Abstract
Limited research in humans suggests that slowly digestible starch may blunt the postprandial increase and subsequent decline of plasma glucose and insulin concentrations, leading to prolonged energy availability and satiety, compared to more rapidly digestible starch. This study examined the postprandial metabolic and appetitive responses of waxy maize starch (WM), a slow-digestible starch. It was hypothesized that the waxy maize treatment would result in a blunted and more sustained glucose and insulin response, as well as energy expenditure and appetitive responses. Twelve subjects (6 men and 6 women) (age, 23 ± 1 years; body mass index, 22.2 ± 0.7 kg/m2; insulin sensitivity [homeostatic model assessment], 16% ± 2%; physical activity, 556 ± 120 min/wk) consumed, on separate days, 50 g of available carbohydrate as WM, a maltodextrin-sucrose mixture (MS), or white bread (control). Postprandial plasma glucose and insulin, energy expenditure, and appetite (hunger, fullness, desire to eat) were measured over 4 hours. Compared to control, the 4-hour glucose response was not different for MS and WM, and the 4-hour insulin response was higher for MS (P < .005) and lower for WM (P < .05). Compared to MS, WM led to lower 4-hour glucose and insulin responses (P < .001). These differences were driven by blunted glucose and insulin responses during the first hour for WM. Postprandial energy expenditure and appetite were not different among treatments. These results support that WM provides sustained glucose availability in young, insulin-sensitive adults.
Keywords: Starch, Glycemic index, Glucose, Insulin, Appetite, Polysaccharide, Humans
1. Introduction
Researchers have begun to examine the utility of using slowly digested starch to influence the postprandial blood glucose and insulin concentrations leading to prolonged energy availability [1]. One application of this might be to improve exercise performance, delay fatigue, and increase physical endurance through extended glucose release [2]. Other applications could be to prolong satiety and improve diabetes management [3].
Starch can be separated into 3 categories based on its digestibility, which is determined by the rate that glucose is released from the starch and then absorbed [4]. Rapidly digestible starch, such as cooked/pregelatinized starch, is enzymatically digested in vitro within 20 minutes. Resistant starch is the residual starch and degradation by-products not digested or absorbed in the small intestine. Resistant starch is fermented by bacteria when it reaches the large intestine and is not a direct source of energy to the body but does contribute energetically through production and absorption of short chain fatty acids [5]. Slowly digested starch (which includes the uncooked cereal starches maize, waxy maize, barley, wheat, and rice) is digested enzymatically in vitro between 20 and 120 minutes. In the case of maize starches, the progress of digestion is from the inside of the starch structure to the outside (inside-out starch digestion) because of the presence of surface pores and channels within the granule [5,6].
There are few reports of data regarding the effects of slowly digestible starch on glucose tolerance, energy expenditure, and appetite. The ingestion of 35 g of available carbohydrate as maize starch or waxy maize starch (WM) (slowly digestible starches) resulted in a smaller increase and longer sustained rise in plasma glucose compared to maltodextrin (a rapidly digestible starch) [2]. The ingestion of a mixture of tapioca and maize starch (slowly digestible starch) lowered the incremental area under the curve for insulin and tended to lower the glucose profile compared to the ingestion of a rapidly digesting starch (a waxy maize-derived starch) [1]. In young healthy women, a meal containing a slowly digestible WM resulted in lower peak concentrations of plasma glucose and insulin compared with a meal containing a rapidly digestible maize starch [5]. In young men, the consumption of uncooked cornstarch (a slowly digested starch) led to blunted plasma glucose and insulin responses. The area under the curve for the slowly digesting starch was lower during the first 120 minutes, but there were no difference after 120 minutes compared to consuming glucose [8]. Similar results were found in a study comparing slowly digesting barley kernels with a white bread control [9]. Concerning appetite, the ingestion of slowly digested barley kernels is reported to cause greater satiety over a 3-hour period compared to white bread. In vitro research documents that waxy maize is a slowly digested starch [6]. We are not aware of any published research examining the metabolic, energy expenditure, and appetitive responses of WM in humans. Thus, the primary purpose of this study is to examine the effects of waxy maize on postprandial plasma insulin and glucose, and secondarily, whole-body energy expenditure and appetite in men and women. We hypothesized that the waxy maize treatment would result in a blunted, more prolonged postprandial glucose and insulin response compared to a maltodextrin-sucrose mixture (MS) (rapidly digestible carbohydrate) or white bread (glycemic control). We also hypothesized that the WM would result in a more prolonged appetitive response compared to the other treatments.
