Summary
Histone deacetylase (HDAC) inhibitors have shown enormous promise for treating various disease states, presumably due to their ability to modulate acetylation of histone and non-histone proteins. Many of these inhibitors contain functional groups capable of strongly chelating metal ions. We demonstrate that several members of one such class of compounds, the hydroxamate-based HDAC inhibitors, can protect neurons from oxidative stress via an HDAC-independent mechanism. This previously unappreciated antioxidant mechanism involves the in situ formation of hydroxamate-iron complexes that catalyze the decomposition of hydrogen peroxide in a manner reminiscent of catalase. We demonstrate that while many hydroxamate-containing HDAC inhibitors display a propensity for binding iron, only a subset form active catalase mimetics capable of protecting neurons from exogenous H2O2. In addition to impacting stroke and neurodegenerative disease research, these results highlight the possibility that HDAC-independent factors might play a role in the therapeutic effects of hydroxamate-based HDAC inhibitors.
Graphical Abstract

Introduction
The deleterious effects of oxidative stress contribute to the neuronal death associated with a variety of brain disorders including Parkinson’s disease, Alzheimer’s disease, and stroke (Uttara et al., 2009). It is believed that these effects can be mitigated by promoting oxidative defense mechanisms through the manipulation of epigenetic factors (Schweizer et al., 2013). This conclusion is due in large part to the effectiveness of small molecule inhibitors of histone deacetylases (HDACs) in various models of neuroprotection (Ryu et al., 2003; Kim et al., 2007; Shein et al., 2009; Butler et al., 2010; Fleiss et al., 2012; Lu et al., 2013). HDACs catalyze the removal of acetyl groups from the ε-nitrogens of lysine residues, a posttranslational modification that can profoundly impact cellular processes ranging from cytoskeletal reorganization (Piperno et al., 1987) to gene expression (Spange et al., 2009). With the exception of the NAD-dependent sirtuins (Class III), HDACs are metalloenzymes (Class I: HDACs 1, 2, 3, and 8; Class II: HDACs 4, 5, 6, 7, 9, and 10: Class IV: HDAC11). This means that the vast majority of known HDAC inhibitors (HDACi) contain a metal-binding group of varied chelating ability (e.g., hydroxamic acids, o-aminoanilides, ketones, etc.) (Pan et al., 2012); however, the chemical properties associated with such functional groups are often overlooked when studying the biological effects of HDAC inhibitors. Herein, we demonstrate that the iron-binding ability of several known hydroxamate-based HDAC inhibitors is necessary, but not sufficient, to explain their capacity to protect neurons from exogenous H2O2. Instead, our data suggests that only a subset of these iron-binding small molecules can form complexes capable of catalyzing the disproportionation of H2O2 into water and the less potent cellular oxidant, molecular oxygen.
Results and Discussion
The Development of Chemical Tools for Studying HDACs
As part of our general interest in using chemical tools to study the function of the various Zn-dependent HDAC isoforms (Gregoretti et al., 2004), we amassed a collection of HDAC inhibitors and analogs, including appropriately designed negative control compounds, belonging to different chemical classes with varying potencies and selectivities (Figure 1). Initially, we tested several of these compounds in a glutathione (GSH) depletion assay that models oxidative stress (Murphy et al., 1990; Ratan et al., 1994; Ratan and Baraban, 1995) and found that many were effective in protecting murine cortical neurons from death (Figure S1). The non-selective hydroxamate-based inhibitors were particularly potent as they conferred full protection at nanomolar concentrations.
Figure 1. Chemical tools for studying the role of HDACs in neuroprotection.
Chemical structures (A) and HDAC potencies (IC50s) (B) of chemical tools used in this study. Absolute potency values can be found in Table S1. *Denotes a negative control compound based on the parent structure. See also Table S1.
Several Hydroxamate-based HDAC Inhibitors Exhibit HDAC-independent Antioxidant Properties
Because the GSH depletion assay requires the use of immature neurons lacking functional NMDA receptors to prevent excitotoxicity (Murphy et al., 1990), we next decided to test the neuroprotective properties of these HDAC inhibitors by subjecting fully mature cortical neurons to H2O2 (Figure 2A), a direct oxidative insult that mimics the concentration of H2O2 released during stroke (Hyslop et al., 1995). Surprisingly, we found that the non-selective inhibitor LBH-589 was able to fully protect neurons from a H2O2 insult though other non-selective inhibitors with similar potencies could not (Figure 2A). We ruled out the possibility of differential toxicity accounting for the observed neuroprotection because in the absence of insult, these compounds did not cause any cell death at the concentrations and time frame employed in the assay (Figure 2C). These results provided the first piece of evidence suggesting that there might be multiple mechanisms by which hydroxamate-based HDAC inhibitors can exert neuroprotective effects.
