Abstract
Background
Alcohol consumption is a known antecedent to cocaine relapse. Through associative conditioning, it is hypothesized that alcohol increases incentive motivation for cocaine and thus the salience of cocaine-related cues, which are important in maintaining drug-taking behavior. Cocaine-using individuals display a robust cocaine cue attentional bias as measured by fixation time during the visual probe task. The purpose of the present study was to evaluate the influence of alcohol administration on cocaine cue attentional bias using eye-tracking technology to directly measure attentional allocation.
Methods
Twenty current cocaine users completed a double-blind, placebo-controlled, within-subjects study that tested the effect of three doses of alcohol (0.00, 0.325, 0.65 g/kg alcohol) on cocaine cue attentional bias using the visual probe task with eye-tracking technology. The participant-rated and physiological effects of alcohol were also assessed.
Results
Participants displayed a robust cocaine cue attentional bias following both placebo and alcohol administration as measured by fixation time, but not response time. Alcohol administration did not influence cocaine cue attentional bias, but increased craving for cocaine in a dose dependent manner. Alcohol produced prototypic psychomotor and participant-rated effects.
Conclusions
Alcohol administration increases cocaine craving but not cocaine cue attentional bias. Alcohol-induced cocaine craving suggests that alcohol increases incentive motivation for cocaine but not the salience of cocaine-related cues.
Keywords: attentional bias, alcohol, cocaine, visual probe task, eye tracking
Introduction
Alcohol consumption is a known antecedent to cocaine relapse. Sixty-two percent of those who report relapsing to cocaine within six months of treatment report consuming alcohol the same day (McKay et al., 1999). In addition to precipitating relapse to cocaine, alcohol and cocaine are commonly used in combination. Fifty-one percent of individuals who meet diagnostic criteria for cocaine dependence also endorse alcohol abuse or dependence (Substance Abuse and Mental Health Services Administration, 2013). Co-administration of alcohol and cocaine outside the laboratory results in greater self-reported alcohol and cocaine consumption than if used separately (Gossop et al., 2006). Human behavioral pharmacology experiments have demonstrated that in cocaine-using individuals, alcohol pretreatment increases choice for cocaine over an alternative monetary reinforcer (Higgins et al., 1996). The mechanism by which alcohol increases cocaine use, however, is not well understood.
Cocaine-related cues are important in maintaining drug-taking behavior (Childress et al., 1999; Kosten et al., 2006; Sinha and Li, 2007; Volkow et al., 2008). Chronic cocaine use sensitizes mesolimbic dopamine pathways associated with the attribution of incentive salience and reward (Robinson and Berridge, 1993; Wise, 1996). Over repeated associative pairings between cocaine and cocaine-related environmental stimuli, incentive salience for cocaine transfers to the cocaine-paired cue, causing the cue to elicit a conditioned motivational state in active cocaine users (Goldstein and Volkow, 2002). This motivational state results in increased cocaine “wanting” and “craving” and therefore attention biased in favor of cocaine-paired cues (Franken, 2003; Robinson and Berridge, 1993; Ryan, 2002). Consequently, cocaine users will selectively attend to cocaine-paired cues in the environment (see Leeman et al., 2014 for review). This allocation of a disproportionate amount of time attending to cocaine-paired stimuli is referred to as attentional bias. Through repeated associative pairings between alcohol and cocaine, alcohol is hypothesized to produce a conditioned motivational state for cocaine (i.e., increased wanting and craving) and further increase cocaine cue attentional bias (Franken, 2003; Robinson and Berridge, 1993).
Montgomery and colleagues (2010) conducted a human laboratory study to systematically evaluate the influence of alcohol on cocaine cue attentional bias. Alcohol (0.0 and 0.4 g/kg) was administered to light cocaine users and non-cocaine-using controls and attentional bias to cocaine and neutral images was measured using response time during the visual probe task. Following placebo, neither cocaine-using individuals nor controls displayed an attentional bias. Following alcohol administration, cocaine-using individuals responded faster to cocaine-related images than neutral images, indicating cocaine cue attentional bias. These results provide evidence that alcohol increases cocaine cue attentional bias in light cocaine users, but not controls.
Response time, however, is an indirect measure of attentional allocation (Field and Cox, 2008). Speed of processing, as measured by a motoric response, only approximates attention immediately prior to the appearance of the probe. Thus, response time does not assess the topography of attention over the duration of the stimulus presentation (Field and Cox, 2008). Response time, particularly in the visual probe task, may not be an optimal measure of attentional bias as internal (Ataya et al., 2012) and test-retest reliability (Marks et al., 2014a; Spiegelhalder et al., 2011) are poor. In contrast, eye-tracking technology directly measures visual attention by recording the direction and duration of gaze (Godijn and Theeuwes, 2003). A number of studies have demonstrated that attention can be directly and reliably measured using eye tracking during the visual probe task (Marks et al., 2014a, 2014b; Miller and Fillmore, 2011; Weafer and Fillmore, 2012). For example, a study conducted in our laboratory demonstrated that cocaine users, but not non-cocaine-using controls, fixate on cocaine-paired images significantly longer than neutral images (Marks et al., 2014b). The influence of acute alcohol administration on cocaine cue attentional bias, however, has not been assessed in a sample of heavy cocaine-using individuals (i.e., the targeted clinical population) or with a direct measure of attention (i.e., eye tracking).
