Abstract
In vitro selection of nucleic acid aptamers, coined SELEX, has led to the discovery of novel therapeutics and aided in the structural and mechanistic understanding of many ligand-biomolecule interactions. A related method, selection with modified aptamers (SELMA), enables selection of DNA aptamers containing bases with a large modification that cannot undergo PCR. A key application of this method is the evolution of aptamers containing carbohydrate modifications. Carbohydrate-binding proteins normally require several copies of the carbohydrate moiety for strong recognition. Whereas it may be difficult to rationally design synthetic scaffolds that cluster glycans in the optimal spacing and orientation for target recognition, SELMA furnishes glycoaptamers with highly optimized glycan clustering, achieving low-nanomolar recognition. Although numerous applications can be envisioned, the protocols and discussions in this article describe procedures involved in applying SELMA to the discovery glycoDNAs that bind to the HIV broadly neutralizing antibody 2G12.
Keywords: SELMA, SELEX, HIV, in vitro aptamer selection, protein-carbohydrate interaction
INTRODUCTION
The massive sequence space and folding possibilities of large nucleic acid libraries make them excellent sources for target-directed discovery. Systematic evolution of ligands by exponential enrichment (SELEX) has been used with remarkable success to evolve aptamers (specific binders) that bind a range of targets, from small molecules to whole cells (Famulok et al., 2007). Selection with modified aptamers (SELMA) is a related approach developed for utilizing a diverse DNA library as a scaffold for glycan presentation to a carbohydrate-binding target (MacPherson et al., 2011).
Multivalent binding is particularly important in carbohydrate-protein interactions. An important example is the carbohydrate-specific recognition of HIV by broadly neutralizing antibodies (bnAbs) and their potential use in vaccine design. 2G12, PG9, and PGT121 are just a few among dozens of bnAbs that have been isolated from HIV-positive individuals (Calarese et al., 2003; Pejchal et al., 2011; Julien et al., 2013). The ability of these antibodies to protect against infection is dependent on binding to a specific cluster of oligosaccharides. Numerous attempts have been made to rationally design synthetic carbohydrate cluster ligands that bind these antibodies, in the hope that such ligands may be useful as vaccines (Wang, 2013); however, these rationally designed carbohydrate clusters lack the structural rigidity and/or proper clustering and presentation of carbohydrates needed to adequately mimic the epitope of the antibody. It was reasoned that a DNA scaffold could evolve to cluster and present carbohydrates in a manner that accurately mimics their multivalent presentation on HIV.
In vitro selection of a glycosylated DNA library presents several challenges, e.g., the chemical step of library glycosylation and enzymatic amplification of a heavily modified DNA library post-selection. The SELMA method addresses these challenges and has been successfully employed to identify glycosylated aptamers with low-nanomolar affinities for 2G12 (Temme et al., 2014). Click chemistry is used to covalently attach azide sugars to an unnatural alkyne-containing uridine derivative. Another important aspect of SELMA is the hairpin loop that links a “phenotypic” segment of glycosylated ssDNA with a “genotypic” dsDNA that is competent for PCR (Ichida et al., 2005). This unit outlines the steps used to successfully generate such a library and implement selection to evolve glycosylated aptamers that bind to a protein target of interest. In Strategic Planning, the antibody target and library/selection design are discussed. The methods have been divided so that the end of each protocol provides a convenient stopping point. The first round of selection consists of: appending the hairpin structure to a purchased or amplified random library (Form A to Form C; Basic Protocol 1), incorporation of alkynyl bases and click attachment of carbohydrates (Form C to Form E; Basic Protocol 2), strand displacement and selection of best glycoDNA binders (Form E to Form G; Basic Protocol 3), and amplification of selected winners and regeneration of the hairpin library (Form F to Form CN+1; Basic Protocol 4). Subsequent rounds of SELMA and library cloning are described in Basic Protocol 5, and sequencing of the library and analysis of the glycosylated aptamers is described in Basic Protocol 6. A Support Protocol is also provided for validating individual steps in Basic Protocols 1 through 3.
STRATEGIC PLANNING
The mAb 2G12 is a carbohydrate-specific antibody that has been shown to bind to multiple Man9 glycans on the surface of the HIV env spike protein gp120. Because of its carbohydrate-specific recognition, 2G12 was targeted in a modified selection procedure. To achieve this goal, the DNA library needed to be modified with a large Man9 glycan, while preserving the possibility of PCR amplification. Based on prior work by Gramlich et al. (2008) and Gierlich et al. (2006, 2007) demonstrating a method for polymerase incorporation of the alkyne-containing 5-ethylnyl-dUTP (EdU) and subsequent glycosylation of the unnatural dsDNA by an azide sugar using click chemistry (Rostovtsev et al., 2002), SELMA was designed to employ click chemistry to attach Man9 azide sugars to an EdU-modified library. Although these large DNA modifications were not well tolerated by PCR enzymes, this problem was overcome by attaching to the modified DNA an unmodified copy composed of natural DNA bearing the same sequence.
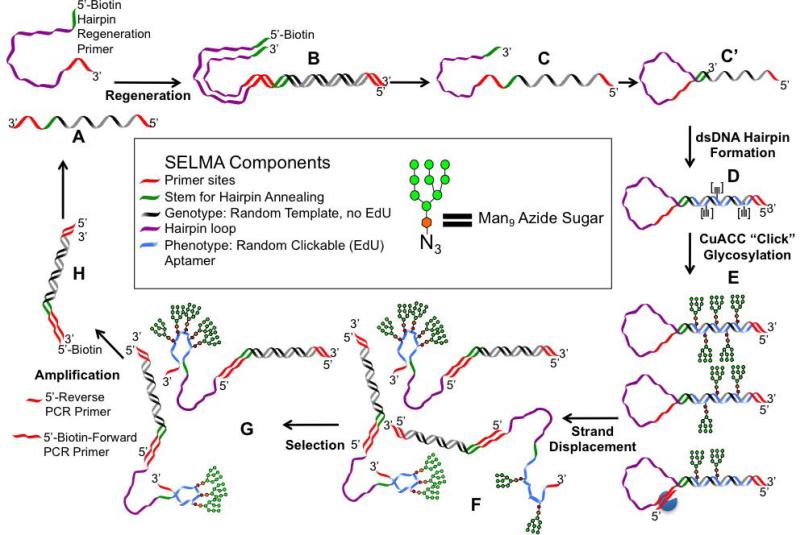
Figure 1 illustrates the individual steps of SELMA and the DNA forms generated at each step. The SELMA library is a ssDNA hairpin closed by a self-complementary stem region (Form C′). The ssDNA hairpin containing a random region is polymerase-extended with EdUTP, dATP, dCTP, and dGTP, and then glycosylated by copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry to afford the glycosylated dsDNA hairpin (Form E). This is opened by a strand-displacing enzyme to make the binding library consisting of glycosylated ss/dsDNA hybrids (Form F). The selected aptamers are amplified by PCR, and then the ssDNA library is regenerated.
Figure 1.
