Abstract
Limited research with rodents and humans suggests that oral ingestion of pinitol (3-O-methyl-d-chiro-inositol) might positively influence glucose tolerance. This double-blinded, placebo-controlled, and cross-over study assessed the effects of acute pinitol supplementation on plasma pinitol concentration, glucose tolerance, insulin sensitivity, and activation of the skeletal muscle insulin receptor. Fifteen older, nondiabetic subjects (62 ± 1 years, mean ± SEM) completed four, 1-day trials. Subjects consumed a non-nutritive beverage with nothing (placebo) or 1 000 mg pinitol. Sixty minutes later, the subjects consumed beverages that were either energy- and carbohydrate-free (Sham) or contained 75 g glucose (OGTT). Blood samples were collected frequently over the 240-min testing period. For the OGTT trials only, vastus lateralis samples were obtained before the placebo and pinitol supplementation and 60 min after consuming the 75 g glucose beverage. Plasma pinitol concentration increased and was maintained for 240 min. Pinitol did not influence the fasting state and 180-min area under the curves for plasma glucose and insulin during the Sham and OGTT trials or hepatic (placebo 0.83 ± 0.08; pinitol 0.80 ± 0.08) and whole-body (placebo 6.10 ± 0.54; pinitol 6.22 ± 0.52) insulin sensitivities. Activation of the muscle insulin receptor was increased by 140% with glucose ingestion (Pre 0.62 ± 0.12; Post 1.49 ± 0.35), but pinitol did not influence this response. These results show that the pinitol supplement was quickly absorbed, but did not acutely influence indices of whole-body glucose tolerance and insulin sensitivity, or the activation of the skeletal muscle insulin receptor in older, nondiabetic humans.
Keywords: d-chiro-inositol, chiro-inositol, myo-inositol
Introduction
Many people experience an increase in fat mass and a decrease in fat-free mass as they advance in age. These age-related changes in body composition may be associated with glucose intolerance and peripheral tissue insulin resistance, which may increase an individual’s susceptibility to diabetes mellitus [1]. Insulin resistance is accompanied by an impairment of the insulin-stimulated glycosylphosphatidylinositol (GPI)/inositol phosphoglycan (IPG) pathway, which can lead to a reduction in GPI hydrolysis of IPG [2]. IPG contains various compounds such as d-chiro-inositol, myo-inositol, glucosamine, galacosamine, and other residues. Myo-inositol is widely distributed in mammalian tissues whereas d-chiro-inositol is relatively rare [3]. The IPG that contains the d-chiro-inositol is a potential second messenger in mediating insulin’s effect on peripheral glucose utilization by activating glycogen synthase and pyruvate dehydrogenase (rate limiting enzymes of oxidative and nonoxidative glucose disposal) [2, 4, 5]. A potential dietary source of d-chiro-inositol is pinitol (3-O-methyl-d-chiro-inositol), a methylated derivative of d-chiro-inositol. Pinitol has been identified in high concentrations in legumes and whole soybeans (1–2% dry weight of whole soybeans) [6, 7].
Limited number of animal [8, 9] and human [10–12] research studies concluded that using pinitol as a dietary supplement improved glycemic control. However, there are animal [8] and human [13, 14] data that show opposing results. Although a positive effect of pinitol on glycemic control was not observed, plasma concentrations [13, 14] and urinary excretions [13, 14] of pinitol were increased over a 4- and 7-week period. There was only a single collection of fasting plasma pinitol at the beginning and end of the study. The plasma pinitol concentration over time following pinitol supplementation is not documented in published literature.
The literature is still inconclusive on the effects of pinitol supplementation on glycemic control. The purposes of this study were to assess the effects of acute pinitol supplementation on appearance of pinitol in the bloodstream (i.e., plasma pinitol concentration), glucose tolerance, and insulin sensitivity during fasting and oral glucose-stimulated hyperglycemic and hyperinsulinemic conditions in older, nondiabetic subjects. Activation of the skeletal muscle insulin receptor was also evaluated. We hypothesized that pinitol supplementation would increase plasma pinitol concentration and the higher concentration would be maintained during the 240-min period of measurements. Also, pinitol supplementation would decrease fasting plasma glucose concentrations and blunt the oral glucose-induced rise in plasma glucose concentrations, as well as increase hepatic and whole body (hepatic plus peripheral tissues) insulin sensitivities and enhance the activation of the skeletal muscle insulin receptor.
Methods and Materials
Subjects
This study included 15 participants recruited from the greater Lafayette, IN region. The inclusion criteria included: 1) age range 50–75 years; 2) body mass index 23 to 33 kg/m2; 3) clinically normal kidney function, liver function, heart function, protein status, and hematological profile; 4) nondiabetic; 5) performance of vigorous exercise <3 times per week for <30 min per session in the 6 months prior to entering the study; 6) willing to stop taking supplements, aspirin, and anti-inflammatory steroid medications starting 4 weeks prior to and during (including nontesting days) the study; and 7) women postmenopausal for >1 year. Before admittance into the study, each subject successfully completed an evaluation that included a self-reported medical history questionnaire, a resting electrocardiogram, and routine blood and urine chemistries that were taken in the fasted state. These results were used for screening purposes only and not as baseline testing measurements. Subjects signed a consent form after receiving complete written and verbal explanations of the study protocol. Each subject received monetary compensation for their time and commitment in the study. The study physician approved the subjects’ eligibility for the study and Purdue University’s Committee on the Use of Human Research Subjects, West Lafayette, IN reviewed and approved the study protocol, consent form, and recruitment tools.
