Abstract
Backdround:
Emerging infectious diseases pose threats to the general human population; including recipients of blood transfusions. Dengue is spreading rapidly to new areas and with increasing frequency of major outbreaks. Screening blood for dengue antigens in dengue-endemic countries would be costly and should, therefore, be recommended only after careful assessment of risk for infection and cost.
Aim:
A prospective study was conducted to establish the magnitude of the threat that dengue poses to blood safety where it is sporadic with seasonal variations, to quantify risk and to assess that whether screening is feasible and cost-effective.
Materials and Methods:
Nonstructural protein 1 (NS1) antigen test was done on 1709 donations during dengue outbreak in the months August to November 2013 as an additional test using Bio-Rad Platelia Dengue NS1AG test kit which is one step sandwich format microplate enzyme immunoassay using murine monoclonal antibodies for capture and revelation. Chi-square test was used to find statistical significance.
Results and Conclusions:
Majority cases were whole blood, replacement, male donors with 76.10% donors in <35 years age group. About 17.85% were single donor platelet donations. NS1 antigen in all donors was negative. In the past, dengue affected mainly children who do not donate blood. With the changing trend, mean age of infection increased affecting the population that does donate blood, further reducing blood donation pool. Further studies need to be done in different geographic regions of the country during dengue transmission season to establish maximum incidence of viremic donations, rates of transfusion transmission and clinical consequences in recipients. If risk is found to be substantial, decision will be taken by the policymakers at what threshold screening should be instituted to ensure safe blood transfusion.
Keywords: Blood donors, dengue, nonstructural protein 1 antigen, screening, transfusion, World Health Organization
Introduction
Emerging infectious diseases pose threats to the general human population, including recipients of blood transfusions. Dengue is a mosquito-borne infection found in tropical and sub-tropical regions around the world. In recent years, transmission has increased predominantly in urban and semi-urban areas and has become a major international public health concern.[1]
The incidence of dengue has grown dramatically around the world in recent decades. Over 2.5 billion people — over 40% of the world's population — are now at risk from dengue. World Health Organization (WHO) currently estimates there may be 50-100 million dengue infections worldwide every year. An estimated 500,000 people with severe dengue require hospitalization each year, a large proportion of who are children. About 2.5% of that affected die.[1] As the numbers continue to increase, the effect on the blood donor attendance will reach levels sufficient enough to impact significantly on the blood supply.
Dengue is spreading rapidly to new areas and with increasing frequency of major outbreaks. The trend has also been observed in various studies toward increasing age among infected patients.[2,3] This will impact blood supply availability as more blood donors are deferred because of dengue infection or exposure to infection. The risk of transmission through transfusion of blood from asymptomatic viremic donors will also increase.
Blood transfusion-related dengue will likely represent only a small proportion of all dengue cases in dengue-endemic countries. Screening blood for dengue antigens in dengue-endemic countries would be costly and should, therefore, be recommended only after careful assessment of risk for infection and cost per blood product-associated dengue infection averted. Therefore, the first step is to quantify this risk in a systematic study. Risk will vary by geographic region and season and may change over time. The initial study should be conducted during the dengue transmission season to identify maximum incidence of viremic donations. This testing would provide a baseline estimate of risk for transmission of infective blood. If the risk is found to be substantial, healthcare providers would need to decide at what threshold screening should be instituted.
Aim
This prospective study was conducted to establish the magnitude of the threat that dengue poses to blood safety where it is sporadic with seasonal variations, to quantify the risk and to assess that whether screening is feasible and cost-effective on the basis of characteristics of local population and seasonality of dengue so that whether it can be recommended to include in the screening tests as other transfusion-transmitted infection.
Materials and Methods
Apart from the mandatory transfusion-transmitted infection tests as per the rules laid down in Drugs and Cosmetic Act, Ministry of Health and Family Welfare, Government of India,[4] nonstructural protein 1 (NS1) antigen test was selected to be performed on all the donations during months of August end to November 2013 as an additional test since there is no Food and Drug Administration-licensed blood donor screening test and NS1 antigen test can be rapid, sensitive and cost-effective test in asymptomatic viremic donors. Blood donors were 18-65 years old and in good health. Tests were done on EVOLIS Automated ELISA System using Bio-Rad Platelia Dengue NS1Ag test. Platelia dengue NS1Ag is a one-step sandwich format micro plate enzyme immunoassay for the qualitative or semi-quantitative detection of dengue virus NS1 antigen in human serum or plasma. The test uses murine monoclonal antibodies for capture and revelation. Kit had specificity of 100.0% (95% confidence interval: 99.4-100.0%) and higher sensitivity of 98.5% in IgG negative samples from primary infection than in IgG positive samples (sensitivity of 85.6%).