Demonstrating a causal relationship between alcohol consumption and cocaine cue attentional bias will provide clinically relevant information about the behavioral mechanisms of cocaine relapse. To do this, a double-blind, placebo-controlled, within-subjects experiment was conducted in which cocaine-using individuals completed the visual probe task with eye-tracking technology after alcohol administration (0.0, 0.325, 0.65 g/kg). Measures of participant-rated drug effects, physiological response (i.e., heart rate, blood pressure), and psychomotor performance (i.e., Digit Symbol Substitution Test) were also included. We hypothesized that following placebo, participants would display an attentional bias to cocaine. We further hypothesized that alcohol would dose-dependently increase cocaine cue attentional bias.
Materials and Methods
Participants
Participants were 20 adults reporting using cocaine and alcohol within the past 30 days. Participants were primarily recruited through word of mouth and postings on community bulletin boards. Prior to participation, all potential participants completed a comprehensive medical-history questionnaire, drug-use questionnaire, mini-mental status examination, and vital sign assessment (see Sevak et al., 2011 for details of this screening process). Potential participants with histories of serious physical disease, current physical disease (e.g., impaired cardiovascular functioning, chronic obstructive pulmonary disease, etc.), seizure, head trauma or CNS tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I, Diagnostic and Statistical Manual of Mental Disorders IV; American Psychiatric Association, 2000) were excluded from participation. All participants were in good health with no contraindications to alcohol. The Institutional Review Board of the University of Kentucky Medical Center approved this experiment and participants gave their written, informed consent before participating. Participants were compensated for their time and effort.
General Procedures
Participants were enrolled as outpatients at the University of Kentucky Laboratory of Human Behavioral Pharmacology (LHBP) for four total sessions (1 practice and 3 experimental). During review of the written informed consent document, participants were informed that they would receive alcohol or placebo in experimental sessions and that the purpose of the study was to determine how alcohol affects physiology, mood, and behavior. Other than this general explanation, participants were given no instruction of what they were “supposed” to do or of what outcomes might be expected. Participants were blind to the type and dose of alcohol administered.
Participants were instructed to abstain from alcohol and drug use (with the exception of non-steroidal anti-inflammatory analgesics and nicotine) for 12 hr and caffeinated products and solid food for 4 hr before a scheduled session. Urine screens were conducted at the outset of every session to detect recent drug use and pregnancy. In order to participate in an experimental session, participants had to provide a urine sample negative for recent amphetamine, benzodiazepine, barbiturate, and opioid use, as well as pregnancy (in female subjects only). If a drug-urine specimen was positive for the presence of amphetamines, benzodiazepines, barbiturates, or opioids, the session was rescheduled. All female participants reported using an effective form of birth control and were not pregnant. Participants completed a field sobriety test and provided a breath sample negative for alcohol prior to each session to ensure that they were not currently intoxicated. To assess for recent tobacco and marijuana use, a handheld carbon monoxide meter was used to determine participant’s carbon monoxide level based on an expired breath sample (Smokerlyzer, Bedfont Scientific, Medford, NH). Carbon monoxide levels had to be less than 10 parts per million (ppm) for session to begin. If an acceptable carbon monoxide level could not be obtained within 1 hr of arrival, the experimental session was rescheduled.
Practice Session
Prior to beginning the experiment proper, participants completed one “practice” session. This practice session was used to familiarize participants with the visual probe task, participant-rated drug-effect questionnaires, and the daily laboratory routine, all of which are described below. It was identical to the experimental sessions with the exception that alcohol was not administered during this session.
Experimental Sessions
Experimental sessions were approximately 7.5 hr long. Table 1 presents an overview of the session timeline. For each session, participants arrived at the laboratory at approximately 0930. After meeting the carbon monoxide criterion, participants who reported smoking tobacco cigarettes were allowed to smoke one observed cigarette in order to reduce the possibility of acute nicotine withdrawal. Participants consumed a standard, low-fat snack with water and completed the pre-session tasks between 0930 and 1000. Participants ingested the drink at approximately 1200 and completed the visual probe task 30 min following drink completion at approximately 1240. Physiological measures, participant-rated drug-effect questionnaires, and Digit Symbol Substitution Test (described below) were collected 30 min prior to drink administration, 15 and 60 min following drink consumption, and then at hourly intervals from 1300 – 1700. Participants were allowed to eat lunch at 1300, which was provided by the LHBP. Participants were released once their breath alcohol concentration (BAC) fell below 20 mg/100 ml and no alcohol effects (physiological or behavioral) were detected 5 hr post-administration. This experimental timeline was devised based on the pharmacokinetics of alcohol to capture the ascending limb of the breath alcohol curve. BALs were expected to peak approximately 40 min after drinking began (Miller and Fillmore, 2011).
Table 1.
Session timeline
| Approximate Hour
|
||||||||
|---|---|---|---|---|---|---|---|---|
| 1130 | 1200 | 1225 | 1240 | 1310 | 1500 | 1600 | 1700 | |
|
| ||||||||
| Alcohol Administration | ■ | |||||||
| Visual Probe Task | ■ | |||||||
| Heart Rate, Blood Pressure | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Drug Effect Questionnaires | ■ | ■ | ■ | ■ | ■ | ■ | ||
| BAC | ■ | ■ | ■ | ■ | ■ | ■ | ||
BAC refers to breath alcohol concentration. Drug Effect Questionnaires include the Cocaine Craving Questionnaire, Drug-Effects Questionnaire, and Digit Symbol Substitution Test.