Study design
A double-blinded, placebo-controlled, cross-over study design was used. Each subject completed four, 1-day trials over a 4-week period (i.e., 1-week between trials); weeks 1 and 2 (Sham trials) were randomized separately from weeks 3 and 4 (oral glucose tolerance test, OGTT trials). Each person received the placebo and pinitol supplements twice (Sham-placebo, Sham-pinitol, OGTT-placebo, OGTT-pinitol).
Dietary control
On the day prior to each of the four trials, each subject was provided all of their foods and beverages (except water) and instructed to only consume those items. The provided menus contained pinitol-free/low-pinitol foods (e.g., no soy or legumes) and a macronutrient distribution of 15% protein, 35% fat, and 50% carbohydrate. Each subject’s total energy intake was estimated using the sex-specific Harris–Benedict equation [15] and a physical activity level factor of 1.5 (sedentary) [16]. The database Nutritionist Pro™ version 1.3 (First Data Bank, INC) was used to structure the menus with the appropriate macronutrient and energy needs of each subject. All foods were prepared and distributed at the metabolic research kitchen, affiliated with Purdue University’s Department of Foods and Nutrition.
Supplementation
During the Sham and OGTT trials, in random order, subjects consumed 30 ml of a saccharin-sweetened, cherry-flavored beverage with nothing (placebo) or 1 000 mg of pinitol dissolved into it. The certificate of chemical analysis of the pinitol supplement (INZITOL™, 3-O-methyl-1,2,4 cis-3,5,6-trans-hexahydroxycyclohexane), provided by Humanetics Corporation (Chanhassen, MN, USA) was documented by using high-performance liquid chromatography with a pinitol purity of 91%. Pinitol was extracted from the wood of a pine tree. Members of the research not involved in the study mixed the beverages with a placebo or pinitol.
Sham and OGTT trial procedures
Each subject reported to the research laboratory in the morning after a 12-h fast. While the subjects were in a fasting state, a catheter was inserted into an antecubital vein. The subjects voided urine, had a fasting blood sample collected (minute 0), and immediately consumed saccharin sweetened beverages without (placebo) or with pinitol added within 5 min. Sixty minutes later, the subjects consumed either an energy- and carbohydrate-free beverage (Sham trials) or 75 g glucose beverage (OGTT trials). Blood for plasma glucose and insulin analyses were collected at minutes 30, 60, 75, 90, 105, 120, 150, 180, 210, and 240. At minutes 60, 120, 180, and 240, blood for plasma pinitol and myo-inositol analyses were also collected. Blood samples for analysis of glucose and insulin were collected into vacutainers containing EDTA, and blood samples for pinitol and myo-inositol were collected into vacutainers containing lithium herparin. All blood samples were centrifuged at 3 000 rpm for 10 min at 4° C. The plasma was dispensed into cryovials and then stored in the −80° C freezer until analyzed.
Muscle samples
During the OGTT trials, two muscle biopsies were performed. After catheterization and before placebo and pinitol supplementation, a small portion of the subjects’ mid-thigh was anesthetized with ~3 ml of a 1% lidocaine solution. Fifteen minutes later, a sample of muscle from the vastus lateralis was obtained by the percutaneous needle biopsy technique (a 6-mm Bergstrom biopsy needle; Microsurgical Instruments) with applied suction [17]. The second muscle biopsy was obtained 60 min post consumption of the 75 g glucose beverage and 120 min after the placebo and pinitol supplementation. This time point was chosen because it corresponded with the expected maximum plasma insulin concentration during an OGTT [18]. The extracted muscle tissues were quickly blotted to remove blood, fat, or connective tissue. The muscle samples were frozen in liquid nitrogen within 90 s of extraction and then stored at −80° C until analyzed.
Urine collection
Timed 240-min urine collections were made between minutes 0 and 240 during each of the four testing days. The total volume of the 240-min urine collection was measured with a graduated cylinder and dispensed into cryovials that were stored in a −80° C freezer until further analyzed. Urinary pinitol and myo-inositol excretions were determined.
Analytical Methods and Calculations
Plasma glucose and insulin
Plasma glucose concentrations were determined by an oxidase method on a COBAS analyzer (Mira Plus, Roche Diagnostic Systems; serial # 31–1718; Indianapolis, IN, USA). Plasma insulin concentrations were analyzed by using enzyme-linked immunosorbent assay (Human insulin ELISA, American Laboratory Products Company, 008-10-1113-01, Windham, NH, USA). For the OGTT trials, the 180-min integrated AUC for glucose and insulin were calculated using the trapezoidal method [19]. Estimates of hepatic and whole-body (hepatic plus peripheral tissues) insulin sensitivities were measured using data from 0 to 120 min and formulas described by Matsuda and DeFronzo [20].