Figure 2. Hydroxamate-based HDAC inhibitors protect murine cortical neurons from oxidative stress through an HDAC-independent mechanism.
(A) Rat E18 cortical neurons were simultaneously treated with 150 μM H2O2 and compounds (30 μM). Cell death was assayed 24h later via an MTT assay. Several negative control compounds based on the structures of known HDAC inhibitors exhibited robust protection of cortical neurons from H2O2-induced death, despite lacking HDAC inhibitory activity. (B) Protection from H2O2 occurs in a dose-dependent manner. (C) Compounds do not induce cell death at the highest concentration (30 μM) and longest time frame (24h) employed in these assays. (D) Protection from H2O2 occurs independent of transcription or translation. Assays were performed in the presence of either 2 μg/mL actinomycin D or 10 μg/mL cycloheximide. Data are shown as mean ± SEM. Significance was established relative to DMSO + H2O2. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 from Tukey post hoc test following a one-way analysis of variance (ANOVA). See also Figure S1.
Upon closer examination, we discovered that the non-selective HDAC inhibitor LBH-589, the HDAC8-selective inhibitor PCI-34051 (Balasubramanian et al., 2008), and the HDAC6-selective inhibitor tubastatin A (Butler et al., 2010) protected neurons from H2O2 at relatively high concentrations (>10 μM) (Figure 2B), results that seemed inconsistent with their exceptional HDAC potencies (IC50s < 15 nM). Furthermore, we recently reported that PCI-34051 protects neurons from GSH depletion via an opaque, but HDAC-independent mechanism (Sleiman et al., 2014), and we hypothesized that such a mechanism might play a role in the neuroprotective effects of LBH-589 and tubastatin A as well. This hypothesis was supported by the fact that other HDAC6- (i.e., BRD3493 and BRD9757) and HDAC6/8-selective (i.e., BRD3954) inhibitors were unable to protect cortical neurons from the deleterious effects of H2O2 (Figure 2A), even at concentrations known to induce tubulin acetylation (Wagner et al., 2013; Olson et al., 2013), the known cellular substrate for HDAC6 (Hubbert et al., 2002).
In order to establish whether or not the inhibition of HDAC6 and/or HDAC8 is involved in neuroprotection, we synthesized BRD3067 and BRD3811 (Olson et al., 2014) as negative control compounds structurally related to tubastatin A and PCI-34051, respectively. These compounds contain a single methyl group ortho to the hydroxamic acid moiety that precludes entry into the enzyme’s active site, but does not interfere with the metal-chelating ability of the hydroxamate. Both negative control compounds protected neurons from H2O2 (Figure 2A) despite their inability to inhibit HDACs (Figure 1B), thus demonstrating that structurally related hydroxamate-based HDAC inhibitors exert neuroprotective effects via an HDAC-independent mechanism. Additionally, we noted that BRD3493 and BRD9287, two analogs of tubastatin A and PCI-34051, respectively, did not exhibit any neuroprotective properties (Figure 2A). These compounds retained the phenyl or indole hydroxamic acid portion of their respective parent compound, but lacked the associated aromatic methylene substituent. We were intrigued as to why such small structural changes would have such dramatic effects on the ability of these compounds to protect neurons from oxidative stress, and we set out to link these structural characteristics to the differential functional responses elicited by these compounds.
Next, we found that LBH-589, tubastatin A, and PCI-34051 were able to protect rat cortical neurons from a H2O2 insult even in the presence of actinomycin D or cycloheximide (Figure 2D), confirming that the mechanism by which these small molecules protect cells from H2O2 is independent of transcription and translation, and thus, not dependent on gene expression changes resulting from HDAC inhibition. Furthermore, we noticed that the neuroprotective effects of PCI-34051 and BRD3811 could be attenuated by the addition of ZnCl2 (unpublished results), suggesting an important role for metal chelation. This hypothesis was further strengthened by the observation that only compounds containing hydroxamic acids, and not other metal-binding groups such as o-aminoanilides or ketones, were protective against H2O2 (Figure 2A). Additionally, it should be noted that the neuroprotective properties of these compounds seem to be limited to oxidative stress as PCI-34051 was unable to protect neurons from other forms of stress including treatment with NMDA, glutamate, 6-OHDA, 3-NP, camptothecin, or staurosporine (unpublished results).