2. Methods and materials
2.1. Subjects
Potential participants were recruited from public advertisements placed in the local newspaper, in local businesses, and in buildings on the Purdue University campus. Study inclusion was based on the following criteria: (1) men and women aged 18 to 29 years; (2) body mass index between 18.5 and 24.9 kg/m2; (3) body fat of less than 27% for women and less than 20% for men; (4) not dieting; (5) weight stability ± 2 kg within the last 3 months; (6) nonsmoker; (7) clinical normalcy for indices of liver and kidney functions and complete blood count (non-anemic); (8) fasting plasma glucose of less than 6.1 mmol/L; and (9) perform at least 1 h/d of aerobic activity on at least 4 d/wk (240 min/wk) for the past 3 months. All women recruited into the study were taking oral or hormonal contraceptives for the past 6 months. Inclusion criteria were chosen based on army criteria for a physically fit individual.
Subject characteristics are shown in Table 1. There were 187 total contacts, of which 45 met the initial telephone screening criteria. Fifteen individuals (7 men, 8 women) met all study criteria and began the study. Twelve subjects (6 men, 6 women) completed all testing days.
Table 1.
Subject characteristics
| Subjects (n = 12) | Men (n = 6) | Women (n = 6) | |
|---|---|---|---|
| Age (y) | 23 ± 1 | 21 ± 1 | 25 ± 1 |
| Height (cm) | 172 ±2 | 176 ± 2 | 168 ±2 |
| Weight (kg) | 65.0 ± 2.1 | 67.0 ± 2.5 | 62.5 ± 3.4 |
| BMI (kg/m2) | 22.2 ± 0.7 | 21.6 ± 1.0 | 22.2 ± 0.9 |
| Body fat (%) | 17.0 ± 2.2 | 10.8 ± 1.3 | 23.2 ± 1.8 |
| Fat mass (kg) | 11.0 ± 1.5 | 7.3 ± 1.1 | 14.8 ± 1.7 |
| Fat-free mass (kg) | 53.7 ± 2.5 | 59.9 ± 2.0 | 47.9 ± 2.0 |
| Fasting glucose (mmol/L) | 4.8 ± 0.1 | 5.1 ± 0.1 | 4.6 ± 0.2 |
| Aerobic activity (min/wk) | 556 ± 120 | 775 ± 207 | 337 ± 44 |
| HOMA (%) | 16 ±2 | 13±2 | 19±2 |
Data are expressed as means ± SEM. BMI indicates body mass index.
All study procedures were approved by the Purdue University Biomedical Institutional Review Board, and all subjects were informed of the purpose, procedures, and potential risks of the study before signing the informed consent document. Each subject received monetary compensation for participation. A clinical trials registration number is not provided because the study was conducted before the requirement for registration was established.
2.2. Experimental design
This study was a randomized, crossover design with 3 treatments. Each subject was tested on 3 separate days over a period of 5 weeks with one or more days between testing days. The clinical phase of this study was accomplished between July 2006 and December 2006.
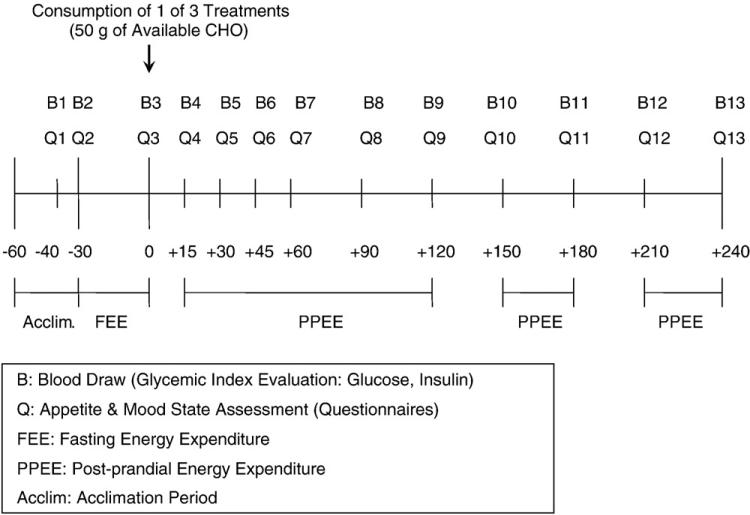
Upon arrival at the laboratory after a 10-hour period of fasting, the subject was asked to void and then rest on a bed in a supine position. A catheter was placed in an antecubetal vein of the nondominant arm and kept patent for the next 5 hours using a saline drip. Twenty and 30 minutes after the catheter was inserted (denoted as minutes –40 and –30 on the timeline in Fig. 1), baseline blood samples were taken and appetite and mood state questionnaires were completed. Resting energy expenditure (fasting state) was then measured using an indirect calorimeter for the next 30 minutes. The subject was then randomly given 1 of 3 treatments (Table 2). Blood sampling and appetite and mood state questionnaires were repeated and resting energy expenditure (postprandial state) was measured over the next 4 hours.