Inositols
Plasma and urine samples were analyzed for pinitol and myo-inositol concentrations by a modification of a previous method [14, 21]. A known amount of allo-inositol (not present in human body) was added to urine and plasma inositol samples as an internal standard before sample purification and the recovery of allo-inositol was verified quantitatively. The purified inositol fractions were derivatized by heating with 100 µL of 10% N-tri-methylsilylimidazole (TMSI) in acetonitrile overnight at 60° C. The derivatized samples were analyzed using electron impact capillary gas chromatography/mass spectrometry with a GCQ (FinniganMAT/ThermoElectron Corporation, San Jose, CA, USA) mass spectrometer system. Typical electron energy was 70 eV with the ion source temperature maintained at 200° C. The individual inositols were separated using a 15 meter DB-1 capillary column. The initial column temperature was set at 100° C (held for 0.1 min) and programmed to 280° C at 10° C per minute. The injector temperature was set at 250° C.
Tyrosine phosphorylation of the insulin receptor β
Total tyrosine phosphorylation of the insulin receptor β was measured in skeletal muscle as described previously [22] by using western blot procedures. Briefly, powdered muscle was homogenized in buffer [50 mM Hepes, 50 mM sodium pyrophosphate, 100 mM NaF, 10 mM EDTA, 10 mM sodium orthovanadate, 1% Triton X-100, and protease and phosphatase (1 and 2) inhibitor cocktails (Sigma, St. Louis, MO, USA)] on ice. After centrifugation for 25 min at 15 000 × g, supernatants were extracted and protein content was detected using a BCA protein assay (Pierce, Rockford, IL, USA). To measure tyrosine phosphorylation of the insulin receptor (IRβ) equal amounts of protein from the muscle homogenate was immunoprecipitated overnight with antiphosphotyrosine agarose beads and homogenization buffer. Resulting immunoprecipitates were separated by SDS-PAGE using 7.5% Tris-HCl gels and then transferred to PVDF membranes, which were probed for total IRβ resulting in bands detecting tyrosine phosphorylation levels of IRβ. The following antibodies were used: insulin receptor (IRβ) from Santa Cruz Biotech (Santa Cruz, CA) and antiphosphotyrosine agarose beads from Sigma (St. Louis, MO, USA).
Body composition
Each subject’s body density was determined by using a whole body plethysmographer (BOD POD®, Life Measurement, Inc., Concord, CA, USA) [23]. The test was done in the morning after a 12-h overnight fast and immediately after the subject had voided urine. The subjects were asked to wear a tight-fitting swimsuit and cap for the test. Body fat percent and fat-free mass were estimated from body density using the two-compartment model equation of Siri [24].
Statistical analyses
Values were reported as mean ± SEM. Data from two subjects were excluded from the Sham trials because they were inadvertently provided with the wrong supplement, which was identified by their urinary pinitol excretion (Sham n = 13, OGTT n = 15). The mean of glucose and insulin concentrations during the sham and AUC of glucose and insulin during the OGTT trials were assessed using a single-factor repeated measure ANOVA with treatment (placebo and pinitol) as a repeated effect in the model. Urinary pinitol and myo-inositol excretions were accessed using a 2-factor repeated measure ANOVA with treatment and trial (Sham and OGTT) as repeated effects in the model. Plasma pinitol and myo-inositol concentrations were assessed using a 3-factor repeated measure ANOVA with time, treatment, and trial as repeated effects in the model with post-hoc (Tukey) comparisons. Tyrosine phosphorylation of the insulin receptor was assessed using a 2-factor repeated measure ANOVA with time (pre and post) and treatment as repeated effects in the model. Statistical significance was assigned if p ≤ 0.05. Data processing and statistical evaluation were performed using Statistical Analyses Systems software (version 9.1, SAS Institute Inc., Cary, NC, USA).
Results
The mean subject characteristics were as follows: 62 ± 1 years, 27.7 ± 0.6 kg/m2 body mass index, 36.2 ± 0.6% body fat, and 50.1 ± 0.9 kg fat-free mass. The 15 subjects were classified as follows: fasting plasma glucose: 10 subjects normal (< 5.6 mmol/l) and 5 subjects impaired (5.6–6.9 mmol/l); 2-h plasma glucose during OGTT: 6 subjects normal (< 7.8 mmol/l) and 9 subjects impaired (7.8–11.0 mmol/l). According to the 2-h plasma glucose during the OGTT, 60% of the study population was considered as having impaired glucose tolerance.
