Abstract
Introduction:
For quite a few years, tranexamic acid (TEA) has been used during total knee arthroplasty (TKA) to reduce blood loss. However, no consensus exits regarding its timing and doses.
Materials and Methods:
We conducted a prospective, randomized double-blinded study of 56 patients in the Indian population undergoing TKA from 2011 to 2012. A dose of 10 mg/kg body weight of TEA (three doses) was given in one group and normal saline was administered in the other.
Results:
The mean blood loss in the TEA unilateral group was 295 mL ± 218 mL and in the placebo group was 482 mL ± 186 mL (P < 0.005). In the bilateral TEA group, the mean blood loss was 596 mL ± 235 mL and in the placebo group was 1349 mL ± 41 mL (P < 0.005).
Conclusion:
The number of patients requiring blood transfusion reduced substantially. There was no increase in the risk of deep vein thrombosis (DVT) and pulmonary embolism. TEA reduces intraoperative and postoperative blood loss and thus reduces the need of allogenic blood transfusion.
Keywords: Blood transfusion, total knee arthroplasty, tranexamic acid
Introduction
Total knee arthroplasty (TKA) alleviates pain and restores physical functioning in arthritic knees. However, it can be associated with significant postoperative bleeding leading to increased morbidity. Blood loss after TKA often amounts to 800-1200 mL.[1,2] Blood transfusion is frequently required but there is always an increased concern about the risks of blood transfusion, which include the transmission of viral diseases, such as human immunodeficiency virus, hepatitis and cytomegalovirus, as well as transfusion reactions.[1,3,4,6,7,8,9,10,11]
Various surgical and anesthetic methods are described, but blood conservation can also be achieved by pharmacological means. After any surgery, the fibrinolytic system is transiently activated[1] and the use of a tourniquet also induces local fibrinolysis. In TKA, postoperative bleeding is attributed to diffuse microvascular bleeding as a result of increased fibrinolytic activity caused by releasing of the tourniquet, which is routinely applied to provide appropriate surgical field during surgery.[1,3,6,8,10,11] Tranexamic acid (TEA) produces antifibrinolytic effects by competitively inhibiting the activation of plasminogen to plasmin.[12,13]
The objective of the proposed study was to determine the role of TEA, an antifibrinolytic agent, in intraoperative and postoperative blood loss and the need of blood transfusion after TKA in the Indian population.
Materials and Methods
After Institutional Review Board approval, a double-blinded, prospective, randomized, controlled study was performed in 56 patients of Indian origin undergoing TKA for primary osteoarthritis of the knee joint between the years 2011 and 2012. Written informed consent was obtained from all the patients. Patients with tricompartmental osteoarthritis of the knee were included in the study. The exclusion criteria were allergy to TEA, rheumatoid arthritis, revision total knee arthroplasty, coagulopathy (preoperative platelet count ≤1,50,000/mm3, BT, PT, CT abnormality), previous history of thromboembolic disease (cerebrovascular accident, deep vein thrombosis, myocardial infarction), severe ischemic heart disease, NYHA class 3 and 4, serum creatinine >1.5 mg/dL, severe pulmonary disease, e.g. FEV1 ≤50% normal, hepatic failure and preoperative anemia (Hb <10 g/dL). The patients were not controlled for intake of drugs that are known to prolong bleeding time.
Randomization was computerized and a total of 56 patients were part of the study. They were further divided into the control unilateral group (CU), control bilateral group (CB), TEA unilateral group (TU) and TEA bilateral group (TB). Thus, there were 14 patients in each group. A clinical orthopedic nurse who otherwise was not part of the study carried out the doses schedule. Preoperative data were entered on an excel sheet. There were no differences between the groups preoperatively and the patients were age and sex matched. Patients in the study group received three intravenous administrations of TEA at a dose of 10 mg/kg of body weight. The first dose was prior to inflation of the tourniquet after induction, the second dose was 4 h after the first dose either in the recovery room or in the ward and the third dose was after 12 h of the first dose. Patients in the control group received normal saline (NS) at the same time intervals, i.e. 0, 4 and 12 h. This was a prospective, randomized, controlled study where the surgeon and the patient were both blinded. Using a computer, with the anesthetist being aware of the selection, the patients were randomized into groups. The surgeon and the patient were both unaware to which group of the four the patient being operated belonged to. A clinical orthopedic nurse carried out the doses schedule and administration of the drugs; she was not part of the study in data recording or interpretation.