SELMA scheme and components. Library forms: (A) Purchased ssDNA library or ssPCR product library; (B) dsDNA library; (C) ssDNA library; (C′ ) ssDNA hairpin library; (D) dsDNA hairpin library; (E) glycosylated dsDNA hairpin library; (F) glycosylated ss/dsDNA hybrid library; (G) selected aptamer library; (H) PCR library.
Care should be taken when designing the primers to avoid hairpin/stem interactions and other common PCR artifacts (Apte and Daniel, 2009). Also, the reverse PCR primer at the 5′ end of the library template strand should be designed without A nucleotides, to avoid adding sites of constant glycosylation after the 3′ terminus of the library's random region. The random region can be adjusted in terms of length or frequency of the modified base. In the case of 2G12, it is believed that three to four gp120 glycans are involved in the interaction, with glycans attached to gp120 polypeptide at points separated by distances of ~9 to 17 Å and making contact with 2G12 at binding sites separated by 20 to 30 Å (Calarese et al., 2003). In practice, a 56-nt library containing a 25-nt random sequence has been sufficient to produce good binders for 2G12. Longer or shorter libraries may be necessary for other target proteins. In addition to the spacing of binding sites on the target protein, one has to consider the size of the glycan. For smaller glycans, it is likely that longer stretches of nucleic acid may be needed to span the same distance between binding sites. The frequency of the glycosylated base in our most successful selection was 15%, corresponding to a starting library with 15% A content in the random sequence template region (and 28.3% each of T, G, and C). This A content should lead to a large fraction of sequences in the starting library containing the desired number of three or four glycosylations (22% and 21%, respectively; Temme et al., 2013). The expected fraction (f) of starting library sequences containing M copies of the modified nucleotide can be calculated, according to the binomial formula, as:
where N is the number of random nucleotides and %A is the fraction of adenosine at each random position of the template strand, expressed in percentage points. A simple Excel file that is helpful for viewing the multivalency profile of a starting library based on these parameters is available (see Internet Resources).
The protocols in this unit describe SELMA for the discovery of glycosylated ssDNA aptamers which bind to a target of interest. The SELMA method can be broken into six distinct Basic Protocols:
BASIC PROTOCOL 1
APPENDING THE HAIRPIN STRUCTURE TO THE RANDOM LIBRARY (FORM A TO FORM C)
The first step of SELMA is to convert the purchased library (or the amplified library from a previous round) from Form A to Form C. The process is begun by annealing the 5′-biotinylated hairpin regeneration primer to the library Form A. Bidirectional polymerase extension produces Form B. After exonuclease I treatment to remove excess primer, the non-biotinylated strand is isolated using streptavidin magnetic beads to afford the full-length ssDNA library, Form C.
Materials
Oligonucleotides for SELMA (Integrated DNA Technologies), both urea PAGE purified:
Library: 5′-CTTGTCGTCTCCTGTGTGCTTNNNNNNNNNNNNNNNNNNNNNNNNNCCCGTACCCGTTAAAACTCCACCTCATAACCGCA-3′
Hairpin regeneration primer: 5′-biotin-CCCGTACCCGAATATAAAATAAAAA TATAAAATATAAAATTGCGGTTATGAGGTGGAGTT-3′
5 U/μl DNA polymerase I, large (Klenow) fragment, with 10× NEBuffer 2 (New England Biolabs, cat. no. M0210)
10 mM dNTP mix (see recipe)
500 mM EDTA, pH 8.0
60 mg/ml Sephadex G-50 slurry in water (see recipe)
20 U/μl exonuclease I (Exo I) and 10× buffer (New England Biolabs, cat. no. M0293)
25:24:1 phenol/chloroform/isoamyl alcohol, saturated with 10 mM Tris, pH 8.0, 1 mM EDTA (Sigma, cat. no. P2069)
Stabilized chloroform
3 M sodium acetate (NaOAc), pH 5.46
100% and 70% (v/v) ethanol
Hydrophilic streptavidin magnetic beads (New England Biolabs, cat. no. S1421, 400 pmol ssDNA/mg)
1× streptavidin binding/wash buffer (see recipe)
100 mM NaOH (freshly prepared and titrated prior to use)
1 M HCl
1 M Tris·Cl, pH 8.0
1.5-ml microcentrifuge tubes
Thermal cycler
Mini-spin columns without medium (e.g., USA Scientific, cat. no. 1415-0600)
Magnetic rack
Tube rotator
NanoDrop spectrophotometer or equivalent
Perform extension to give Form B
-
Prepare the following annealing reaction:
- 20 μl 10× NEBuffer 2
- 10 μl 10 μM library
- 12 μl 10 μM hairpin regeneration primer
- 150 μl Milli-Q water.
Anneal the primer in a thermal cycler using an annealing ramp of 95°C to 45°C at a rate of 6 sec/°C.
-
Prepare the extension reaction by adding:
- 4 μl 10 mM dNTP mix (final 200 μM each)
- 4 μl Klenow fragment (20 U).
Incubate 15 min at 25°C in the thermal cycler.
Add 6 μl of 500 mM EDTA, pH 8.0, to quench the reaction, then incubate at 75°C for 20 min in the thermal cycler to denature the enzyme.
Desalt reaction mix
-
4.
Add 1 ml Sephadex G-50 slurry to each of two mini-spin columns and centrifuge 2 min at 750 × g, room temperature, in a benchtop centrifuge to remove excess water and pack the columns. Discard the flowthrough.
-
5.
Load 100 μl extension reaction per spin column, place columns in clean 1.5-ml microcentrifuge tubes, and centrifuge 2 min at 750 × g, room temperature, to collect the desalted reaction mix.
Remove excess primer
-
6.Prepare the exonuclease digestion by combining:
- 20 μl 10× Exo I reaction buffer
- 178 μl desalted dsDNA library (Form B)
- 2 μl Exo I (40 U).
- Incubate 30 min at 37°C in the thermal cycler.Some loss in reaction volume can be expected during the desalting process. The reaction volume can be adjusted with water, if necessary.
Perform phenol/chloroform extraction and ethanol precipitation
-
7.Add 200 μl phenol/chloroform/isoamyl alcohol and vortex briefly until an emulsion forms. Centrifuge 5 min at 10,000 × g, room temperature, to separate layers.The use of a basified mixture is required to keep dsDNA in the aqueous layer.
-
8.
Transfer the aqueous layer to a new 1.5-ml tube and repeat extraction to ensure complete deactivation of enzymes.
-
9.
Extract the aqueous layer three times with 200 μl stabilized chloroform to remove any traces of phenol.
-
10.Adjust volume of the extracted library to 360 μl with Milli-Q water.The library volume will vary based on pipetting efficiency during extractions.
-
11.
Add 40 μl of 3 M NaOAc, pH 5.46, and 1000 μl of 100% ethanol, and place on ice for 20 min.
-
12.
Centrifuge 10 min at 20,000 × g, 4°C. Remove supernatant by pipet, taking care to not disturb or discard the DNA pellet.
-
13.Add 1 ml ice-cold 70% ethanol, vortex, and centrifuge 5 min at 20,000 × g, 4°C. Carefully remove supernatant by pipet.Analysis by PAGE at this point should reveal a single clean band, corresponding to the 120-bp dsDNA library Form B (see Support Protocol).