Plasma pinitol and myo-inositol
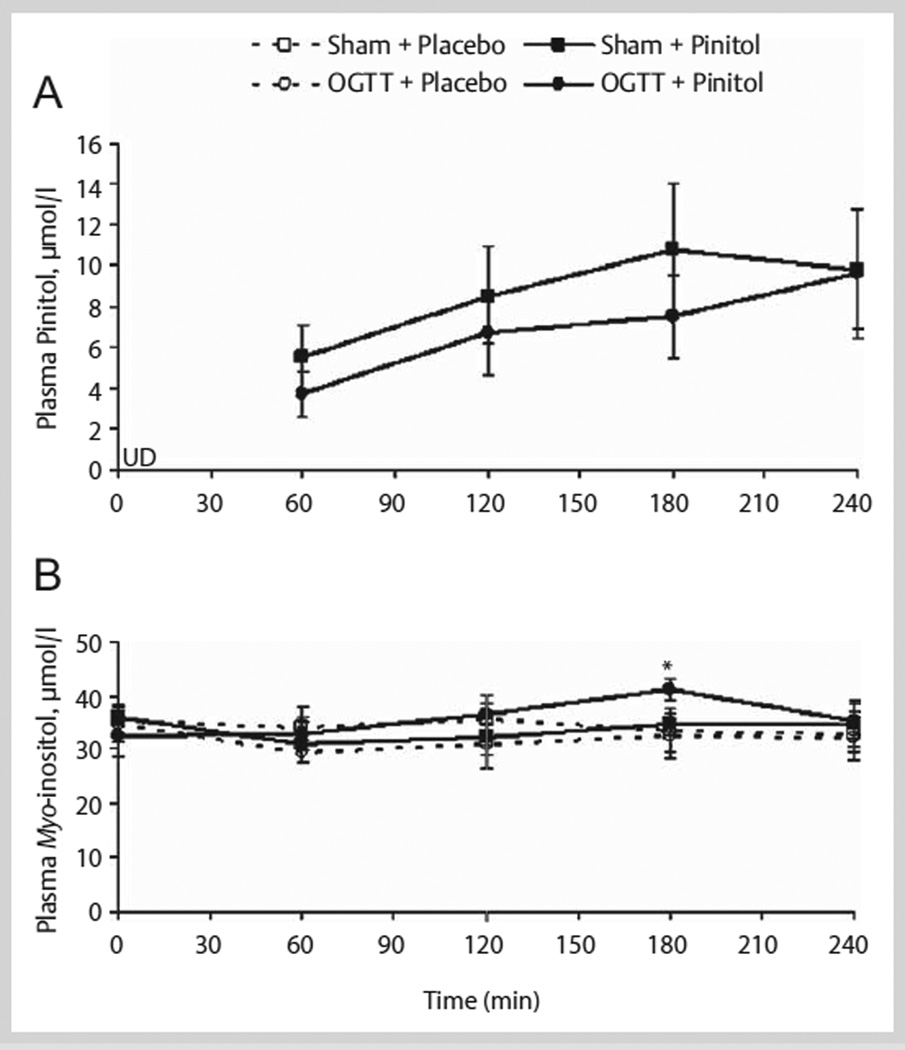
A complete profile (minutes 0, 60, 120, 180, and 240) of plasma pinitol concentration was determined in a subset of 7 subjects. For the other 8 subjects, the plasma collected before (minute 0) and after pinitol supplementation (minute 180) during the Sham and OGTT trials was used to confirm the presence of pinitol. Fasting plasma pinitol concentrations were below the level of detection (minute 0). After placebo supplementation, plasma pinitol remained undetected during the Sham and OGTT trials’ 240-min testing period. However, 60 min after pinitol supplementation, plasma pinitol became detectable and was maintained during the Sham and OGTT trials’ 240-min testing period (n = 7, Fig. 1). Pinitol absorption into the bloodstream, after pinitol supplementation, was confirmed in the other 8 subjects at minute 180 during the Sham (9.9 ± 1.7 µmol/l) and OGTT (12.5 ± 1.9 µmol/l) trials. At minute 0, plasma pinitol concentration was below the threshold of detection in these 8 subjects. Plasma myo-inositol was detected before (fasting, minute 0) and after placebo and pinitol supplementation during the Sham and OGTT trials (n = 7; Fig. 1). At minute 180, plasma myo-inositol concentration was different between the placebo and pinitol supplementation only during the OGTT trials (Fig. 1).
Fig. 1.
(A) Plasma pinitol and (B) myo-inositol concentrations over 240 min before and after acute placebo and 1 000 mg pinitol supplementation during Sham and OGTT in a subset of healthy older subjects (n = 7). Placebo and pinitol were given at minute 0 (after minute 0 blood draw) and the Sham and 75 g glucose beverages were given at minute 60 (after minute 60 blood draw). * p = 0.04, OGTT + Placebo vs. OGTT + Pinitol. UD = undetected.