Not All Hydroxamate-based HDAC Inhibitors Are Strong Binders of Iron
As it is well established that Fenton chemistry can play a critical role in deleterious oxidative stress (Kohen and Nyska, 2002; Prousek, 2007; Thomas et al., 2009), and these compounds are neuroprotective at the approximate concentration of free chelatable cellular iron (Fiedler et al., 2007), we reasoned that the metal chelating property of these hydroxamic acids is crucially important for protecting neurons from a H2O2 insult. To test this hypothesis, we measured the ability of each of these compounds to bind iron using an in vitro calcein assay (Figure 3) and found that several of them could bind metals in cells (Figure S2A) (Cabantchik et al., 1996). Briefly, upon binding to iron, the fluorescence of calcein is partially quenched. Compounds that are strong chelators of iron can effectively compete with calcein for the available iron, ultimately decreasing the amount of iron-bound calcein and increasing calcein fluorescence. Somewhat surprisingly, we found that not all hydroxamic acid-containing compounds were able to effectively bind iron (Figure 3). For instance, BRD3493 and BRD9287, the analogs of tubastatin A and PCI-34051, respectively, that did not exhibit any neuroprotective properties, also did not effectively compete with calcein for free chelatable iron. Not surprisingly, the compounds that were the most effective iron binders were also the best at protecting cortical neurons from an Fe/8-hydroxyquinoline complex, a membrane permeable iron insult (Jonas and Riley, 1991) (Figure S2B). This effect could be due to either the sequestration of redox active iron within the cell, or the rapid stripping of iron from 8-hydroxyquinoline to form cell-impermeable complexes. Regardless, these studies confirm the results of our in vitro iron-binding assays.
Figure 3. HDAC inhibitors display differential abilities to bind iron.
In vitro calcein fluorescence was partially quenched upon the addition of FeSO4. Several, but not all, compounds were able to restore calcein fluorescence through competitive iron binding. Data are shown as mean ± SEM. Significance was established relative to DMSO + FeSO4. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 from Tukey post hoc test following a one-way analysis of variance (ANOVA). See also Figure S2.
The Ability to Bind Iron is Not Sufficient to Protect Neurons From H2O2
While all compounds protective in the H2O2 neuroprotection assay were effective chelators of iron, the non-selective inhibitor scriptaid and its structurally related negative control nullscript were able to bind iron and protect cells from an iron insult (Figure 3 and Figure S2B), but were unable to protect neurons from H2O2 (Figure 2). Therefore, we deemed iron chelation necessary, but not sufficient to protect neurons from exogenous H2O2. It is possible that a reduction in Fenton chemistry plays a small role in the neuroprotective effects of these compounds; however, based on the scriptaid and nullscript results, we hypothesized that the complexes formed upon iron chelation might be exerting a more profound effect than simply the act of sequestering iron itself.