Visual Probe Task
Fixation time data were collected using T60-XL and X2-60 eye trackers (Tobii Technology, Sweden). Two different 60-hertz eye tracker models were utilized due to upgrades to technology over time, but eye-tracking model does not significantly influence fixation-time data (Marks et al., 2014a). Individual participants completed all sessions on the same eye-tracker model. Attentional bias was measured using the visual probe procedure described previously (Marks et al., 2014b).
For each trial, two 13 cm x 18 cm images (a cocaine-related image and a matched neutral image) were presented side-by-side, 3 cm apart, on a computer screen for 1000 milliseconds (ms). The amount of time (ms) fixating on the cocaine and neutral image was measured. Upon offset of the image pair, a visual probe (X) appeared either on the left or the right side of the screen, in the same location as one of the previously presented images. The amount of time (ms) to respond to the probe was measured. Participants were instructed respond as quickly as possible to the probe by pressing one of two response keys indicating on which side the probe appeared. Participants completed ten practice trials to ensure that they understood the task requirements.
Critical task stimuli were ten cocaine images matched with ten neutral images (i.e. non-cocaine-related). Cocaine images contained crack or powder cocaine as well as related paraphernalia. Neutral images were matched by the investigators on the number of objects in the image, the size of those objects, and the color scheme. Images were presented four times each once for each of the four image/probe combinations (i.e. left and right image locations and visual probe locations). In addition, 40 filler trials consisting of ten pairs of additional neutral images were intermixed with the test trials, for a total of 80 trials. Stimuli in filler trials were a separate set of neutral images (e.g. shoes, telephone) unrelated in content to the cocaine images or their matched, neutral images. The task required approximately 3.5 minutes to complete.
The primary outcome variables were fixation time to cocaine and neutral images and response time to probe location (ms). Participants completed the visual probe task operated using E-prime experiment generation software (Psychology Software Tools, Pittsburgh, PA) on a PC.
Participant-Rated Drug-Effect Questionnaires
Cocaine Craving Questionnaire
The modified Cocaine Craving Questionnaire consists of three items (Dudish-Poulsen and Hatsukami, 1997). Participants were asked to rate how much they want, need, and crave cocaine at the moment using a five-point rating scale. Responses to these three questions were summed to create a Cocaine Craving Total Score.
Drug-Effect Questionnaire
The Drug-Effect Questionnaire consists of nine items that were presented one at a time. Participants were asked to rate each item by placing a slider along a 100-unit visual analog scale (VAS) anchored with Not at All on the left and Extremely on the right. The items included were: Anxious, Drink More, Drunk, High, Light-Headed, Sedated, Sick, Sleepy, and Stimulated.
Digit Symbol Substitution Test
The Digit Symbol Substitution Test (DSST) is widely used in human behavioral pharmacology research to assess changes in psychomotor performance following drug administration (McLeod et al., 1982). Participants were asked to match geometric patterns displayed on the computer screen with corresponding numbers on a numeric keypad. Participants had 90 s to enter as many geometric patterns as possible. The dependent measures were the number of geometric patterns the participant attempted to enter (i.e., trials attempted) and the number of patterns the participant entered correctly (i.e., trials correct).
Physiological Measures
Breath alcohol concentration was measured using Alco-Sensor (Model F99-01, Intoximeters Inc., St. Louis, MO). Blood pressure and heart rate were recorded using an automated digital vital-signs monitor (1000 Vital Signs monitor, Critikon Company LLC, Tampa, FL). Physiological measures were assessed immediately prior to drink administration, 15, and 30 minutes, and then hourly following drink administration for the remainder of the 5 hr session. Participants were not given feedback regarding BAC measurements during testing. Specific physiological safety criteria were in place to terminate participation (i.e., blood pressure > 180/120 mmHg and heart rate > 130 bpm), but no participants were excluded for exceeding these parameters.
Alcohol Doses and Administration
Alcohol (0.0, 0.325, and 0.65 g/kg) was administered under double-blind conditions in a randomized order. Each dose was administered on a separate test session and sessions were separated by at least 24 hr. These doses produce peak BACs of approximately 0, 50, and 80 mg/100 ml, respectively. Participants were tested on the ascending limb of BACs that produce a range of behaviorally active effects but do not disrupt the measurement of attentional bias (Miller and Fillmore, 2011, Schoenmakers et al., 2008). The alcohol doses were mixed in a ratio of 3 parts lemon-lime soda to 1 part alcohol (0.325 or 0.65 g/kg). The placebo dose was mixed in a ratio of 3 parts lemon-lime soda to 1 part water to match the volume of the active dose. To better mask conditions in all drinks, 3 ml of alcohol was floated on top and the glass was sprayed with an alcohol mist (Fillmore and Vogel-Sprott, 1998). The placebo condition was selected to control for expectancy effects, compensatory reactions, and the conditioned stimulus-related properties of alcohol administration cues, thereby better isolating the pharmacological effects of alcohol on outcome measures (Marczinski and Fillmore, 2005). The entire drink was presented in one insulated, opaque tumbler. Participants had 10 min to consume the drink.
Data Analysis
To test the hypothesis that alcohol dose dependently increased cocaine cue attentional bias, fixation time and response time during the visual probe task were analyzed using a two-factor, repeated measures analysis of variance (ANOVA; StatView, Cary, NC). Cue type (cocaine and neutral) and alcohol dose (0.0, 0.325, and 0.65 g/kg) were the factors. If a statistically significant effect was observed, the mean-square error term was used to conduct Fisher’s Least Significant Difference post hoc tests to compare cue type at each dose.
