Abstract
Background:
Laboratory health care workers (HCWs) may become infected through their occupation with blood-borne pathogens. The aims of this study were determining the seroprevalence of hepatitis B virus (HBV) and the protection offered by HBV vaccine in medical laboratory HCWs.
Materials and Methods:
A descriptive cross-sectional study was carried out on 203 employers of clinical laboratories. Participant data were obtained through a questionnaire, and the level of antigens and antibodies were measured by enzyme-linked immunosorbent assay (ELISA).
Results:
All of the subjects were negative for HBV infection. Forty-seven (23.2%) were not immune, 126 (62.0%) were relatively immune, and 30 (14.8%) were highly immune.
Conclusion:
Hepatitis B infection is infrequent in laboratory HCWs in Isfahan.
Keywords: Health care worker, hepatitis B infection, occupational exposure
Introduction
Hepatitis B virus (HBV) infection is a major public health problem worldwide. More than two billion people have been infected with HBV and approximately, 400 million people are carriers of the virus.[1] Iran is a country in which hepatitis B prevalence is intermediate. About 2.6% of the Iranian individuals are HBV carriers, and 67.8% of chronic hepatitis patients are positive for at least one of the serologic markers of HBV.[2] According to the World Health Organization and the center for disease control (CDC), more than 85 million health care workers (HCWs) worldwide have been reported to be continuously exposed to injury with contaminated sharp medical devices.[3] HBV contract risk in HCWs is four-times more than general adult population. The highest rates are seen among dentists, physicians, laboratory workers, dialysis workers, cleaning service employees, and nurses. Protection (anti-hepatitis B surface antibody (HBs Ab) level ≥ 10 mIU/mL) following first, second, and third doses of the recombinant vaccine has been mentioned nearly 25%, 75%, and 95%, respectively. Despite the availability of an effective vaccination program, HBV infection continues to be an important occupational hazard worldwide.[4,5] According to CDC and occupational health and administration protocols, every patient should be thought as a probable source of HBV infection.[6] Scanty data are available about the prevalence of HBV antibody among laboratory workers in Iran. The present study was carried out to evaluate the frequency and risk factors of infection with HBV and assessment of the anti-HBs status among laboratory workers in Isfahan, Iran.
Materials and Methods
This descriptive cross-sectional study was performed in 203 medical laboratory technologists (laboratory workers responsible for analyzing body fluids), medical laboratory technicians (laboratory workers responsible for sampling, preparing specimens), and laboratory staff (laboratory workers responsible for cleaning surface and equipment) who had the potential for high risk exposures. The study was conducted under approvement of Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. The study was performed between July and September 2010. Study was approved by the ethics committee of IUMS, Iran (Research project number: 288178).
Sample was collected on voluntary basis using simple random sampling method. The laboratory HCWs were employed in the hospitals and medical laboratory centers in Isfahan city. An inclusion criterion was work history of more than 6 months in medical laboratories. Questionnaire were filled out by the subjects to collect personal and occupational injuries within the last 12 months including splashes to mucous, previous hepatitis history, and other contributing factors. Five ml of venous blood was obtained from each participant and separated serum was sent to the laboratory of the Infectious Diseases and Tropical Medicine Research Center in a cold chain. HBV markers (Hepatitis B core antibody, HBs Ab, and hepatitis B surface antigen) were measured by means of ELISA (DIAPRO kits, diagnostic bio probes s.r.j, Italy) according to the manufacturer recommendations. The antibody titers were classified into three groups: 0-10 mIU/mL, which was considered as no or weak immunity (no responder), 10-100 mIU/mL were termed relative immunity (adequate responder) and above 100 mIU/mL was termed high immunity (excellent responder).[6] Statistical analysis was performed using SPSS software (Version 19, 2010, SPSS Inc., Chicago, IL, USA). Descriptive statistical method was used for data analysis.