HDAC Inhibitors Display Catalase-like Activity in the Presence of Iron
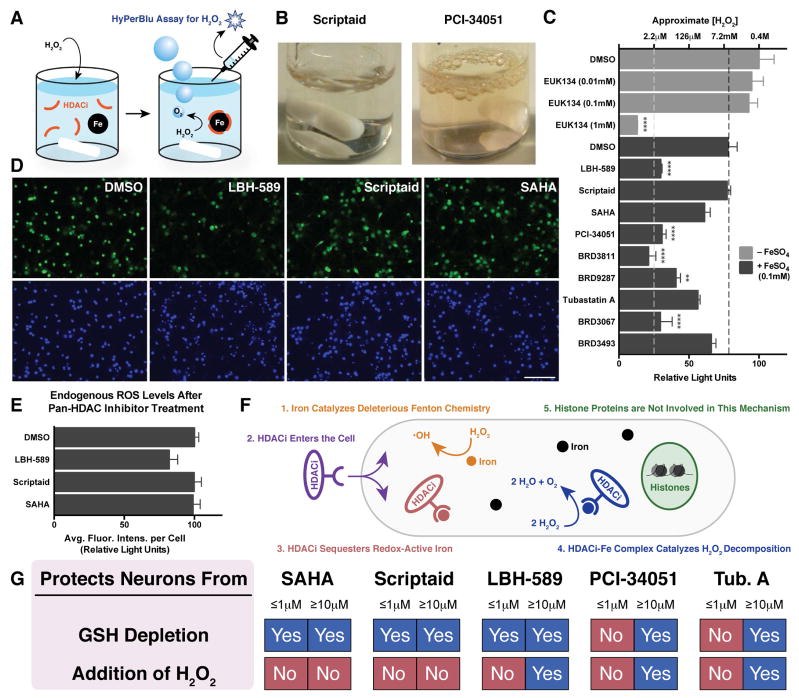
Inspired by previous reports (Baker et al., 1998; Rauen et al., 2004; Sustmann et al., 2007), we hypothesized that some of these hydoxamic acid-containing compounds might form catalase mimetic complexes upon binding intracellular iron, thus facilitating the degradation of H2O2 and making them particularly well-suited to protect neurons from a H2O2 insult. To test this idea, we performed an in vitro assay (Figure 4A) that uses chemiluminescent detection of H2O2 (Figure S3A) to measure changes in H2O2 concentration and compared these hydroxamate-iron complexes to the known catalase mimetic EUK 134 (Baker et al., 1998). Iron sulfate (FeSO4) itself causes a slight reduction in H2O2 levels after 2h. However, when FeSO4 is combined with certain hydroxamic acid-containing HDAC inhibitors or analogs, these compounds serve as ligands for the iron and form complexes leading to the rapid decomposition of H2O2 (Figure 4C). Although the most potent complexes caused the final concentration of H2O2 to fall below the linear range of the assay (Figure S3B), we were able to estimate that 0.00005 equivalents of one of these iron complexes (e.g., iron complexes utilizing LBH-589 or PCI-34051 as ligands) relative to H2O2 is able to reduce the levels of oxidant by six orders of magnitude (from >1 M to ~1 μM) in only 2h. Importantly, in the absence of additional FeSO4, these compounds do not cause the decomposition of H2O2 (Figure S3C), thus indicating that it is the complexes, and not the ligands themselves that are responsible for performing the catalysis.
Figure 4. Hydroxamate-based HDAC inhibitor-iron complexes catalyze the decomposition of H2O2 and mitigate intracellular ROS levels.
(A) Schematic depicting an in vitro H2O2 decomposition assay. (B) Reactions employing hydroxamate-based HDACi-iron complexes capable of catalyzing the disproportionation of H2O2 into water and gaseous O2 produce effervescence. (C) Some hydroxamate-based HDAC inhibitors (HDACi), in combination with FeSO4, are capable of drastically reducing in vitro levels of H2O2 while others cannot. The black dotted line indicates the decomposition of H2O2 resulting from DMSO + FeSO4 only. Concentrations below the white dotted line fall outside the linear range of this assay. Data are shown as mean ± SEM. Significance was established relative to DMSO + FeSO4. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 from Tukey post hoc test following a one-way analysis of variance (ANOVA). (D) Rat cortical neurons were treated with HDAC inhibitors at 30 μM for 24h before endogenous levels of intracellular ROS were determined using the ROS-sensitive fluorescein derivative CM-H2DCFDA. The scale bar denotes 100 μm. (E) Quantification of endogenous ROS levels shows that the catalase mimetic properties of LBH-589 can reduce endogenous ROS levels. Data are shown as mean ± SEM. (F) Schematic depiction of a model for an HDAC-independent neuroprotection mechanism (G) Summary of neuroprotective effects at either low (≤1μM) or high (≥10μM) concentrations. See also Figures S3 and S4.
It is important to note that not all hydroxamic acids are capable of serving as a precursor to a catalase-mimetic complex. Some do not bind iron strongly (e.g., BRD9287, BRD3493, and BRD9757) while others are strong iron binders yet the complexes they form are unable to perform the catalysis (e.g., scriptaid and nullscript). While the exact structures of these hydroxamate-based catalase mimetic complexes have not yet been elucidated, all competent ligands share similar structural features. These ligands are invariably either an aryl or cinnamyl hydroxamic acid with a pendant substituent. For reasons that are presently unclear, alkyl and alkenyl hydroxamic acids in the presence of iron do not display catalase-like activity.