Data from the participant-rated drug-effect questionnaires and DSST were analyzed using a two factor, repeated-measures ANOVA with dose (0.0, 0.325, and 0.65 g/kg) and time (Pre, 15, 60, 120, 180, 240, 300 min post alcohol administration) as the factors. Physiological measures were analyzed in the same manner with the exception that an additional time point (30 min post alcohol administration) was included in the time factor. If a statistically significant effect was observed, the mean-square error term was used to conduct Fisher’s Least Significant Difference post hoc tests to compare doses at each time point. The alpha level was set at p ≤ 0.05 for all analyses.
Previous studies have observed a positive correlation between attentional bias to illicit substances and craving (see Field et al., 2009; Leeman et al., 2014). Therefore, correlations were conducted between cocaine cue attentional bias and peak ratings of cocaine craving, under each dose condition. The Shapiro-Wilk test was used to assess the normality of residuals. Pearson product-moment correlations were conducted if residuals were normally distributed and Spearman’s rank correlations were conducted if residuals were not normally distributed. A Bonferroni corrected p-value of 0.01 was used for all correlations.
Results
Demographics
Twenty-four individuals were enrolled in the protocol. Four participants did not complete all four sessions due to drop out or failure to comply with study rules. Twenty individuals completed the protocol. Table 2 presents demographics and substance use history. All participants reported current cocaine use (preferred routes primarily smoked and intranasal) and 19 of the 20 participants provided a urine sample positive for cocaine at some point during participation. Furthermore, 14 participants provided urine samples positive for cocaine at all four sessions and five more participants provided at least two samples positive for cocaine. Twelve participants met cocaine dependence and one met cocaine abuse criteria on the computerized version of the Structured Clinical Interview for the Diagnostic and Statistical Manual-IV (SCID). All participants reported alcohol use in the past 30 days. Five met criteria for alcohol dependence and two met criteria for alcohol abuse on the SCID. Participants did not meet criteria for abuse or dependence on any substances other than cocaine, alcohol, and nicotine.
Table 2.
Baseline Demographic and Substance Use Variables
| Measure | Mean | Standard Deviation | Range |
|---|---|---|---|
| Age | 40.5 | 6.1 | 29 – 51 |
| Sex (# male) | 10 | ||
| Race | |||
| African American | 16 | ||
| Caucasian | 3 | ||
| Other | 1 | ||
| Years of Education | 12.2 | 1.4 | 8 – 14 |
| Weight (kg) | 75.3 | 12.3 | 53.5 – 95.0 |
| Cigarettes per day (n=17) | 7.1 | 20.8 | 0.2 – 20.0 |
| FTND | 2.5 | 2.2 | 0 – 6 |
| Standard alcohol drinks per week | 17.1 | 20.8 | 1.0 – 88.0 |
| MAST | 7.9 | 9.8 | 0 – 32 |
| DAST | 8.6 | 4.9 | 3–20 |
| Cocaine | |||
| Days used past week | 2.4 | 1.4 | 0 – 5 |
| Days used past month | 9.0 | 5.9 | 1 – 20 |
| Years used | 14.2 | 7.7 | 3 – 34 |
| Other: Days used past month | |||
| Amphetamines (n=1) | 8 | ||
| Benzodiazepines (n=1) | 3 | ||
| Marijuana (n=15) | 12.1 | 11.2 | 1 – 30 |
| Opioids (n=4) | 7.8 | 9.1 | 1 – 21 |
Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al., 1991); Michigan Alcoholism Screening Test (MAST; Selzer, 1971); Drug Abuse Screening Test (DAST; Skinner, 1992)
Visual Probe Task
For fixation time, a significant main effect of image type, F(1,19) = 17.9, p < 0.05, was detected. Individuals fixated on cocaine-related images significantly longer than neutral images, demonstrating a large cocaine cue attentional bias across all dose conditions, as determined by the Cohen’s d effect size (Table 3). Neither the main effect of dose, F(2,38) = 0.1, p > 0.05, nor the interaction of dose and image type, F(2,38) = 0.0, p > 0.05, were significant.
Table 3.
Fixation and Response Time to Cocaine and Neutral Images
Mean fixation time and response time (milliseconds; ms) and Cohen’s d effect size for cocaine and neutral images during the visual probe task following 0.0, 0.325, and 0.65 g/kg alcohol.
| Dose (g/kg) | Fixation Time | Response Time | ||||
|---|---|---|---|---|---|---|
| Cocaine | Neutral | d | Cocaine | Neutral | d | |
| 0.0 | 358.0 (25.9) | 270.2 (23.8) | 0.79 | 458.7 (15.4) | 464.9 (14.6) | 0.1 |
| 0.325 | 348.1 (30.9) | 259.0 (28.9) | 0.67 | 462.0 (15.5) | 468.1 (14.1) | 0.1 |
| 0.65 | 353.8 (23.4) | 259.3 (24.4) | 0.88 | 471.0 (17.0) | 477.5 (16.4) | 0.1 |
For response time, the ANOVA did not reveal a main effect of image type, F(1,19) = 1.6, p > 0.05, dose, F(2,19) = 0.6, p > 0.05, or an interaction of image type and dose, F(2,38) = 0.0, p > 0.05. However, the ANOVA revealed a significant main effect of dose, F(2,19) = 4.4, p < 0.05, for the percent of errors made while responding to probe locations. On average, the error rate for incorrectly responding to the probe location was 1.0% following placebo, 1.5% following 0.325 g/kg alcohol, and 2.9% following 0.65 g/kg alcohol dose. The main effect of image type, F(1,19) = 1.7, p > 0.05, and the interaction of dose and image type, F(2,38) = 1.6, p > 0.05, on percent of errors made while responding to the probe locations, however, were not significant.