Anesthesia was standardized and all the patients received general anesthesia with femoral nerve block. A bolus of 20 mL, 0.5% Bupivacaine was administered through the catheter at insertion followed by a continuous infusion started postoperatively. Femoral nerve block catheter was removed on the second postoperative day. A dose of 2 g Inj. Cefazolin was given intravenously shortly before the operation. A tourniquet was placed around the upper thigh and inflated to 300 mmHg after exsanguinating with an esmarch bandage. The tourniquet was released before skin closure. Coagulation was achieved with a cautery. An anterior midline skin incision was used followed by the subvastus approach in all the cases. All patients were operated by a single orthopedic surgeon using flexion — balancing instrumentation (Zimmer FBI — USA) and the same implants (Zimmer Nexgen — USA). Antibiotic-impregnated Palacos cement was used in all the cases. The hole created for the intramedullary guide of the femur was occluded with bone before implantation of the femoral component. In each knee, one intraarticular drain (10-gauge) was used and connected to a vacuum drain bottle. For postoperative analgesia, intravenous patient-controlled analgesia (IVPCA) was administered using Fentanyl citrate. Postoperative analgesic techniques were used so as to keep the Visual Analogue Score (VAS) less than or equal to 3 on a 10-point scale (0-no pain, 10-worst imaginable pain). All the knees were placed in compressive bandages. The patients were asked to perform a mechanical ankle pumping exercise regimen for DVT prophylaxis as soon as possible. Physiotherapy was started on the first day after surgery, and all drains were removed 48 h postoperatively.
Measuring the volume in the suction apparatus and estimating blood loss by weighing the sponges recorded the approximate blood loss at the end of surgery. Postoperative blood loss was recorded from the drain bottle at 24 h and on drain removal at 48 h. The blood Hb concentration was determined preoperatively on the morning of first, second, fourth and seventh postoperative days. For both lower limbs, Venous Color Doppler was performed in all the patients on the fifth postoperative day or earlier if the patient was symptomatic. All our patients received DVT prophylaxis in the form of inj. dalteparin sodium 5000 IU SC for 5 days or tablet rivaroxaban 10 mg for 10 days. Along with this, a mechanical DVT prophylaxis in the form of pump or DVT stockings was given.
The indication of blood transfusion in our department was not set at a specific value of Hb concentration or hematocrit levels. Each transfusion was prescribed with regard to patient's cardiovascular history, present status and fall in Hb level, rate of blood loss and age. As a rule of thumb, we considered that blood transfusion was indicated at an Hb concentration below 8 g/dL. Also, the physicians who ordered blood transfusion were blinded in the study; they were not aware as to which group the patient being treated belonged to as the clinical orthopedic nurse who otherwise was not part of the study carried out the doses schedule and drug administration. Blood transfusions were recorded as the number of units of packed erythrocytes. We determined differences in the mean age, preoperative hemoglobin, volume of drained blood, decrease in hemoglobin postoperatively and the mean number of transfused units between the TEA and the control groups.
All continuous data are expressed as mean and standard deviation (SD) and Chi square test was used for statistical analysis. P < 0.05 was considered significant.
Results
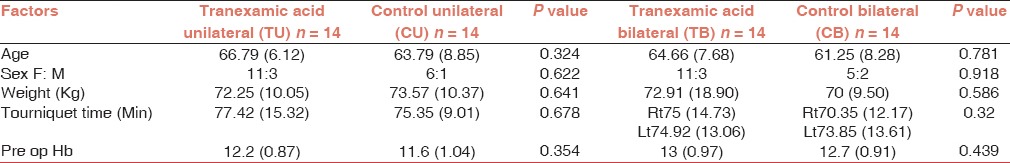
Mean age in the control unilateral group and the TEA unilateral group was 63.79 years and 66.79 years, respectively. Mean age in the control bilateral group and the TEA group was 61.25 years and 64.66 years, respectively [Table 1].
Table 1.
Preoperative data: Values in mean (SD)
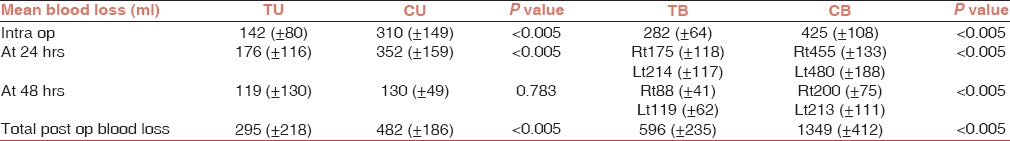
The mean (±SD) postoperative volume of drained blood was lower (P < 0.005) in patients receiving TEA (295 ± 218 mL) in the unilateral group than in the control unilateral group (482 ± 186 mL). The mean (±SD) postoperative blood loss was lower in patients receiving TEA (596 ± 235 mL) in the bilateral group than in the control bilateral group (1349 ± 412 mL). But, there was no significant difference in blood loss at 48 h in the unilateral TEA group and the unilateral control group. Also, intraoperative blood loss was lower (P < 0.005) in patients receiving TEA in the unilateral as well as bilateral groups compared with the control group [Table 2].
