Abstract
Objective
To describe variability in end-of-life practices among tertiary care pediatric intensive care units (PICUs) in the U.S.
Design
Secondary analysis of data prospectively collected from a random sample of patients (n=10,078) admitted to PICUs affiliated with the Collaborative Pediatric Critical Care Research Network (CPCCRN) between December 4, 2011 and April 7, 2013.
Setting
Seven clinical centers affiliated with the CPCCRN
Patients
Patients included in the primary study were <18 years of age, admitted to a PICU, and not moribund on PICU admission. Patients included in the secondary analysis were those who died during their hospital stay.
Interventions
None.
Measurements and Main Results
Two hundred and seventy-five (2.7%, range across sites 1.3%–5.0%) patients died during their hospital stay; of these, 252 (92%, 76%–100%) died in a PICU. Discussions with families about limitation or withdrawal of support occurred during the initial PICU stay for 173 (63%, 47%–76%, p=0.27) patients who died. Of these, palliative care was consulted for 67 (39%, 12%–46%); pain service for 11 (6%, 10 of which were at a single site); and ethics committee for 6 (3%, from 3 sites). Mode of death was withdrawal of support for 141 (51%, 42%–59%), failed CPR for 53 (19%, 12%–28%), limitation of support for 46 (17%, 7%–24%), and brain death for 35 (13%, 8%–20%); mode of death did not differ across sites (p=0.58). Organ donation was requested from 101 (37%, 17%–88%, p<0.001) families. Of these, 20 (20%, 0%–64%) donated. Sixty-two (23%, 10%–53%, p<0.001) deaths were medical examiner cases. Of non-medical examiner cases (n=213), autopsy was requested for 79 (37%, 17%–75%, p<0.001). Of autopsies requested, 53 (67%, 50%–100%) were performed.
Conclusions
Most deaths in CPCCRN-affiliated PICUs occur after life support has been limited or withdrawn. Wide practice variation exists in requests for organ donation and autopsy.
Keywords: Pediatrics, Death, End-of-life care, Organ donation, Autopsy
INTRODUCTION
In developed countries, most pediatric deaths occur in intensive care settings (1–3). Severely ill children are admitted to pediatric intensive care units (PICUs) to receive potentially curative therapies. However, for some children, these therapies are eventually found to be ineffective in achieving the desired outcome of survival with an acceptable quality of life (4). This often initiates a transition from invasive interventions to comfort care and pursuit of a dignified death. Because this transition often takes place in PICUs, pediatric intensivists have an important role in discussing end-of-life care with families, facilitating decision making, treating the child’s symptoms, and managing death and its immediate aftermath.
Recommendations and guidelines describing best practices for some aspects of end-of-life care have been published (5–7). Despite this guidance, variability in end-of-life practice in PICUs has been documented among continents (8), countries (9), cities within countries (10–12) and hospitals within cities (13). For example, death after limitation or withdrawal of support is less common in South American PICUs than those in North America, Europe or Australia (8). Parents’ level of information about end-of-life decision-making and clinicians’ documentation of decisions in medical records are greater in northern European countries than southern European countries (9). Within one Brazilian city, three PICUs report rates of limitation and withdrawal of support that vary more than 3-fold, and increasing rates of parental participation in end-of-life decision making and medical record documentation of decisions over time (13). Little data is available regarding variability in end-of-life practice in PICUs in the U.S. (12, 14). However, one recent report suggests that decisions to limit or withdraw support occur less often in PICUs with no trainees, and for Black children (12).
Identifying aspects of end-of-life care that vary across PICUs in the U.S. is important because variability in care often signals a need for greater evidence or education regarding best practices. If variability in care is identified, further research can be conducted to understand the potential reasons behind it such as patient preferences, clinician attitudes, or variability in access to care (15,16). The objective of this study is to describe the extent of variability in end-of-life practices among tertiary care PICUs in the U.S. The PICUs evaluated are those affiliated with the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN).
METHODS
Design and Setting
The study was a secondary analysis of data prospectively collected from a random sample of patients (n=10,078) admitted to PICUs affiliated with the CPCCRN between December 4, 2011 and April 7, 2013 (17). The CPCCRN consists of seven clinical centers which have approximately 17,000 PICU admissions each year (18, 19). Each center contributed 12% to 16% of the sample. The study was approved by the Institutional Review Board at each site and the requirement for parental permission was waived.
Study Population
Patients were eligible for inclusion in the primary study if they were less than 18 years of age and admitted to a general medical or cardiac PICU. There were no separate surgical or neurological PICUs. Patients were excluded if they had a previous PICU admission during the current hospitalization or if their vital signs were incompatible with life for at least the first 2 hours after PICU admission (i.e., moribund patients). Patients were included in this secondary analysis if they died during their hospitalization.