The reaction of several of these catalase-mimetic complexes, such as the one formed from PCI-34051 and iron, with H2O2 is so vigorous that the product of that reaction (i.e., molecular oxygen) is immediately visualized as obvious effervescence (Figure 4B) (Fung and Petrishko, 1973). This is in stark contrast to complexes formed by compounds like scriptaid, which do not catalyze the decomposition of H2O2 (Figure 4C), and thus do not cause effervescence (Figure 4B). Importantly, the results of our in vitro H2O2 decomposition assay are in accord with the results of our H2O2 neuroprotection assay, that is to say that the compounds that form complexes capable of catalyzing the decomposition of H2O2 in vitro are also those that protect cortical neurons from a H2O2 insult. Although the iron complex of BRD9287 did show some catalase-like activity in vitro (Figure 4C), it did not in cells (Figure 2A). This is likely due to in vitro conditions driving the formation of the catalase mimetic complex. In cells, the low concentration of free chelatable iron coupled with the low affinity of BRD9287 for iron (Figure 3) is likely to preclude the formation of the active catalyst, thus explaining the inability of BRD9287 to protect neurons from a H2O2 insult (Figure 2A). Therefore, we propose that hydroxamate-containing molecules must 1) display a propensity for binding cellular iron, and 2) form iron-complexes capable of catalyzing the disproportionation of H2O2 in order for these compounds to exhibit sufficient antioxidant properties to protect cells from exogenous H2O2.
When compared to the state of the art commercially available catalase mimetic EUK 134 (Baker et al., 1998), a Mn-complex whose antioxidant properties have been shown to drastically extend the life of C. elegans (Melov et al., 2000), the hydroxamate-iron complexes described here display similar catalase-like activity. In fact, they are approximately 10 times more potent than EUK 134, as 1 mM EUK 134 was necessary to produce a decrease in H2O2 concentration comparable to the decrease produced by 0.1 mM of the hydroxamate-iron complexes (Figure 4C). Furthermore the in situ formation of catalase mimetic complexes from hydroxamates confers two obvious advantages over simply utilizing a Mn complex like EUK 134, 1) it obviates the need to introduce toxic metals into the cells, and 2) it has the added benefit of sequestering any excess redox-active iron that may be engaging in deleterious Fenton chemistry.
Next, we assayed the effects of hydroxamate-based HDAC inhibitors and associated analogs on cellular levels of reactive oxygen species (ROS) using CM-H2DCFDA, a fluorescein derivative whose fluorescence intensity is increased in the presence of ROS (Figure S4). Initially, when rat cortical neurons are treated with H2O2, levels of cellular ROS appear to decrease (Figure S4). This somewhat counterintuitive result can be rationalized by a rapid H2O2-induced increase in antioxidant defense mechanisms. Compounds that form catalase mimetic complexes in vitro and protect neurons from a H2O2 insult either delay or completely prevent this initial decrease in fluorescence, presumably by sufficiently lowering intracellular H2O2 levels (Figure S4). In time, the antioxidant defense mechanisms of cells not treated with catalase mimetics are overwhelmed and the cells ultimately succumb to the swelling and rupture characteristic of H2O2-induced necrosis. Finally, we treated cells for 24h (in the absence of H2O2) with several non-selective HDAC inhibitors to assess changes in endogenous levels of ROS. While LBH-589 was able to lower endogenous ROS levels, the two compounds of this series that did not form catalase mimetic complexes in vitro (i.e., scriptaid and SAHA) could not (Figures 4D and 4E), thus confirming that iron sequestration alone is not sufficient to reduce endogenous ROS levels.
Significance
Our data suggest that hydroxamate-based HDAC inhibitors can exert neuroprotective effects via an HDAC-dependent mechanism, an HDAC-independent mechanism, or a combination of both (Figure 4G). At low nM concentrations near their HDAC IC50s, non-selective inhibitors, such as scriptaid, can protect neurons from GSH depletion via what appears to be an HDAC-dependent mechanism, a conclusion that is supported by the fact that no such protection is observed when the structurally related negative control compound nullscript is employed (Figure S1). Other HDAC inhibitors, such as PCI-34051, protect neurons from oxidative stress at concentrations much greater than their HDAC IC50s, and these compounds engage in an HDAC-independent mechanism outlined in Figure 4F. Upon entering the cell, these hydroxamic acids bind to free cellular iron, potentially mitigating deleterious Fenton chemistry, but more importantly, forming complexes capable of catalyzing the disproportionation of H2O2 into water and O2. Hydroxamate-containing compounds that exhibit catalase-like activity but not HDAC inhibitor activity, such as BRD3811, may prove to be useful in the treatment of diseases such as stroke as these compounds have powerful antioxidant properties, and presumably, will not suffer from the HDAC-related toxicities that have hampered the development of HDAC inhibitors as therapeutics. Of course, exploiting such catalase-like antioxidant effects for therapeutic purposes would necessitate achieving a sufficiently high concentration of hydroxamate in vivo. The discovery that hydroxamate-based HDAC inhibitors can exert neuroprotective effects by forming catalase mimetic complexes in cells lays the foundation for future studies regarding the HDAC-independent effects of these important compounds on a variety of disease states involving oxidative stress including immunological disorders and cancer.