Table 2.
Mean blood loss
The mean hemoglobin decrease 24 h postoperatively was lower (P < 0.005) in patients receiving TEA unilateral (1 ± 0.02 g/dL) than in the control unilateral group (2.15 ± 0.42 g/dL). The mean hemoglobin decrease 24 h postoperatively was lower (P < 0.005) in patients in the TEA (2 ± 0.16 g/dL) bilateral group than in the control bilateral group (3.89 ± 0.09 g/dL) [Figure 1].
Figure 1.
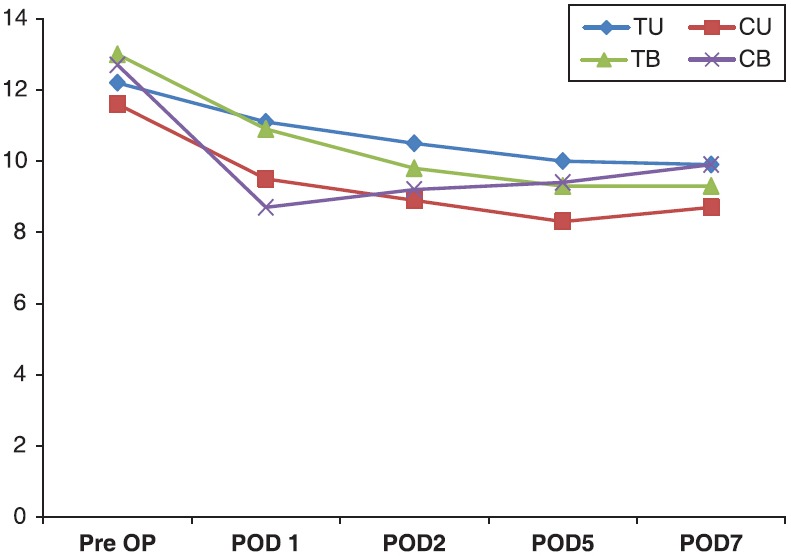
Fall in the level of hemoglobin, X-axis: Value of Hb in gm%, Y-axis: Day of stay in hospital
Only one patient required blood transfusion in the TEA unilateral group compared with the control unilateral group, in which nine patients required blood transfusion (P < 0.005). In the bilateral group, two among the TEA group received blood transfusion as compare with the control group, in which all patients required blood transfusion (P < 0.005). The mean number of transfused units was lower (P < 0.001) in patients in the TEA unilateral group (0.07 units) and TEA bilateral group (0.23 units) than in the control unilateral group (1.07 units) and control bilateral group (2.42 units). During their stay in the hospital, the patients were monitored to record occurrences of any complications. Three patients among the TEA group had evidence of DVT; of them, two were in the unilateral group and one was in the bilateral group. Two patients from the control group also had evidence of DVT, and both of them were from the bilateral group. But, the difference was not significant. Also, DVT was detected in the small vessels of the leg below the level of the popliteal vein. None of our patients had pulmonary embolism during their stay in the hospital.
Discussion
TKA is associated with considerable blood loss, at times accounting to 800-1200 mL, and may require postoperative transfusion.[2] Blood transfusion involves transfusion-associated risks, such as immunologic reactions, transmission of disease, coagulopathy, possible overtransfusion and volume overload. Additional cost is also a rising concern.
Different strategies have been established to decrease the risk of allogenic blood transfusion in the postoperative patient;[14,15,16,17] however, they are expensive procedures with logistic problems in many hospitals. Multiple surgical variables affect postoperative blood loss. Techniques such as tourniquet release after wound closure and bandage proved blood loss decrease in many studies.[18,19,20] A meta analysis concluded that tourniquet release to perform surgical hemostasis before closure was related to an increased bleeding of 229 mL on average.[19] Plugging the intramedullary canal of the femur after introduction of the intramedullary instrumentation guide significantly reduced blood loss in TKA up to 17%.[21] Blood loss decreased in (all-component cemented) TKA due to the hemostatic effect of cement polymerization on the exposed bone.[22] The use of drainage raised the transfusion rate in a meta analysis of 18 prospective, randomized controlled trials.[23,24]
TEA is a synthetic antifibrinolytic drug that acts by competitively inhibiting the activation of plasminogen to plasmin used to prevent bleeding. There are various methods of administering TEA to reduce blood loss in TKA: Intramuscular, oral, intravenous and intraarticular.[25] The time taken for maximum plasma levels of TEA to reach ranges from 30 min for intramuscular to 5-15 min for intravenous administration. An intravenous injection for patients undergoing TKA is the best method for rapidly increasing and maintaining the therapeutic concentration of TEA.