Remove unwanted biotinylated strand to give Form C
-
14.
Prewash 0.25 mg streptavidin magnetic beads with 150 μl of 1 × streptavidin binding/wash buffer in a 1.5-ml tube. Vortex well, then place on a magnetic rack and remove the buffer.
-
15.
Dissolve biotinylated dsDNA library pellet in 200 μl of 1 × streptavidin binding/wash buffer and add to the prewashed beads. Incubate 30 min at room temperature with rotation in a tube rotator, then remove the supernatant on the magnetic rack.
-
16.
Add 150 μl of 1 × streptavidin binding/wash buffer to the beads, vortex well, then remove the buffer on the magnetic rack. Repeat wash cycle two additional times, discarding the buffer after each cycle.
-
17.
Elute the non-biotinylated ssDNA library (Form C) by adding 40 μl of 100 mM NaOH to the beads. Mix by pipetting, then let sit 4 min at room temperature. Place on the magnetic rack and transfer the supernatant to a fresh tube containing 4 μl of 1 M HCl and 1 μl of 1 M Tris·Cl, pH 8.0. Vortex to mix.
-
18.
Determine the concentration of the non-biotinylated ssDNA library on a NanoDrop Spectrophotometer. Store product up to 6 months at −20°C.
BASIC PROTOCOL 2
INCORPORATION OF ALKYNYL BASES AND CLICK ATTACHMENT OF CARBOHYDRATES (FORM C TO FORM E)
In this protocol, the ssDNA library Form C is heated to ensure proper annealing of the self-complementary stem region, forming the ssDNA hairpin Form C′ structure (Figure 1). The alkynyl dsDNA hairpin library Form D is then completed by polymerase incorporation of the unnatural EdUTP together with the natural triphosphates dATP, dCTP, and dGTP. The alkynes are then glycosylated using click chemistry to afford the glycosylated dsDNA hairpin library Form E.
Materials
ssDNA library Form C (see Basic Protocol 1)
BST DNA polymerase, large fragment, with 10× ThermoPol reaction buffer (New England Biolabs, M0275)
10 mM EdUTP mix (see recipe)
Liquid nitrogen
Nitrogen gas
Argon gas (optional)
100 mM HEPES-KOH, pH 8.0, with 0.5% (v/v) Triton X-100
50 mM azide sugar (see recipe)
10 mM CuSO4 (see recipe)
10 mM THPTA (see recipe)
(+)-Sodium l-ascorbate
Thermal cycler
Two 50-ml, two-neck, pear-shaped flasks with ground-glass joints and pointed bottom (e.g., Chemglass, cat. no. CG-1558-14)
Gas/vacuum manifold
500-μl tubes
SpeedVac concentrator
Rubber septum
Additional reagents and equipment for desalting (see Basic Protocol 1)
Perform polymerase extension with EdUTP to give Form D
- Prepare the library hairpin folding reaction by combining 21.5 μl starting ssDNA library Form C with 2.5 μl of 10× Thermopol buffer. Incubate 15 sec at 95°C in a thermal cycler, then cool to ambient temperature and place on ice.The reaction should contain 30 to 40 pmol starting library. The rate of cooling does not seem to affect the annealing reaction.
Add 0.5 μl of 10 mM EdUTP mix and 0.5 μl BST DNA polymerase, then incubate 2 min at 60°C in the thermal cycler to give Form D.
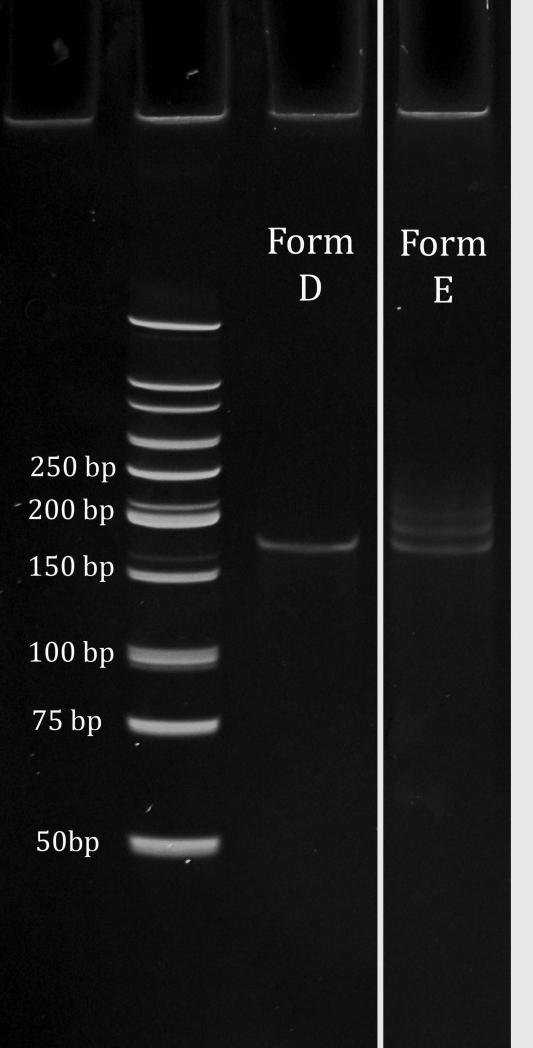
- Add 25 μl Milli-Q water and desalt using a G-50 mini-spin column (see Basic Protocol 1, steps 4 and 5).At this point, PAGE analysis should show one clean band at ~190 bp (see Support Protocol).
Perform click glycosylation to give Form E
-
4.Prepare oxygen-free water by one of the following methods:
- Freeze-pump-thaw method (preferred): Freeze 500 μl water in a two-neck flask under inert atmosphere using liquid nitrogen. Next, using a gas/vacuum manifold, apply a vacuum to the flask and allow the water to thaw under static vacuum. Finally, backfill the flask with nitrogen. Repeat freeze-pump-thaw cycle three additional times, then maintain the inert atmosphere for the remainder of the procedure.
- Argon bubbling method: Bubble argon through water for 30 min to displace oxygen.
The reaction will proceed best if oxygen-free water is used. -
5.Prepare three 500-μl tubes containing the following:
- Tube 1:
- Remaining volume of desalted dsDNA hairpin library (step 3)
- 2 μl 100 mM HEPES-KOH, pH 8.0, with 0.5% (v/v) Triton X-100
- 1 μl 50 mM azide sugar
- Tube 2:
- 5 μl 10 mM CuSO4
- 5.5 μl 10 mM THPTA
- Tube 3:
- 1.98 mg sodium ascorbate
-
6.
Place tubes 1 and 2 in a SpeedVac concentrator and condense to dryness.
-
7.
Cut off the caps, then place all three tubes in a second two-neck flask and purge the flask with inert gas for 5 min.
-
8.While inert gas is flowing into the flask through one neck, perform the following steps by sticking the pipet tip through the other (open) neck of the flask.
- Dissolve the contents of tube 3 in 100 μl degassed water (step 5) to make a 100 mM solution of sodium ascorbate.