Data Collection
Details of data collection are provided elsewhere (17). Briefly, trained research assistants collected data by prospective record review, direct observation, and discussion with bedside clinicians. Data for this analysis included socio-demographics; baseline Pediatric Cerebral Performance Category scores (PCPC) (20) and Functional Status Scale scores (FSS) (21); pupillary reflexes on PICU admission; primary and secondary PICU admission diagnoses; and variables related to end-of-life care. Socio-demographics included sex, age at PICU admission, race, ethnicity, and payer type. PCPC is a scale for assessing general cognitive function of pediatric patients (20). Scores are 1 for normal, 2 for mild disability, 3 for moderate disability, 4 for severe disability, 5 for coma or vegetative state, and 6 for brain death. FSS is a scale for assessing pediatric functional status in 6 domains including mental, sensory, communication, motor function, feeding and respiratory status (21). Total FSS scores range from 6–30 and are categorized as 6–7 (good), 8–9 (mildly abnormal), 10–15 (moderately abnormal), 16–21 (severely abnormal), and >21 (very severely abnormal). Baseline PCPC and FSS scores were derived from historical information and represent the child’s status prior to the hospital admission.
Variables related to end-of-life care included whether the child’s family participated in a discussion with clinicians about limitation or withdrawal of support during their child’s PICU stay; if such a discussion occurred, whether the palliative care service, pain service or institutional ethics committee were consulted; and whether a decision was made during the initial PICU stay to limit or withdraw support. Other variables related to end-of-life care included the date, time and location of death; mode of death; whether organ donation was offered and organs donated; whether the death was deemed a medical examiner case; and whether autopsy was requested and performed. Location of death was defined as PICU, general ward, or other hospital location. Mode of death was defined as death after limitation of support, withdrawal of support, failed cardiopulmonary resuscitation (CPR) or brain death. Limitation of support included precluding the use of one or more of the following interventions: mechanical ventilation, vasoactive medications, cardiac compressions, extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD), or renal replacement therapy. Withdrawal of support included discontinuing the use of one or more of the following interventions: mechanical ventilation, vasoactive medications, fluids or feeding, ECMO or VAD, renal replacement therapy, or other interventions with death as the expected outcome.
Statistical Analysis
Data are expressed as absolute counts and percentages, or, in the case of continuous data, summarized using the median and interquartile range (25th–75th percentiles). We evaluated the association between key characteristics and site using Pearson chi-square or Fisher’s exact test. A significance level of 0.05 was used for all analyses. No adjustments were made for multiple comparisons in this descriptive study. However, we restricted statistical comparisons of data across sites to variables that are relevant for all deaths. All statistical analyses were performed in SAS® version 9.3 (SAS Institute, Cary, NC).
RESULTS
A total of 275 (2.7%, range across sites 1.3%–5.0%) patients from the primary study sample died during their hospital stay. Socio-demographic characteristics and baseline PCPC and FSS scores of patients who died are shown in Table 1. One hundred and forty-two (52%) patients were male and 122 (45%) were less than one year of age. Approximately one-third of patients had missing race and ethnicity data due to incomplete documentation of these variables in the medical record. One hundred and seventy-two (63%) patients had normal PCPC scores and 181 (66%) had good FSS scores at baseline.
Table 1.
Patient Characteristics
| Parameter | Deaths (N=275) |
|---|---|
| Male | 142 (52%) |
| Age at PICU Admission | |
| < 1 month | 54 (20%) |
| 1 month to < 12 months | 68 (25%) |
| 12 months to < 12 years | 104 (38%) |
| >= 12 years | 49 (18%) |
| Race | |
| American Indian or Alaska Native | 6 (2%) |
| Asian | 10 (4%) |
| Black or African American | 59 (21%) |
| Native Hawaiian or Other Pacific Islander | 1 (0%) |
| Caucasian | 103 (37%) |
| Unknown or Not Reported | 96 (35%) |
| Ethnicity | |
| Hispanic or Latino | 68 (25%) |
| Not Hispanic or Latino | 123 (45%) |
| Unknown or Not Reported | 84 (31%) |
| Payer Type | |
| Missing | 12 (4%) |
| Commercial | 72 (26%) |
| Government | 183 (67%) |
| Other | 8 (3%) |
| Baseline PCPC | |
| 1 – Normal | 172 (63%) |
| 2 – Mild disability | 48 (17%) |
| 3 – Moderate disability | 27 (10%) |
| 4 – Severe disability | 23 (8%) |
| 5 – Coma/vegetative | 5 (2%) |
| Baseline FSS Score | |
| Good (6–7) | 181 (66%) |
| Mild (8–9) | 36 (13%) |
| Moderate (10–15) | 46 (17%) |
| Severe (16–21) | 7 (3%) |
| Very Severe (>21) | 5 (2%) |
Discussions between families and clinicians about limitation or withdrawal of support occurred during the initial PICU stay for 173 (63%, range across sites 47%–76%, p=0.27) patients who died. Among those who discussed limitation or withdrawal of support, palliative care was consulted for 67 (39%, 12%–46%), pain service for 11 (6%, 10 of which were at a single site), and institutional ethics committee for 6 (3%, from 3 sites). Also, among those who discussed limitation or withdrawal of support, 159 (92%, 80–100%) made a decision to limit or withdraw support during the initial PICU stay.