Results
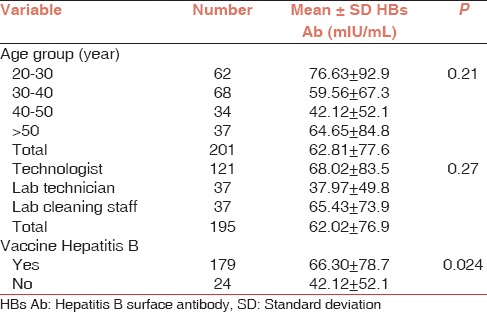
A total of 203 laboratory workers were included in this study. Demographic characteristics of subjects are shown in Table 1. The age ranges of participants were from 20 to 69 years with the mean age (mean ± standard deviation) 35.8 ± 9.5. Duration of the profession as laboratory workers was between 1 and 29 years, and the mean duration of working was 16.7 ± 11.8 years. At the time of sampling, 92.3% of subjects had completed the program of vaccination earlier. Of the 203 participants studied, 1.1% had a history of viral hepatitis A infection, 75.1% had hand abrasions, 66.8% skin wounds, and 29.8% had a history of eye splashing. Most of our subjects (86.4%) had received their last HBV vaccine dose within 10 years, and 62.4% had received it within 5 years. Protective anti-HBs Ab titers were seen in 100% of the cases who were completely vaccinated < 5 years before the study and in 87.1% of those who had received a complete course of vaccination 5-10 years. All of the participants were assessed negative for HBV infection, and no risk factors could be evaluated. The relation between age, job, vaccination, and mean anti-HBs titer was not statistically significance [Table 2].
Table 1.
Characteristics of study participants
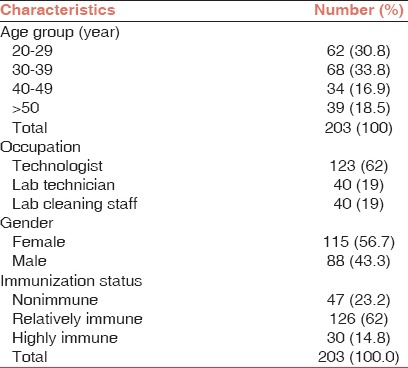
Table 2.
Relation between age, job, vaccination, and anti-HBs status in participants
Discussion
Hepatitis B is a serious occupational blood-borne disease that can be transmitted from patients to HCWs. It can cause chronic asymptomatic carrier condition for a long time before the development of the symptomatic liver disease. Therefore, HCWs with HBV infection may be unaware of their disease or carrier condition and infect other people.[7] In this study, none of the laboratory workers showed the presence of serological markers of HBV infection. In previous studies, protective anti-HBs response rates after immunization against HBV exceeded 90% but some factors such as older age, immunodeficiency, obesity, and chronic liver disease can reduce the efficiency rate of antibody response.[3] In our study, 76.8% of HBV vaccine compliant subjects were in relatively immune and highly immune groups while other studies have shown vaccination coverage varying between 18% and 85%.[4] The highest level of HBs-Ab was in the technologist group (68.02 ± 83.5), and the lowest antibody concentration was in lab technologists (37.97 ± 49.8). Decreasing antibody titration of serologic response rate to hepatitis vaccination in HCWs may be due to some less effective vaccines than other similar vaccines.[8,9] In our study, most of the responders were in the 20-29 years age group and the lowest titer was observed in the 60-69 years age group. However, this was not statistically significant (P = 0.2). Many studies confirm the opposite relation between age at primary vaccination and anti-HBs positive status, as older persons have a weak immunity against HBV immunization.[1,5] Although none of the participants were infected with HBV, the most positive eye splash injuries in our study were in the nonimmune group. Previous similar study fluids splashing to mucous membrane and needle stick injuries were the most frequent causes of occupational exposure in nursing practices.[9] Many studies have shown that needle stick injuries among HCWs, especially in nurses and lab technicians, had the highest frequency, but most of them were in the immune group.[10,11] For HBs Ab, we did not find a significant relation between occupational groups and immunization status. In another study carried out in Iran, 43% of HCWs were exposed to infectious body fluids, while in several similar studies from Singapore, Greece, Denmark, and Egypt, the rate of exposure among HCWs was different (7.5%, 0.01%, 37.6%, and 3%, respectively) based on job category.[9,10,11,12]
Possible explanations for the observed high exposure frequencies are less skilled HCWs, a high load of patients, insufficient protective devices, unexpected movements in patients, and performing the usual protocols by less proficient employees.[9] As a result, focused programs for teaching risks of occupational contacts with body fluids, the requirement of vaccination and postexposure management should be performed in hospitals. An 18-year follow-up study on HCWs vaccinated against HBV in Italy has indicated that with effective seroprotection, more than 85% of healthy adult participants did not need a booster dose for 10 years after primary immunization.[6] Furthermore, we found that the protective anti-HBs Ab titers were seen in all of the subjects that completed vaccination program <5 years ago and in 87.1% of those who had received a complete course of vaccination 5-10 years ago. Other similar studies from India and Iran have shown that nearly 95% of subjects with complete vaccination <5 years before, also 58% and 13.9% of cases who had been vaccinated within 5-10 years ago were protected, respectively.[13]
This effective protection of immunological memory persists at least for 5-10 years, but further studies on primary vaccination in adolescence are justified for the assessment of HBs-Ab status in subjects starting to work.[2,6] Finally, one of the restrictions of this study was that our data were gathered by volunteers. Therefore, our results may not describe the whole community of Iranian laboratory HCWs. Further immunologic and molecular investigation in HBV vaccinated subjects with a low level of the anti-HBs titer is needed about the possible low-level viremia and causes of lower efficiency in laboratory workers.