Fig 1.
Testing day protocol. Values from –60 to +240 represent the time in minutes relative to when the subject started to consume one of the test foods.
Table 2.
Experimental treatments
| White bread control | Maltodextrin | Waxy maize | |
|---|---|---|---|
| General characteristics: | |||
| Energy (kJ/treatment) | 1151 | 875 | 921 |
| Protein (g/treatment) | 7.5 | 0 | 0 |
| Available CHO (g/treatment) | 50 | 50 | 50 |
| Fat (g/treatment) | 3.8 | 0 | 0 |
| Weight (g/treatment) | 103.7 | 110 | 110 |
| Viscosity at 60 rpm (centipoise) | N/A | 215 | 224 |
| Formulation | N/A | Maltodextrin = 38.2g Sucrose = 11 g Mira Gel® 463 Tate and Lyle = 4.8g Water = 55.6 g |
Mira Gel® 463 Tate and Lyle = 2.2g Waxy maize = 51.7 g Water = 55.1 g Sucralose = 0.3 g |
CHO indicates cholesterol; N/A, not applicable.
2.3. Treatments
The subjects consumed 50 g of available carbohydrates as faster-digesting, 78%:22% MS or slower-digesting, uncooked WM (Table 2). Both products were incorporated into a gel matrix composed of nonstarch hydrocolloids. Waxy maize starch was provided by Tate & Lyle (Decatur, Ill). Water was added to the gel mixtures so that the mixtures were in a drinkable form. These mixtures were compared to a white bread control (Wonder-Classic brand white bread, with the crust removed [Interstate Bakeries Corporation; Kansas City, Mo]), which was frozen and then defrosted before each test day. The weight of each treatment to be consumed was calculated based on the amount of available carbohydrate. In the case of the white bread control, available carbohydrate was the “carbohydrate, by difference” value from the USDA National Nutrient Database for Standard Reference [10]. Viscosities of the test meals were equalized, using a Brookfield Viscometer (Brookfield Engineering Laboratories; Middleboro, Mass) to ensure that viscosity did not affect the differences in glycemic response between the products. Water was provided to the participants for each treatment so that the amount of water consumed was equal across treatments, including the water added to treatments to decrease viscosity. This totaled 240 mL of water with each treatment.
2.4. Body composition
During screening, each subject's body volume and mass were measured once using a plethysmography system (BOD-POD, Life Measurement Instruments, Inc, Concord, Calif); whole-body density (mass/volume) was determined, from which estimates of fat mass and fat-free mass were made. Standing height (with the subjects wearing socks) was measured using a stadiometer. Body mass index was calculated from body mass and height (kilogram per square meter).
2.5. Physical activity
During screening, each subject completed a validated physical activity recall questionnaire addressing all moderate to vigorous aerobic activity performed over the previous 3-month period [11].
2.6. Resting energy expenditure
Fasting- and postprandial-state whole-body oxygen uptake and carbon dioxide production were measured using an indirect calorimeter (MedGraphics Cardiopulmonary Diagnostics Systems; MedGraphics Corporation, St Paul, Minn) during the times indicated in Fig. 1. Energy expenditures at these times were estimated using the Weir Equation [12].
2.7. Appetite
A visual analog scale questionnaire [13] was used to assess the appetitive perceptions of hunger, fullness, and desire to eat at the times denoted in Fig. 1. A 13-point linear scale with end anchors of “not at all” and “extremely” was used to assess each perception. The participants circled the vertical dash along the horizontal line corresponding to their feelings at that moment. The results are reported using arbitrary units.