- Dissolve the contents of tube 2 in 9 μl degassed water and transfer to tube 1. Mix by pipetting.
- Add 1 μl from tube 3 to tube 1 and mix by pipetting.
For a video of this procedure, see Video 1. Care should be taken to maintain adequate inert gas flow to prevent oxygen from contaminating the reaction. -
9.
Seal the flask with a rubber septum and purge the system with inert gas for 5 min.
-
10.Allow the reaction to sit for 3 hr at room temperature under a N2 atmosphere.The final reagent concentrations are 20 mM HEPES-KOH, pH 8.0, 0.1% Triton X-100, 5 mM azide sugar, 5 mM CuSO4, 5.5 mM THPTA, and 10 mM sodium ascorbate. It has been noted that higher sodium concentrations will slow the reaction.
-
11.Desalt using a G-50 mini-spin column (see Basic Protocol 1, step 4 and 5). Store product up to 6 months at −20°C.PAGE analysis of the glycosylated dsDNA hairpin library (Form E) will reveal an increase in molecular weight compared to Form D. Form E will appear as a broader band due to the heterogeneous library (Fig. 2).
Figure 2.
Glycosylation of a library with a 25-nt random region and 15% A content. Lane 1: Low-molecular-weight ladder. Lane 2: dsDNA hairpin library Form D. Lane 3: Man9-cyclohexylglycosylated dsDNA hairpin Form E. Separate bands can also be visualized when the library is glycosylated with the smaller glycan Man4-cyclohexyl.
BASIC PROTOCOL 3
STRAND DISPLACEMENT AND SELECTION OF BEST GLYCODNA BINDERS (FORM E TO FORM G)
To successfully employ SELMA, the glycosylated “phenotypic” ssDNA must be displaced from the natural “genomic” DNA and folded for presentation to the antibody or target of choice. Strand displacement is arguably the most important step in the entire SELMA procedure, as suboptimal strand displacement will lead to oligomerization of the hairpin (see Critical Parameters and Troubleshooting). First, a primer is annealed to the hairpin loop of the glycosylated dsDNA, and polymerase extension leads to strand displacement of the glycosylated ssDNA. Then the glycosylated ss/dsDNA hybrid library Form F is folded and presented to the antibody target for selection. The antibody with its bound library fraction is then captured using protein A magnetic beads and the non-bound library is washed away.
Materials
Sephadex G-50 slurry in binding buffer (see recipe)
Desalted, glycosylated dsDNA hairpin library Form E (see Basic Protocol 2)
8 U/μl BST 2.0 WarmStart DNA polymerase with 10× ThermoPol reaction buffer (New England Biolabs, cat. no. M0538)
10 μM forward primer: 5′ -TGCGGTTATGAGGTGGAGTT-3′
10 mM dNTP mix (see recipe)
Binding buffer + 0.02% Tween-20 (see recipe)
550 nM 2G12 antibody (Polymun Scientific)
Protein A magnetic beads (e.g., Dynabeads Protein A, Invitrogen Life Technologies, cat. no. 10001D)
Protein A elution buffer (see recipe)
Mini-spin column without medium (e.g., USA Scientific, cat. no. 1415-0600)
PCR tubes
Thermal cycler
1.5-ml low-adhesion tubes (e.g., USA Scientific)
Rotator in 37°C incubator or room
Magnetic rack
Boiling water bath
Perform strand displacement to give Form F
- Add 1 ml Sephadex G-50 slurry in binding buffer (not water) to a mini-spin column and position in a benchtop centrifuge. Do not start centrifuge.This column is for buffer exchange and is not the same as the desalting column.
- Prepare the following primer annealing mix in a PCR tube on ice:
- 25-35 μl desalted glycosylated dsDNA hairpin library
- 5 μl 10× Thermopol reaction buffer
- 6 μl 10 μM forward primer
- Milli-Q water to 48 μl.
Place in a preheated 65°C thermal cycler and let the reaction heat for 10 sec.
As quickly and efficiently as possible, and without removing the tube from the thermal cycler, add 1 μl of 10 mM dNTP mix and 1 μl of 8 U/μl BST WarmStart, and mix with a 20-μl pipet. Close the PCR tube lid and the thermal cycler and incubate for 5 min.
At 3 min into the incubation, start the benchtop centrifuge containing the spin column and centrifuge for 2 min at 750 × g, room temperature. Place the column in a fresh 1.5-ml tube.
- After a total of 5 min at 65°C, very quickly pipet the reaction mix onto the column and centrifuge 2 min at 750 × g, room temperature, to yield the glycosylated ss/dsDNA hybrid library Form F.For strand displacement verification techniques, see Support Protocol. For a discussion of side reactions that are observed, see Critical Parameters and Troubleshooting.
Adjust the volume of the buffer-exchanged product to 50 μl using binding buffer + 0.02% Tween-20, then incubate 2 min at 70°C in the thermal cycler.
Remove tube from the thermal cycler and cool to ambient temperature to fold the glycoDNA.
Transfer the folded, glycosylated ss/dsDNA hybrid library to a 1.5-ml low-adhesion tube.
Perform antibody selection to give Form G
-
10.
Add 5 μl of 550 nM 2G12 antibody to the library (final 50 nM 2G12) and incubate 1 hr at 37°C with rotation.
-
11.
During incubation, prewash 1.5 mg protein A magnetic beads with prewarmed (37°C) binding buffer + 0.02% Tween-20 in a 1.5-ml low-adhesion tube. Vortex well, then place on a magnetic rack and remove the buffer.
-
12.
Transfer antibody-library mixture to the tube containing prewashed beads and incubate 45 min at 37°C with rotation.
-
13.
Place tube on the magnetic rack and remove the supernatant.
-
14.
Wash beads with 150 μl prewarmed (37°C) binding buffer + 0.02% Tween-20. Pipet up and down to wash, then return tube to the magnet and remove the buffer. Repeat one to two times, discarding the buffer after each cycle.
-
15.Add 30 μl protein A elution buffer and place tube in a boiling water bath for 2 min to elute bound selection winners. Place tube on the magnet, and remove and save the supernatant. Store up to 1 week at −20°C.The supernatant will be used to amplify the selected winners and regenerate the second-round library.
SUPPORT PROTOCOL
CONFIRMATION OF HAIRPIN FORMATION AND STRAND DISPLACEMENT
One of the more challenging aspects of Basic Protocols 2 and 3 is determining whether or not each step was successful. This protocol should be attempted before SELMA is carried through multiple cycles. The manipulations undertaken here are performed on non-glycosylated DNA, and thus are not useful for selection. However, performing them will familiarize you with the method and help avoid potential problems. PAGE monitoring of non-glycosylated SELMA steps is encouraged due to the inherent multivalency of the glycosylated library, creating faint and dilute individual bands.
In this protocol, samples for Forms B and D are first reserved for PAGE analysis. Next, a sample of dsDNA hairpin library Form D is subjected to endonuclease digestion to confirm the presence of the hairpin loop. In addition, hairpin formation is verified by adding primer to dsDNA hairpin library Form D. Finally, the ss/dsDNA hybrid form of the library is monitored before and after exonuclease digestion to confirm the success of strand displacement.