Specific limitations of support during the initial PICU stay included limitation of cardiac compressions for 106 (39%) patients who died, vasoactive medications for 76 (28%), ECMO or VAD for 68 (25%), mechanical ventilation for 64 (23%), and renal replacement therapy for 57 (21%). Specific withdrawals of support during the initial PICU stay included discontinuation of mechanical ventilation for 115 (42%) patients who died, vasoactive medications for 78 (28%), fluids or feeding for 66 (24%), ECMO or VAD for 25 (9%), renal replacement therapy for 19 (7%), and other interventions for 10 (4%).
Ultimately, the mode of death was withdrawal of support for 141 (51%, range across sites 42%–59%) patients, failed CPR for 53 (19%, 12%–28%), limitation of support for 46 (17%, 7%–24%), and brain death for 35 (13%, 8%–20%) (Figure 1). There were no significant differences in mode of death across sites (p=0.58). PICU admission characteristics for patients experiencing each mode of death are shown in Table 2. The most common primary PICU admission diagnosis for patients with withdrawal of support was congenital heart disease (n=47, 33%); for patients with failed CPR was congenital heart disease (n=19, 36%); for patients with limitation of support was respiratory failure (n=20, 43%); and for patients with brain death was neurologic condition (n=19, 54%), 13 of whom had a primary or secondary diagnosis of trauma. Of 35 patients with brain death, 22 (63%) had fixed dilated pupils on admission. Overall, 199 (72%) patients who died had chronic illness present on PICU admission. Chronic illness was present in 114 (81%) with withdrawal of support, 38 (83%) with limitation of support, 37 (70%) with failed CPR, and 10 (29%) with brain death.
Figure 1.
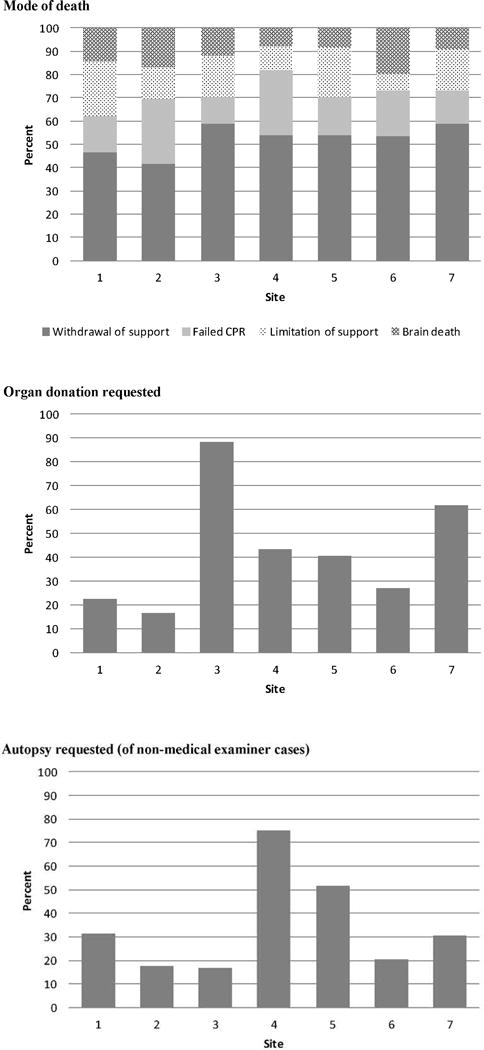
Mode of death was not different across sites (p=0.58). Requests for organ donation varied across sites (17%–88%, p<0.001). Requests for autopsy varied across sites (17%–75%, p<0.001).
Table 2.