2.8. Blood sampling, and glucose and insulin analyses
Thirteen blood samples were taken during each testing period (Fig. 1). The samples were collected in test tubes containing EDTA and centrifuged at 3000 rpm and –4°C for 15 minutes. The plasma was then extracted from the tube and aliquots stored in microcentrifuge tubes at –80°C for future analyses. Plasma glucose concentration was measured by enzymatic colorimetry, using an oxidase method on a COBAS Integra 400 analyzer (Roche Diagnostic Systems, Indianapolis, Ind). Plasma insulin concentration was measured by an electrochemiluminescence immunoassay method on the Elecsys 2010 analyzer (Roche Diagnostic Systems).
2.9. Calculations and statistical analyses
Areas under the curve (AUC) for postprandial appetite, energy expenditure, plasma glucose, and plasma insulin were calculated using the trapezoidal rule [13]. Glycemic index was calculated over a 2-hour period (traditional calculation) and a 4-hour period (total time of testing) using the following equation [15]:
| (1) |
The homeostatic model assessment (HOMA), a measure of insulin sensitivity, was calculated using the following equation and conversion factors [16]:
| (2) |
All values are expressed as means ± SEM. Glucose data from one subject were excluded because their baseline concentration was inexplicably high for one of the trials. Repeated-measures analysis of variance (ANOVA) with least significant difference pairwise comparisons were performed on all of the parameters and P < .05 considered statistically significant. A power calculation was performed using a repeated-measures ANOVA that showed greater than 95% power to detect differences in 4-hour plasma glucose and insulin concentrations between treatments. Statistical analyses were performed using SPSS (Version 12.0; SPSS, Chicago, Ill).
3. Results
3.1. Glycemic index
The glycemic index of each treatment is shown in Table 3. At 2 and 4 hours, MS was not different and WM was lower (P < .05) compared to the white bread control. WM was lower than MS at 2 and 4 hours (P < .005).
Table 3.
Glycemic index after the consumption of the study treatments in 12 subjects
| Calculated glucose control | White bread control | Maltodextrin | Waxy maize | |
|---|---|---|---|---|
| Glycemic index (2-h) | 100 ± 0a (100) | 71 ± 0a (71) | 163 ± 37a (106) | 63 ± 11b (58) |
| Glycemic index (4-h) | 100 ± 0a (100) | 71 ± 0a (71) | 127 ± 27a (89) | 60 ± 11b (64) |
Values are means ± SEM (median). Values within a row with a different superscript letter differ (P < .05). Glycemic index was calculated over both a 2- and 4-hour period, using the equation as follows: GI = [(postprandial incremental AUC ofthe test treatment/postprandial incremental AUC of the white bread control) × 100]/1.4. The correction factor 1.4 was used because of the use of white bread as a control rather than glucose [14].
3.2. Glucose
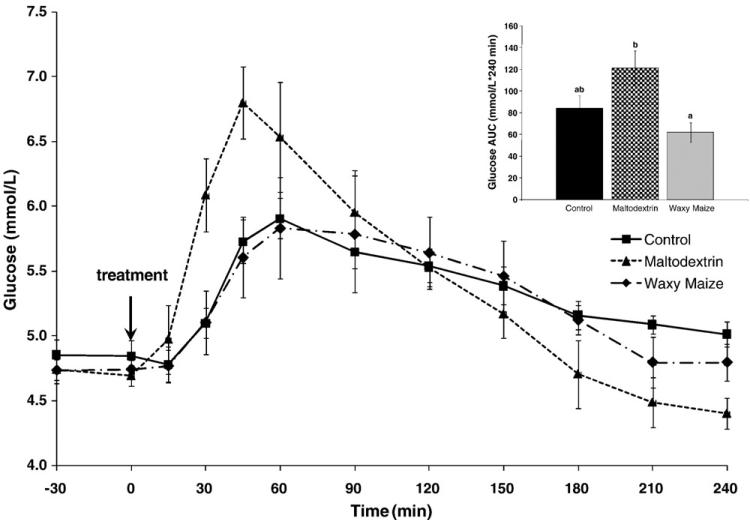
Consumption of the white bread control led to a gradual rise in plasma glucose, reaching a peak concentration of 5.91 ± 0.16 mmol/L at 60 minutes, followed by a gradual lowering toward baseline over the 4-hour period (Fig. 2). The postprandial rise in plasma glucose resulting from MS was higher and faster, with a peak concentration of 6.80 ± 0.28 mmol/L at minute 45. The glucose response of WM was comparable to the white bread control. The peak glucose concentration of WM was 5.83 ± 0.39 mmol/L at minute 60. The composite glucose AUC response over the 4-hour period was not different between the white bread control (84 ± 12 mmol/L per 240 minutes) and the other treatments (Fig. 2). However, the WM led to lower glucose AUC (62 ± 9 mmol/L per 240 minutes) vs MS (121 ± 16 mmol/L per 240 minutes; P < .001). Comparable results were shown for the glucose AUC response during the first 60 minutes: control, MS, and WM, 36 ± 6ab, 78 ± 11a, and 25 ± 4b mmol/L per 60 minutes, respectively (different superscript letters differ, P < .05). There were no differences in hourly glucose AUC responses among the treatments during hours 2, 3, and 4.·
Fig. 2.