Participant-Rated Drug Effects
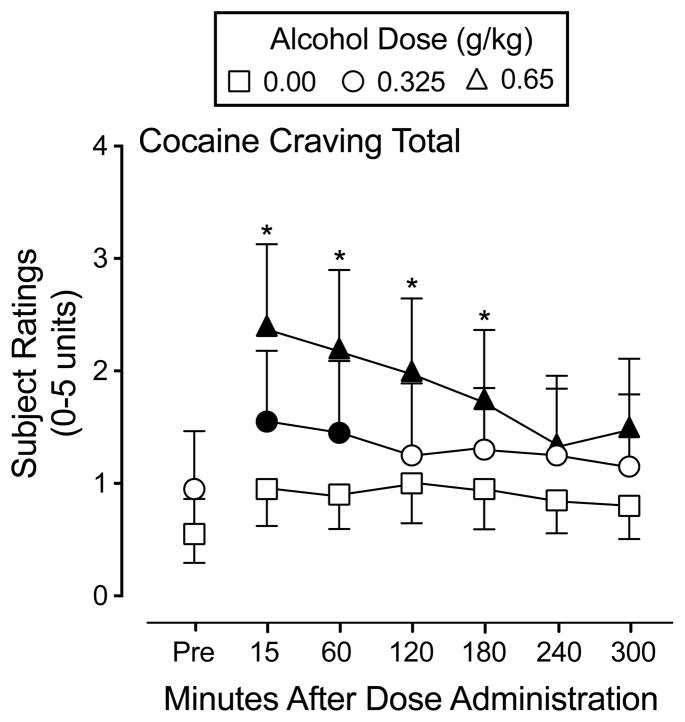
Alcohol produced a prototypical constellation of participant-rated drug effects, as a function of dose and time. A significant interaction of dose and time was observed on the Cocaine Craving Questionnaire Total score, F(12,228) = 3.4, p < 0.05. Following the 0.325 g/kg alcohol dose, participant ratings increased significantly from placebo and were still discernible from placebo 60 minutes after alcohol administration. Following the 0.65 g/kg alcohol dose, the magnitude and duration of participant ratings increased relative to placebo and to the 0.325 g/kg dose. Figure 1 shows participant ratings for Cocaine Craving Total. Peak cocaine craving did not correlate significantly with cocaine cue attentional bias as measured by fixation time following placebo (r = 0.54, p = 0.02), the 0.325 g/kg (r = −0.29, p = 0.21), or the 0.65 g/kg dose (r = 0.14, p = 0.56) or response time following placebo (r = 0.04, p = 0.87), the 0.325 g/kg (r = 0.07 p = 0.78), or the 0.65 g/kg dose (r = 0.19, p = 0.42).
Fig. 1.
Mean rating of Cocaine Craving Total from the Cocaine Craving Questionnaire following administration of alcohol (0.00, 0.325, 0.65 g/kg). Symbols represent the mean of twenty (20) participants. Filled symbols indicate a significant difference from placebo (0.00 g/kg) at that time point. An asterisk indicates a significant difference between 0.65 g/kg and the corresponding 0.325 g/kg alcohol dose. Error bars indicate one standard error of the mean (SEM).
A significant interaction of dose and time, F(12,228) = 1.8–8.4, p’s < 0.05, was observed for eight items on the Drug-Effect Questionnaire: Anxious, Drink More, Drunk, Lightheadedness, High, Sedated, Sleepy, and Stimulated. Figure 2 shows participant ratings for three representative items: High, Drink More, and Drunk.
Fig. 2.
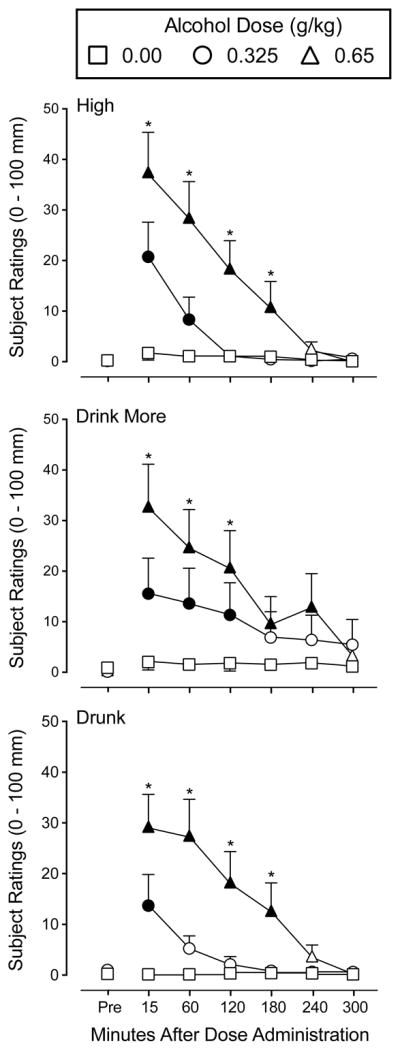
Mean rating of the items High, Drink More, and Drunk, from the Drug-Effect Questionnaire following administration of alcohol (0.00, 0.325, 0.65 g/kg). Other details are as in Fig. 1.