Admission Characteristics by Mode of Death
| Parameter | Failed resuscitation N = 53 |
Withdrawal of support N = 141 |
Limitation of support N = 46 |
Brain death N = 35 |
|---|---|---|---|---|
| Age at PICU admission | ||||
| < 1 month | 14 (26%) | 33 (23%) | 7 (15%) | 0 (0%) |
| 1 month to < 12 months | 16 (30%) | 32 (23%) | 11 (24%) | 9 (26%) |
| 12 months to < 12 years | 19 (36%) | 48 (34%) | 19 (41%) | 18 (51%) |
| >= 12 years | 4 (8%) | 28 (20%) | 9 (20%) | 8 (23%) |
| Baseline PCPC | ||||
| 1 – Normal | 30 (57%) | 94 (67%) | 19 (41%) | 29 (83%) |
| 2 – Mild disability | 13 (25%) | 21 (15%) | 10 (22%) | 4 (11%) |
| 3 – Moderate disability | 3 (6%) | 12 (9%) | 10 (22%) | 2 (6%) |
| 4 – Severe disability | 4 (8%) | 12 (9%) | 7 (15%) | 0 (0%) |
| 5 – Coma/vegetative | 3 (6%) | 2 (1%) | 0 (0%) | 0 (0%) |
| Baseline FSS score | ||||
| Good (6–7) | 36 (68%) | 94 (67%) | 20 (43%) | 31 (89%) |
| Mild (8–9) | 7 (13%) | 20 (14%) | 7 (15%) | 2 (6%) |
| Moderate (10–15) | 7 (13%) | 22 (16%) | 15 (33%) | 2 (6%) |
| Severe (16–21) | 1 (2%) | 3 (2%) | 3 (7%) | 0 (0%) |
| Very Severe (>21) | 2 (4%) | 2 (1%) | 1 (2%) | 0 (0%) |
| Primary acute diagnosis | ||||
| Respiratory | 14 (26%) | 39 (28%) | 20 (43%) | 6 (17%) |
| Cancer | 2 (4%) | 3 (2%) | 4 (9%) | 0 (0%) |
| Cardiovascular Disease – Acquired | 8 (15%) | 28 (20%) | 8 (17%) | 9 (26%) |
| Cardiovascular Disease – Congenital | 19 (36%) | 47 (33%) | 6 (13%) | 1 (3%) |
| Gastrointestinal disorder | 3 (6%) | 6 (4%) | 1 (2%) | 0 (0%) |
| Hematologic disorder | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Musculoskeletal Condition | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Neurologic | 3 (6%) | 14 (10%) | 5 (11%) | 19 (54%) |
| Miscellaneous | 3 (6%) | 4 (3%) | 0 (0%) | 0 (0%) |
| Primary or secondary acute trauma | 2 (4%) | 7 (5%) | 0 (0%) | 13 (37%) |
| Chronic diagnosis(es) at PICU admission | 37 (70%) | 114 (81%) | 38 (83%) | 10 (29%) |
Length of PICU and hospital stay for patients with each mode of death is shown in Table 3. Patients with brain death had the shortest PICU length of stay (median 3 days, IQR 2–6 days) and hospital length of stay (median 3 days, IQR 2–6 days). Regarding location of death, 252 (92%, range across sites 76%–100%) patients died in a PICU, 12 (4%, 0%–18%) in a general hospital ward, and 11 (4%, 0%–8%) in another hospital location.
Table 3.
Hospital and PICU Length of Stay by Mode of Death
| Statistic | Failed resuscitation N = 53 |
Withdrawal of support N = 141 |
Limitation of support N = 46 |
Brain death N = 35 |
|---|---|---|---|---|
| Length of hospital stay (days) | ||||
| Min, Max | 0, 225 | 0, 391 | 0, 286 | 1, 87 |
| Median (IQR) | 14 (4, 39) | 11 (3, 24) | 22 (4, 73) | 3 (2, 6) |
| Length of initial PICU stay (days) | ||||
| Min, Max | 0, 168 | 0, 139 | 0, 231 | 0, 20 |
| Median (IQR) | 5 (1, 24) | 6 (2, 15) | 4 (1, 14) | 3 (2, 6) |
IQR: 25th and 75th percentiles
Organ donation was requested from 101 (37%, range across sites 17%–88%, p<0.001) patients who died (Figure 1). Of these, 20 (20%, 0%–64%) donated. Organs donated included heart for 11 patients, liver for 14, kidneys for 19, pancreas for 5, intestines for 3, and lung for one. Donation after brain death occurred for 15 (75%, from 6 sites) and after cardiac death for 5 (25%, from 3 sites). Sixty two (23%, 10%–53%, p<0.001) deaths were deemed medical examiner cases. Of non-medical examiner cases (n=213), autopsy was requested for 79 (37%, 17%–75%, p<0.001). Of autopsies requested, 53 (67%, 50%–100%) were performed.