However, there is no consensus regarding the timing and dose of TEA. Also, few studies speak of its use in the Indian population.[26] Clinical studies report that TEA reduces blood loss or transfusion requirements when given on deflation of the tourniquet and with a repeat dose postoperatively.[27,28] Some authors recommend two doses of TEA, one on induction and another dose shortly before release of the tourniquet.[29] Others recommend a dose of 15 mg/kg TEA at the time of cementing of the prosthesis,[30] and yet others recommend a 10 mg/kg bolus dose followed by a dose of 1 mg/kg/h.[31] Tanaka et al. described that TEA given preoperatively and on deflation of the tourniquet reduced blood loss compared with when given only preoperatively or only on deflation of the tourniquet without increasing the risk of thromboembolic complications.[25] They also thought that hemostatic control was better when TEA was administered before surgery rather than on deflation of the tourniquet, and suggested that suppression of fibrinolysis from the beginning of the operation may be more effective than only later at the time of peak hyperfibrinolysis.[25]
Pharmacokinetic studies[27,32] indicate that a dose of 20 mg/kg TEA is suitable for TKA. A therapeutic level can be maintained for approximately 8 h after surgery, and this covers the period of hyperfibrinolysis in cases of increased blood loss. It has been reported that 65% of drainage volume occurs in the first 8 h postoperatively.[2,33]
Numerous studies have reported that TEA reduces blood loss by 30-50%. Our study confirms that administration of TEA reduces blood loss in TKA by 50%. These differences in the amount of reduction of blood loss may be difficult to compare owing to differences in surgical techniques and the times for recording blood loss.
In our study, TEA reduced the blood transfusion rates and number of blood units transfused to one-third when compared with the control group. We found that TEA reduced the amount of blood transfused from 1.07 units to 0.07 units in the unilateral group (P < 0.005) and 2.42 units to 0.23 units in the bilateral group in comparison with the control group (P < 0.005). All 14 patients of the bilateral control group required blood transfusion compared with only 23% (2/14) patients in the TEA group. More than half (9/14) of the patients from the unilateral control group required transfusion as compared with only 14% (1/14) patients in the TEA group. There was no significant increase in risk of DVT in the TEA group. None of our patients had symptomatic pulmonary embolism.
Our study has few limitations;firstly, we did not monitor plasminogen levels, D-dimer and fibrin degradation products. This would have given us a direct evidence of fibrinolysis and antifibrinolytic activity. Secondly, we did not investigate our patients for pulmonary embolism.
Conclusion
We conclude that three doses of IV TEA (10 mg/kg) — first dose given before inflation of tourniquet and subsequently 4 and 12 h following the first dose — is effective in reducing blood loss in TKA in both unilateral as well as bilateral cases in the Indian population. TEA reduces intraoperative blood loss and postoperative blood loss. It reduces the need of blood transfusion and therefore the associated risks of transfusion. In our study, we observed no increase in the risk of deep vein thrombosis and pulmonary embolism.
However, larger prospective, randomized, double-blind trials are needed to further assess whether this promising strategy to reduce bleeding and the need for blood transfusion in patients undergoing TKA is safe with regard to thromboembolic complications.
