | 10.1 (3.22–31.60) | <0.001 | 0.07 (0.02–0.38) | 0.2 |
| Side of stone | 0.6 (0.29–1.26) | 0.19 | – | |
| Size of stone | 2.82 (1.42–5.62) | 0.002 | 0.24 (0.06–0.98) | 0.47 |
| Hydronephrosis | 3.71 (1.72–8.01) | <0.001 | 2.18 (0.57–8.33) | 0.26 |
| PFS | 45.8 (15.5–135.5) | <0.001 | 27.05 (5.79–126.5) | <0.001 |
| TRS | 14.2 (5.44–37.27) | <0.001 | 8.3 (2.15–32.1) | 0.002 |
Variables included in the multivariate model were those significant on univariate analysis.
Discussion
Various treatment methods are available for ureteric stones, varying from active surveillance or minimally invasive management to open or laparoscopic intervention. Avoiding operative stress, or merely compliance with medication, are of concern to both the physician and patient. However, the problem is how and when to make the decision. Delaying this decision can be associated with an increased risk of complications. Therefore, predicting the possibility of spontaneous stone passage is the key to deciding to opt for active surveillance of a ureteric stone.
We evaluated the results of expectant treatment in patients with ureteric stones of ⩽10 mm, and the factors associated with a high possibility of spontaneous stone passage. The most widely studied factor for the spontaneous passage of a ureteric stone is stone size. Preminger et al. [7] reported a steady proportional relationship between the chance of stone passage and its size. The same concept was previously reported, before the era of ESWL, by Hubner et al. [8] in their meta-analysis of 2704 patients. Other studies showed a SPR of 76–100% for stones of ⩽5 mm in diameter, and 0–60% for stones of ⩾5 mm [9,10]. In the present study the overall SPR was 77.9% over an observational period of 45 days. The SPR was significantly higher for stones of ⩽5 mm than for those of >5–10 mm (P = 0.001).
Another factor that was reported in different studies was the site of the stone. In 2002, Coll et al. [9] related the spontaneous passage of a ureteric stone to its location, and reported a SPR of 48%, 60% and 75% for the proximal, middle and distal ureter, respectively. In 2013, the European Association of Urology published their guidelines, stating a SPR for ureteric stones of 25%, 45% and 70% for the upper, middle and lower ureter, respectively [5].
In the present study, although there was a high and significant correlation between stone size and location and SPR, these two factors were not significant in the multivariate analysis.
Many authors advocated intervention to remove the stone if there was no spontaneous passage within 4 weeks of the start of symptoms [3,8,11]. This would support the preservation of kidney function, as otherwise there might be renal deterioration. However, in the present analysis, the SPR for stones of <5 mm was up to 98% at 42 days after the first presentation. Therefore, as long as the kidney status is stable, we recommend a longer follow-up of up to 6 weeks after the onset of symptoms.
Smoking habit has been assessed in some studies as a possible influencing factor in spontaneous stone expulsion. Nicotine is said to alter ureteric peristalsis, probably through its effect on the cholinergic receptors present in the ureter. Recently, Fazlioglu et al. [12] reported that smoking had no effect. Similarly, we found no significant difference between smokers and non-smokers in the present study.
In their prospective study of 265 patients, Sfoungaristos et al. [2] found that the WBC count was a highly significant contributing factor for predicting spontaneous stone passage. They hypothesised that the interaction between the stone and ureteric mucosa could result in an inflammatory process at the stone site, which would be greater if the stone could move, and less if the stone was impacted and incapable of moving, thus eliciting an inflammatory reaction. However, the present results did not give the same conclusions and the serum WBC count did not seem to be a significant factor in the multivariate analysis.
The detection of the TRS mostly depends on stone size, being more evident with small ureteric stones, and it is an indicator of ureteric obstruction [13]. Hence we assessed it as a possible predictor. The absence of the TRS was significantly associated with a greater SPR (P < 0.001). Similar results were reported by Mokhless et al. [14], as they noted that an increased TRS and degree of hydronephrosis were associated with the failure of stone passage, and in which case the need for intervention was increased. Another factor is the PFS, which was reported, in combination with unilateral ureteric dilatation on spiral CT, to be associated with a 97% positive predictive value for stone disease [15]. The absence of both these features was associated with a 93% negative predictive value to exclude stone disease [16]. Again, we found PFS to be a highly significant predictor of spontaneous stone passage.