This study was approved by the Institutional Ethics Committee.
Statistical analysis
Chi-square test was used to find statistical significance.
Results
During the months of August to November 2013, blood center at Tertiary-Care Hospital in Northern India performed additional test of NS1 antigen on the 1709 donations of which 1404 (82.15%) were whole blood 450 ml donations while 305 (17.85%) were SDP donations (P < 0.001).
Majority of cases (1705; 99.97%) were replacement donors with only few cases (04, 0.23%) of voluntary donations (P < 0.001).
Out of 1709 donors, 1680 (98.30%) were male donors and 29 (1.70%) were female donors (P < 0.001) [Table 1].
Table 1.
Age and sex wise distribution of donors
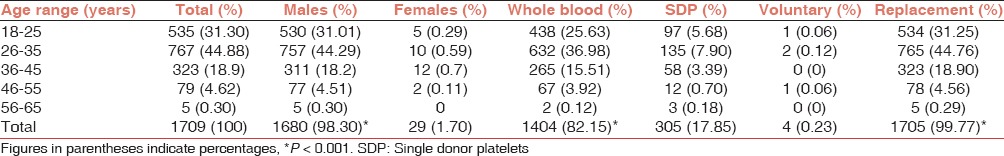
Mean age of donors was 30.51 ± 7.80 years (range of 18-58 years). Mean age of male and female donors was 30.43 ± 7.80 years and 34.93 ± 7.82 years, respectively.
Analyzing blood-group wise donation, highest percentage (37.62%, 643) was found to be “B” positive donors followed by “O” positive donors (27.56%, 471), “A” positive (19.92%, 341), “AB” positive (9.08%, 155). Frequency of Rh negative donors in decreasing order were B negative (2.35%, 40), “O” negative (1.76%, 30) “A” negative (1.23%, 21) and “AB” negative (0.48%, 8).
Number of donations in various age groups is significantly associated with gender. In the age group categorization, highest donations (767, 44.88%) were made in the age range of 26-35 years followed by 18-25 years age group (535, 31.30%) and 36-45 years age groups (323, 18.9%). The age group 46-55 and 56-65 years had the lowest donations as 4.62% (79) and 0.30% (5), respectively. In the different age groups, males were the predominant donors ranging from 96.28% to 100%, while the maximum females (3.72%) were in the age group of 36-45 year (P < 0.001). Same trend was observed for whole blood versus SDP donations and voluntary versus replacement donations [Table 1].
All 1709 samples were found to be negative for NS1 antigen test.
Discussion
Severe dengue (previously known as dengue hemorrhagic fever) was first recognized in the 1950s during dengue epidemics in the Philippines and Thailand. Today, severe dengue affects most Asian and Latin American countries and has become a leading cause of hospitalization and death among children in these regions.[1]
Before 1970, only nine countries had experienced severe dengue epidemics. The disease is now endemic in more than 100 countries in Africa, the Americas, the Eastern Mediterranean, South-east Asia, and the Western Pacific. The American, South-east Asia and the Western Pacific regions are the most seriously affected. Cases across the Americas, South-east Asia and Western Pacific have exceeded 1.2 million cases in 2008 and over 2.3 million in 2010. Recently, the number of reported cases has continued to increase. In 2010, 1.6 million cases of dengue were reported in the Americas alone, of which 49,000 cases were severe dengue. Not only is the number of cases increasing as the disease spreads to new areas, but explosive outbreaks are occurring.[1] Increased spread is also seen into suburban and rural areas.[5]
The first documented transfusion-associated case of dengue occurred during a local outbreak in MaWan, Hong Kong, in 2002, an area that is not endemic for dengue. The second documentation of transfusion transmission was a transmission cluster reported from Singapore.[6]
Earlier, dengue affected mainly children, a population who do not donate blood. With the changing trend, mean age of infection increased affecting youths and young adults-population that does donate blood, further reducing blood donation pool.
Data of this study showed predominance of male donors (98.3%) over female donors (1.70%) with the majority of donors (76.18%) of <35 years age group. These findings are similar to findings of Ribas-Silva and Andressa[7] who reported 63.8% of male donors with 30% of donors within 30-39 years age group.