Urine pinitol and myo-inositol
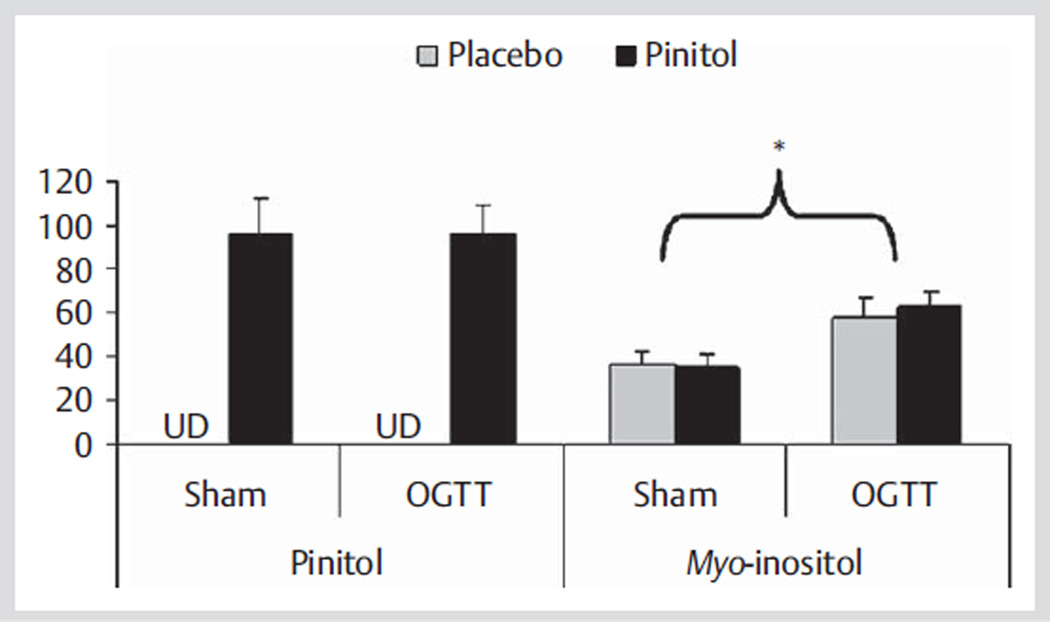
Urinary pinitol excretion was undetected after placebo supplementation and was detectable after pinitol supplementation during Sham and OGTT trials (Fig. 2). Urinary myo-inositol excretion was not influenced by pinitol supplementation, but was 69% higher during the OGTT (60.2 µmol/240 min) versus Sham (35.7 µmol/240 min) trials (Fig. 2).
Fig. 2.
Urinary pinitol and myo-inositol excretions over 240 min after acute placebo and 1 000 mg pinitol supplementation during Sham (n = 13) and OGTT (n = 15) trials in healthy older subjects. * p < 0.0001, Sham vs. OGTT. UD = undetected.
Plasma glucose and insulin
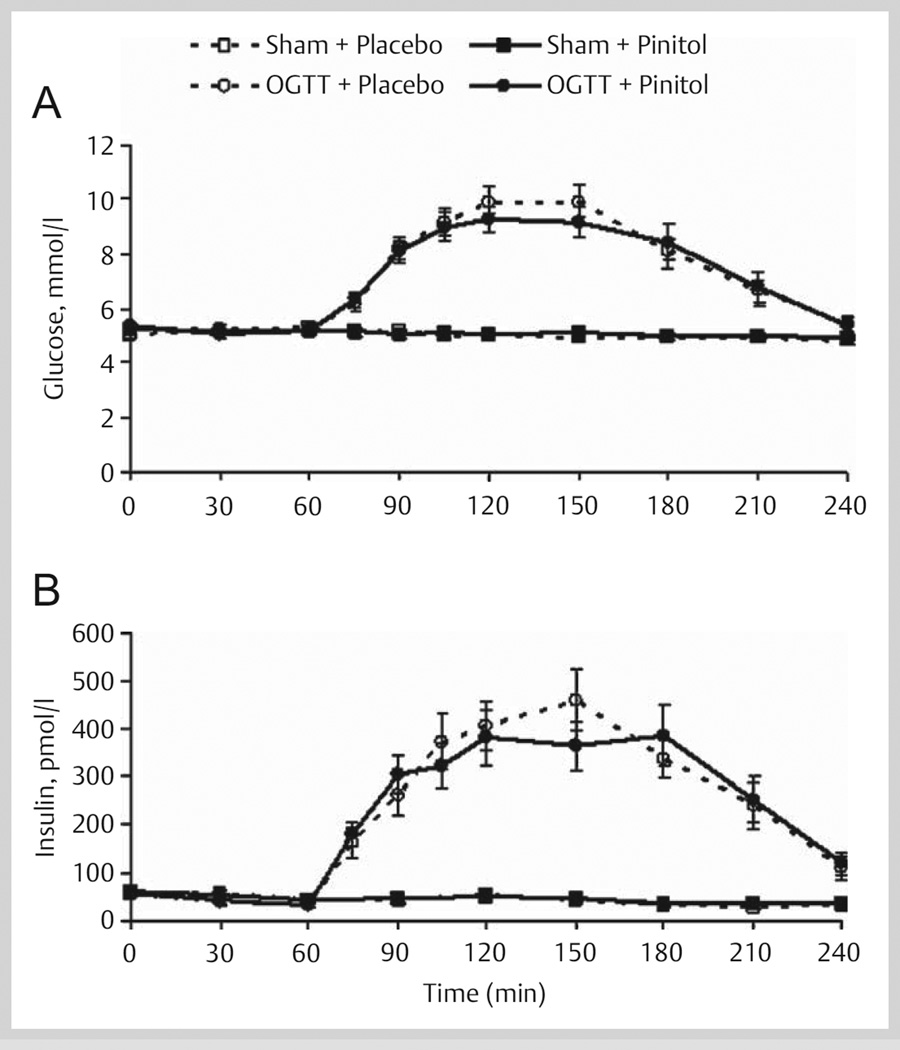
During the Sham trials, mean glucose and insulin concentrations were not influenced by pinitol supplementation during the 240-min fasting testing period (Fig. 3). During the OGTT trials, the subjects reached hyperglycemic and hyperinsulinemic states. However, pinitol supplementation did not blunt the oral glucose-induced plasma glucose AUC (placebo, 493 ± 46 and pinitol, 480 ± 59 mmol/l·180 min) and insulin AUC (placebo, 47 ± 6 and pinitol, 46 ± 6 nmol/l·180 min) (Fig. 3). There were no differences between placebo and pinitol supplementation for estimates of hepatic (placebo, 0.83 ± 0.08 and pinitol, 0.80 ± 0.08) and whole body (placebo, 6.10 ± 0.54 and pinitol, 6.22 ± 0.52) insulin sensitivities.