Additional Materials (also see Basic Protocols 1, 2, and 3)
10 U/μl mung bean nuclease with 10× reaction buffer (New England Biolabs, M0250)
10% polyacrylamide gel in 1× Tris-borate-EDTA (TBE) electrophoresis buffer Ethidium bromide or equivalent stain for polyacrylamide gels
Perform Basic Protocol 1 in its entirety, but reserve 2 μl of resuspended dsDNA library Form B (see Basic Protocol 1, step 15) for PAGE analysis before adding the remaining sample to the streptavidin beads.
Using the Form C library obtained in step 1, perform Basic Protocol 2 (steps 1 to 3) to make 40 μl of dsDNA hairpin Form D. Reserve 4 μl for PAGE analysis before proceeding.
- Prepare the following endonuclease reaction:
- 8 μl desalted dsDNA hairpin (step 2)
- 1 μl 10× mung bean nuclease reaction buffer
- 1 μl 10 U/μl mung bean nuclease.
Incubate 30 min at 30°C in a thermal cycler and reserve on ice for PAGE analysis.
- Prepare the following annealing reaction:
- 20 μl desalted dsDNA hairpin (step 2 above)
- 5 μl 10 mM forward primer
- 5 μl Milli-Q water
- 2.5 μl 10× Thermopol reaction buffer
Mix with a pipet and reserve 5 μl of primer-annealed library on ice for PAGE analysis.
Perform strand displacement on the primer-annealed library as described (see Basic Protocol 3, steps 3 to 6) to make the ss/dsDNA hybrid library Form F. Reserve 10 μl of buffer-exchanged product on ice for PAGE analysis before continuing to exonuclease digestion.
- Prepare the following exonuclease digestion:
- 8 μl strand displacement (step 5 above)
- 1 μl Exo I buffer
- 1 μl Exo I.
Incubate 30 min at 37°C in the thermal cycler and reserve on ice for PAGE analysis.
- Analyze samples on a 10% polyacrylamide gel in 1× TBE electrophoresis buffer, using ethidium bromide to visualize the bands.The dsDNA library Form B (step 1) will appear as a tight band at 120 bp. The ds-DNA hairpin library Form D (step 2) will appear as a tight band at ~190 bp. The endonuclease-treated dsDNA hairpin Form D (step 3) will appear as a tight band at 56 bp, from the stem region to the end of the reverse primer. Incomplete endonuclease digestion will produce a smear above the 56 bp band. The dsDNA hairpin library Form D with primer annealed in the hairpin loop (step 4) will appear as a tight band at ~220 bp. The ss/dsDNA hybrid library Form F (step 5) will appear as a band at ~550 bp. Finally, the exonuclease-treated ss/dsDNA hybrid library Form F (step 6) will appear as a tight band corresponding to 80 bp, the same size as the PCR product.All migration distances are based on a 10% gel composed of 29:1 acrylamide/bisacrylamide. In non-denaturing polyacrylamide gels, hairpin loops and ssDNA may migrate differently depending on acrylamide percent and crosslinking.
BASIC PROTOCOL 4
AMPLIFICATION OF SELECTED WINNERS AND HAIRPIN REGENERATION (FORM F TO FORM CN+1)
The final step of each round of SELMA is PCR amplification of the selected winners and rebuilding of the hairpin structure in the (N+1)-generation library. Two amplification primers, one of which is biotinylated, are used to amplify the selected winners. Caution should be taken in the protocol design to ensure that any antibody co-eluted with the DNA from Basic Protocol 3 does not interfere with successful PCR (see Critical Parameters and Troubleshooting). PCR in SELMA serves two purposes: to amplify the library winners and to act as a tool for evaluating the recovery and enrichment of the library. To avoid artifacts, it is critical that PCR not be taken beyond the exponential amplification period. For this reason, a pilot PCR is required prior to whole-library PCR amplification. The product of the full-scale PCR is the next-generation library in Form G, lacking the hairpin. The ssDNA hairpin is then rebuilt through several steps, yielding form CN+1.
Materials
Winners of selection (see Basic Protocol 3)
Phusion Hot Start II High-Fidelity DNA polymerase with 5× HF Buffer (Thermo, cat no. F-549)
10 μM biotinylated forward primer: 5′-biotin-TGCGGTTATGAGGTGGAGTT-3′
10 μM reverse primer: 5′-CTTGTCGTCTCCTGTGTGCTT-3′
10 mM dNTP mix (see recipe)
6× loading buffer (see recipe)
1.5% agarose gel containing ethidium bromide
Low-molecular-weight DNA ladder
20 U/μl exonuclease I (Exo I; New England Biolabs, cat no. M0293)
2× streptavidin binding/wash buffer (see recipe)
10μM library hairpin regeneration primer: 5′-biotin-CCCGTACCCGAATATAAAATAAAAATATAAAATATAAAATTGCGGTTATGAGGTGGAGTT-3′
200- and 500-μl PCR tubes
Thermal cycler
Additional reagents and equipment for agarose gel electrophoresis, and for removing biotinylated DNA strands and regenerating the library (see Basic Protocol 1)
Amplify selected library sequences to give Form H
- In a PCR tube, prepare the following PCR master mix:
- Eluted winners of selection
- 40 μl 5× HF Buffer
- 10 μl 10 μM biotinylated forward primer
- 10 μl 10 μM reverse primer
- Milli-Q water to 194 μl.
- In a second PCR tube, prepare the pilot PCR reaction:
- 48.5 μl PCR master mix (step 1)
- 1.0 μl 10 mM dNTP mix
- 0.5 μl (1 U) Phusion DNA polymerase.
- Mix by pipet, then aliquot 5 μl into each of ten 200-μl PCR tubes labeled according to cycle number.Ten cycles is good starting point for monitoring following the first round of selection.
- Use the following thermal cycling program for SELMA:
1 cycle: 30 sec at 98°C 30 cycles: 5 sec at 98°C 20 sec at 64°C 8 sec at 72°C Remove individual tubes at the end of cycles 10, 12, 14, 16, 18, 20, 22, 24, 26, and 28. Place on ice, add 1 μl of 6× loading buffer, and mix. Load samples into separate wells of a 1.5% agarose gel containing ethidium bromide. Load a primer sample and a low-molecular-weight DNA ladder for referencing. Perform electrophoresis at 100 V for 20 min.
- Analyze the progress of PCR on a UV light box. Choose a cycle that shows product during the exponential growth period.Excessive cycles can result in side reactions (see Critical Parameters and Troubleshooting).
- Set up the main PCR reaction as follows:
- 145.5 μl PCR master mix (step 1)
- 3 μl 10 nM dNTP mix
- 1.5 μl (3 U) Phusion DNA polymerase.
- Mix by pipet.