Glucose response over 4 hours after the consumption of the study treatments in 11 subjects. Area under the curve graph for the glucose response where different letters represent statistically significant differences (P < .05).
3.3. Insulin
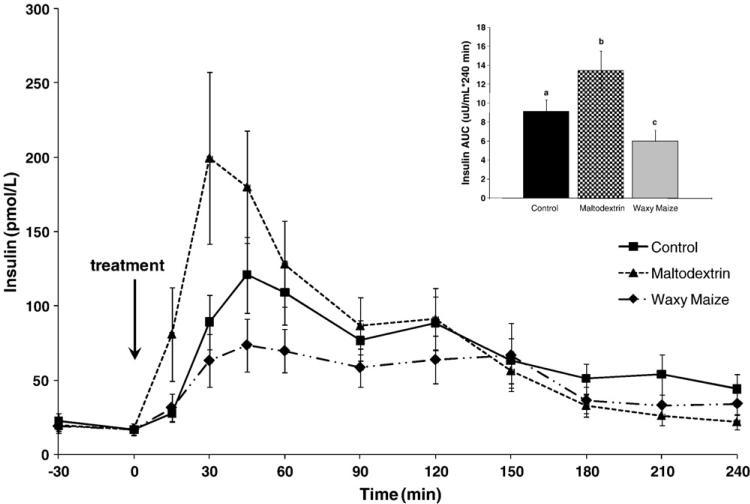
Ingestion of the white bread control led to a gradual rise in plasma insulin, reaching a peak concentration of 121 ± 25 pmol/L at 45 minutes (Fig. 3). Compared to the white bread control, MS led to a greater and faster increase in plasma insulin, reaching a peak concentration of 200 ± 58 pmol/L at 30 minutes, and WM led to lower peak (74 ± 18 pmol/L) that occurred at 45 minutes. The insulin AUC responses over 4 hours were different among the 3 treatments. Compared to control (9.13 ± 1.25 nmol/L per 240 minutes), the MS response was higher (13.43 ± 2.09 nmol/L per 240 minutes; P < .005) and the WM response lower (5.98 ± 1.17 nmol/L per 240 minutes; P < .05). The largest difference among treatments occurred during the first 60 minutes. Specifically, the insulin AUC response of the first 60 minutes was lower after the WM treatment (2.44 ± 0.56 nmol/L per 60 minutes) compared to MS (8.61 ± 1.68 nmol/L per 60 minutes; P < .001) and tended to be lower than the control (3.96 ± 0.66 nmol/L per 60 minutes; P = .100), although glucose response profiles were similar. There were no differences in hourly insulin AUC responses among the treatments during hours 2 and 3; however, in hour 4, compared to the control (1.06 ± 0.23 nmol/L per 240 minutes), both MS (0.31 ± 0.14 nmol/L per 240 minutes) and WM (0.52 ± 0.14 μU /mL per 60 minutes) were lower, but MS was not different from WM.
Fig. 3.
Insulin response over 4 hours after the consumption of the study treatments in 12 subjects. Area under the curve graph for the glucose response where different letters represent statistically significant differences (P < .05).