On the DSST, a significant interaction of dose and time was observed for the number of trials attempted, F(12,228) = 2.9, p < 0.05, and number of trials correct, F(12,228) = 2.6, p < 0.05. The 0.325 and 0.65 g/kg doses significantly decreased the number of trials attempted and correct (data not shown).
Physiological Measures
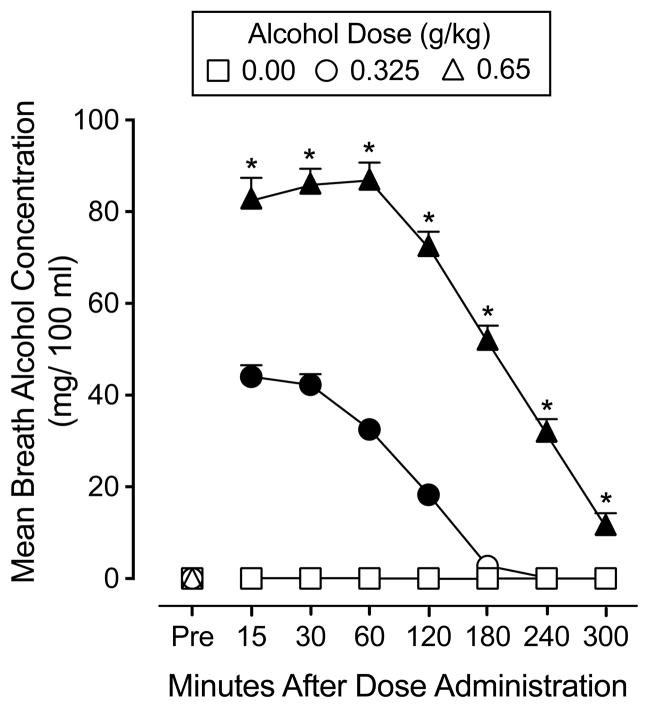
A significant interaction of dose and time, F(14,266) = 174.9, p < 0.05, was observed for BAC. BAC increased and decreased as an orderly function of alcohol dose and time. No detectable BACs were observed in the placebo condition. Following the 0.325 mg/kg dose, the average peak BAC was 44.0 mg/100 ml (SEM = 2.3). Following the 0.65 mg/kg dose, the average peak BAC was 87.1 mg/100ml (SEM = 3.8). Figure 3 displays BAC dose-response and time-course functions under two the active alcohol conditions.
Fig. 3.
Mean breath alcohol concentration following administration of alcohol (0.00, 0.325, 0.65 g/kg) across the 5 hr session. Error bars indicate one standard error of the mean (SEM).
A significant interaction of dose and time was observed for systolic, F(14,266) = 2.7, p < 0.05, and diastolic blood pressure, F(14,266) = 2.0, p < 0.05, and heart rate, F(14,266) = 2.9, p < 0.05. Average peak systolic blood pressure was 129.3 (2.4 SEM) following placebo, 129.6 (2.1 SEM) following the 0.325 mg/kg dose, and 129.5 (2.4 SEM) following the 0.65 mg/kg dose. Average peak diastolic blood pressure was 80.2 (2.2 SEM) following placebo, 79.2 (1.8 SEM) following the 0.325 mg/kg dose, and 78.4 (1.6 SEM) following the 0.65 mg/kg dose. Changes in systolic and diastolic blood pressure were transient and not clinically significant. Average peak heart rate was 76.9 (2.9 SEM) following placebo, 78.1 (3.1 SEM) following the 0.325 mg/kg dose, and 84.1 (3.5 SEM) following the 0.65 mg/kg dose. Heart rate also increased and decreased as a function of time.
Discussion
This human laboratory study is the first to report that alcohol dose dependently increased self-reported cocaine craving. On average, craving peaked 15 minutes following drink completion, prior to completion of the visual probe task. Peak craving did not correlate significantly with cocaine cue attentional bias, however a positive trend was observed following placebo, but not alcohol consumption. Cognitive models of attentional bias hypothesize a reciprocal relationship between craving and attentional bias (Franken, 2003; Ryan, 2002). Attentional bias promotes craving, craving enhances attentional bias, and both contribute to drug-taking behavior (see Field et al., 2009). By inference, the magnitude of craving should correlate with attentional bias. Previous studies have observed a significant positive correlation between attentional bias and cocaine craving, although none measured alcohol-induced cocaine craving (e.g., Field et al., 2009; Leeman et al., 2014, Rosse et al., 1997). Mogg and colleagues (2003) similarly observed that the relationship between fixation duration to cigarette cues and ratings of attractiveness of those cues operates independently of self-reported urge to smoke a cigarette. Likewise, the present results demonstrate that under specific conditions, motivational states can be dissociated from one another. These findings are clinically informative as they suggest that even if an individual does not report cocaine craving, cocaine-related cues might still remain salient.
In line with the first hypothesis, following placebo, participants displayed a large magnitude cocaine cue attentional as measured by fixation time during the visual probe task. This result replicates previous studies demonstrating that fixation time is a sensitive measure of cocaine cue attentional bias (Marks et al., 2014a; 2014b). In contrast to the second hypothesis, the magnitude of cocaine cue attentional bias did not increase in a dose-dependent manner. Instead, attentional bias remained unchanged across alcohol doses. Alcohol produced prototypical psychomotor (e.g., impaired performance on the DSST) and participant-rated effects (e.g., increased VAS ratings of Drink More, Drunk, and Stimulated) in a dose and time dependent manner as has been observed in previous studies in which alcohol was administered to cocaine-using individuals (Higgins et al., 1993; Montgomery et al., 2010).