The annual incidence of dengue infection in Singapore was 180.6/100,000 population in 2007, with the highest incidence in the segment of the population older than 15 years. It is estimated that dengue infection has reduced the blood donor pool by 0.2%; this excludes donors deferred for symptoms related to dengue and exposure to dengue, which is estimated to lose a further 11% of donors in 2007.[8]
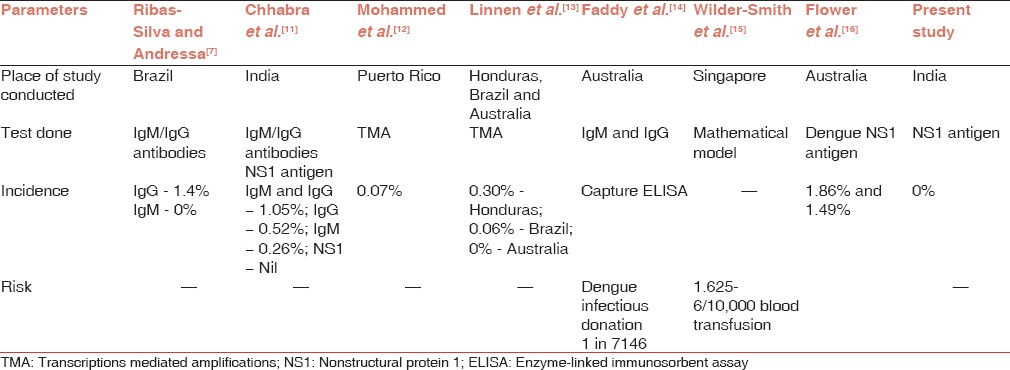
The dengue NS1 antigen-capture ELISA is useful for detection of dengue early in the disease. Although it is useful in the 1st week of the disease and provides evidence of the presence of the virus, its effectiveness in screening blood donors is yet to be established. Subclinical dengue infection rates vary by population, specific outbreak, and area examined.[9,10] During the 2013 dengue outbreak, we tried to demonstrate the clinical or subclinical infection rate in blood donors in population from where majority of patients reach to our hospital, by NS1 antigen detection. It was in accordance with studies done by Chhabra et al.,[11] Mohammed et al.,[12] and Linnen et al.[13] [Table 2]. Study by Ribas-Silva and Andressa[7] also showed the absence of antidengue IgM in their research suggesting that blood donors were not actively infected with the dengue virus. Zero percent seroprevalence in our study can be attributed to thorough screening of blood donors as per the guidelines to defer donors due to the history of fever or prodromal symptoms. However, this strategy did add strain on the inventory available to meet clinical demand for fresh blood component especially platelet apheresis.
Table 2.
Incidence of dengue positivity in blood donors in various studies
The relatively low sensitivity and specificity of blood safety measures based on donor history has long been debated. In addition, there is often a negative effect on the donor of being deferred, and many donors do not return even if the deferral is temporary.[17] Introducing such additional testing measures during outbreaks of dengue is likely to exacerbate the problems of dwindling the donor attendance and decreasing blood collection.
The efficiency of transmission depends on a combination of factors: Amount and stability of the virus, volume of viremic blood transfused and immune status of the recipient. Although dengue viremia titers in vertebrate hosts are usually in the range of 105 -109 copies/mL,[18] it is likely that lower levels and shorter duration of viremia occur during asymptomatic infection compared with dengue fever or dengue hemorrhagic fever. Mohammed et al.[12] and Linnen et al.[13] demonstrated the presence of viral loads of at least 2 × 103-8 × 107 copies/mL and 12-4.2 × 104 copies/mL, respectively, in healthy blood donors.
In countries with high dengue prevalence, the introduction of a screening test or pathogen reduction process is therefore likely to prevent infections through blood transfusion, but the proportion would be very low in comparison to the total number of infections.
Dengue carries a high economic burden on society, in terms of medical costs and control measures, as well as reduced workforce productivity.[19] Though importance of thorough screening as per the guidelines cannot be underestimated which offer a similar level of safety and is cost effective but introduction of quantitative or molecular serological methods, during the outbreaks, to determine the detection of the dengue virus in blood donors should be established so that the safety of blood transfusions is guaranteed.