Supplementary Material
Highlights.
Several, but not all, HDAC inhibitors display HDAC-independent antioxidant properties
Not all hydroxamates bind iron equally well
Several HDAC inhibitor-iron complexes catalyze the disproportionation of H2O2 in vitro
In vitro catalase-like activity correlates with capacity to protect neurons from H2O2
Acknowledgments
We would like to thank T. Hasaka for technical help with imaging experiments, and L. Gaffney for help with illustrations. Furthermore, we would like to thank R. Becker, J. Biag, M. Walk, and M. Wëiwer for helpful discussions. This work was supported by the Stanley Medical Research Institute, the Burke Foundation, the National Institute of Health (Grant P01 NIA AG014930, Project 1 to RRR), a Miriam and Sheldon Adelson Medical Research Foundation Grant (to RRR), and a Goldsmith Foundation post-doctoral fellowship (to SFS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic Combined Superoxide Dismutase/Catalase Mimetics are Protective as a Delayed Treatment in a Rat Stroke Model: A Key Role for Reactive Oxygen Species in Ischemic Brain Injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- Balasubramanian A, Ramos J, Luo W, Sirisawad M, Verner E, Buggy JJ. A Novel Histone Deacetylase 8 (HDAC8)-specific Inhibitor PCI-34051 Induces Apoptosis in T-cell Lymphomas. Leukemia. 2008;22:1026–1034. doi: 10.1038/leu.2008.9. [DOI] [PubMed] [Google Scholar]
- Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational Design and Simple Chemistry Yield a Superior, Neuroprotective HDAC6 Inhibitor, Tubastatin A. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik ZI, Glickstein H, Milgram P, Breuer W. A Fluorescence Assay for Assessing Chelation of Intraceullular Iron in a Membrane Model System and in Mammalian Cells. Anal Biochem. 1996;233:221–227. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- Fiedler A, Reinert T, Morawski M, Brückner G, Arendt T, Butz T. Intracellular Iron Concentration of Neurons With and Without Perineuronal Nets. Nucl Instr Meth Phys Res B. 2007;260:153–158. [Google Scholar]
- Fleiss B, Nilsson MKL, Blomgren K, Mallard C. Neuroprotection by the Histone Deacetylase Inhibitor Trichostatin A in a Model of Lipopolysaccharide-sensitised Neonatal Hypoxic-ischaemic Brain Injury. J Neuroinflamm. 2012;9:1–15. doi: 10.1186/1742-2094-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung DC, Petrishko DT. Capillary Tube Catalase Test. Appl Microbiol. 1973;26:631–632. doi: 10.1128/am.26.4.631-632.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV. Molecular Evolution of the Histone Deacetylase Family: Functional Implications of Phylogenetic Analaysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a Microtubule-Associated Deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of Striatal H2O2 by Microdialysis Following Global Forebrain Ischemia and Reperfusion in the Rat: Correlation with the Cytotoxic Potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- Jonas SK, Riley PA. The Effect of Ligands on the Uptake of Iron by Cells in Culture. Cell Biochem Funct. 1991;9:245–253. doi: 10.1002/cbf.290090406. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone Deacetylase Inhibitors Exhibit Anti-inflammatory and Neuroprotective Effects in a Rat Permanent Ischemic Model of Stroke: Multiple Mechanisms of Action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for their Quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Lu J, Frerich JM, Turtzo LC, Li S, Chiang J, Yang C, Wang X, Zhang C, Wu C, Sun Z, Niu G, Zhuang Z, Brady RO, Chen X. Histone Deacetylase Inhibitors are Neuroprotective and Preserve NGF-mediated Cell Survival Following Traumatic Brain Injury. Proc Natl Acad Sci. 2013;110:10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of Life-span with Superoxide Dismutase/catalase Mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature Cortical Neurons are Uniquely Sensitive to Gutamate Toxicity by Inhibition of Cystine Uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- Olson DE, Wagner FF, Kaya T, Gale JP, Aidoud N, Davoine EL, Lazzaro F, Wëiwer M, Zhang YL, Holson EB. Discovery of the First Histone Deacetylase 6/8 Dual Inhibitors. J Med Chem. 2013;56:4816–4820. doi: 10.1021/jm400390r. [DOI] [PubMed] [Google Scholar]