DISCUSSION
Findings from our study suggest that mortality rates for infants and children admitted to tertiary care PICUs in the U.S. for the first time during a hospitalization are low, ranging from 1.3% to 5% across CPCCRN-affiliated sites. Our observed mortality rate is similar to that recently reported from other U.S. PICUs (14). About three-quarters of the deaths in our study occurred among patients with chronic illnesses at PICU admission, and about one-third among patients with reduced cognitive and/or functional status at baseline. For patients who died during their hospitalization, discussions with families regarding options for end-of-life care (e.g., limitation or withdrawal of support) were common during the initial PICU stay, with little variability in the frequency observed across sites. Most of these discussions took place without the assistance of palliative care, pain service, or ethics committee consultation.
The most common form of limitation of support observed in our study was avoidance of cardiac compressions. Other research suggests that withholding cardiac compressions is most often, but not always, accomplished by establishing a formal “do not resuscitate” order (14); however, we did not collect detailed information on how orders were entered. The most common form of withdrawal of support observed in our study was discontinuation of mechanical ventilation; however again, the process by which mechanical ventilation was discontinued was not collected. Others have described that discontinuation of mechanical ventilation in PICU patients occurs most often by extubation, or removal of the patient from the ventilator while leaving the endotracheal tube in place; terminal weaning (i.e., stepwise decrease in ventilator support) occurs less often (8, 22, 23).
Over two-thirds (68%) of deaths in our study occurred after support was limited or withdrawn, with minimal variability observed across sites. This percentage is similar to other recent reports from PICUs in North America, northern Europe and Australia (2, 8, 14, 24). For example, a study describing the epidemiology of death in PICUs at five U.S. teaching hospitals found that 70% occurred after limitation or withdrawal of support (14). Similarly, 69% of deaths among patients with advanced cardiac disease hospitalized in a tertiary care PICU in Boston occurred after withdrawal of disease-directed therapy (2). Sixty-five percent of deaths in a multidisciplinary PICU in the United Kingdom (24) and 74% in a multidisciplinary PICU in Australia (8) occurred after limitation or withdrawal of support. In contrast, a recent study from Spain (3) found that only 31% of PICU deaths occurred after limitation or withdrawal of support, and another from Brazil (11) found that only 44% occurred without CPR. The literature also suggests that the percentage of PICU deaths occurring after limitation or withdrawal of support has increased over time worldwide. For example, in the 1980s, limitation and withdrawal of support preceded 32% of deaths in a PICU in Washington, DC (25), and 6% of deaths in PICUs in Brazil (13).
Respiratory failure and congenital heart disease were the most common PICU admission diagnoses among patients who died in our study. These diagnoses predominated in three of the four categories of mode of death including limitation of support, withdrawal of support, and failed CPR. Brain death occurred most often among patients with a neurological disorder, primarily trauma. These findings are similar to those of Lee et al (12) who retrospectively explored end-of-life care in 30 U.S. PICUs using data obtained from VPS, LLC (Milwaukee, WI), a multi-institutional pediatric critical care clinical database. Patients were categorized as dying with limitations (i.e., do not resuscitate, limitation of support, or withdrawal of support) versus no limitations; patients with brain death were excluded. In Lee’s study, respiratory failure and cardiovascular disorders were the most common admission diagnoses among patients dying with and without limitations in support. Similar to our findings in patients experiencing brain death, others have also reported a high frequency of traumatic injury on admission for such patients (8, 26). Variability in PICU admission characteristics for each mode of death could not be adequately evaluated across sites in our study due to insufficient sample size in some categories.
PICU patients who do not survive their hospitalization most often die in the PICU rather than another hospital location. Length of PICU stay is shortest for patients with brain death (overall range 0–20 days) who are frequently admitted to PICUs with profound neurological deficits (e.g., fixed dilated pupils). However for patients with other modes of death, PICU length of stay is often prolonged with maximum length of stay observed in our study ranging from 139 days for patients from whom support is withdrawn to 231 days for patients with limitations. These prolonged lengths of stay prior to death emphasize the need for adequate treatment of pain and suffering, and provision of family support; these patients are likely the ones to benefit most from use of palliative care, pain team, and other support services in the PICU setting.