Acknowledgements
The authors acknowledge the contribution of anaesthesia consultant, Dr Shalini Saxena, Department of Anaesthesia, P.D. Hinduja hospital.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Risberg B. The response of the fibrinolytic system in trauma. Acta Chir Scand Suppl. 1985;522:245–71. [PubMed] [Google Scholar]
- 2.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty.Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561–5. [PubMed] [Google Scholar]
- 3.Janssens M, Joris J, David JL, Lemaire R, Lamy M. High-dose aprotinin reduces blood loss in patients undergoing total hip replacement surgery. Anesthesiology. 1994;80:23–9. doi: 10.1097/00000542-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Fiebig E. Safety of the blood supply. Clin Orthop Relat Res. 1998;30:6–18. doi: 10.1097/00003086-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gascón P, Zoumbos NC, Young NS. Immunologic abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984;100:173–7. doi: 10.7326/0003-4819-100-2-173. [DOI] [PubMed] [Google Scholar]
- 6.Kruithof EK, Nicolosa G, Bachmann F. Plasminogen activator inhibitor 1: Development of a radioimmunoassay and observations on its plasma concentration during venous occlusion and after platelet aggregation. Blood. 1987;70:1645–53. [PubMed] [Google Scholar]
- 7.Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33:794–7. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara M, Sakahashi H. Effect of application of a tourniquet on bleeding factors in dogs. J Bone Joint Surg Am. 1967;49:1345–51. [PubMed] [Google Scholar]
- 9.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–90. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 10.Klenerman L, Chakrabarti R, Mackie I, Brozovic M, Stirling Y. Changes in haemostatic system after application of a tourniquet. Lancet. 1977;1:970–2. doi: 10.1016/s0140-6736(77)92276-0. [DOI] [PubMed] [Google Scholar]
- 11.Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63:461–5. [PubMed] [Google Scholar]
- 12.Andersson L, Nilsson IM, Niléhn JE, Hedner U, Granstrand B, Melander B. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol. 1965;2:230–47. doi: 10.1111/j.1600-0609.1965.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 13.Dubber AH, Mcnicol GP, Douglas AS. Amino methyly cyclohexane carboxylic acid (AMCHA), a new synthetic fibrinolytic inhibitor. Br J Haematol. 1965;11:237–45. doi: 10.1111/j.1365-2141.1965.tb06583.x. [DOI] [PubMed] [Google Scholar]
- 14.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Feagan BG, Wong CJ, Kirkley A, Johnston DW, Smith FC, Whitsitt P, et al. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. A randomized, controlled trial. Ann Intern Med. 2000;133:845–54. doi: 10.7326/0003-4819-133-11-200012050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Friederichs MG, Mariani EM, Bourne MH. Perioperative blood salvage as an alternative to predonating blood for primary total knee and hip arthroplasty. J Arthroplasty. 2002;17:298–303. doi: 10.1054/arth.2002.30409. [DOI] [PubMed] [Google Scholar]
- 17.Woolson ST, Wall WW. Autologous blood transfusion after total knee arthroplasty: A randomized, prospective study comparing predonated and postoperative salvage blood. J Arthroplasty. 2003;18:243–9. doi: 10.1054/arth.2003.50058. [DOI] [PubMed] [Google Scholar]
- 18.Hersekli MA, Akpinar S, Ozkoc G, Ozalay M, Uysal M, Cesur N, et al. The timing of tourniquet release and its influence on blood loss after total knee arthroplasty. Int Orthop. 2004;28:138–41. doi: 10.1007/s00264-004-0550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rama KR, Apsingi S, Poovali S, Jetti A. Timing of tourniquet release in knee arthroplasty. Meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:699–705. doi: 10.2106/JBJS.F.00497. [DOI] [PubMed] [Google Scholar]
- 20.Widman J, Isacson J. Surgical hemostasis after tourniquet release does not reduce blood loss in knee replacement. A prospective randomized study of 81 patients. Acta Orthop Scand. 1999;70:268–70. doi: 10.3109/17453679908997805. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Saleh J, Gardiner E, Devadoss VG, Howell FR. Plugging the intramedullary canal of the femur in total knee arthroplasty: Reduction in postoperative blood loss. J Arthroplasty. 2000;15:947–9. doi: 10.1054/arth.2000.8592. [DOI] [PubMed] [Google Scholar]
- 22.Mylod AG, France MP, Muser DE, Parsons JR. Perioperative blood loss associated with total knee arthroplasty. A comparison of procedures performed with and without cementing. J Bone Joint Surg Am. 1990;72:1010–2. [PubMed] [Google Scholar]
- 23.Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86-A:1146–52. doi: 10.2106/00004623-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007:CD001825. doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83:702–5. doi: 10.1302/0301-620x.83b5.11745. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop. 2011;45:148–52. doi: 10.4103/0019-5413.77135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: A prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–40. [PubMed] [Google Scholar]
- 28.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 29.Hynes M, Calder P, Scott G. The use of tranexamic acid to reduce blood loss during total knee arthroplasty. Knee. 2003;10:375–7. doi: 10.1016/s0968-0160(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 30.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: A prospective randomised controlled trial of 29 patients. Knee. 2006;13:106–10. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–25. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974;7:375–80. doi: 10.1007/BF00558210. [DOI] [PubMed] [Google Scholar]
- 33.Prasad N, Padmanabhan V, Mullaji A. Comparison between two methods of drain clamping after total knee arthroplasty. Arch Orthop Trauma Surg. 2005;125:381–4. doi: 10.1007/s00402-005-0813-7. [DOI] [PubMed] [Google Scholar]