- Change the thermal cycling program to reflect the optimized cycle number (X) for appropriate amplification and run as follows:
1 cycle: 30 sec at 98°C X cycles: 5 sec at 98°C 20 sec at 64°C 8 sec at 72°C 1 cycle: 5 min at 72°C. Add 1.5 μl (30 U) Exo I and incubate in a thermal cycler for 30 min at 37°C and then 20 min at 80°C to remove excess primers and denature the enzymes.
Add 150 μl of 2× streptavidin binding/wash buffer.
Remove biotinylated strand to give Form A
-
10.
Transfer product to 0.25 mg prewashed streptavidin magnetic beads and proceed as described (see Basic Protocol 1, steps 14 to 17).
Regenerate library to give Form CN+1
-
11.Perform regeneration of the hairpin library by following Basic Protocol 1 with the following changes:
- In step 1, prepare the following annealing reaction:
- 10 μl 10× NEBuffer 2
- 45 μl library template from PCR
- 6 μl 10 μM hairpin regeneration primer
- Milli-Q water to 96 μl.
- In step 2, use 2 μl each of dNTPs and Klenow.
- In steps 4 and 5, use a single desalting column.
- In step 6, perform the exonuclease reaction on a 100-μl scale.
- Use a NanoDrop to verify and characterize the recovery of dsDNA Form B and ssDNA Form C.After the ssDNA library is recovered, the second round of selection can begin.If achieving a high recovery of the library becomes difficult during subsequent rounds of selection, steps 3 to 13 of Basic Protocol 1 (including phenol/chloroform extraction and ethanol precipitation) may be replaced with steps 8 to 10 of this protocol.
BASIC PROTOCOL 5
SUBSEQUENT ROUNDS OF SELMA AND LIBRARY CLONING
Once the first round of SELMA has been completed, Basic Protocols 1 to 4 can be repeated to enrich the library with the tightest binders. Although the protocol for subsequent SELMA rounds is nearly identical to the first round, some additional steps are taken to avoid artifacts and ensure enrichment. A negative selection against protein A magnetic beads is included. Stringency is increased to force the emergence of tighter binders. Additionally, PCR artifacts may necessitate PAGE purification of the PCR product. When selections are finished, the library's PCR product is cloned and sequenced.
Determine the amount of library Form C on a NanoDrop spectrophotometer, as noted in step 12 of Basic Protocol 4. The amount of DNA library used after the first selection round should be ~10 pmol. It is often adequate to use ¼ to ½ of the product of Basic Protocol 4 in the next round of selection.
Having reduced the amount of library in the second generation, it is also advisable to reduce the amount of primer used in step 2 of Basic Protocol 3 from 60 pmol to 15 pmol.
After bringing the next generation library through step 6 of Basic Protocol 3, prepare a counterselection against protein A magnetic beads. Add the product of step 6 to 0.75 mg prewashed protein A magnetic beads and incubate 45 min at 37°C. Remove the supernatant and use it for selection with the antibody. It is advisable to do this counterselection in all remaining rounds.
In step 10 of Basic Protocol 3, a lower antibody concentration can be used to increase stringency/evolutionary pressure on the selection. Begin reducing the antibody concentration after the third round of selection.
In step 14 of Basic Protocol 3, more vigorous washing (i.e., increased number of bead washing cycles, increased number of times the beads are aspirated and blown out of the tip) can be used to increase stringency on the selection.
After PCR in step 7 of Basic Protocol 4, the library may need to be purified by preparative PAGE. If higher-molecular-weight bands are visible in a polyacrylamide gel of the library PCR product even at low cycle numbers, the library should be purified by preparative PAGE to remove these artifacts and prevent them from overwhelming the library (see Critical Parameters and Troubleshooting).
- Repeat SELMA for seven to ten rounds.Nearly complete library convergence has been observed in as few as seven rounds.
- When selections are complete, clone and sequence the PCR library Form H.Colony PCR (Woodman, 2008) is recommended when selecting colonies for sequencing. Blue-white colony screening can give a false negative result on an insert of this size (56 bp). False negatives may favor a particular population of the aptamer library, threatening to eliminate it from the sequence pool.
BASIC PROTOCOL 6
SYNTHESIS AND ANALYSIS OF GLYCOSYLATED APTAMERS
This procedure describes the analysis of selected glycosylated aptamers obtained from SELMA. After the library has been cloned and sequenced, the library population is analyzed with appropriate software programs. Complementary ssDNA oligos of promising candidates are commercially synthesized. A short primer is annealed to the synthesized oligo, and polymerase extension is used to incorporate the clickable EdUTP. Following glycosylation using click chemistry, the glycosylated ssDNA is purified by urea PAGE.
Materials
Complementary oligo templates (where N is the random region or desired aptamer to be analyzed):
5′-CTTGTCGTCTCCTGTGTGCTTNNNNNNNNNNNNNNNNNNNNNNNNNCCCGTACCCG-3′
Stem primer: 5′-CGGGTACGGG-3′
5 U/μl DNA polymerase I, large (Klenow) fragment, with 10× NEBuffer 2 (New England Biolabs, cat. no. M0210)
10 mM EdUTP mix (see recipe)
12% urea polyacrylamide gel
Acridine orange staining solution
Thermal cycler
Additional reagents and equipment for desalting and ethanol precipitation (see Basic Protocol 1), for click glycosylation (see Basic Protocol 2), and for urea PAGE
Organize aptamer sequences using a nucleic acid database (e.g., Biology WorkBench) and use it to check aptamers for sequence alignment and library convergence.
Check library members for structural similarity using a prediction algorithm, e.g., mfold (Zuker, 2003; see Internet Resources).
- Purchase stem primer and oligos complimentary to the desired glycoDNA sequence.The complementary oligo should start with the reverse primer sequence, then the random region, and end with the complement of the stem region.
- To synthesize individual clones, prepare the following annealing reaction:
- 20 μl 10× NEBuffer 2
- 10 μl 10 μM oligo template
- 20 μl 10 μM stem primer
- 142 μl Milli-Q water.
Anneal the primer in a thermal cycler using the annealing ramp program: 95°C to 25°C at a rate of 10 sec/°C.
- Prepare the following extension reaction:
- 192 μl annealing reaction (from step 4)
- 4 μl 10 mM EdUTP mix (final 200 μM each)
- 4 μl Klenow (20 U).
Incubate 15 min at 25°C in the thermal cycler.
Desalt and ethanol precipitate the reaction (see Basic Protocol 1, steps 4-5 and 10-13).
Click glycosylate the dsDNA (see Basic Protocol 2, steps 4-11).
- Purify the desired glycosylated ssDNA product using a 12% urea polyacrylamide gel. Visualize ssDNA bands with acridine orange staining and excise the desired fully clicked ssDNA product.Large sugar modifications (i.e., Man9 and Man4) provide sufficient size changes in oligonucleotides that it is possible to resolve and purify the product from incomplete click products, template strand, and other impurities.
Radiolabel the purified glycosylated ssDNA oligos with 32P and study the aptamer/antibody interaction using a filter binding assay as in Temme et al. (2014).
REAGENTS AND SOLUTIONS
Use Milli-Q-purified water or equivalent in all recipes and protocol steps.