Among families of children who died, the percentage asked to donate organs (37%) varied considerably across sites (17%–88%), as did the percentage of those asked who actually donated (20%, 0%–64%). Overall, 7% of patients who died donated organs. Although the number of requests and the number of donations are available in our dataset, the number of deceased patients who were ultimately eligible to donate (i.e., eligible deaths) is not. Eligible deaths are defined by the Organ Procurement and Transplantation Network (OPTN) as deaths of patients who are legally declared brain dead according to hospital policy and are absent of certain infections and malignancies; however it is recognized by OPTN that this definition does not include all eligible donors (27). Due to lack of data on eligible deaths, consent rates (i.e., percentage of eligible donors who are consented) cannot be determined. Webster and colleagues (28) report consent rates of 69.2% for pediatric donors in the U.S. If we assume that all patients in our study whose families were asked to donate were indeed eligible donors, our findings suggest that our overall consent rate is low; however, it is also possible that some families were asked about their willingness to donate before full evaluation and subsequently their child was determined to be ineligible. In Webster’s report (28), hospitals with level I trauma programs and pediatric critical care medicine fellowship programs had higher numbers of eligible donors and higher consent rates than hospitals without these programs. All CPCCRN clinical centers have level 1 trauma programs and pediatric critical care fellowship programs; thus the presence or absence of these programs does not account for the variability in organ donation practices observed across sites. Regardless of the cause, the variability in organ donations among families from whom organ donation was requested suggests that further research and education is needed to optimize identification of eligible donors and the consent process.
The percentage of non-medical examiner cases for which an autopsy was requested (37%) varied across sites (17%–75%); non-medical examiner cases are those in which autopsy is optional for families. Of those requested, the percentage that had an autopsy performed (67%) also varied across sites (50%–100%). Overall, 25% of non-medical examiner cases underwent autopsy. Recent reports describe decreasing rates of pediatric autopsy (29) despite studies suggesting that autopsy continues to provide important information in a third to nearly half of cases (30, 31). Various methods for improving autopsy rates have been described such as enhanced clinician education and a family-centered approach to consent (31), and assistance of local Decedent Affairs Offices (32). Variability in the rate of performance of autopsies that are not required by a medical examiner suggests an opportunity for improved practice.
Strengths of this study include the random selection of patients from a national network of tertiary care PICUs with geographic variability, as well as the prospective collection of data on end-of-life practices. Limitations of this study include a lack of information regarding why various end-of-life decisions were made such as limitation or withdrawal of support; and the possibility that some family-clinician discussions about limitation or withdrawal of support were missed during data collection. Detail is also lacking in our dataset to evaluate reasons behind practice variability for specific aspects of end-of-life care such as organ donation and autopsy rates. Other limitations include the collection of baseline (i.e., pre-hospitalization) PCPC and FSS scores from historical data, and the inability to evaluate differences across sites for some clinically important variables due to an insufficient number of subjects in some categories. Also, although prior research suggests some racial disparities in pediatric end-of-life care in PICUs (12), we were unable to further explore this issue within our dataset due to missing race and ethnicity data from the medical records of about one-third of patients, and the known confounding of race and ethnicity by site within the CPCCRN (18). Notably, PICU patients who may have been discharged home to die are not included in this dataset.
CONCLUSION
Mortality rates in tertiary care PICUs in the U.S. are low with over two-thirds of deaths occurring after limitation or withdrawal of support. Variability in rates of organ donation and autopsy across sites suggests the need for further research and education in these areas.
Acknowledgments
Financial Support: The study was supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: U10HD050096, U10HD049981, U10HD063108, U10HD063106, U10HD063114, U10HD049983, U10HD050012 and U01HD049934.
The Authors wish to acknowledge the contributions of the following individuals:
Teresa Liu, MPH, CCRP; University of Utah
Jeri Burr, MS, RN-BC, CCRN; University of Utah
Jean Reardon, MA, BSN, RN; Children’s National Medical Center
Aimee La Bell, MS, RN; Phoenix Children’s Hospital