3.4. Postprandial energy expenditure
The postprandial energy expenditure following the white bread control treatment increased acutely to peak at 30 minutes (1.076 ± 0.172 kJ/min at 30 minutes), then decreased gradually. The MS response was not different than for the white bread control; it rose quickly with a peak at 30 minutes (0.724 ± 0.314 kJ/min at 30 minutes) and then fell gradually. The WM response occurred later, with a peak at 90 minutes (0.352 ± 0.402 kJ/min at 90 minutes) before gradually decreasing. However, there were no differences in composite postprandial energy expenditure (4-hour AUC) among MS, WM, and the control.
3.5. Appetite
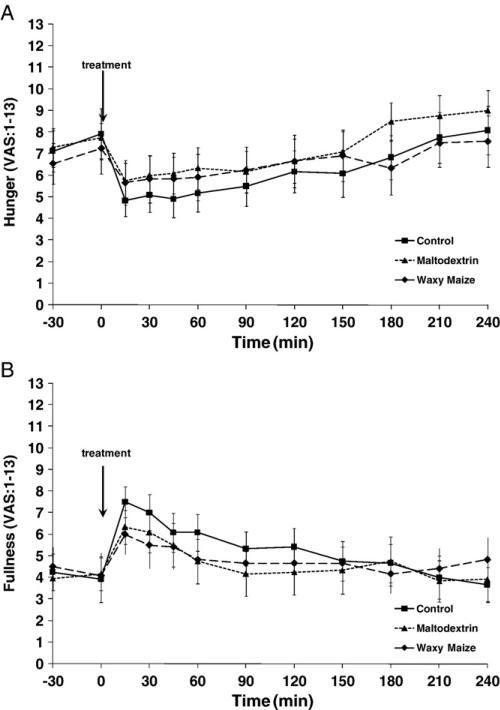
Hunger (Fig. 4A) and desire to eat (data not shown) decreased, and fullness (Fig. 4B) increased within 15 minutes after the consumption of each of the treatments and then progressively returned to baseline over time. These responses measured by AUC were not different among the 3 treatments (data not reported).
Fig. 4.
Hunger and fullness responses over 4 hours after the consumption of the study treatments in 12 subjects. (A) Hunger. (B) Fullness.
4. Discussion
These results indicate that the consumption of uncooked WM, a slowly digestible starch, leads to lower postprandial glucose and insulin concentrations but has no effect on postprandial energy expenditure and appetite compared to the consumption of rapidly digesting MS. These findings are similar to those of Wachters-Hagedoorn et al [8] who reported that the consumption of 50 g of available carbohydrate from a slowly digestible starch, uncooked corn starch led to smaller glucose and insulin AUC compared to 50 g of glucose.
Both uncooked normal maize starch and WM are characterized as slowly digesting starches because of their structures, which allow digestion from the inside out and a side-by-side slow digestion of the lamellar semicrystalline and amorphous layers in the granule. WM contains a greater amount of amylopectin compared with normal maize starch. The branched structure of amylopectin, which is believed to be responsible for the slow digestion effect, is densely packed in WM with perhaps somewhat less well-developed crystallites due to slightly shorter external linear chains, as well as branches that become intertwined and are more difficult to break apart. This apparently results in a steady release of glucose over an extended period [6].
The differential glucose and insulin responses between WM and MS suggest that waxy maize might be a suitable choice of carbohydrate when a slower, more prolonged release of energy is desired. In this regard, it is important to note that the participants in this study were young, lean, physically fit individuals. Our HOMA value of 16% ± 2.4% confirms that our volunteers were highly insulin sensitive. For comparison, a HOMA value of more than 47% indicates a lowered insulin sensitivity [16]. Waxy maize starch might be especially useful in food preparation for athletes. A food that resulted in a slower release of energy could eliminate the potential for hypoglycemia after ingestion of a quickly digesting food. Previous research has shown that ingesting a more complex carbohydrate with a slower release of available carbohydrate results in a higher blood glucose concentration during exercise than more quickly digesting starches [2,17,18]. This higher blood glucose concentration throughout exercise can result in a longer, enhanced performance during exercise [19]. Further research is required to evaluate the effects of waxy maize on macro-nutrient utilization and performance during exercise. The elimination of hypoglycemia could also be useful in diabetes management, where a more controlled glucose response could help improve diabetes management and disease outcomes [7].
In this study white bread was used as a control treatment to determine a glycemic index value for the treatment containing waxy maize compared to the treatment without waxy maize. It was expected that the glycemic response of white bread would follow a course similar to that of maltodextrin; however, our results show a course more similar to the treatment containing waxy maize. This may be due to the storage conditions of the white bread. It is hypothesized that freezing and defrosting white bread may decrease the area under the curve of the glucose response [20]. It is also possible that other nutrients in the bread, besides starch, may have contributed to the lower glycemic response [21].