2G12 binding buffer
242.28 mg Tris base (20 mM final)
876.6 mg NaCl (150 mM final)
24.1 mg MgSO4 (2 mM final)
Adjust to pH 7.5 with HCl
Bring to 100 ml with water
Store up to 6 months at −20°C
2G12 binding buffer + 0.02% (v/v) Tween-20
Add 200 μl of 10% (v/v) Tween-20 to 100 ml 2G12 binding buffer (see recipe). Store up to 6 months at −20°C.
Azide sugar, 50 mM
5 mg Man9 cyclohexyl azide sugar (C60H101N3O46) or other azide sugar, as desired 62.5 μl Milli-Q water
Store up to several months at −20°C
The integrity of the azide should be monitored after long storage periods, ideally by NMR.
CuSO4,, 10 mM
100 mM stock solution:
249.7 mg cupric sulfate pentahydrate
10 ml Milli-Q water
Store up to 1 month at −20°C
10 mM working solution:
Dilute 10 μl stock solution with 90 μl water
Store up to 1 month at −20°C
dNTP mix, 10 mM
10 mM each dATP, dCTP, dGTP, and dTTP in Milli-Q water
Store up to 1 year at −20°C
EdUTP mix, 10 mM
10 mM 5-ethynyl-dUTP (Jena Bioscience)
10 mM each dATP, dCTP, and dGTP
Milli-Q water
Store up to 1 year at −20°C
Loading buffer, 6×
8.8 ml 30% glycerol in water
1.2 ml 50× Tris-acetate-EDTA (TAE) buffer (6× final)
Protein A elution buffer
242.28 mg Tris base (20 mM final)
876.6 mg NaCl (150 mM final)
200 μl 10% (v/v) Tween-20 (0.02% final)
Adjust to pH 8 with HCl
Bring up to 100 ml with water
Store up to 6 months at −20°C
Sephadex G-50 slurry in binding buffer, 60 mg/ml
1.8 g Sephadex G-50 fine (GE Healthcare Life Sciences, cat. no. 17-0042-01)
30 ml 2G12 binding buffer (see recipe)
Store up to 1 week at 20°C or 1 month at 4°C
Sephadex G-50 slurry in water, 60 mg/ml
1.8 g Sephadex G-50 fine (GE Healthcare Life Sciences, cat. no. 17-0042-01)
30 ml Milli-Q water
Store up to 1 week at 20°C or 1 month at 4°C
For 2× buffer
3 mg Tris base (20 mM final)
11.7 g NaCl (2 M final)
58.4 mg EDTA (2 mM final)
100 μl Tween-20 (0.1% [v/v] final)
Adjust pH to 7.5 with HCl
Bring up to 100 ml with water
Store up to 6 months at −20°C
For 1× buffer, dilute with water prior to use.
Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), 10 mM
100 mM stock solution:
434.5 mg THPTA
10 ml water
Store up to 1 year at −20°C
10 mM working solution:
Dilute 10 μl stock solution with 90 μl water
Store up to 1 year at −20°C
COMMENTARY
Background Information
In vitro selection has been used to generate many rare and useful RNA and DNA aptamers. Additionally, in vitro selection using polymerase-compatible modified nucleotides have been reported (Keefe and Cload, 2008). Many such modifications are useful for extending the in vivo half-life of a therapeutic aptamer. For instance, 2′-amino and 2′-fluoro substitutions of pyrimidines have been used in SELEX to discover aptamers that resist nuclease digestion in human serum. These developments have streamlined the delivery of aptamers to the clinic. However, any modifications used in SELEX must be reliably amplified by a polymerase to create a second-generation enriched library. SELMA was developed as an alternative to SELEX that can be used for in vitro selection of heavily modified aptamers. It enables the incorporation of large “un-PCRable” modifications to the DNA sequence. While the ssDNA scaffold may make contact with the molecular target of interest, in the case of a carbohydrate-binding protein as target, the carbohydrate modifications themselves are central to the binding interaction being studied, and the DNA itself may or may not interact directly with the target protein. SELMA was used successfully to discover glycosylated aptamers with lownanomolar affinities for the HIV bnAb 2G12.
Critical Parameters and Troubleshooting
Validation of SELMA (Support Protocol)
Before undergoing a full round of SELMA, the success of each step should be verified as described in the Support Protocol. Native PAGE migration distances of library forms D to G are difficult to predict. To verify that the structure contains the hairpin, it should be viewed on a gel both before and after addition of the strand displacement primer. The primer-annealed hairpin will migrate as a single band above the hairpin alone. ss/dsDNA hybrids also migrate as unexpectedly large species on native PAGE.
Click reaction (Basic Protocol 2)
In this demanding application of click chemistry (multiple alkynes, high yield needed, large modifications, use of EdU), a low THPTA ligand:copper ratio of ~1:1 is required to obtain sufficent catalyst activity. Under these conditions, DNA is prone to oxidative damage if the reaction is done in open air, and the reaction is likely to terminate prematurely. Therefore, it is best to take reasonable precautions to exclude oxygen. The use of an oxygen-free glovebox or perfect Schlenk technique is helpful, but not necessary; however, the use of degassed solvents and a low-oxygen inert atmosphere are a minimum precautions (for a video demonstration, see Video 1). The reaction can be optimized further by varying time, temperature, reagent concentrations, choice of ligand, pH, and buffer/salt concentrations with model oligos.
Strand displacement (Basic Protocol 3)
As noted in the protocol, a suboptimal strand displacement procedure can lead to artifacts that compromise the library. BST DNA polymerase is a very robust enzyme, and controlling its action is critical. Even on ice, BST DNA polymerase can extend the forward primer within the hairpin prematurely if dNTPs are available. At cold temperatures, the polymerase will not have sufficient energy to displace the glycosylated strand, and instead seems to process around the hairpin loop, leading to oligomeric hairpin repeats in the library. For this reason, the dNTPs and fresh polymerase are added to the strand displacement reaction only after it has been heated to 65°C. BST WarmStart is used as an additional precaution. If oligomeric laddering is encountered in the PCR product, preparative PAGE should be used to purify the product. If this becomes a recurring problem in a given selection, preparative PAGE purification of the PCR product should be incorporated in each of the first few rounds of selection, until significant enrichment has occurred.
Buffer exchange (Basic Protocol 3)
Once strand displacement is complete, it is critical that the Form F library be buffer-exchanged and not desalted. Desalting will destabilize the duplex region, allowing the re-invasion of the glycosylated strand to displace the non-glycosylated strand, reverting the library to Form E.
Selection conditions (Basic Protocol 3)
The selection temperature has proven to be of utmost importance (Temme et al., 2014). Selections done at room temperature have resulted in selection products with very high multivalency but mediocre binding. Increasing the selection temperature to 37°C results in selection winners with a 100-fold improvement in Kd and multivalency more closely matching the number of binding sites in the target protein. However, since a temperature of 37°C may not be optimal for every protein target binding site, adjusting the temperature should be considered if the desired results are elusive. Each protein target may require optimization of binding buffer or equilibration time.