Margaret Villa, RN; Children’s Hospital Los Angeles and Mattel Children’s Hospital
Jeni Kwok, JD; Children’s Hospital Los Angeles
Ann Pawluszka, BSN, RN; Children’s Hospital of Michigan
Monica S. Weber, RN, BSN, CCRP; University of Michigan
Alan C. Abraham, BA, CCRC; University of Pittsburgh Medical Center
Mary Ann DiLiberto, BS, RN, CCRC; Children’s Hospital of Philadelphia
Chris Feudtner, MD, PhD, MPH; Children’s Hospital of Philadelphia
Copyright form disclosures: Dr. Meert is employed by the Cincinnati Children’s Hospital (one-time consultancy visit unrelated to manuscript) and received support for article research from the National Institutes of Health (NIH). Her institution received grant support from the NIH. Dr. Keele is employed by Pediatric Anesthesia Associates Medical Group. Dr. Morrison served as a board member for the American Academy of Pediatrics (Editorial Board membership), consulted for Glaxo Smith Klein (Data monitoring committee), and lectured for Elizabeth Seton Hospital for Children (invited speaker). Dr. Berg received support for article research from the NIH. His institution received grant support from NICHD. Dr. Dalton served as a board member for ELSO and PCICS; lectured for ThermoFisher, rEVO biologics, and Maquet; received royalties and support for the development of educational presentations from the Society of Critical Care Medicine (SCCM); and received support for article research from the NIH. Her institution received grant support from the NIH. Dr. Newth received support for article research from the NIH. His institution received grant support from the NIH. Dr. Harrison lectured for the SCCM and received support for article research from the NIH. His institution received grant support and support for travel from the NIH. Dr. Wessel received support for article research from the NIH. His institution received grant support from the NIH. Dr. Shanley received support for travel from the NIH; consulted for Case, U-MN, and U-Cinc (Ext Advisory Board-CTSA); and received support for article research from the NIH. His institution received grant support from the NIH (CSTA). Dr. Carcillo received support for article research from the NIH. His institution received grant support, support for travel, support for manuscript writing/review, and other support. Dr. Clark received support for article research from the NIH. Dr. Clark and her institution received grant support from the NIH/NICHD (Collaborative Pediatric Critical Care Research Network grant). Dr. Holubkov served as a board member for Pfizer, Inc. and Inno Clinical Outcomes LLC (DSMB Board Member); served as biostatistical consultant for Physicians Committee for Responsible Medicine and St. Jude Medical, Inc.; and received support for article research from the NIH. Dr. Holubkov and his institution received grant support (Biostatistician for CPCCRN Network) and support for travel (Grant also pays for CPCCRN network meetings) from NIH/NICHD. Dr. Jenkins disclosed government work. Dr. Dean received support for article research from the NIH. His institution received grant support and support for travel from the NIH. Dr. Pollack is employed by the Children’s National and Phoenix Chld Hosp and received support for article research from the NIH. His institution received grant support and support for travel form the NIH (NIH grant listed in the manuscript). Dr. Doctor consulted for Novartis and Viasys and received support for article research from the NIH. His institution received support for travel from NICHD and received grant support from the NIH, AHA, Children’s Discovery Institute, and Doris Duke Foundation.
References
- 1.Fontana MS, Farrell C, Gauvin F, et al. Modes of death in pediatrics: Differences in the ethical approach in neonatal and pediatric patients. J Pediatr. 2013;162:1107–1111. doi: 10.1016/j.jpeds.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Morrell E, Wolfe J, Scheurer M, et al. Patterns of care at end of life in children with advanced heart disease. Arch Pediatr Adolesc Med. 2012;166:745–748. doi: 10.1001/archpediatrics.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launes C, Cambra FJ, Jordan I, et al. Withholding or withdrawing life-sustaining treatments: An 8-yr retrospective review in a Spanish pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:e383–e385. doi: 10.1097/PCC.0b013e31820aba5b. [DOI] [PubMed] [Google Scholar]
- 4.Goh AY, Mok Q. Identifying futility in a paediatric critical care setting: A prospective observational study. Arch Dis Child. 2001;84:265–268. doi: 10.1136/adc.84.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics. Section on Hospice and Palliative Medicine and Committee on Hospital Care: Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics. 2013;132:966–972. doi: 10.1542/peds.2013-2731. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Paediatrics and Child Health. Withholding or withdrawing life sustaining treatment in children: A framework for practice. 2004 Available at: http://www.gmc-uk.org/witholding.pdf_40818793.pdf Accessed March 24, 2015.