- Olson DE, Udeshi ND, Wolfson NA, Pitcairn CA, Sullivan ED, Jaffe J, Svinkina T, Natoli T, Liu X, Paulk J, McCarren P, Barker D, Howe E, Lazzaro F, Gale JP, Zhang Y-L, Subramanian A, Fierke C, Carr SA, Holson EB. An Unbiased Approach to Identify Endogenous Substrates of “Histone” Deacetylase 8. ACS Chem Biol. 2014 doi: 10.1021/cb500492r. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Cao J, Xu W. Selective Histone Deacetylase Inhibitors. Anti-Cancer Agent Med Chem. 2012;12:247–270. doi: 10.2174/187152012800228814. [DOI] [PubMed] [Google Scholar]
- Piperno G, LeDizet M, Chang XJ. Microtubules Containing Acetylated Alpha-tubulin in Mammalian Cells in Culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prousek J. Fenton Chemistry in Biology and Medicine. Pure Appl Chem. 2007;79:2325–2338. [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. Oxidative Stress Induces Apoptosis in Embryonic Cortical Neurons. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Baraban JM. Apoptotic Death in an in vitro Model of Neuronal Oxidative Stress. Clin Exp Pharmacol Physiol. 1995;22:309–310. doi: 10.1111/j.1440-1681.1995.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Rauen U, Li T, Sustmann R, De Groot H. Protection Against Iron- and Hydrogen Peroxide-dependent Cell Injuries by a Novel Synthetic Iron Catalase Mimic and its Precursor, the Iron-free Ligand. Free Radic Biol Med. 2004;37:1369–1383. doi: 10.1016/j.freeradbiomed.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR. Histone Deacetylase Inhibitors Prevent Oxidative Neuronal Death Independent of Expanded Polyglutamine Repeats via an Sp1-dependent Pathway. Proc Natl Acad Sci. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Meisel A, Märschenz S. Epigenetic Mechanisms in Cerebral Ischemia. J Cerebr Blood F Met. 2013;33:1335–1346. doi: 10.1038/jcbfm.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shein NA, Grigoriadis N, Alexandrovich AG, Simeonidou C, Lourbopoulos A, Polyzoidou E, Trembovler V, Mascagni P, Dinarello CA, Shohami E. Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury. FASEB J. 2009;23:4266–4275. doi: 10.1096/fj.09-134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Olson DE, Bourassa MW, Karuppagounder SS, Zhang YL, Gale J, Wagner FF, Basso M, Coppola G, Pinto JT, Holson EB, Ratan RR. Hydroxamic Acid-Based Histone Deacetylase (HDAC) Inhibitors Can Mediate Neuroprotection Independent of HDAC Inhibition. J Neurosci. 2014;34:14328–14337. doi: 10.1523/JNEUROSCI.1010-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of Non-histone Proteins Modulates Cellular Signalling at Multiple Levels. Int J Biochem Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Sustmann R, Korth HG, Kobus D, Baute J, Seiffert KH, Verheggen E, Bill E, Kirsch M, de Groot H. Fe(III) Complexes of 1,4,8,11-tetraaza[14]annulenes as Catalase Mimetics. Inorg Chem. 2007;46:11416–11430. doi: 10.1021/ic700961b. [DOI] [PubMed] [Google Scholar]
- Thomas C, Mackey MM, Diaz AA, Cox DP. Hydroxyl Radical is Produced via the Fenton Reaction in Submitochondrial Particles Under Oxidative Stress: Implications for Diseases Associated with Iron Accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FF, Olson DE, Gale JP, Kaya T, Wëiwer M, Aidoud N, Thomas M, Davoine EL, Lemercier BC, Zhang YL, Holson EB. Potent and Selective Inhibition of Histone Deacetylase 6 (HDAC6) Does Not Require a Surface-binding Motif. J Med Chem. 2013;56:1772–1776. doi: 10.1021/jm301355j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.