PCR (Basic Protocol 4)
It is very important to stop the PCR reaction before the primers are exhausted. If the library is overamplified, hairpin repeat artifacts from unsuccessful strand displacements will begin to take over the library. Pilot PCR is essential for determining the optimal cycle number to avoid this problem. The buildup of such artifacts in later rounds of selection, if encountered, can be eliminated by preparative PAGE purification of the PCR product.
As the selection progresses, the enrichment of the library should be monitored by the PCR recovery after selection. If the library is becoming enriched in good binders, fewer PCR cycles should be required to recover the library from round to round, when selection stringency is held constant. Increased stringency will increase the number of PCR cycles required for library recovery.
Purification of individual sequences for binding studies (Basic Protocol 6)
When using a very large modification such as Man9, purifying the fully glycosylated clone away from the template and incomplete click impurities is easily achieved using urea PAGE. If the modification is small, however, resolving the product from the template may be challenging. Although costly, biotinylated template/streptavidin beads can be used to remove the template, but the modified ssDNA should be further purified by HPLC or urea PAGE prior to use in filter binding assays.
Anticipated Results
Similar to other methods of in vitro aptamer selection, the results of SELMA depend on the population of sequences in the library that can interact favorably with the target antibody. The selection conditions (e.g., buffer, temperature, and time) play a vital role in the selection results and should be optimized (see MacPherson et al., 2011; Temme et al., 2013, 2014). The experimenter must pay careful attention to avoid selecting aptamers that bind to unwanted targets, e.g., protein A beads. In some cases, it may also be desirable to perform counterselections by preparing “unclicked” library, and discarding the fraction that binds to the target without the modification, although this has not been necessary for selections with carbohydrate-binding antibodies.
The PCR cycles required to recover the library should decrease after several rounds of selection. Typically, the number of PCR cycles necessary to amplify the library decreases from 24-26 in the first round to 17-18 after a few rounds. After observing this kind of enrichment, it is reasonable to decrease the target antibody concentration two-fold, with similar concentration decreases after similar additional enrichment. It may also be useful in later rounds to increase the stringency further by adding a competitor (such as mannose, in the case of 2G12). When a plateau in recovery is reached, or when the number of PCR cycles necessary for recovery is low (e.g., 10-12), the library should be sequenced. If the library has not converged (for example, to 1-5 dominant sequences or sequence families among 48 sequence reads), then further rounds of selection with increased stringency are probably necessary.
Time Considerations
Designing the library and obtaining the necessary starting materials should take 1 to 2 weeks. Each round of selection can be completed in a 14- to 16-hr day, although the procedure can be stopped at the end of Basic Protocols 1 to 4 for convenience. Seven to ten rounds of selection can be finished in 2 weeks to 2 months. Cloning, sequencing, sequence analysis, and binding assays will take about 2 to 4 weeks.
Supplementary Material
Acknowledgments
The authors thank Drs. Iain MacPherson, Satoru Horiya, and Marcus Long for helpful discussions, and Dr. Hermann Katinger and Polymun Scientific for the gift of mAb 2G12. The financial support of Brandeis University and the National Institutes of Health (R01 AI090745 and R01 AI113737) is gratefully acknowledged.
Footnotes
How to cite this article:
Temme, J. S. and Krauss, I. J. 2015. SELMA: Selection with modified aptamers. Curr. Protoc. Chem. Biol. 7:73-92. doi: 10.1002/9780470559277.ch140233
Internet Resources
http://workbench.sdsc.edu/ Biology WorkBench is a useful nucleic acid sequence database used for organizing and analyzing aptamer sequences.
http://mfold.rna.albany.edu/?q=mfold The mfold web server offers nucleic acid secondary structure prediction algorithms.
http://people.brandeis.edu/~kraussi/tools.html Provides an Excel file that is useful for predicting the multivalency profile of the starting library, and the video for running the click reaction under inert atmosphere.
Literature Cited
- Apte A, Daniel S. PCR primer design. Cold Spring Harbor Protoc. 2009;2009:pdb.ip65. doi: 10.1101/pdb.ip65. [DOI] [PubMed] [Google Scholar]
- Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Famulok M, Hartig JS, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007;107:3715–3743. doi: 10.1021/cr0306743. [DOI] [PubMed] [Google Scholar]
- Gierlich J, Burley GA, Gramlich PME, Hammond DM, Carell T. Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org. Lett. 2006;8:3639–3642. doi: 10.1021/ol0610946. [DOI] [PubMed] [Google Scholar]
- Gierlich J, Gutsmiedl K, Gramlich PME, Schmidt A, Burley GA, Carell T. Synthesis of highly modified DNA by a combination of PCR with alkyne-bearing triphosphates and click chemistry. Chem. Eur. J. 2007;13:9486–9494. doi: 10.1002/chem.200700502. [DOI] [PubMed] [Google Scholar]
- Gramlich PME, Wirges CT, Gierlich J, Carell T. Synthesis of modified DNA by PCR with alkyne-bearing purines followed by a click reaction. Org. Lett. 2008;10:249–251. doi: 10.1021/ol7026015. [DOI] [PubMed] [Google Scholar]
- Ichida JK, Zou K, Horhota A, Yu B, McLaughlin LW, Szostak JW. An in vitro selection system for TNA. J. Am. Chem. Soc. 2005;127:2802–2803. doi: 10.1021/ja045364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J-P, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, Ramos A, Diwanji DC, Pejchal R, Cupo A, Katpally U, Depetris RS, Stanfield RL, McBride R, Marozsan AJ, Paulson JC, Sanders RW, Moore JP, Burton DR, Poignard P, Ward AB, Wilson IA. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog. 2013;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe AD, Cload ST. SELEX with modified nucleotides. Curr. Opin. Chem. Biol. 2008;12:448–456. doi: 10.1016/j.cbpa.2008.06.028. [DOI] [PubMed] [Google Scholar]
- MacPherson IS, Temme JS, Habeshian S, Felczak K, Pankiewicz K, Hedstrom L, Krauss IJ. Multivalent glycocluster design through directed evolution. Angew. Chem. Int. Ed. 2011;50:11238–11242. doi: 10.1002/anie.201105555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Doores KJ, Walker LM, Khayat R, Huang P-S, Wang S-K, Stanfield RL, Julien J-P, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scan-lan CN, Wong C-H, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Temme JS, Drzyzga MG, MacPherson IS, Krauss IJ. Directed evolution of 2G12-targeted nonamannose glycoclusters by SELMA. Chem. Eur. J. 2013;19:17291–17295. doi: 10.1002/chem.201303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme JS, MacPherson IS, DeCourcey JF, Krauss IJ. High temperature SELMA: Evolution of DNA-supported oligomannose clusters which are tightly recognized by HIV bnAb 2G12. J. Am. Chem. Soc. 2014;136:1726–1729. doi: 10.1021/ja411212q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-X. Synthetic carbohydrate antigens for HIV vaccine design. Curr. Opin. Chem. Biol. 2013;17:997–1005. doi: 10.1016/j.cbpa.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman ME. Direct PCR of intact bacteria (colony PCR). Curr. Protoc. Microbiol. 2008;9:A.3D.1–A.3D.6. doi: 10.1002/cpmc.14. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.