The present study has some limitations. First, the exact measurement of stone size needs to be validated, as we relied on the simple visual estimation of the largest stone diameter on UHCT. Second, because few studies have used the TRS and PFS as predictors of stone passage, and that our results emphasise their significance, further studies with more patients are needed.
In conclusion, many factors could influence the spontaneous passage of a ureteric stone in the course of expectant treatment. However, the absence of PFS and TRS are significant predictors for stone passage. These signs, that can be easily identified at the initial evaluation of the patient, should be considered when deciding to use expectant management.
Conflict of interest
None.
Source of funding
None.
STONES/ENDOUROLOGY
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Picozzi S.C., Marenghi C., Casellato S., Ricci C., Gaeta M., Carmignani L. Management of ureteral calculi and medical expulsive therapy in emergency departments. J Emerg Trauma Shock. 2011;4:70–76. doi: 10.4103/0974-2700.76840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sfoungaristos S., Kavouras A., Katafigiotis I., Perimenis P. Role of white blood cell and neutrophil counts in predicting spontaneous stone passage in patients with renal colic. BJU Int. 2012;110:E339–E345. doi: 10.1111/j.1464-410X.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- 3.Sfoungaristos S., Kavouras A., Perimenis P. Predictors for spontaneous stone passage in patients with renal colic secondary to ureteral calculi. Int Urol Nephrol. 2012;44:71–79. doi: 10.1007/s11255-011-9971-4. [DOI] [PubMed] [Google Scholar]
- 4.Morse R.M., Resnick M.I. Ureteral calculi. Natural history and treatment in an era of advanced technology. J Urol. 1991;145:263–265. doi: 10.1016/s0022-5347(17)38310-6. [DOI] [PubMed] [Google Scholar]
- 5.Turk C, Knoll T, Petrik A, et al. European Association of Urology (EAU) Guidelines Office. European Association of Urology Guidelines on Urolithiasis, 28th ed., In: EAU Annual Congress, Milan; 2013. ISBN 978-90-79754-70-60.
- 6.Park C.H., Ha J.Y., Kim C.I., Kim K.S., Kim B.H. Relationship between spontaneous passage rates of ureteral stones less than 8 mm and serum C-reactive protein levels and neutrophil percentages. Korean J Urol. 2013;54:615–618. doi: 10.4111/kju.2013.54.9.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preminger G.M., Tiselius H.G., Assimos D.G., Alken P., Buck A.C., Gallucci M. Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–1631. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Hubner W.A., Irby P., Stoller M.L. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24:172–176. doi: 10.1159/000474289. [DOI] [PubMed] [Google Scholar]
- 9.Coll D.M., Varanelli M.J., Smith R.C. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002;178:101–103. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 10.Ueno A., Kawamura T., Ogawa A., Takayasu H. Relation of spontaneous passage of ureteral calculi to size. Urology. 1977;10:544–546. doi: 10.1016/0090-4295(77)90097-8. [DOI] [PubMed] [Google Scholar]
- 11.Miller O.F., Kane C.J. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688–690. doi: 10.1097/00005392-199909010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Fazlioglu A., Salman Y., Tandogdu Z., Kurtulus F.O., Bas S., Cek M. The effect of smoking on spontaneous passage of distal ureteral stones. BMC Urol. 2014;14:27. doi: 10.1186/1471-2490-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Nakshabandi N.A. The soft-tissue rim sign. Radiology. 2003;229:239–240. doi: 10.1148/radiol.2291020690. [DOI] [PubMed] [Google Scholar]
- 14.Mokhless I., Zahran A.-R., Youssif M., Fouda K., Fahmy A. Factors that predict the spontaneous passage of ureteric stones in children. Arab J Urol. 2012;10:402–407. doi: 10.1016/j.aju.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdodru T., Aker O., Kaplancan T., Erodlu E. Predictive role of non-contrast spiral computerized tomography on spontaneous passage of ureteral stones. Int Braz J Urol. 2002;28:516–521. [PubMed] [Google Scholar]
- 16.Dalrymple N.C., Verga M., Anderson K.R., Bove P., Covey A.M., Rosenfield A.T. The value of unenhanced helical computerized tomography in the management of acute flank pain. J Urol. 1998;159:735–740. [PubMed] [Google Scholar]