Fig. 3.
(A) Plasma glucose and (B) insulin concentrations before and after acute placebo and 1 000 mg pinitol supplementation during Sham (n = 13) and OGTT (n = 15) trials in healthy older subjects. The placebo and pinitol were given at minute 0 (after minute 0 blood draw) and the sham and 75 g glucose beverages were given at minute 60 (after minute 60 blood draw).
Tyrosine phosphorylation of the insulin receptor β
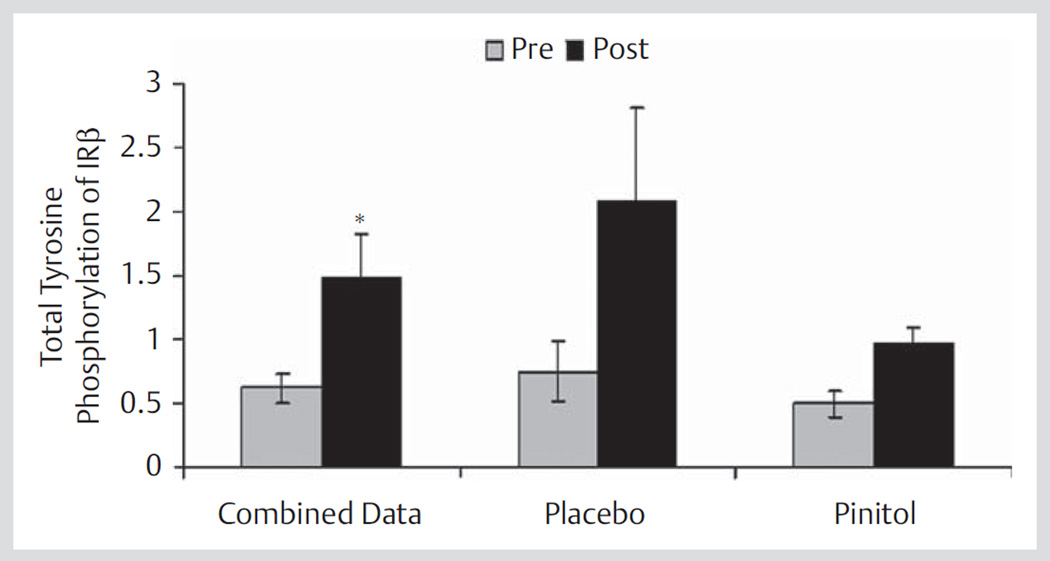
Muscle samples from a subset of 7 subjects were analyzed for activation of the insulin receptor. Over time, from the fasting (pre) to the oral glucose-induced hyperglycemic and hyperinsulinemic state (post), the activation of the insulin receptor was increased by 140% (Fig. 4). However, tyrosine phosphorylation of the insulin receptor was not influenced by pinitol supplementation (Fig. 4) and further analyses were not completed.
Fig. 4.
Activation of the insulin receptor before (pre) placebo and 1 000 mg pinitol supplementation and 60 min after (post) 75 g glucose beverage in a subset of healthy older subjects (n = 7). The combined data contains all of the subjects for the pre (pre-placebo plus pre-pinitol, n = 14) and post (post-placebo plus post-pinitol, n = 14). * p = 0.02, Pre versus Post. IR = insulin receptor.
Discussion
To our knowledge, this is the first study to evaluate plasma pinitol concentration over time acutely following pinitol supplementation during fasting and oral glucose stimulated states. Our data show that glucose stimulation was not necessary for the increased concentration of pinitol in the bloodstream. For both physiological states, following acute pinitol supplementation, the pinitol concentration was increased in the bloodstream within 60 min, maintained in the bloodstream over a 240-min testing period, and excreted in the urine. The mean 96 µmol/240 min urinary pinitol excretion was 1.9% of the amount of pinitol consumed during the testing period. These observations support previous research studies [13, 14] that documented pinitol supplementation was an integral contributor to the increase in plasma pinitol concentrations and urinary pinitol excretions. The results of the current study suggest that acute pinitol supplementation does not influence fasting and oral glucose-induced rise in plasma glucose and insulin concentrations in older, nondiabetic adults. These data support the findings from another acute pinitol supplementation study in mice. Bates et al. [8] reported that acute oral administration of pinitol (100 mg/kg) to normal nondiabetic and hyperinsulinemic obese-diabetic mice (model of type 2 diabetes) did not influence fasting plasma glucose and insulin concentrations over 4 and 6 h, respectively. Also, the normal non-diabetic mice did not experience a change in plasma glucose and insulin after a 4-h glucose tolerance test. However, there are animal [8, 9] data that show acute changes in plasma glucose concentrations after pinitol supplementation, but plasma insulin concentrations were not affected. In comparison, the only other human acute pinitol supplementation study evaluated various doses of pinitol (1 200 mg and 600 mg) at different time points (0, 60, 120, 180 min) prior to the meal tolerance test (rice, 50 g carbohydrate). These humans were diabetic and their plasma insulin AUC did not change during supplementation, but the plasma glucose AUC was lowered when supplemented at a dose of 1 200 mg of pinitol 60 min prior to a meal tolerance test. When pinitol (600 and 1 200 mg) was consumed at minutes 0, 60 (only 600 mg), 120, and 180, its influence on plasma glucose AUC was insignificant. Plasma pinitol concentrations and the effect of pinitol during the fasting state were not assessed. The subjects in the current study were supplemented with a comparable dose of pinitol (1 000 mg) 60 min prior to an oral glucose tolerance test (75 g glucose beverage). However, null findings were obtained between the placebo and pinitol supplementation and its effects on plasma glucose and insulin AUC.