Conclusion
In the absence of a mandatory screening test for blood donations in India, the continuation of the dengue management strategy during future outbreaks is warranted. Since dengue carries a high economic burden on society, in terms of medical costs and control measures, as well as reduced workforce productivity, alternative approaches need to be explored such as implementation of a suitable screening test or pathogen reduction, which may offer a similar level of safety. With “large swathes of densely populated regions coinciding with very high suitability for disease transmission,” Asia bore 70% of the apparent infections, Africa contributed about 16% of the global dengue infections and the Americas 14%.[20] With dengue becoming increasingly common in India and the world,[1] these alternative approaches will be needed in the future.
It is recommended that further studies should to be done in different geographic regions of the country during the dengue transmission season to establish maximum incidence of viremic donations, rates of transfusion transmission and clinical consequences in recipients. If the risk is found to be substantial, policy makers and healthcare providers would need to decide at what threshold screening should be instituted to ensure safe blood transfusion keeping in mind about economic resources and healthcare priorities of the country.
Acknowledgment
We acknowledge Bio-Rad to support this prospective study in knowing the effects of emerging infectious disease on blood safety.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.World Health Organization Dengue and Severe Dengue. Media Centre Fact Sheet. WHO, 2013. [Last accessed on 2014 Feb 08]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006;3:92. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan E, Siddiqui J, Shakoor S, Mehraj V, Jamil B, Hasan R. Dengue outbreak in Karachi, Pakistan, 2006: experience at a tertiary care center. Trans R Soc Trop Med Hyg. 2007;101:1114–9. doi: 10.1016/j.trstmh.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Malik V. 16th ed. Lucknow: Eastern Book Company; 2003. In Drugs and Cosmetic Act 1940; pp. 279–303. [Google Scholar]
- 5.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–42. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 6.Dengue Viruses. Transfusion. [Last accessed on 2013 Jul 23];2009 49(Suppl):67–9S. Available from: http://www.aabb.org/resources/bct/eid/Documents/67s.pdf . [Google Scholar]
- 7.Ribas-Silva RC, Andressa AE. Dengue antibodies in blood donors. [Last accessed on 2013 Jul 15];Rev Bras Hematol Hemoter Sao Paulo. 2012 34 doi: 10.5581/1516-8484.20120048. Available from: http://www.dxdoi.org/105581/1516-848420120048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19:66–77. doi: 10.1111/j.1365-3148.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Figueroa L, Rigau-Perez JG, Suarez EL, Reiter P. Risk factors for dengue infection during an outbreak in Yanes, Puerto Rico in 1991. Am J Trop Med Hyg. 1995;52:496–502. doi: 10.4269/ajtmh.1995.52.496. [DOI] [PubMed] [Google Scholar]
- 10.McBride WJ, Mullner H, LaBrooy JT, Wronski I. The 1993 dengue 2 epidemic in Charters Towers, North Queensland: Clinical features and public health impact. Epidemiol Infect. 1998;121:151–6. doi: 10.1017/s0950268898001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhabra C, David R, Singh SN, Khan MK, Negi PS, Hussian SH, et al. Additional dengue testing helps in prevention transfusion transmitted infections - Experience from a tertiary care hospital. Vox Sang. 2013;105(Suppl 1):65–299. doi: 10.1111/vox.12048. [Google Scholar]
- 12.Mohammed H, Linnen JM, Muñoz-Jordán JL, Tomashek K, Foster G, Broulik AS, et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48:1348–54. doi: 10.1111/j.1537-2995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 13.Linnen JM, Vinelli E, Sabino EC, Tobler LH, Hyland C, Lee TH, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48:1355–62. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 14.Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT, et al. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013;19:787–9. doi: 10.3201/eid1905.121664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilder-Smith A, Chen LH, Massad E, Wilson ME. Threat of dengue to blood safety in dengue-endemic countries. Emerg Infect Dis. 2009;15:8–11. doi: 10.3201/eid1501.071097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flower RL, Fryk J, Hyland C, McBride J, Ritchie S, Faddy H. Dengue fever viral exposure rates among Australian blood donors during local outbreaks. Vox Sang. 2011;101:96. [Google Scholar]
- 17.Halperin D, Baetens J, Newman B. The effect of short-term, temporary deferral on future blood donation. Transfusion. 1998;38:181–3. doi: 10.1046/j.1537-2995.1998.38298193102.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen LH, Wilson ME. Non-vector transmission of dengue and other mosquito-borne flaviviruses. Dengue Bull. 2005;29:18–30. [Google Scholar]
- 19.Guha-Sapir D, Schimmer B. Dengue fever: New paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]