- 7.Truog RD, Cist AFM, Brackett SE, et al. Recommendations for end-of-life care in the intensive care unit: The Ethics Committee of the Society of Critical Care Medicine. Crit Care Med. 2001;29:2332–2348. doi: 10.1097/00003246-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Moore P, Kerridge I, Gillis J, et al. Withdrawal and limitation of life-sustaining treatments in a paediatric intensive care unit and review of the literature. J Paediatr Child Health. 2008;44:404–408. doi: 10.1111/j.1440-1754.2008.01353.x. [DOI] [PubMed] [Google Scholar]
- 9.Devictor DJ, Nguyen DT. The Working Group on Ethics of the European Society of Pediatric and Neonatal Intensive Care. Forgoing life-sustaining treatments in children: A comparison between northern and southern European pediatric intensive care units. Pediatr Crit Care Med. 2004;5:211–215. doi: 10.1097/01.PCC.0000123553.22405.E3. [DOI] [PubMed] [Google Scholar]
- 10.Althabe M, Cardigni G, Vassallo JC, et al. Dying in the intensive care unit: Collaborative multicenter study about forgoing life-sustaining treatment in Argentine pediatric intensive care units. Pediatr Crit Care Med. 2003;4:164–169. doi: 10.1097/01.pcc.0000059428.08927.a9. [DOI] [PubMed] [Google Scholar]
- 11.Lago PM, Piva J, Garcia PC, et al. End-of-life practices in seven Brazilian pediatric intensive care units. Pediatr Crit Care Med. 2008;9:26–31. doi: 10.1097/01.PCC.0000298654.92048.BD. [DOI] [PubMed] [Google Scholar]
- 12.Lee KJ, Tieves K, Scanlon MC. Alterations in end-of-life support in the pediatric intensive care unit. Pediatrics. 2010;126:e859–e864. doi: 10.1542/peds.2010-0420. [DOI] [PubMed] [Google Scholar]
- 13.Kipper DJ, Piva JP, Garcia PCR, et al. Evolution of the medical practices and modes of death on pediatric intensive care units in southern Brazil. Pediatr Crit Care Med. 2005;6:258–263. doi: 10.1097/01.PCC.0000154958.71041.37. [DOI] [PubMed] [Google Scholar]
- 14.Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD. Epidemiology of death in the PICU at five U.S. teaching hospitals. Crit Care Med. 2014;42:2101–2108. doi: 10.1097/CCM.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truog RD. Variability in end-of-life care – How much is too much? Pediatr Crit Care Med. 2005;6:368–369. doi: 10.1097/01.pcc.0000161618.76811.d8. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson DJ, Truog RD. The luck of the draw: physician-related variability in end-of-life decision-making in intensive care. Intensive Care Med. 2013;39:1128–1132. doi: 10.1007/s00134-013-2871-6. [DOI] [PubMed] [Google Scholar]
- 17.Pollack M, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, and Mortality from Pediatric Intensive Care. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000001081. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willson DF, Dean JM, Newth C, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN) Pediatr Crit Care Med. 2006;7:301–307. doi: 10.1097/01.PCC.0000227106.66902.4F. [DOI] [PubMed] [Google Scholar]
- 19.Willson DF, Dean JM, Meert KL, et al. Collaborative Pediatric Critical Care Research Network: Looking back and moving forward. Pediatr Crit Care Med. 2010;11:1–6. doi: 10.1097/PCC.0b013e3181c01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 21.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zawistowski CA, DeVita MA. A descriptive study of children dying in the pediatric intensive care unit after withdrawal of life-sustaining treatment. Pediatr Crit Care Med. 2004;5:216–223. doi: 10.1097/01.pcc.0000123547.28099.44. [DOI] [PubMed] [Google Scholar]
- 23.Burns JP, Mitchell C, Outwater KM, et al. End-of-life care in the pediatric intensive care unit after the forgoing of life-sustaining treatment. Crit Care Med. 2000;28:3060–3066. doi: 10.1097/00003246-200008000-00064. [DOI] [PubMed] [Google Scholar]
- 24.Sands R, Manning JC, Vyas H, et al. Characteristics of deaths in paediatric intensive care: A 10-year study. Nurs Crit Care. 2009;14:235–240. doi: 10.1111/j.1478-5153.2009.00348.x. [DOI] [PubMed] [Google Scholar]
- 25.Mink RB, Pollack MM. Resuscitation and withdrawal of therapy in pediatric intensive care. Pediatrics. 1992;89:961–963. [PubMed] [Google Scholar]
- 26.Garros D, Rosychuk RJ, Cox PN. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. 2003;112:e371–e379. doi: 10.1542/peds.112.5.e371. [DOI] [PubMed] [Google Scholar]
- 27.Organ Procurement and Transplantation Network. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf Accessed March 24, 2015.
- 28.Webster PA, Markham L. Pediatric organ donation: A national survey examining consent rates and characteristics of donor hospitals. Pediatr Crit Care Med. 2009;10:500–504. doi: 10.1097/PCC.0b013e318198b06b. [DOI] [PubMed] [Google Scholar]
- 29.Thaker HM, Vernon DD. The autopsy: Underutilized weapon in the pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:675–676. doi: 10.1097/PCC.0b013e3182071277. [DOI] [PubMed] [Google Scholar]
- 30.Narayanan A, Thorburn K, Baines P. Autopsies in children continue to reveal unanticipated discrepancies between autopsy findings and antemortem clinical diagnoses. Arch Dis Child. 2009;94:645. doi: 10.1136/adc.2008.150417. [DOI] [PubMed] [Google Scholar]
- 31.von Dessauer B, Velozo L, Benavente C, et al. Postmortem studies in the contemporary pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:617–621. doi: 10.1097/PCC.0b013e3182071266. [DOI] [PubMed] [Google Scholar]
- 32.Haque AK, Patterson RC, Grafe MR. High autopsy rates at a university medical center. What has gone right? Arch Pathol Lab Med. 1996;120:727–732. [PubMed] [Google Scholar]