There are also chronic (ranging from 72 h to 13 weeks) pinitol supplementation studies that have observed the glucose lowering effect of pinitol in nondiabetic and diabetic rodents and humans [8, 10–12], while others have observed no differential response [13, 14]. There are many different possible reasons for discrepancies in the data. Previous studies purchased the pinitol from different companies, the extraction methods were different, and the source of pinitol (i.e., soybeans, plant Bougainvillea spectabilis, and wood from the pine tree) varied for each study. Also, the percent of pinitol purity and the amount of d-chiro-inositol and other contaminants in the source of pinitol were not reported in many of the studies. Other discrepancies may be explained by the population studied, duration of the studies, and also other medications taken with pinitol. There is evidence that metformin, a hypoglycemic agent, may enhance the action of insulin by improving insulin-mediated release of the IPG containing d-chiro-inositol, which helps with peripheral glucose utilization [5, 25]. When pinitol was consumed in conjunction with a hypoglycemic agent, there was a reduction in plasma glucose concentrations in overweight individuals with type 2 diabetes [10–12]. However, this response was not observed in a similar population when pinitol was consumed without any other medications [14]. It is possible that the pinitol and hypoglycemic agent synergically or synergistically improved plasma glucose and insulin concentrations in individuals with type 2 diabetes. Future research is warranted to investigate if pinitol is more effective with a hypoglycemic agent in controlling hyperglycemia.
It is still unclear how pinitol may influence glucose metabolism. Pinitol might be converted to d-chiro-inositol, but this conversion is not conclusive [13, 14]. Also, pinitol may influence glucose uptake into cells similar to insulin [8]. Incubation of L6 muscle cells (derived from rat skeletal muscle) with pinitol (independent of insulin) increased glucose uptake by 41% after 10 min, 34% after 4 h, and 15% after 24 h [8]. It is unknown if the glucose uptake percent was decreased due to lower concentrations of pinitol and d-chiro-inositiol concentrations over time. In addition, it is unknown if pinitol directly or indirectly (conversion of pinitol to d-chiro-inositol) contributed to the glucose uptake. Pinitol and d-chiro-inositol concentrations were not measured in the study [8]. The current study did not observe any influences of pinitol on the activation of the insulin receptors, which would have been similar to an insulin effect on glucose metabolism. However, there was an increase in the activation of the insulin receptors after an oral glucose-stimulated load. Future research is warranted to further explore pinitol and d-chiro-inositol in the muscle and its mechanisms.
Interestingly, urinary myo-inositol excretion was higher during the OGTT trials than the sham trials, which suggests that urinary myo-inositol excretion is responsive to the acute creation of hyperglycemic and hyperinsulinemic states. This finding supports previous observations that urinary myo-inositol excretion is increased in people with poor glycemic control [21] and the suggestion that it may be a sensitive marker of glucose intolerance [26]. Also, glucose stimulation in conjunction with pinitol increased plasma myo-inositol concentrations. It is possible that pinitol influences plasma myo-inositol concentrations. However, myo-inositol has never been measured in other pinitol supplementation studies and more research is necessary.
Conclusion
Findings from this study improve our understanding of the acute appearance of pinitol in the bloodstream and excretion of pinitol after oral pinitol supplementation, and support that pinitol does not influence glucose tolerance, insulin sensitivity, and activation of the insulin receptor in older, nondiabetic humans. There are still gaps in pinitol research that need to be explored before any conclusion can be drawn about the effectiveness of pinitol supplementation on glycemic control.
Acknowledgments
Sincere thanks go to the volunteers who participated in this study. We are grateful to the undergraduate and graduate students in the Nutrition, Fitness, and Aging Laboratory for their dedicated support of this research. We also thank Connie Bonham for analyzing the inositol samples on the Gas chromatography/mass spectrometry machine; Arthur Rosen, MD for providing medical coverage; Robert Drennan, MD for interpreting electrocardiograms; Andreana Robertson for help with statistical analyses; and Zonda Birge, MS, and Anne Mahon, PhD, RD, for providing technical assistance to the project. Also, we would like to thank Humanetics Corporation for providing the pinitol powder. Support: NIH P50 AT00477, Purdue University Lynn and Doctoral Fellowships, and Phillis Izant gift funds.