Previous alcohol administration studies measuring fixation time have only evaluated alcohol and tobacco cue attentional bias. Those studies have produced inconsistent results that varied as a function of dose, substance-related cue, and severity of substance use. As was observed in the present study, acute alcohol administration failed to modify attentional bias under a variety of experimental conditions (Fernie et al., 2012; Miller and Fillmore, 2011; Roberts and Fillmore, 2014; Weafer and Fillmore, 2012). In contrast, other studies have reported that alcohol administration modified attentional bias, either by increasing (Fernie et al., 2012; Field et al., 2005; Schoenmakers et al., 2008) or decreasing (Roberts and Fillmore, 2014; Weafer and Fillmore, 2012) fixation time to alcohol-related images.
The stability of attentional bias across the alcohol doses suggests that cocaine cue attentional bias, as measured by fixation time, is a relatively stable behavioral trait in active, cocaine-using individuals. Supporting this notion, a previous study conducted in our laboratory demonstrated that fixation time is stable across repeated measurements ranging from 7 to 336 days (Marks et al., 2014a). The stability of fixation time, therefore, might make cocaine cue attentional bias a difficult target to modify. Future studies should identify the parameters under which fixation time to cocaine-related cues might be manipulated.
Worth noting is that cocaine cue attentional bias was only measured on the ascending limb or peak of the blood alcohol curve. Attentional bias during the descending limb should be investigated. Cognitive impairment, subjective intoxication, and craving are typically diminished on the descending limb of the blood alcohol curve, relative to the same BAC on the ascending limb of the curve (Fillmore et al., 2005; Roberts et al., 2012; Schweizer and Vogel-Sprott, 2008). Improved cognitive functioning and diminished subjective intoxication likely precipitate renewed drug taking and thus the salience of substance-related cues might be enhanced (Roberts and Fillmore, 2014). As an example, Roberts and Fillmore (2014) demonstrated that on the ascending limb of the BAC, participants reported an increased desire to consume alcohol, however alcohol cue attentional bias was absent. In contrast, on the descending limb, desire to drink was attenuated and alcohol cue attentional bias was present. Measuring cocaine cue attentional bias on the descending limb of the blood alcohol curve might similarly increase cocaine cue attentional bias and decrease cocaine craving.
The alcohol use history of the participants is another parameter that might have influenced the present results. Many participants reported binge-drinking episodes (i.e., 4 or more drinks in one occasion if female and 5 or more drinks if male), regularly achieving blood alcohol concentrations substantially higher than that produced by the 0.65 g/kg dose in the present study. Indeed, participant ratings of alcohol effects were modest and remained in the lower half of the visual analog scale. Whether higher doses of alcohol (i.e., doses that more closely match the discriminative stimulus properties of alcohol use outside the laboratory) might affect cocaine cue attentional bias is unknown. Relatedly, it is unknown how alcohol administration might influence attentional bias in individuals dependent upon both cocaine and alcohol. Previous research suggests that cocaine and alcohol dependent individuals attend to cocaine-related images differently than cocaine-only dependent individuals (Marks et al., 2015).
In contrast to fixation time, a cocaine cue attentional bias was not detected by differences in response time to cocaine and neutral images following placebo or alcohol administration. Other visual probe studies have similarly failed to detect cocaine cue attentional bias through response time in the absence of moderators such as alcohol administration or post-traumatic stress disorder (Marks et al., 2014a, 2014b; Tull et al., 2011). However, slower response time in the presence of cocaine-related images has previously been demonstrated using the modified Stroop task, which is an indirect measure of attentional bias (see Leeman et al., 2014). Only one previous study assessed the influence of alcohol on response time using the visual probe task (Montgomery et al., 2010). In that study, light cocaine users did not display a cocaine cue attentional bias following placebo. Following alcohol administration (0.40 g/kg), cocaine cue attentional bias was modestly increased. Alcohol, however, did not increase cocaine craving in that study. Methodological differences likely contribute to the discrepant results between studies. First, Montgomery and colleagues (2010) enrolled light cocaine users and utilized a between-subject design.
The present study enrolled heavier cocaine users and utilized a within-subject design. Second, attentional bias was measured 10 minutes following drink completion in the Montgomery and colleagues study (2010) as opposed to 30 minutes following drink completion in the present study. Third, the duration of image presentation was 500 ms in the Montgomery and colleagues (2010) study and 1000 ms in the present study. Alcohol dose, severity of use, timing of attentional bias assessment, and duration of image presentation have all been demonstrated to influence attentional bias (Field and Cox, 2008; Roberts and Fillmore, 2014). The present experiment was the first human laboratory study to systematically evaluate the influence of acute alcohol administration on cocaine cue attentional bias, as measured with eye tracking, in cocaine-using individuals. A robust cocaine cue attentional bias was detected by fixation time, but not response time. This finding is consistent with previous research reporting a large magnitude cocaine cue attentional bias (Leeman et al., 2014). Alcohol did not increase cocaine cue attentional bias, however, alcohol did increase self-reported cocaine craving. The dissociation between alcohol-induced cocaine craving and cocaine cue attentional bias suggests that alcohol influences motivation for cocaine (i.e., wanting) but not the salience of cocaine-related cues. These findings are consistent with clinical recommendations that individuals trying to abstain from cocaine use should also abstain for alcohol use. Future studies should continue to explore the experimental parameters and individual differences that mediate the relationship between alcohol and cocaine, especially to gain insight into alcohol-precipitated cocaine relapse. The influence of alcohol on other behaviors related to incentive motivation and cue salience, such as impaired inhibitory control, should also be considered.