Conclusion
In summary, our study showed that HBV infection among laboratory workers is infrequent, and the probability of infection from laboratory workers is certainly low. However, using personal protective equipment, reporting exposures and executing a planned vaccination program for all laboratory HCWs are highly recommended.
Acknowledgements
The authors would like to thank all the HCWs who volunteered and cooperated. The authors are grateful to research council of IUMS for financial support of this study.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Zamani F, Fallahian F, Hashemi F, Shamsaei Z, Alavian SM. Immune response to hepatitis B vaccine in health-care workers. Saudi J Kidney Dis Transpl. 2011;22:179–84. [PubMed] [Google Scholar]
- 3.Prüss-Ustün A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482–90. doi: 10.1002/ajim.20230. [DOI] [PubMed] [Google Scholar]
- 4.FitzSimons D, François G, De Carli G, Shouval D, Prüss-Ustün A, Puro V, et al. Hepatitis B virus, hepatitis C virus and other blood-borne infections in healthcare workers: Guidelines for prevention and management in industrialised countries. Occup Environ Med. 2008;65:446–51. doi: 10.1136/oem.2006.032334. [DOI] [PubMed] [Google Scholar]
- 5.Dannetun E, Tegnell A, Torner A, Giesecke J. Coverage of hepatitis B vaccination in Swedish healthcare workers. J Hosp Infect. 2006;63:201–4. doi: 10.1016/j.jhin.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Floreani A, Baldo V, Cristofoletti M, Renzulli G, Valeri A, Zanetti C, et al. Long-term persistence of anti-HBs after vaccination against HBV: An 18 year experience in health care workers. Vaccine. 2004;22:607–10. doi: 10.1016/j.vaccine.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Deuffic-Burban S, Delarocque-Astagneau E, Abiteboul D, Bouvet E, Yazdanpanah Y. Blood-borne viruses in health care workers: Prevention and management. J Clin Virol. 2011;52:4–10. doi: 10.1016/j.jcv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Das HS, Sawant P, Shirhatti RG, Vyas K, Vispute S, Dhadphale S, et al. Efficacy of low dose intradermal hepatitis B vaccine: Results of a randomized trial among health care workers. Trop Gastroenterol. 2002;23:120–1. [PubMed] [Google Scholar]
- 9.Janbakhsh A, Sayad B, Vaziri S, Aieni P. Serologic response to hepatitis B vaccine in health care workers, kermanshah Iran. J Res Med Sci. 2005;10:147–9. [Google Scholar]
- 10.Hadadi A, Afhami S, Karbakhsh M, Esmailpour N. Occupational exposure to body fluids among healthcare workers: A report from Iran. Singapore Med J. 2008;49:492–6. [PubMed] [Google Scholar]
- 11.Azap A, Ergönül O, Memikoglu KO, Yesilkaya A, Altunsoy A, Bozkurt GY, et al. Occupational exposure to blood and body fluids among health care workers in Ankara, Turkey. Am J Infect Control. 2005;33:48–52. doi: 10.1016/j.ajic.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Adegboye AA, Moss GB, Soyinka F, Kreiss JK. The epidemiology of needlestick and sharp instrument accidents in a Nigerian hospital. Infect Control Hosp Epidemiol. 1994;15:27–31. doi: 10.1086/646814. [DOI] [PubMed] [Google Scholar]
- 13.Jha AK, Chadha S, Bhalla P, Saini S. Hepatitis B infection in microbiology laboratory workers: Prevalence, vaccination, and immunity status. Hepat Res Treat 2012. 2012:520362. doi: 10.1155/2012/520362. [DOI] [PMC free article] [PubMed] [Google Scholar]