Footnotes
During the time the research was conducted Dr. April J. Stull was a doctoral student and currently she is a postdoctoral fellow at Pennington Biomedical Research Center, Division of Nutrition and Chronic Disease and Botanicals Research Center, 6400 Perkins Rd., Baton Rouge, LA 70808, USA
References
- 1.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 2.Larner J. d-chiro-inositol – its functional role in insulin action and its deficit in insulin resistance. Int J Exp Diabetes Res. 2002;3:47–60. doi: 10.1080/15604280212528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larner J, Huang LC, Schwartz CF, Oswald AS, Shen TY, Kinter M, Tang GZ, Zeller K. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and d-chiroinositol. Biochem Biophys Res Commun. 1988;151:1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]
- 4.Ortmeyer HK, Bodkin NL, Hansen BC, Larner J. In vivo d-chiroinositol activates skeletal muscle glycogen synthase and inactivates glycogen phosphorylase in rhesus monkeys. Nutr Biochem. 1995;6:499–503. [Google Scholar]
- 5.Saltiel AR. Second messengers of insulin action. Diabetes Care. 1990;13:244–256. doi: 10.2337/diacare.13.3.244. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DV, Dougherty DE, Smilth AE. Cyclitols in soybean. J Agric Food Chem. 1982;30:456–458. doi: 10.1021/jf00111a011. [DOI] [PubMed] [Google Scholar]
- 7.Streeter JG, Strimbu CE. Simultaneous extraction and derivatization of carbohydrates from green plant tissues for analysis by gas-liquid chromatography. Anal Biochem. 1998;259:253–257. doi: 10.1006/abio.1998.2675. [DOI] [PubMed] [Google Scholar]
- 8.Bates SH, Jones RB, Bailey CJ. Insulin-like effect of pinitol. Br J Pharmacol. 2000;130:1944–1948. doi: 10.1038/sj.bjp.0703523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayanan CR. Pinitol – A new anti-diabetic compound from the leaves of Bougainvillea Spectabilis. Curr Sci. 1987;56:139–141. [Google Scholar]
- 10.Kang MJ, Kim JI, Yoon SY, Kim JC, Cha IJ. Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetes mellitus. J Med Food. 2006;9:182–186. doi: 10.1089/jmf.2006.9.182. [DOI] [PubMed] [Google Scholar]
- 11.Kim JI, Kim JC, Kang MJ, Lee MS, Kim JJ, Cha IJ. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: a randomized controlled study. Eur J Clin Nutr. 2005;59:456–458. doi: 10.1038/sj.ejcn.1602081. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Yoo KH, Kim JH, Seo YT, Ha BW, Kho JH, Shin YG, Chung CH. Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res Clin Pract. 2007;77(Suppl 1):S247–S251. doi: 10.1016/j.diabres.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 13.Campbell WW, Haub MD, Fluckey JD, Ostlund RE, Jr, Thyfault JP, Morse-Carrithers H, Hulver MW, Birge ZK. Pinitol supplementation does not affect insulin-mediated glucose metabolism and muscle insulin receptor content and phosphorylation in older humans. J Nutr. 2004;134:2998–3003. doi: 10.1093/jn/134.11.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis A, Christiansen M, Horowitz JF, Klein S, Hellerstein MK, Ostlund RE., Jr Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care. 2000;23:1000–1005. doi: 10.2337/diacare.23.7.1000. [DOI] [PubMed] [Google Scholar]
- 15.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington D.C.: Carnegie Institute of Washington; 1919. [Google Scholar]
- 16.Shetty PS, Henry CJ, Black AE, Prentice AM. Energy requirements of adults: an update on basal metabolic rates (BMRs) and physical activity levels (PALs) Eur J Clin Nutr. 1996;50(Suppl 1):S11–S23. [PubMed] [Google Scholar]
- 17.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 18.Campbell WW, Ostlund RE, Jr, Joseph LJ, Farrell PA, Evans WJ. Relationships of plasma C-peptide and gender to the urinary excretion of inositols in older people. Horm Metab Res. 2001;33:44–51. doi: 10.1055/s-2001-12626. [DOI] [PubMed] [Google Scholar]
- 19.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Ostlund RE, Jr, MacGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR. d-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci USA. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2007;292:C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 23.MacCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 24.Siri W. Techniques for measuring body composition. Washington, D.C.: National Academy of Sciences; 1961. Body Composition from fluid spaces and density: analysis of methods; pp. 223–224. [Google Scholar]
- 25.Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Apridonidze T, He N, Nestler JE. Metformin therapy increases insulin-stimulated release of d-chiro-inositol-containing inositolphosphoglycan mediator in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:242–249. doi: 10.1210/jc.2003-030437. [DOI] [PubMed] [Google Scholar]
- 26.Sarashina G, Yamakoshi M, Noritake M, Takahashi M, Kure M, Katsura Y, Shiomi H, Tsuboi I, Kawazu S, Yamagata F, Tominaga M, Matsuoka T. A study of urinary myo-inositol as a sensitive marker of glucose intolerance. Clin Chim Acta. 2004;344:181–188. doi: 10.1016/j.cccn.2004.02.026. [DOI] [PubMed] [Google Scholar]