The decision to continue testing for 4 hours after the white bread, MS, and WM were consumed was based, in part, on the findings of Zhang et al [6]. They determined, using an in vitro model, that the glucose and insulin responses to WM should extend postprandially much longer than for fast digesting starches, and more than 2 hours were needed to document the differential responses [6]. Although the results of our study in humans indicates that the differential responses between the MS and WM primarily occurred during the first 60 min postprandial, future studies are needed to document the complete glucose and insulin response profiles. This will require continuing testing beyond 4 hours and until glucose and insulin concentrations return to baseline in all treatments.
Our findings indicated no difference in hunger or satiety among the treatments, but these results should be viewed with caution until confirmed. Granfeldt et al studied satiety responses to slowly digesting barley treatments compared to a white bread control. Barley products resulted in a higher satiety than white bread, which was found to be inversely correlated with lower glucose responses [9]. Our small sample size (n = 12) may have contributed to the inability to find a difference in hunger, desire to eat, and fullness. The observed powers for hunger, desire to eat, and fullness were found to be 0.4, 0.6, and 0.3, respectively. It is also possible that the results were affected by the food form; for example, beverages elicit a smaller satiety response than solid foods [22-24]. Each of the treatments containing starch was in a gel/semisolid form, whereas the white bread control was a solid food. We found no differences in satiety between the gel (test treatments) and solid (white bread) treatments. However, this may have been confounded by the low palatability and distaste of the treatments containing the starch as commented by the volunteers.
It has been stated that the use of 10 subjects or less may be an insufficient number to determine a reliable glycemic index in a food [25]. It is also recommended that the glycemic index be tested 2 to 3 times in each subject for a food to reduce within-subject variability [25]. Our small subject size, and only testing each treatment once in each subject, may have led to glycemic index values that are much more variable than if we had a larger sample size or repeated testing. Although our sample size (n = 12) is small, the randomized crossover design allowed us to calculate a repeated-measures ANOVA leading to more than 95% power to detect differences in our primary outcomes, 4-hour insulin and glucose, among the treatments. In spite of our small sample size, significant differences were still observed. Thus, our sample size appears to be adequate to identify differences in postmeal glucose and insulin responses.
An additional limitation pertains to the treatment compositions. The MS treatment was composed of 78% maltodextrin and 22% sucrose; thus, it is not appropriate to attribute the responses to maltodextrin alone or the differential glucose and insulin responses among treatments to the type of starch. It is highly likely that the sucrose in the MS might have affected the postprandial responses of the MS treatment. The glycemic indices of maltodextrin, sucrose, and fructose are 100, 58, and 12, respectively. The sucrose might have reduced the difference between the glycemic indices of the MS and WM treatments. Although our results are consistent with previous research in other types of slow digestible starches, they should be viewed as preliminary, and the precision and accuracy of the glycemic and insulinemic responses of waxy maize confirmed with other research.
In conclusion, these results establish in humans that the consumption of WM leads to blunted postprandial glucose and insulin responses, potentially leading to a more steady supply and release of energy over a period, compared to the rapidly digesting starch maltodextrin.
Acknowledgment
The authors would like to thank the study participants for their dedication and compliance to the study; Kristin Duke, project coordinator and research technician for the study, for her involvement with subject recruitment, screening, scheduling, and testing; Arthur Rosen who provided medical coverage; and Doug Maish who performed all catheter insertions and provided clinical laboratory services. This study was funded through the US Army, Natick Soldier RDECOM, Combat Feeding Directorate, Natick, Mass. Support for Heather Leidy was also provided, in part, by an Ingestive Behavior Research Center postdoctoral fellowship from Purdue University.
Abbreviations
- Au
arbitrary units
- ANOVA
analysis of variance
- AUC
area under the curve
- CHO
carbohydrate
- HOMA
homeostatic model assessment
- MS
maltodextrin-sucrose mixture
- WM
waxy maize starch
Footnotes
1.4 is the correction factor when using white bread as the control instead of glucose [14].