Acknowledgments
Role of Funding Source: This research was supported by NIDA Grants R01 DA025032 and R01 DA025591 [CRR], T32 DA035200 [CRR, KRM], TL1 TR000115 [KRM], as well as by internal funding from the University of Kentucky [WWS]. These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121:148–151. doi: 10.1016/j.drugalcdep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentation. Drug Alcohol Depend. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Fernie G, Christiansen P, Cole JC, Rose AK, Field M. Effects of 0.4g/kg alcohol on attentional bias and alcohol-seeking behavior in heavy and moderate social drinkers. J Psychopharmacology. 2012;26:1017–1025. doi: 10.1177/0269881111434621. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Alcohol increases cognitive biases for smoking cues in smokers. Psychopharmacology. 2005;180:63–72. doi: 10.1007/s00213-005-2251-1. [DOI] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IHA. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psycho Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol Drugs. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addict Behav. 2000;25:99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. The relationship between exogenous and endogenous saccades and attention. In: Hyona J, Radach R, Deubel H, editors. The Minds Eye: Cognitive and Applied Aspects of Eye Movement Research. Elsevier; New York: 2003. pp. 3–26. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction. 2006;101:1292–1298. doi: 10.1111/j.1360-0443.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Bickel WK, Hughes JR, Lynn M, Capeless MA. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology. 1993;111:285–294. doi: 10.1007/BF02244943. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent subjects. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Robinson CD, Waters AJ, Sofuoglu M. A crucial review of the literature on attentional bias in cocaine use disorder and suggestions for future research. Exp Clin Psychopharmacol. 1204;22:469–483. doi: 10.1037/a0037806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: effects of inhibition and activation on behavior. Psychopharmacology (Berl) 2005;181:337–346. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR. Test-retest reliability of eye tracking during the visual probe task in cocaine-using adults. Drug Alcohol Depend. 2014a;145:235–237. doi: 10.1016/j.drugalcdep.2014.09.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR. The magnitude of drug attentional bias is specific to substance use disorder. Psychol Addict Behav. 2015 doi: 10.1037/adb0000084. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Roberts W, Stoops WW, Pike E, Fillmore MT, Rush CR. Fixation time is a sensitive measure of cocaine cue attentional bias. Addiction. 2014b;109:1501–1508. doi: 10.1111/add.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Rutherford MJ, Cacciola JS, McLellan AT. The relationship of alcohol use to cocaine relapse in cocaine dependent patients in an aftercare study. J Stud Alcohol. 1999;60:176–180. doi: 10.15288/jsa.1999.60.176. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Ins. 1982;14:463–466. [Google Scholar]
- Miller MA, Fillmore MT. Persistence of attentional bias toward alcohol-related stimuli in intoxicated social drinkers. Drug Alcohol Depend. 2011;117:184–189. doi: 10.1016/j.drugalcdep.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Field M, Atkinson AM, Cole JC, Goudie AJ, Sumnall HR. Effects of alcohol preload on attentional bias towards cocaine-related cues. Psychopharmacology (Berl) 2010;210:365–375. doi: 10.1007/s00213-010-1830-y. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT. Attentional bias to alcohol-related stimuli as an indicator of changes in motivation to drink. Psychol Addict Behav. 2014 doi: 10.1037/adb0000005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Drinking to distraction: does alcohol increase attentional bias in adults with ADHD? Exp Clin Psychopharmacol. 2012;20:107–117. doi: 10.1037/a0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Johri S, Kendrick K, Hess AL, Alim TN, Miller M, Deutsch SI. Preattentive and attentive eye movement during visual scanning of a cocaine cue: correlation with intensity of cocaine cravings. J Neuropsychiatry Clin Neurosci. 1997;9:91–93. doi: 10.1176/jnp.9.1.91. [DOI] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp Clin Psychopharmacol. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl) 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacology. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Vansickel AR, Stoops WW, Glaser PEA, Hays LR, Rush CR. Discriminative-stimulus, subject-rated, and physiological effects of methamphetamine in humans pretreated with aripiprazole. J Clin Psychopharmacol. 2011;31:470–480. doi: 10.1097/JCP.0b013e318221b2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Jähne A, Kyle SD, Beil M, Doll C, Feige B, Riemann D. Is smoking-related attentional bias a useful marker for treatment effects? Behav Med. 2011;37:26–34. doi: 10.1080/08964289.2010.543195. [DOI] [PubMed] [Google Scholar]
- Tull MT, McDermott MJ, Gratz KL, Coffey SF, Lejuez CW. Cocaine-related attentional bias following trauma cue exposure among cocaine dependent in-patients with and without post-traumatic stress disorder. Addiction. 2011;106:1810–1818. doi: 10.1111/j.1360-0443.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health, 2013. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2014. pp. 11–18. ICPSR35509-v1. Available at: http://www.icpsr.umich.edu/cgi-bin/sdaterms. [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute alcohol effects on attentional bias in heavy and moderate drinkers. Psychol Addict Behav. 2012;27:32–41. doi: 10.1037/a0028991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]