References
- 1.Seal CJ, et al. Postprandial carbohydrate metabolism in healthy subjects and those with type 2 diabetes fed starches with slow and rapid hydrolysis rates determined in vitro. Br J Nutr. 2003;90(5):853–64. doi: 10.1079/bjn2003972. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DE, Brotherhood JR, Miller JB. Plasma glucose levels after prolonged strenuous exercise correlate inversely with glycemic response to food consumed before exercise. Int J Sport Nutr. 1994;4(4):361–73. doi: 10.1123/ijsn.4.4.361. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann U, Robin F. Slowly digestible starch—it's structure and health implications: a review. Trends Food Sci Technol. 2008;18:345–55. [Google Scholar]
- 4.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46(Suppl 2):S33–50. [PubMed] [Google Scholar]
- 5.Zhang G, Venkatachalam M, Hamaker BR. Structural basis for the slow digestion property of native cereal starches. Biomacromolecules. 2006;7(11):3259–66. doi: 10.1021/bm060343a. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Ao Z, Hamaker BR. Slow digestion property of native cereal starches. Biomacromolecules. 2006;7(11):3252–8. doi: 10.1021/bm060342i. [DOI] [PubMed] [Google Scholar]
- 7.Ells LJ, et al. Postprandial glycaemic, lipaemic and haemostatic responses to ingestion of rapidly and slowly digested starches in healthy young women. Br J Nutr. 2005;94(6):948–55. doi: 10.1079/bjn20051554. [DOI] [PubMed] [Google Scholar]
- 8.Wachters-Hagedoorn RE, et al. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J Nutr. 2006;136(6):1511–6. doi: 10.1093/jn/136.6.1511. [DOI] [PubMed] [Google Scholar]
- 9.Granfeldt Y, et al. Glucose and insulin responses to barley products: influence of food structure and amylose-amylopectin ratio. Am J Clin Nutr. 1994;59(5):1075–82. doi: 10.1093/ajcn/59.5.1075. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Agriculture, A.R.S. USDA National Nutrient Database for Standard Reference, Release 20, in Nutrient Data Laboratory Home Page. 2007.
- 11.Kohl HW, et al. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127(6):1228–39. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 12.Weir JDV. New methods for calculating metabolic rate with specific reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66(2):159–65. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 14.Wolever TM, et al. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54(5):846–54. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 15.Wolever TM, et al. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57(3):475–82. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- 16.Carmina E, Lobo RA. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2004;82(3):661–5. doi: 10.1016/j.fertnstert.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DE, Brotherhood JR, Brand JC. Carbohydrate feeding before exercise: effect of glycemic index. Int J Sports Med. 1991;12(2):180–6. doi: 10.1055/s-2007-1024664. [DOI] [PubMed] [Google Scholar]
- 18.Guezennec CY, et al. The role of type and structure of complex carbohydrates response to physical exercise. Int J Sports Med. 1993;14(4):224–31. doi: 10.1055/s-2007-1021168. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, et al. The effects of pre-exercise starch ingestion on endurance performance. Int J Sports Med. 1996;17(5):366–72. doi: 10.1055/s-2007-972862. [DOI] [PubMed] [Google Scholar]
- 20.Burton P, Lightowler HJ. The impact of freezing and toasting on the glycaemic response of white bread. Eur J Clin Nutr. 2008;62(5):594–9. doi: 10.1038/sj.ejcn.1602746. [DOI] [PubMed] [Google Scholar]
- 21.Priebe MG, et al. An explorative study of in vivo digestive starch characteristics and postprandial glucose kinetics of wholemeal wheat bread. Eur J Nutr. 2008;47(8):417–23. doi: 10.1007/s00394-008-0743-6. [DOI] [PubMed] [Google Scholar]
- 22.Tieken SM, et al. Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults. Horm Metab Res. 2007;39(5):389–94. doi: 10.1055/s-2007-976545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattes RD, Rothacker D. Beverage viscosity is inversely related to postprandial hunger in humans. Physiol Behav. 2001;74(4-5):551–7. doi: 10.1016/s0031-9384(01)00597-2. [DOI] [PubMed] [Google Scholar]
- 24.Leathwood P, Pollet P. Effects of slow release carbohydrates in the form of bean flakes on the evolution of hunger and satiety in man. Appetite. 1988;10(1):1–11. doi: 10.1016/s0195-6663(88)80028-x. [DOI] [PubMed] [Google Scholar]
- 25.Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61(Suppl 1):S122–31. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]